Abstract
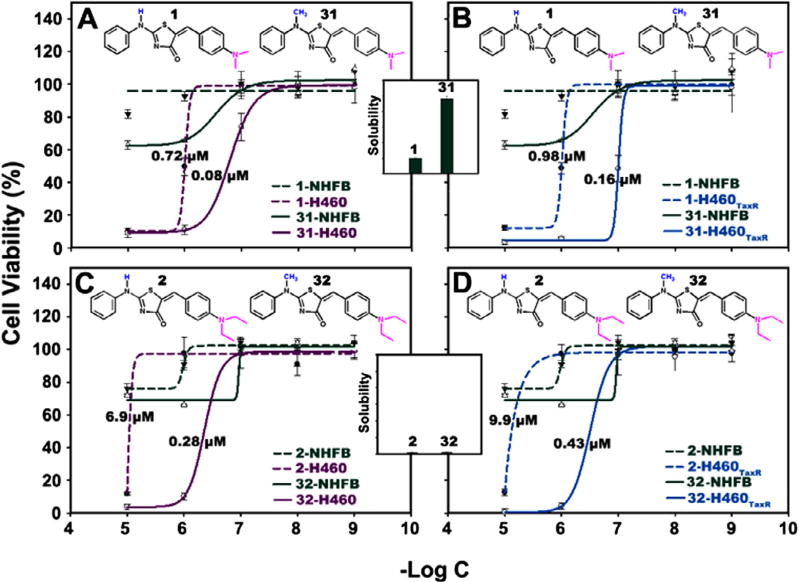
Thiazolidinone compounds 1–3 are lead compounds that have cytoselective toxicity toward non-small cell lung cancer (NSCLC) cells and drug-resistant NSCLC cells while showing low toxicity to normal human fibroblasts (NHFB). However, this class of compounds generally has a very low aqueous solubility (~0.1 µg/ml). In order to improve both solubility and anti-cancer activity, we designed and synthesized two lead-optimization libraries and investigated these libraries using simultaneous high-throughput solubility and cytotoxicity assays. By all-around modifications on R1, R2 and R3 substitutions, consecutive library synthesis, and testing, we improved the aqueous solubility (5-fold improvement in solubility, from 0.1 to 0.5 µg/ml) and anti-cancer activity (10-fold improvement in EC50 from 0.72–0.98 µM to 0.08–0.16 µM) in the new lead thiazolidinone compound 31.
Keywords: NSCLC, Thiazolidinone, Aqueous solubility, Cytotoxicity, Lead optimization
Because of its persistent low survival rate, lung cancer remains a top global threat.1 Therapeutic agents for lung cancer, especially for drug-resistant non-small cell lung cancer (NSCLC) are urgently needed. We previously reported a series of thiazolidinone compounds2 that targeted tubulin and heat shock proteins.3,4 These compounds can inhibited growth of NSCLC cells and the drug-resistant NSCLC cells with EC50 values around 1.0 µM while exhibiting a low toxicity to normal human fibroblasts (>100 µM). However, these compounds generally have a low aqueous solubility. Solubility is crucial in the success of a drug candidate.5 Compounds with low solubility not only cause problem for in vitro and in vivo assays, but also add significant burdens to drug development. In order to optimize lead compounds, exploring rapid and effective approaches for optimization of multi-parameters in a compound is necessary. This article reports our efforts to optimize both aqueous solubility and anti-cancer activity of leading thiazolidinone compounds using combinatorial lead optimization libraries.
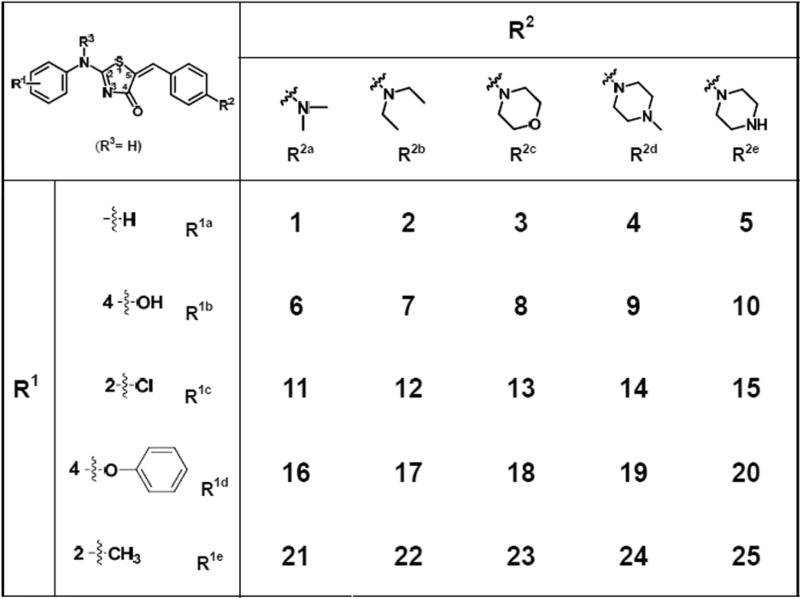
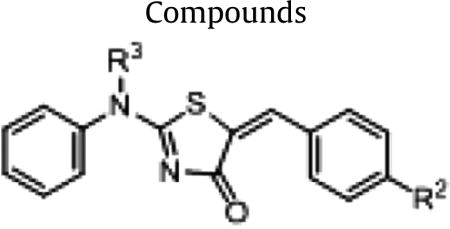
In order to improve aqueous solubility of thiazolidinone compounds, we first incorporated more polar R2 groups and diverse R1 groups (Fig. 1) in a combinatorial lead-optimization library (Library 1). Library 1 containing 25 members were synthesized using an existing method2 with slight modifications (Section 1 in the SI). The crude yields of all products were ranging from 50% to 90%. All compounds were purified by column chromatography to a purity of ≥95% by LC/UV214nm and characterized by 1H NMR and ESI-MS (Section 2 in the SI).
Figure 1.
Chemical structures of Library 1 (R3 = H).
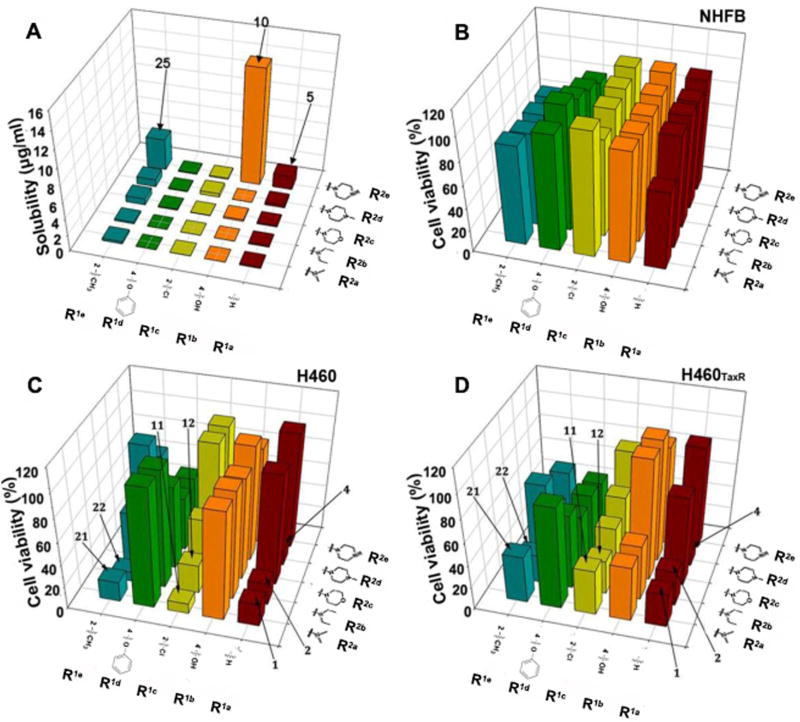
Compounds in Library 1 were screened for their aqueous solubility using a high throughput solubility assay (Section 3 in the SI) and for their cytoselective toxicity in NSCLC cell line H460, drug-resistant NSCLC cell line H460TaxR, and normal human fibroblasts (NHFB) using SRB assay. (Sections 4 and 5 in the SI). Since a single concentration of 10 µM best distinguishes the anti-cancer activities of this class of compounds from our preliminary experiments, we selected this concentration for an initial screening. The aqueous solubility results (Fig. 2A) showed that besides R2e substitution (Compounds 5, 10 and 25) there was little improvement in aqueous solubility for compounds in this library. The R2e substitution with an amino group adds an H-donor, which is responsible for an improved solubility. Compounds in this group also show a lower computed logP than compounds with other substitutions (Section 6 in SI) consistent with the improved solubility. The cytotoxicity of these compounds to normal cell NHFB was generally low (Fig. 2B). However, only compounds 1, 2, 4, 11, 12, 21 and 22 exhibited good cytoselective anti-cancer effects in H460 and H460TaxR cells. But their solubility remained poor. Compounds with R2a and R2b (–NMe2 and –NEt2) groups have better cytoselective anti-cancer activities compared to those compounds with a ring structure (R2c, R2d, and R2e, Section 6 in the SI). We found a consistent SAR trend with our previously finding that a –NMe2 group at 4-position is required for optimal cytoselective anti-cancer activity,2 while R1 tolerates more diverse substitutions at various positions.
Figure 2.
Aqueous solubility and in vitro anti-cancer activities of compounds in Library 1 (R3 = H). Aqueous solubility of compounds (A) was determined using a method described in Supporting information. Cytotoxicity of compounds in normal human fibroblast (NHFB) (B), H460 (C) and H460TaxR (D) were measured using SRB method with a compound concentration of 10 µM. Cell viability in DMSO was designated as 100%.
Compounds 5, 10 and 25 had the largest improvement in aqueous solubility. Substituting group at 4-position with ring (morpholine, N-methyl piperazine, piperazine) structures increased the number of hydrogen bond donors and acceptors in these molecules. The number of H-bonds was a key factor in determining the solubility.6,7 Unfortunately, they all had a reduced anticancer activity. The altered number of the hydrogen bonds and hydrophobic regions in these molecules might cause a deviation from the optimal pharmacophore we discovered previously2 in active compounds.
Since the purpose of this work was to optimize both aqueous solubility and cytoselective anti-cancer activity, the first library did not achieve our goal. To accomplish our original goal, we made another compounds library (Library 2) to further explore the effects of R3 modifications. By reviewing Library 1 screening results, we noticed that more active compounds in Library 1 had R1a as a hydrogen atom. Therefore, we selected this group in designing Library 2.
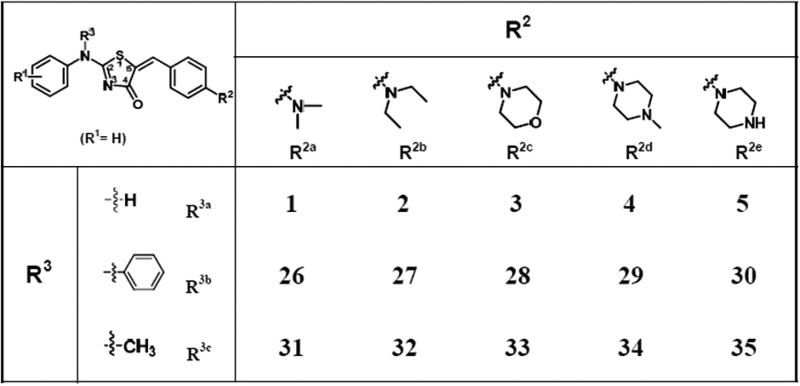
In Library 2,we synthesized 10 compounds 26–35 using a similar synthesis protocol (Section 1 in SI). All compounds were also purified using column chromatography to a purity of ≥95% (LC/UV214nm) and characterized by 1H NMR and ESI-MS (Section 2 in SI). In order to compare effects of R3, we also listed data from compounds 1–5 (R3a = H) from Library 1 (Fig. 3). These compounds were different only in the R3 position. Compounds 1–5 have R3 substitution as a –H group, 26–30 as –Ph group, and 31–35 as –Me group.
Figure 3.
Chemical structures of Library 2 (R1 = H).
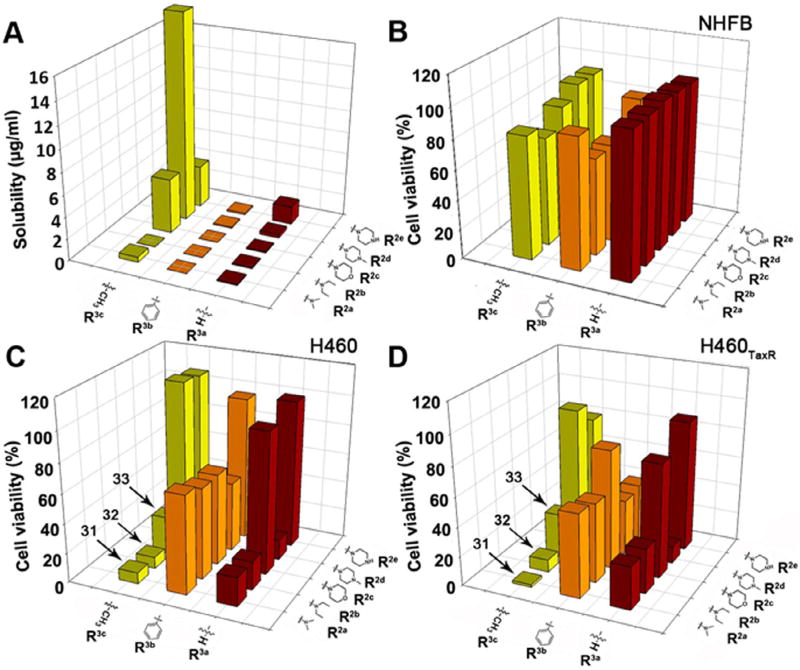
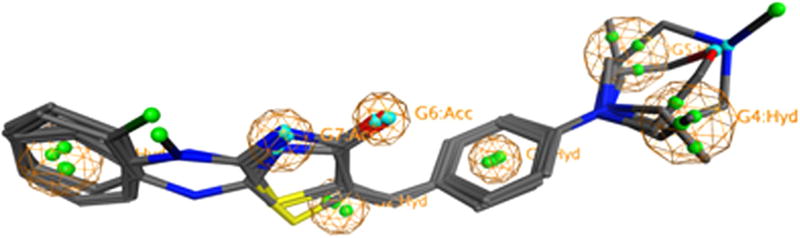
The aqueous solubility results (Fig. 4A) showed that R3 have played a more important role in determining compound solubility. Larger (R3b = Ph) or smaller (R3a = H) groups did not improve solubility. When R3c = CH3, the aqueous solubility of compounds exhibited improvements. This modification turned the nitrogen into a tertiary amine. Although a larger substitution like –Ph also turned the nitrogen into a tertiary amine, it simultaneously increases the logP of the molecule. Therefore, the aqueous solubility was not enhanced. Previous reports revealed that the tertiary amine substitution enhanced both the aqueous solubility8,9 and the anti-cancer activity.10,11 Compounds 31 and 32 also showed a potent cytoselective anti-cancer activity in H460 and H460TaxR cell lines at a single concentration (10 µM) of compounds. To explain why some compounds have the anti-cancer activity, we conducted a computational study generating a pharmacophore using 10 active compounds. The pharmacophore showed a requirement for two hydrogen bond acceptor regions and three hydrophobic regions. (Fig. 5) These pharmacophore features match well with what we previously reported on anti-cancer thiazolidinone compounds.2
Figure 4.
Aqueous solubility and in vitro anti-cancer activities of compounds in Library 2 (R1 = H). Aqueous solubility of compounds (A) was determined using a method described in Supporting information. Cytotoxicity of compounds in normal human fibroblast (NHFB) (B), H460 (C) and H460TaxR (D) were measured using SRB methods using with a compound concentration of 10 µM. Cell viability in DMSO was designated as 100%.
Figure 5.
Anti-cancer pharmacophore generated using the 10 active molecules. The graph shows the four most active (2, 4, 12, 33) compounds aligned. Green dots represent hydrophobic features; blue regions represent hydrogen bond acceptors.
To further evaluate effect of R3 substitutions on compound’s anti-cancer efficacy, we determined EC50 values of compounds in Library 2. Physicochemical properties and experimental data for compounds with R3 modifications were summarized in Table 1. Compound 31 exhibited the largest improvement compared to compound 1 in both aqueous solubility (5-fold) and cytoselective toxicity toward NSCLC cell line H460 (EC50 0.08 µM) and its drug resistant variant H460TaxR (EC50 0.16 µM) cells (Fig. 6A and B). It also exhibited less toxicity to normal cell NHFB (EC50 >100 µM) (Fig. 6A and B). Compound 32 did not improve solubility compared to 2 (Fig. 6C and D) while compound 33 did not maintain anti-cancer activity (EC50 8.9 and 2.9 µM) although its solubility was significantly better than 3. A computational pharmacophore study was conducted and results showed that the less active compounds such as 33, 34, and 35 exhibited weak anti-cancer effects because they did not possess the unique pharmacophore we identified previously.2
Table 1.
Solubility and anti-cancer activity of compounds with diverse R2 and R3 substitutions
|
Clog Pa | Solubilityb (µg/ml) |
EC50c (µM) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| R2 | R3 | H460 | H460TaxR | NHFB | |||
| 1 | –N(CH3)2 | –H | 4.3 | 0.1 | 0.72 ± 0.02 | 0.98 ± 0.03 | >100 |
| 2 | –N(CH2CH3)2 | –H | 5.3 | 0.1 | 6.9 ± 0.28 | 9.9 ± 0.35 | >100 |
| 3 |
|
–H | 3.6 | 0.1 | >100 | >100 | >100 |
| 4 |
|
–H | 4.1 | 0.35 | 1.4 ± 0.14 | 2.5 ± 0.46 | 6.4 ± 0.05 |
| 5 |
|
–H | 3.5 | 1.68 | >100 | >100 | >100 |
| 26 | –N(CH3)2 |
|
5.7 | 0 | >100 | >100 | >100 |
| 27 | –N(CH2CH3)2 |
|
6.8 | 0.01 | >100 | >100 | >100 |
| 28 |
|
|
5.0 | 0.01 | >100 | >100 | >100 |
| 29 |
|
|
5.6 | 0.11 | >100 | >100 | >100 |
| 30 |
|
|
5.0 | 0.15 | >100 | >100 | >100 |
| 31 | –N(CH3)2 | –CH3 | 4.3 | 0.50 | 0.08 ± 0.06 | 0.16 ± 0.05 | >100 |
| 32 | –N(CH2CH3)2 | –CH3 | 5.6 | 0.01 | 0.28 ± 0.05 | 0.43 ± 0.03 | >100 |
| 33 |
|
–CH3 | 3.5 | 5.06 | 8.9 ± 0.08 | 2.9 ± 0.05 | >100 |
| 34 |
|
–CH3 | 4.1 | 18.91 | >100 | >100 | >100 |
| 35 |
|
–CH3 | 3.5 | 3.89 | >100 | >100 | >100 |
Note:
Clog P was computed by using Accord 6.1 (Accelrys, San Diego, CA, USA).
Albendazole was used as negative control and carbamazepine, ranitidine and verapamil as positive controls for solubility assay.
DMSO was used as a negative control in cytotoxicity assay.
Figure 6.
Comparison of effects of R3 on the cytotoxicity and solubility of compounds. Dose-dependent cytotoxicity and aqueous solubility for selected compounds with –H and −CH3 as R3 group in cell lines H460 (A, C) and H460TaxR (B, D). Aqueous solubility was shown in insets.
Compounds with R3b substitution (R3b = Ph) all have larger ClogP values. This resulted in a poor aqueous solubility due to increased hydrophobicity and a low anti-cancer activity due to unfavorable pharmacophore as discussed in previous sections. Compounds with R3c substitution exhibited an improved solubility compared with compounds with R3a substitution. This can be explained by the conversion of a secondary amine to a tertiary amine.9 This modification also resulted in an improvement in anti-cancer activity (10-fold) showing a significant advance in this round of optimization.
In summary, using progressive optimization library approach, we improved both aqueous solubility and cytoselective anti-cancer activity of lead compound 1. We demonstrate that lead optimization library approach combined with solubility and cytoselective toxicity screenings is an effective approach to optimize both compound solubility and anti-cancer activity. Although further optimization, especially for solubility, is still needed, a new lead compound 31, with a 5-fold enhanced solubility and 10-fold improved anti-cancer activity is a promising compound for further investigations.
Supplementary Material
Acknowledgments
We thank Lei Yang, Jianbo Jia and Yan Mu for technical assistance.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2015.03.016.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Cancer Res. 2014;74:2913. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Wu S, Zhai S, Liu A, Sun Y, Li R, Zhang Y, Ekins S, Swaan PW, Fang B, Yan B. J. Med. Chem. 2008;51:1242. doi: 10.1021/jm7012024. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Liu X, Li X, Li C, Zhou H, Yan B. J. Pharm. Sci. 2013;122:223. doi: 10.1254/jphs.13064fp. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Zhai S, Li L, Li X, Zhou H, Liu A, Su G, Mu Q, Du Y, Yan B. Biochem. Pharmacol. 2013;86:351. doi: 10.1016/j.bcp.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Di L, Fish PV, Mano T. Drug Discovery Today. 2012;17:486. doi: 10.1016/j.drudis.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Dayam R, Aiello F, Deng J, Wu Y, Garofalo A, Chen X, Neamati N. J. Med. Chem. 2006;49:4526. doi: 10.1021/jm051296s. [DOI] [PubMed] [Google Scholar]
- 7.Lipinski CA. J. Pharmacol. Toxicol. Methods. 2000;44:235. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 8.Muanprasat C, Sonawane N, Salinas D, Taddei A, Galietta LJ, Verkman A. J. Gen. Physiol. 2004;124:125. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurimoto A, Ogino T, Ichii S, Isobe Y, Tobe M, Ogita H, Takaku H, Sajiki H, Hirota K, Kawakami H. Bioorg. Med. Chem. 2004;12:1091. doi: 10.1016/j.bmc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Go M-L, Leow JL, Gorla SK, Schüller AP, Wang M, Casey PJ. J. Med. Chem. 2010;53:6838. doi: 10.1021/jm1002843. [DOI] [PubMed] [Google Scholar]
- 11.Ramanujulu PM, Yang T, Yap S-Q, Wong F-C, Casey PJ, Wang M, Go M-L. Eur. J. Med. Chem. 2013;63:378. doi: 10.1016/j.ejmech.2013.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.