Abstract
Levodopa-induced dyskinesia (LID) is commonly seen in Parkinson's disease patients treated with levodopa. This side effect is usually encountered after long duration of treatment, but occasionally, this may be seen even after few days or months of treatment. LID is broadly classified as peak-dose dyskinesia, wearing-off or off-period dyskinesia, and diphasic dyskinesia. Pathogenesis of LID is complex, and different neurotransmitters such as dopamine, glutamine, adenosine, and gamma-aminobutyric acid play important role altering the normal physiology of direct and indirect pathway of cortico-basal ganglia-thalamic loop responsible for fine motor control. Treatment of LID requires careful history taking and clinical examination to find the type of dyskinesia as different approach is required for different types. Changes in dopaminergic medication including continuous dopaminergic stimulation are very helpful in the management of peak-dose dyskinesia. Different types of surgical approaches including unilateral pallidotomy and deep brain stimulation have given very good result in patients, who cannot be managed by medications alone. The surgical management of LID is dealt with in detail in another review in this series.
Keywords: Dopamine, dyskinesia, levodopa, Parkinson's disease
INTRODUCTION
Levodopa is the most effective drug for treating Parkinson's disease (PD), but its long-term use is complicated by motor fluctuations and dyskinesia.[1] Dyskinesia may be mild at the beginning but may progress to become a disabling symptom and may interfere with quality of life. Different types of movement disorders are seen in levodopa-induced dyskinesia (LID) including chorea, ballism, dystonia, myoclonus, or combination of any of these movements. These dyskinesias are seen in the neck, facial muscles, jaw, tongue, hip, shoulder, trunk, and limb or may appear as involuntary flexion of toes.[2]
CLINICAL PHENOMENOLOGY
LID may have different clinical phenomenology, but broadly speaking, they are of three types: peak-dose dyskinesia, wearing-off or off-period dyskinesia, and diphasic dyskinesia, of which peak-dose dyskinesia is the most common and diphasic dyskinesia is least common [Table 1].[3] A patient may have one type of dyskinesia or a combination of two or three types. Luquin et al. reported dyskinesia in 168 of 220 PD patients receiving levodopa treatment. One hundred and fifty-two patients had on dyskinesia, 31 patients had diphasic dyskinesia and 60 patients had off-period dyskinesia. Eighty-four patients had one type, 68 patients had two types, and 16 patients had three types of dyskinesia. Most common types of dyskinesia were chorea (n = 113), dystonic posturing of the limbs (n = 63), repetitive movement of the limbs (n = 24), craniocervical dystonia (n = 15), blepharospasm (n = 6), mixed movement disorders (n = 9), myoclonus (n = 6), and tics (n = 1).[4]
Table 1.
Different types of levodopa-induced dyskinesia and medical management
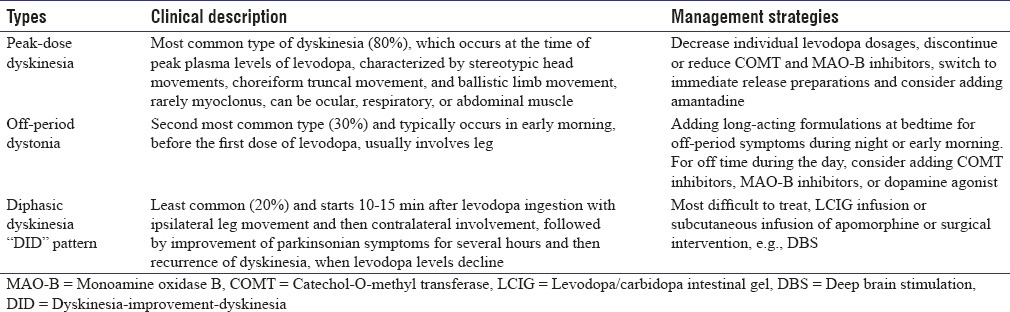
Peak-dose dyskinesia is seen at high plasma drug level and was first described by Cotzias et al.[1] The phenomenology is characterized by choreiform movements, but patients may have any other movement disorders such as dystonia, myoclonus, and ballism, which usually involve upper limbs, trunk, and orofacial muscles.[3,4] In some patients, dyskinesia may have “square-wave response,” which is characterized by symptoms starting with on phase and lasting till medications wear off. Isolated ballism occurs rarely and majority of the patients present as a part of severe chorea.[5] Peak-dose dyskinesia may be exacerbated by anxiety and emotional stress. Rest tremor and peak-dose dyskinesia are considered quite specific for PD, and they are not seen in other movement disorders. Other forms of peak-dose dyskinesia have been described such as ocular, belly dancer's, and respiratory dyskinesia.[6,7] In ocular dyskinesia, involuntary and upward eye movements are seen, which usually occur with peak-dose limb dyskinesia. In one study, 16% (5 of 32) of advanced PD patients had abnormal involuntary eye movements during on state, which disappeared during the off state.[8] Carecchio et al. reported abdominal dyskinesia (belly dancer's dyskinesia) with levodopa in a 72-year-old woman with PD. The dyskinesia started around 30 min after each dose of levodopa and lasted for about 3 h.[7] Rice et al. reported respiratory dyskinesia coinciding with peak levodopa effect in two patients. Patients complained of irregularities of respiratory rate and depth beginning 80–90 min after levodopa.[9] This type of dyskinesia is due to chorea of respiratory muscles and abnormality of central control of ventilatory rhythm. Myoclonus is rarely seen in peak-dose dyskinesia and should raise a suspicion of other movement disorders such as cortico-basal-ganglionic degeneration.[10] PD patients having dementia are more likely to have myoclonus, which can be spontaneous, or action induced and usually multifocal. Myoclonus usually appears within 10–20 min of levodopa administration and disappears when patient fully improves. Off-period dyskinesia is due to long-standing chronic levodopa therapy and seen when the serum level of levodopa is least. They usually manifest as abnormal spasm of body parts, which most commonly affect foot or leg and rarely present on the arm or trunk. Physicians must differentiate off-period dyskinesia from foot dystonia, which is seen, in young-onset PD patients.[11] They are commonly seen in early morning; however, it can also be seen in other off state throughout the day. In one study, diphasic dyskinesia and off dystonia were reported to be in continuation.[12] Diphasic dyskinesia is seen when serum level of levodopa is going up or down coinciding with two peaks of abnormal movements, one present at the onset of drug effect and another present at the end of drug effect.[11] Movements frequently involve lower limb and they are usually dystonic type; however, rarely, there can be a combination of chorea and dystonia. Repetitive abnormal movements of the lower limb compatible with dystonia and ballism have also been reported.[4,13] Patients may show improvement in dyskinesia in between during the on state. The classical description of this type of dyskinesia is “dystonia (dyskinesia)-improvement-dystonia (dyskinesia).”[12] Some patients may also present autonomic dysfunction such as cardiac arrhythmias during the period of dyskinesia.
OTHER TYPES OF DYSKINESIA IN PARKINSON'S DISEASE
PD patients on treatment with levodopa may also have other types of dyskinesia including stimulation-induced dyskinesia (SID) and graft-induced dyskinesia (GID). SID has been reported in patients who receive subthalamic nucleus (STN)-deep brain stimulation (STN-DBS) surgery. SID is considered to be a good prognostic sign for optimal lead placement.[14] Usually, SID occurs within 1 month after surgery. In one study, 40 contacts of 16 electrodes (15 patients) causing SID were analyzed.[15] Most common site of dyskinesia was contralateral lower limb and dystonia was commonly reported. In another study, 4 of 179 STN-DBS patients had SID.[16] This type of dyskinesia was also labeled as “brittle” STN-associated dyskinesia. Interestingly, none of 75 patients undergoing globus pallidus interna (GPi)-DBS (GPi-DBS) developed SID. One STN-DBS patient, who developed SID, underwent GPi-DBS and his SID was successfully reversed. GID is an abnormal dyskinetic movement in off state seen in PD patients receiving transplantation of fetal tissues.[17] Serotonergic neurons are considered to mediate the GID. In one study, systemic administration of 5-HT1A agonist, buspirone, completely suppressed the GID.[18]
EPIDEMIOLOGY AND RISK FACTORS
Symptoms at onset of dyskinesia are more likely to start on the side of the body, which was first effected by motor symptoms, but it can also start in other body parts such as craniobulbar region.[19] The prevalence of LID increases with disease and treatment duration, and usually, it takes approximately 3–5 years after administrating levodopa for developing the dyskinesia [Figure 1]. In a 5-year study in one hundred patients of PD treated with levodopa, 49% had dyskinesia.[20] In another study, 55% PD patients had dyskinesia after 6 years of treatment with levodopa.[21] Patients having advanced PD symptoms tend to have more dyskinesia. In a study, no PD patient with Hoehn and Yahr (H and Y) stage 1 or 1.5 had motor complications even after 10 years, whereas 60% patients with H and Y stages 4 or 5 had dyskinesia.[22]
Figure 1.
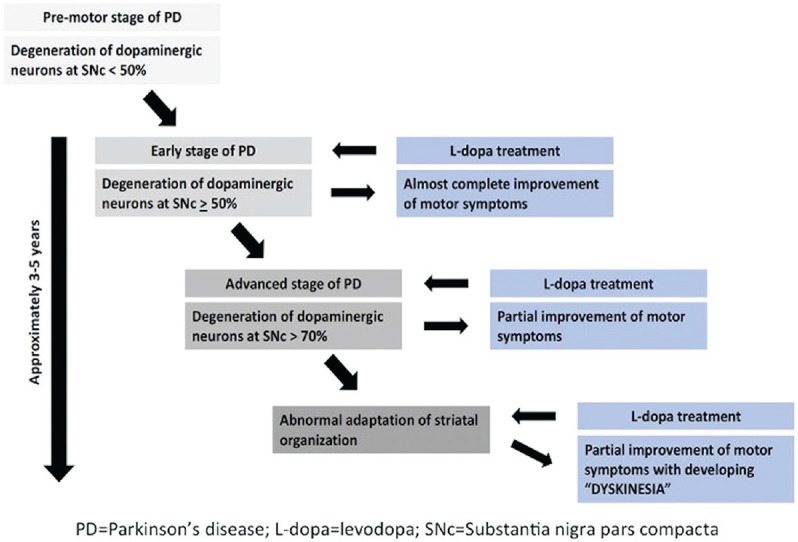
Time sequence of developing levodopa-induced dyskinesia in Parkinson's disease
There are many factors responsible for dyskinesia in a PD patient. Age at onset of PD symptoms and duration of disease are most important factors. Therefore, patients who present symptoms of PD at younger age are greatest risk for developing LID. In a study, 70% of PD patients who developed onset of symptoms of PD between 40 and 49 years had dyskinesia after 5 years of treatment in comparison to 42% of PD patients who developed onset of symptom of PD between 50 and 59 years.[23] Higher cumulative dosage of levodopa is another risk factor for developing LID. In a randomized, double-blind, placebo-controlled trial, 16.5% of PD patients who received 600 mg/day of levodopa treatment for 40 weeks developed dyskinesia, whereas only 3.3% and 2.3% of PD patients receiving 300 mg/day and 150 mg/day, respectively, developed dyskinesia.[24] It has been reported that treatment regimen avoiding pulsatile stimulation of dopamine receptors decreases the risk of dyskinesia. In a recent study, predictive factors of dyskinesia were analyzed.[25] Predictive factors in order of severity were younger age at onset of PD symptoms, higher levodopa usage, low body weight, natives of North American geographic region, levodopa/carbidopa/entacapone treatment, female gender, and more severe Unified PD Rating Scale (UPDRS) Part II score. Predictors of wearing-off dyskinesia also included more severe UPDRS Part III score. Risk of developing dyskinesia or wearing-off was closely linked to levodopa dose. Important genetic associations have also been identified as a risk factor such as DRD2 and DRD2Taq1A polymorphism.[26,27] In one study, DRD3p. S9G polymorphism was associated with diphasic dyskinesia and not with peak-dose dyskinesia.[28] Studies have showed that high daily levodopa dosage and longer duration of treatment are associated with high risk of developing dyskinesia. However, a recent study provided the evidence that motor fluctuation and dyskinesia are associated with disease progression rather than duration of exposure to levodopa therapy.[29] Experimental studies have also proven that early initiation of levodopa treatment is best option to delay the molecular changes associated with dyskinesia;[30] however, this finding needs to be confirmed in the larger study.
PATHOGENESIS
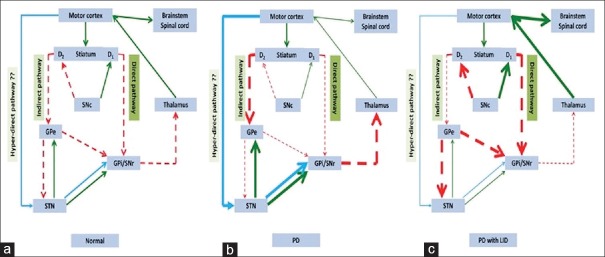
The pathogenesis of LID is not well understood.[2] Loss of nigral dopaminergic neurons creates abnormalities in the connectivity between the motor cortex and the striatum and establishes a functional disturbance in basal ganglia leading to the generation of involuntary abnormal movements [Figure 2]. The level and duration of drug exposure that is required to induce dyskinesia is regulated by the extent of the degeneration.[31,32] Normal humans or monkeys do not develop dyskinesia when treated with pharmacological doses of levodopa for long duration of time, by contrast PD patients, and 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-exposed monkeys with high degree of nigral degeneration develop dyskinesia rapidly after levodopa treatment.[33] Typically, dyskinesia and motor fluctuations are temporally related with rise and fall in plasma levodopa level. As the disease advances, the same dosage of levodopa required to relieve parkinsonian symptoms may also cause dyskinesia. It is not clear, what causes this altered response pattern; however, there is wide consensus that disturbances of both pre- and post-synaptic nigrostriatal dopamine transmission lead to these motor complications.[34] Presynaptic disturbances generate large dopamine fluctuation in the brain and postsynaptic changes result into abnormal responses in dopaminergic neurons.[35] Progressive degeneration of presynaptic nigral neurons results in loss of dopamine storage capacity causing sudden rise in dopamine level leading to peak-dose dyskinesia and sudden decline in dopamine level causing wearing-off dyskinesia. The presynaptic hypothesis has been further supported by positron emission tomography studies using the reversible D2 ligand, (11C) raclopride, to estimate dopamine release. De la Fuente-Fernandez et al. used this technique to show large fluctuation in striatal dopamine level following standard oral levodopa dosage in PD patients experiencing motor fluctuations.[36] Loss of nigrostriatal dopaminergic neurons causes plastic changes postsynaptically and supersensitivity of postsynaptic dopaminergic neurons [Figure 3].[37] Several other nondopaminergic systems including glutamatergic, gamma-aminobutyric acid-ergic, serotonergic, histaminergic, adenosine, and cannabinoid receptors have been postulated to play an important role in the development of LID.[38] Glutamatergic pathway acts through N-methyl-D-aspartate (NMDA) receptors, and amantadine used as antidyskinesia drug acts by blocking these receptors.
Figure 2.
The models of basal ganglia in normal condition, Parkinson's disease and Parkinson's disease with levodopa-induced dyskinesia. (a) The model of basal ganglia in normal condition. Normal dopaminergic input from substantia nigra pars compacta (SNc) influences the motor movement through dopaminergic receptors D1 (direct pathway) and D2 (indirect pathway). Basically, dopaminergic stimulation on D1 receptor facilitates motor movement while stimulation on D2 receptor inhibits motor movement. In addition, hyperdirect pathway may also inhibit motor movement. (b) In Parkinson's disease (PD), loss of dopaminergic input from SNc leads to underactivity of the direct pathway and overactivity of the indirect pathway. Finally, the glutamatergic output from thalamus is reduced and decreases the motor movement. In addition, the role of hyper-direct pathway in PD is still unknown; however, the hyperdirect pathway might increase its activity in PD. (c) After long-term administration of levodopa concomitant with more degree of loss of striatal dopamine, the interconnections within nigrostriatal circuit change in the opposite directions, overactivity of the direct pathway, and underactivity of the indirect pathway produces an excessive motor movement named “levodopa-induced dyskinesia.” In addition, the role of hyperdirect pathway in PD with LID is not exactly known; however, the hyperdirect pathway might decrease the activity in PD with LID stage. PD = Parkinson's disease; LID = levodopa-induced dyskinesia; GPe = Globus pallidus externa; GPi = Globus pallidus interna; SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; STN = Subthalamic nucleus; Green arrow = excitatory output; Red arrow with dashed line = inhibitory output
Figure 3.
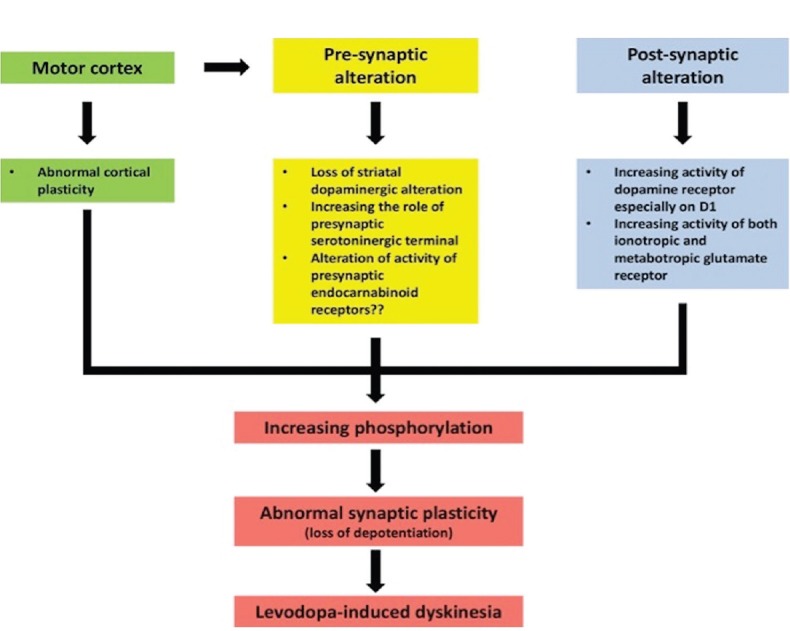
Possible pathophysiology of levodopa-induced dyskinesia. The pathophysiology of levodopa-induced dyskinesia could be classified into three levels. The first is cortical level, the second is presynapses within striatum, and the third is postsynapses within striatum. At the cortical level, it has an abnormal cortical plasticity resulting the abnormal of glutamatergic output to the striatum. Subsequently, at the presynaptic level, there are many alterations such as loss of striatal presynaptic dopaminergic terminal and increasing the role of presynaptic serotoninergic terminal. When these alterations are occurred coupling with administration of levodopa, the pulsatile release of the dopamine might occur. Another presynaptic alteration is the alteration of activity of endocannabinoid receptors that might increase the glutamatergic activity. According to the postsynaptic alterations, there is increased activity of dopaminergic receptor, especially on D1 receptor and increasing the activity of both metabotropic and ionotropic glutamatergic receptors. The result of the alterations of all levels is changing the intracellular signaling pathway leading to increase the phosphorylation that creates the abnormal synaptic plasticity in term of losing the ability to create the depotentiation. Finally, the onset of inappropriate control of motor function and the dyskinesia occurs
RATING SCALES FOR DYSKINESIA
Different scales and instruments have been used to provide objective assessment of LID and its impact on overall quality of life. UPDRS (Part IV) is helpful in assessment of different aspects of dyskinesia, but it does not include the anatomical distribution of dyskinesia in different body parts.[39] There are other scales used to assess LID, including the “Rush Dyskinesia Rating Scale” (RDRS), “Unified Dyskinesia Rating Scale,” and “Clinical Dyskinesia Rating Scale” (CDRS).[40] There are different instruments to measure quality of life, including 39-item “Parkinson's Disease Questionnaire” (PDQ-39) and “Parkinson's Disease Quality of Life Scale.”[41] Patients’ self-evaluation diaries such as “Hauser diary” have been used to know the effect of drugs used to treat LID, but the compliance to diary completion and accuracy is extremely challenging.[42] Several quantitative instrumental techniques have been developed to quantify dyskinesia including wearing devices, accelerometers, and position transducers.[43]
PREVENTION AND MANAGEMENT ISSUES OF LEVODOPA-INDUCED DYSKINESIA
Theoretical consideration that levodopa may accelerate neuronal degeneration due to oxidative stress led to levodopa-sparing therapy.[44] In a 5-year study, cumulative incidence of dyskinesia was 20% in the ropinirole group and 45% in the levodopa group.[45] In another study, 54% of patients in the levodopa group and 25% of patients in the pramipexole group had dyskinesias.[46] However, initial treatment with levodopa had lower incidences of freezing, somnolence, and edema. CALM-PD trial showed significant less number of PD patients with dyskinesia who took pramipexole in comparison to levodopa (9.9% vs. 30.7%).[47] However, the Movement Disorder Society evidence-based review panel concluded that there was insufficient evidence to support the use of pramipexole extended release to delay or treat LID.[48] They also concluded that ropinirole and ropinirole extended-release preparations are effective in prevention of dyskinesia, but there is insufficient evidence for their use in treatment of dyskinesia. Rotigotine transdermal patch has been used to manage PD patients with dyskinesia, but data on using for prevention of dyskinesia are insufficient.[3,48] In addition, the evidence-based review concluded that rasagiline, a long-acting monoamine oxidase B (MAO-B) inhibitor, is insufficient for preventing and treating LID.[48]
TREATMENT
Treating PD patients presenting with LID, physicians need detailed assessment regarding time of dyskinesia, type of dyskinesia, and current medications. Treatment of dyskinesia requires changes in current dopaminergic and nondopaminergic medications, trials of new drugs as well as neurosurgical interventions. Using different therapeutic approaches is based on the type of dyskinesia, and individualization of therapy is the most prudent approach.
Dopaminergic drugs
Using frequent smaller dosage of levodopa and fractionation is helpful to minimize peak-dose dyskinesia. If patients are receiving long-acting formulations, such as controlled release, switching to immediate-release formulations may be beneficial. Reducing or discontinuing the dosages of MAO-B inhibitor or catechol-O-methyl transferase (COMT) inhibitors will also be helpful for such patients.[49] Off-period dystonia usually occurs at night or early morning, and adding a long-acting formulation at bedtime may be very helpful. Addition of COMT inhibitors, MAO-B inhibitor, or long-acting dopamine agonists can also be very helpful.[50] For sudden or unpredictable off, apomorphine (continuous subcutaneous infusion) can be considered, which is a highly selective dopamine agonist acting at D1 and D2, and provides a rapid onset of action.[51] There are no randomized controlled studies of continuous subcutaneous apomorphine infusion (CSAI). García Ruiz et al. reviewed data (open-label studies) from 82 PD patients with severe motor fluctuations treated for at least 3 months with CSAI.[52] The study showed improvement in mean daily off time of 38-80%. Most common adverse events were skin nodules, neuropsychiatric problems (confusion, hallucination, and hypersexuality), and systemic reactions (sedation, nausea, and orthostatic hypotension).
Treatment of diphasic dyskinesia is difficult and challenging. Initial strategy should be to decrease the dosage of levodopa and increase the dosage of dopamine agonist. Levodopa/carbidopa intestinal gel (LCIG) may also be helpful in controlling parkinsonian symptoms and dyskinesia in such patients.[53] LCIG is delivered by a pump and the cartridge containing the levodopa (2 g) and carbidopa (500 mg), which is changed daily. There is good evidence to show that LCIG reduces daily off time and increases on time without dyskinesia.
New formulations of levodopa
Several newer formulations of levodopa are in different stages of development. The Accordion Pill is a novel formulation of levodopa/carbidopa, which dissolves in stomach and slowly releases over 12 h. Subcutaneous levodopa formulation, ND0612, which delivers up to 360 mg of levodopa over 24 h is currently undergoing phase II studies. CVT-301 is an inhalable formulation of levodopa, and therapeutic plasma levodopa level reaches within 5–10 min of administration. This formulation has been found to be particularly useful as a rescue medication in sudden and/or severe off. Another novel formulation of levodopa, ODM 101 (contains levodopa/carbidopa/entacapone), has showed a significant increase in on time without dyskinesia.[54]
Nondopaminergic treatments
Amantadine
Amantadine is an NMDA antagonist and considered the most effective drug used for LID. Small-randomized, placebo-controlled trials have showed its efficacy in decreasing the frequency and severity of dyskinesia without decreasing the on time.[55,56] The standard dosages are 200–300 mg daily. Although some researchers have suggested that patients treated with this drug may experience rebound in dyskinesia after using this medication for a long period of time, a recent study published by Wolf et al. has documented its long-term efficacy.[57] They treated 32 PD patients experiencing LID with amantadine for 1 year and then randomized them to continue the drug or switch to placebo for 3 weeks. Patients in the placebo arm experienced worsening of dyskinesia. Potential side effects of amantadine are sedation, hallucinations, confusion, edema of feet, myoclonus, livedo reticularis, and corneal edema.[58]
Antiepileptic drugs
Levetiracetam has been found to be effective in reducing LID in MPTP-induced macaques.[59] Several open-label studies and placebo-controlled trials have also shown the efficacy of levetiracetam in reducing severity of dyskinesia.[60] In a multicenter double-blind, placebo-controlled, crossover trial in 38 PD patients, there was a significant reduction in LID at doses of 500 and 1000 mg/day.[61] The exact mechanism of action is not known, but the proposed mechanism includes its action at multiple sites including phosphorylated kinases in the striatum.[62] Topiramate has also been found to reduce LID in rat and nonprimate human model by modulating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, but worsening of dyskinesia was reported in a small randomized, double-blind, crossover trial in 15 patients.[63] Zonisamide has been used as antidyskinetic drug following positive result in several studies. In a randomized, double-blind, placebo-controlled trial on 422 patients, zonisamide was effective in reducing off time in PD patients with wearing-off dyskinesia.[64] The effect of zonisamide is mediated by multiple modes of action, including inhibition of glutamate release, MAO-B inhibition, and increase in dopamine synthesis.[3]
Antipsychotic drugs
Atypical antipsychotics, clozapine and quetiapine, have been used in the treatment of LID.[48] Several open-label studies and one double-blind, placebo-controlled trial reported the efficacy of clozapine in reducing the severity of LID.[65] The proposed mechanism of action of clozapine includes antagonistic binding to striatal dopamine receptor type 2A and serotonin receptor type 2A (5-HT2A). The common reported side effects are agranulocytosis, sialorrhea, somnolence, seizures, orthostatic hypotension, and myocarditis. Quetiapine is also used to control LID and the postulated mechanism of action is through antagonistic binding to 5HT2A receptor. In a double-blind study, 50 mg of quetiapine showed minimal reduction in dyskinesia, but patients reported significant drowsiness and sedation.[66] There was no antidyskinetic effect of 25 mg of quetiapine.
Safinamide
Mechanisms of action of safinamide, an alpha-aminoamide, are glutamate release inhibition and MAO-B inhibition. In a phase III trial, safinamide 100 mg or placebo was tried as add on to a single dopamine agonist over 24 weeks’ period.[67] Motor score as measured by UPDRS III, total score improved in patients taking safinamide 100 mg over placebo. Investigators concluded that safinamide 100 mg can be a good option to add with dopamine treatment in PD patients as adding other agents such as levodopa/carbidopa/entacapone may lead to more dyskinesia. In another study, 549 PD patients (all had off time >1.5 h/day) were randomized (274 safinamide and 275 placebo), and among them, 245 (89.4%) receiving safinamide and 241 receiving placebo completed the study.[68] The mean daily on time increased by + 1.42 (standard deviation [SD] = 2.80) hours in safinamide group and by + 0.57 (SD = 2.47) hours in the placebo group (P < 0.001). The authors concluded that safinamide is an effective adjunct to levodopa in PD patients with motor fluctuation. Major side effects are headache, nausea, and pain abdomen.
Istradefylline
Istradefylline, an adenosine A2A antagonist, has been registered in Japan as an adjunctive therapy for PD patients who currently receive levodopa.[69] However, its effect on motor fluctuation and dyskinesia is controversial. A recent randomized, double-blind, placebo-controlled study conducted in 373 PD patients with motor fluctuation showed a significant reduction in off time, but the most common adverse effect was dyskinesia.[70]
Eltoprazine
Despite degeneration of dopaminergic neurons, PD patients also have degeneration of serotoninergic neurons in raphe nuclei. Stimulation of 5-HT1A presynaptic receptor can theoretically reduce dopamine release causing reduction in dyskinesia.[71] Eltoprazine, a selective partial agonist for 5HT1A and 5HT1B receptors, has shown antidyskinesia property in animal model.[49] This drug was tested in 22 PD patients with dyskinesia in a randomized, double-blind, placebo-controlled pilot trial. The result showed that eltoprazine 5 mg significantly reduced the area under the curves of CDRS and RDRS, but there was no change in UPDRS III score.[72] Further trials are still ongoing to confirm its efficacy in PD patients with motor fluctuation and dyskinesia.
Other experimental drugs and future perspectives
Several new treatment options targeting different neurotransmitters are being studied for the management of LID. Noradrenergic receptor antagonist (fipamezole), endocannabinoid receptor antagonist (rimonabant), opioid receptor antagonist (nalbuphine), and histamine H3 hetero-receptor agonist (imepip and imifit) have been tried in nonhuman primate model and their efficacy is being studied in human.[73,74,75,76]
SUMMARY
LID is common and difficult to treat condition in PD patients. Clinical phenomenology of LID is variable, but three main types are peak-dose dyskinesia, wearing-off or off-period dyskinesia, and diphasic dyskinesia. The exact pathogenesis is not known, but both presynaptic and postsynaptic mechanisms operate in the pathogenesis of LID. Management of LID depends on identifying the type of dyskinesia and treating it accordingly. Reduction of levodopa dose is helpful in minimizing peak-dose dyskinesia while addition of long-acting formulations is effective in wearing-off dyskinesia. Infusion therapy (LCIG or subcutaneous infusion of apomorphine) as well as surgical intervention is helpful to combat all kinds of dyskinesia. Amantadine and clozapine have also been used successfully. Many newer drugs have emerged for the treatment of LID targeting different mechanism of actions, including multiple neurotransmitters.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967;276:374–9. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
- 2.Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with parkinson's disease: Filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–17. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- 3.Vijayakumar D, Jankovic J. Drug-induced dyskinesia, part 1: Treatment of levodopa-induced dyskinesia. Drugs. 2016;76:759–77. doi: 10.1007/s40265-016-0566-3. [DOI] [PubMed] [Google Scholar]
- 4.Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa-induced dyskinesias in parkinson's disease: Clinical and pharmacological classification. Mov Disord. 1992;7:117–24. doi: 10.1002/mds.870070204. [DOI] [PubMed] [Google Scholar]
- 5.Hametner E, Seppi K, Poewe W. The clinical spectrum of levodopa-induced motor complications. J Neurol. 2010;257(Suppl 2):S268–75. doi: 10.1007/s00415-010-5719-9. [DOI] [PubMed] [Google Scholar]
- 6.LeWitt PA. Conjugate eye deviations as dyskinesias induced by levodopa in Parkinson's disease. Mov Disord. 1998;13:731–4. doi: 10.1002/mds.870130421. [DOI] [PubMed] [Google Scholar]
- 7.Carecchio M, Collini A, Comi C, Cantello R, Bhatia KP, Monaco F. Levodopa-induced belly dancer's dyskinesias in Parkinson's disease: Report of one case. Mov Disord. 2010;25:1760–2. doi: 10.1002/mds.23345. [DOI] [PubMed] [Google Scholar]
- 8.Grötzsch H, Sztajzel R, Burkhard PR. Levodopa-induced ocular dyskinesia in Parkinson's disease. Eur J Neurol. 2007;14:1124–8. doi: 10.1111/j.1468-1331.2007.01919.x. [DOI] [PubMed] [Google Scholar]
- 9.Rice JE, Antic R, Thompson PD. Disordered respiration as a levodopa-induced dyskinesia in parkinson's disease. Mov Disord. 2002;17:524–7. doi: 10.1002/mds.10072. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S. The spectrum of levodopa-induced dyskinesias. Ann Neurol. 2000;47(Suppl 1):S2–9. [PubMed] [Google Scholar]
- 11.Nutt JG. Levodopa-induced dyskinesia: Review, observations, and speculations. Neurology. 1990;40:340–5. doi: 10.1212/wnl.40.2.340. [DOI] [PubMed] [Google Scholar]
- 12.Marconi R, Lefebvre-Caparros D, Bonnet AM, Vidailhet M, Dubois B, Agid Y. Levodopa-induced dyskinesias in Parkinson's disease phenomenology and pathophysiology. Mov Disord. 1994;9:2–12. doi: 10.1002/mds.870090103. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletti A, Mostile G, Nicoletti G, Arabia G, Iliceto G, Lamberti P, et al. Clinical phenotype and risk of levodopa-induced dyskinesia in parkinson's disease. J Neurol. 2016;263:888–94. doi: 10.1007/s00415-016-8075-6. [DOI] [PubMed] [Google Scholar]
- 14.Moyer JT, Danish SF. Stimulation-induced dyskinesias inform basal ganglia models and the mechanisms of deep brain stimulation. J Neurosci. 2007;27:1799–800. doi: 10.1523/JNEUROSCI.5359-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z, Li Y, Li J, Zhang Y, Zhang X, Zhuang P, et al. Stimulation-induced dyskinesia in the early stage after subthalamic deep brain stimulation. Stereotact Funct Neurosurg. 2010;88:29–34. doi: 10.1159/000260077. [DOI] [PubMed] [Google Scholar]
- 16.Sriram A, Foote KD, Oyama G, Kwak J, Zeilman PR, Okun MS. Brittle dyskinesia following STN but not GPi deep brain stimulation. Tremor Other Hyperkinet Mov. 2014;4:242. doi: 10.7916/D8KS6PPR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane EL. Clinical and experimental experiences of graft-induced dyskinesia. Int Rev Neurobiol. 2011;98:173–86. doi: 10.1016/B978-0-12-381328-2.00007-9. [DOI] [PubMed] [Google Scholar]
- 18.Politis M, Oertel WH, Wu K, Quinn NP, Pogarell O, Brooks DJ, et al. Graft-induced dyskinesias in Parkinson's disease: High striatal serotonin/dopamine transporter ratio. Mov Disord. 2011;26:1997–2003. doi: 10.1002/mds.23743. [DOI] [PubMed] [Google Scholar]
- 19.Fabbrini G, Defazio G, Colosimo C, Suppa A, Bloise M, Berardelli A. Onset and spread of dyskinesias and motor symptoms in Parkinson's disease. Mov Disord. 2009;24:2091–96. doi: 10.1002/mds.22703. [DOI] [PubMed] [Google Scholar]
- 20.Sweet RD, McDowell FH. Five years’ treatment of parkinson's disease with levodopa. therapeutic results and survival of 100 patients. Ann Intern Med. 1975;83:456–63. doi: 10.7326/0003-4819-83-4-456. [DOI] [PubMed] [Google Scholar]
- 21.Barbeau A. High-level levodopa therapy in severely akinetic parkinsonian patients: Twelve years later. In: Rinne UK, Klinger M, Stammer G, editors. Parkinson's Disease: Current Progress, Problems and Management. Amsterdam: Elsevier; 1980. pp. 229–39. [Google Scholar]
- 22.Schrag A, Quinn N. Dyskinesias and motor fluctuations in parkinson's disease. A community-based study. Brain. 2000;123(Pt 11):2297–305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]
- 23.Ku S, Glass GA. Age of Parkinson's disease onset as a predictor for the development of dyskinesia. Mov Disord. 2010;25:1177–82. doi: 10.1002/mds.23068. [DOI] [PubMed] [Google Scholar]
- 24.Brodsky MA, Park BS, Nutt JG. Effects of a dopamine agonist on the pharmacody-namics of levodopa in Parkinson's disease. Arch Neurol. 2010;67:27–32. doi: 10.1001/archneurol.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, et al. Factors predictive of the development of levodopa-induced dyskinesia and wearing-off in parkinson's disease. Mov Disord. 2013;28:1064–71. doi: 10.1002/mds.25364. [DOI] [PubMed] [Google Scholar]
- 26.Zappia M, Annesi G, Nicoletti G, Arabia G, Annesi F, Messina D, et al. Sex differences in clinical and genetic determinants of levodopa peak- dose dyskinesias in Parkinson's disease: An exploratory study. Arch Neurol. 2005;62:601–5. doi: 10.1001/archneur.62.4.601. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Liu ZL, Chen B. Association study of dopamine D2, D3 receptor gene polymorphisms with motor fluctuations in PD. Neurology. 2001;56:1757–9. doi: 10.1212/wnl.56.12.1757. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Cho J, Lee EK, Park SS, Jeon BS. Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson's disease. Mov Disord. 2010;26:73–9. doi: 10.1002/mds.23400. [DOI] [PubMed] [Google Scholar]
- 29.Cilia R, Akpalu A, Sarfo FS, Cham M, Amboni M, Cereda E, et al. The modern pre-levodopa era of parkinson's disease: Insights into motor complications from sub-saharan africa. Brain. 2014;137:2731–42. doi: 10.1093/brain/awu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marin C, Aguilar E, Mengod G, Cortés R, Obeso JA. Effects of early vs. late initiation of levodopa treatment in hemiparkinsonian rats. Eur J Neurosci. 2009;30:823–32. doi: 10.1111/j.1460-9568.2009.06877.x. [DOI] [PubMed] [Google Scholar]
- 31.Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–77. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- 32.Iravani MM, McCreary AC, Jenner P. Striatal plasticity in parkinson's disease and L-dopa induced dyskinesia. Parkinsonism Relat Disord. 2012;18(Suppl 1):S123–5. doi: 10.1016/S1353-8020(11)70038-4. [DOI] [PubMed] [Google Scholar]
- 33.Boyce S, Rupniak NM, Steventon MJ, Iversen SD. Nigrostriatal damage is required for induction of dyskinesias by L-DOPA in squirrel monkeys. Clin Neuropharmacol. 1990;13:448–58. doi: 10.1097/00002826-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Cenci MA. Presynaptic mechanisms of l-DOPA-induced dyskinesia: The findings, the debate, and the therapeutic implications. Front Neurol. 2014;5:242. doi: 10.3389/fneur.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metman LV, Konitsiotis S, Chase TN. Pathophysiology of motor response complications in Parkinson's disease: Hypotheses on the why, where, and what. Mov Disord. 2000;15:3–8. doi: 10.1002/1531-8257(200001)15:1<3::aid-mds1003>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 36.De la Fuente-Fernandez R, Schulzer M, Mak E, Calne DB, Stoessl AJ. Presynaptic mechanisms of motor fluctuations in Parkinson's disease: A probabilistic model. Brain. 2004;127:888–99. doi: 10.1093/brain/awh102. [DOI] [PubMed] [Google Scholar]
- 37.Klawans HL, Goetz C, Nausieda PA, Weiner WJ. Levodopa-induced dopamine receptor hypersensitivity. Trans Am Neurol Assoc. 1977;102:80–3. [PubMed] [Google Scholar]
- 38.Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007;22:1379–89. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- 39.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified parkinson's disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 40.Goetz CG, Stebbins GT, Chung KA, Hauser RA, Miyasaki JM, Nicholas AP, et al. Which dyskinesia scale best detects treatment response? Mov Disord. 2013;28:341–6. doi: 10.1002/mds.25321. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Martin P, Jeukens-Visser M, Lyons KE, Rodriguez-Blazquez C, Selai C, Siderowf A, et al. Health-related quality of- life scales in Parkinson's disease: Critique and recommendations. Mov Disord. 2011;26:2371–80. doi: 10.1002/mds.23834. [DOI] [PubMed] [Google Scholar]
- 42.Hauser RA, Deckers F, Lehert P. Parkinson's disease home diary: Further validation and implications for clinical trials. Mov Disord. 2004;19:1409–13. doi: 10.1002/mds.20248. [DOI] [PubMed] [Google Scholar]
- 43.Hoff JI, van den Plas AA, Wagemans EA, van Hilten JJ. Accelerometric assessment of levodopa-induced dyskinesias in parkinson's disease. Mov Disord. 2001;16:58–61. doi: 10.1002/1531-8257(200101)16:1<58::aid-mds1018>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Jankovic J, Bressman S, Dauer W, Kang UJ. Clinical and scientific perspectives on movement disorders: Stanley Fahn's contributions. Mov Disord. 2015;30:1862–9. doi: 10.1002/mds.26445. [DOI] [PubMed] [Google Scholar]
- 45.Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa.056 Study Group. N Engl J Med. 2000;342:1484–91. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- 46.Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, et al. Pramipexole vs. levodopa as initial treatment for parkinson disease: A 4-year randomized controlled trial. Arch Neurol. 2004;61:1044–53. doi: 10.1001/archneur.61.7.1044. [DOI] [PubMed] [Google Scholar]
- 47.Parkinson Study Group. A randomized controlled trial comparing pramipexole with levodopa in early Parkinson's disease: Design and methods of the CALM-PD study. Clin Neuropharmacol. 2000;23:34–44. doi: 10.1097/00002826-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, et al. The movement disorder society evidence-based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S2–S41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 49.Mazzucchi S, Frosini D, Bonuccelli U, Ceravolo R. Current treatment and future prospects of dopa-induced dyskinesias. Drugs Today (Barc) 2015;51:315–29. doi: 10.1358/dot.2015.51.5.2313726. [DOI] [PubMed] [Google Scholar]
- 50.Rascol O, Perez-Lloret S, Ferreira JJ. New treatments for levodopa-induced motor complications. Mov Disord. 2015;30:1451–60. doi: 10.1002/mds.26362. [DOI] [PubMed] [Google Scholar]
- 51.LeWitt PA. Subcutaneously administered apomorphine: Pharmacokinetics and metabolism. Neurology. 2004;62(6 Suppl 4):S8–11. doi: 10.1212/wnl.62.6_suppl_4.s8. [DOI] [PubMed] [Google Scholar]
- 52.García Ruiz PJ, Sesar Ignacio A, Ares Pensado B, Castro García A, Alonso Frech F, Alvarez López M, et al. Efficacy of long-term continuous subcutaneous apomorphine infusion in advanced Parkinson's disease with motor fluctuations: A multicenter study. Mov Disord. 2008;23:1130–6. doi: 10.1002/mds.22063. [DOI] [PubMed] [Google Scholar]
- 53.Timpka J, Fox T, Fox K, Honig H, Odin P, Martinez-Martin P, et al. Improvement of dyskinesias with l-dopa infusion in advanced Parkinson's disease. Acta Neurol Scand. 2016;133:451–8. doi: 10.1111/ane.12483. [DOI] [PubMed] [Google Scholar]
- 54.Poewe W, Antonini A. Novel formulations and modes of delivery of levodopa. Mov Disord. 2015;30:114–20. doi: 10.1002/mds.26078. [DOI] [PubMed] [Google Scholar]
- 55.Snow BJ, Macdonald L, McAuley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson's disease: A double blind, placebo-controlled study. Clin Neuropharmacol. 2000;23:82–5. doi: 10.1097/00002826-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Ory-Magne F, Corvol JC, Azulay JP, Bonnet AM, Brefel-Courbon C, Damier P, et al. Withdrawing amantadine in dyskinetic patients with parkinson disease: The AMANDYSK trial. Neurology. 2014;82:300–7. doi: 10.1212/WNL.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 57.Wolf E, Seppi K, Katzenschlager R, Hochschorner G, Ransmayr G, Schwingenschuh P, et al. Long-term antidyskinetic efficacy of amantadine in Parkinson's disease. Mov Disord. 2010;25:1357–63. doi: 10.1002/mds.23034. [DOI] [PubMed] [Google Scholar]
- 58.Vijverman AC, Fox SH. New treatments for the motor symptoms of Parkinson's disease. Expert Rev Clin Pharmacol. 2014;7:761–77. doi: 10.1586/17512433.2014.966812. [DOI] [PubMed] [Google Scholar]
- 59.Bezard E, Hill MP, Crossman AR, Brotchie JM, Michel A, Grimée R, et al. Levetiracetam improves choreic levodopa-induced dyskinesia in the MPTP-treated macaque. Eur J Pharmacol. 2004;485:159–64. doi: 10.1016/j.ejphar.2003.11.065. [DOI] [PubMed] [Google Scholar]
- 60.Tousi B, Subramanian T. The effect of levetiracetam on levodopa induced dyskinesia in patients with Parkinson's disease. Parkinsonism Relat Disord. 2005;11:333–4. doi: 10.1016/j.parkreldis.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Stathis P, Konitsiotis S, Tagaris G, Peterson D. Levetiracetam for the management of levodopa-induced dyskinesias in Parkinson's disease. Mov Disord. 2011;26:264–70. doi: 10.1002/mds.23355. [DOI] [PubMed] [Google Scholar]
- 62.Du H, Nie S, Chen G, Ma K, Xu Y, Zhang Z, et al. Levetiracetam ameliorates L-DOPA-induced dyskinesia in hemiparkinsonian rats inducing critical molecular changes in the striatum. Parkinsons Dis 2015. 2015 doi: 10.1155/2015/253878. 253878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobylecki C, Burn DJ, Kass-Iliyya L, Kellett MW, Crossman AR, Silverdale MA. Randomized clinical trial of topiramate for levodopa-induced dyskinesia in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:452–5. doi: 10.1016/j.parkreldis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Murata M, Hasegawa K, Kanazawa I, Fukasaka J, Kochi K, Shimazu R. Zonisamide improves wearing-off in Parkinson's disease: A randomized, double-blind study. Mov Disord. 2015;30:1343–50. doi: 10.1002/mds.26286. [DOI] [PubMed] [Google Scholar]
- 65.Durif F, Debilly B, Galitzky M, Morand D, Viallet F, Borg M, et al. Clozapine improves dyskinesias in Parkinson disease: A doubleblind, placebo-controlled study. Neurology. 2004;62:381–8. doi: 10.1212/01.wnl.0000110317.52453.6c. [DOI] [PubMed] [Google Scholar]
- 66.Katzenschlager R, Manson AJ, Evans A, Watt H, Lees AJ. Low dose quetiapine for drug induced dyskinesias in Parkinson's disease: A double blind cross over study. J Neurol Neurosurg Psychiatry. 2004;75:295–7. [PMC free article] [PubMed] [Google Scholar]
- 67.Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt MH, Chirilineau D, et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson's disease. Mov Disord. 2014;29:1273–80. doi: 10.1002/mds.25961. [DOI] [PubMed] [Google Scholar]
- 68.Schapira AH, Fox SH, Hauser RA, Jankovic J, Jost WH, Kenney C, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: A randomized clinical trial. JAMA Neurol. 2017;74:216–24. doi: 10.1001/jamaneurol.2016.4467. [DOI] [PubMed] [Google Scholar]
- 69.Mizuno Y, Kondo T. Adenosine A2A receptor antagonist istradefylline reduces daily off time in Parkinson's disease. Mov Disord. 2013;28:1138–41. doi: 10.1002/mds.25418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vorovenci RJ, Antonini A. The efficacy of oral adenosine A2A antagonist istradefylline for the treatment of moderate to severe Parkinson's disease. Expert Rev Neurother. 2015;15:1383–90. doi: 10.1586/14737175.2015.1113131. [DOI] [PubMed] [Google Scholar]
- 71.Cenci MA, Lindgren HS. Advances in understanding L-DOPA-induced dyskinesia. Curr Opin Neurobiol. 2007;17:665–71. doi: 10.1016/j.conb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Svenningsson P, Rosenblad C, Af Edholm Arvidsson K, Wictorin K, Keywood C, Shankar B, et al. Eltoprazine counteracts l-dopa-induced dyskinesias in Parkinson's disease: A dosefinding study. Brain. 2015;138(Pt 4):963–73. doi: 10.1093/brain/awu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lewitt PA, Hauser RA, Lu M, Nicholas AP, Weiner W, Coppard N, et al. Randomized clinical trial of fipamezole for dyskinesia in parkinson disease (FJORD study) Neurology. 2012;79:163–9. doi: 10.1212/WNL.0b013e31825f0451. [DOI] [PubMed] [Google Scholar]
- 74.Van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, et al. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J. 2005;19:1140–2. doi: 10.1096/fj.04-3010fje. [DOI] [PubMed] [Google Scholar]
- 75.Potts LF, Park ES, Woo JM, Dyavar Shetty BL, Singh A, Braithwaite SP, et al. Dual?-agonist/μ-antagonist opioid receptor modulation reduces levodopa-induced dyskinesia and corrects dysregulated striatal changes in the nonhuman primate model of parkinson disease. Ann Neurol. 2015;77:930–41. doi: 10.1002/ana.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez-Ramirez J, Johnston TH, Visanji NP, Fox SH, Brotchie JM. Histamine H3 receptor agonists reduce L-dopa-induced chorea, but not dystonia, in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov Disord. 2006;21:839–46. doi: 10.1002/mds.20828. [DOI] [PubMed] [Google Scholar]