Abstract
Introduction:
Acute intermittent porphyria (AIP) is an inherited metabolic disease characterized by disordered heme biosynthesis. There is no recent study reported from India.
Materials and Methods:
It was a retrospective, observational study. Clinical records of patients of AIP with acute porphyric attacks admitted from April 2008 to December 2016 were analyzed.
Results:
Fifteen AIP patients constituted of eight females and seven males were analyzed. Mean age at presentation was 34.33 ± 15.86 years. Thirteen patients (86.67%) had acute flaccid paralysis (AFP). All of them had peripheral neuropathy. These patients concomitantly had abdominal pain, seizure, encephalopathy, autonomic hyperactivity, history of passage of dark urine, and electrolyte abnormality (hyponatremia) in various combinations. Abdominal pain was the presenting symptom in 11 (73.33%) patients. Seven (46.67%) patients had seizure episodes. Five patients (33.33%) had hyponatremia at presentation. Significantly higher percentage of them had seizure at presentation or during hospital stay (P = 0.007). These patients also had evidence of autonomic hyperactivity in the form of higher pulse rate, systolic and diastolic blood pressure at presentation. They had prolonged duration of hospital stay as well (P = 0.016). Eleven patients had partial recovery and rest four patients (26.67%) had in-hospital mortality.
Conclusion:
Patients had severe neurological involvement manifesting mainly as AFP and seizure episodes. We recommend screening for AIP in patients presenting with features of AFP along with any combination of clinical/laboratory manifestations such as abdominal pain, seizure, encephalopathy, autonomic hyperactivity, passage of dark urine, and hyponatremia. Electrolyte abnormality in the form of hyponatremia was an important severity marker.
Keywords: Acute flaccid paralysis, acute intermittent porphyria, hyponatremia, peripheral neuropathy, seizure
INTRODUCTION
Porphyrias, inherited metabolic disorders secondary to enzyme defects in the pathway for heme biosynthesis, are associated with the generation of excess of porphyrin intermediates or their precursors.[1] Depending on the major site of expression of the underlying enzyme deficiency, these disorders are divided into hepatic and erythropoietic forms. However, from a general physician's point of view, it is more appropriate to classify the porphyrias into acute and nonacute forms, thereby emphasizing the presence or absence of potentially life-threatening acute porphyric attacks. There are four types of porphyrias in which acute attacks can occur: acute intermittent porphyria (AIP), hereditary coproporphyria, variegate porphyria (VP), and aminolevulinic acid (ALA) dehydratase deficiency porphyria.[1,2,3,4,5] AIP is the most frequently encountered porphyria. It is inherited in autosomal dominant pattern with variable penetrance. Therefore, genetic mutation frequencies for AIP is higher than the prevalence of clinically overt cases.[1,2,3,4,5,6] Various studies report that 90% of individuals with putative genetic mutation for AIP or VP are clinically latent.[1,2] Studies report wide variability in prevalence also. In a European 3-year prospective study, the prevalence of clinically overt AIP was 5.4 cases per million.[7] There is no large-scale epidemiological study undertaken on this disease from India though small case series have been reported mainly from the parts of Northwestern India. These patients present with varied symptomatology. As clinical symptoms observed during an acute porphyric attack involve multiple systems, these patients may report to number of doctors, including physicians, gastroenterologists, surgeons, gynecologists, and neurologists. Abdominal pain is the most common presenting clinical feature. Vomiting, autonomic instability, convulsions, and weakness of limbs are other clinical manifestations noted in these patients.[1,2,3,4,5,6] Symptom complex is so variable and misleading that this condition is often misdiagnosed. A high index of suspicion is required for accurate diagnosis and management. There is no recent study reported from the Indian subcontinent describing clinical profile and hospital course of acute porphyric attack requiring hospital care in these patients. We thus decided to undertake this descriptive study to report the demographic, clinical, biochemical features and hospital course of AIP patients with acute attacks.
MATERIALS AND METHODS
This retrospective, observational study was undertaken at Postgraduate Institute of Medical Education and Research, Chandigarh, a tertiary care referral hospital in Northern India. This institute caters to health needs of people of Chandigarh, Punjab, Himachal Pradesh, Haryana, Uttar Pradesh, Rajasthan, Uttarakhand, and Jammu - Kashmir. Around 82,014 patients were admitted while 89,550 patients attended emergency services of this tertiary care hospital in the year 2015–2016.
Clinical records of patients of AIP with acute porphyric attacks admitted from April 2008 to December 2016 were reviewed. We recorded all relevant patients’ details in a predesigned proforma. The medical record library uses the International Statistical Classification of Diseases and Related Health Problems-10 system for classification of diseases. A unique code number is assigned to each disease. We retrieved medical records of AIP patients using the specific code number assigned to this disease entity and recorded data of AIP patients presenting with acute attacks. AIP was diagnosed on the basis of typical clinical history, suggestive of acute neurovisceral attacks including pain abdomen, dysautonomia, neuropsychiatric manifestations, and urine porphobilinogen (PBG) positivity.[1,2,3,4,5,6] Various parameters recorded were sociodemographic profile and clinical variables at the time of admission including any past history of acute attacks, potential precipitating factors, clinical symptoms, and physical findings. Laboratory data included serum electrolytes, renal function and liver function test. Watson–Schwartz test was employed for urine PBG detection. Reports of imaging modalities including ultrasonography (USG), contrast-enhanced computed tomography, and magnetic resonance imaging (MRI) of relevant organs if done were noted. Hospital course of each patient was recorded specifically documenting type of treatment offered, complications developed, and finally hospital outcome.
Statistical analysis
Statistical analysis was performed using the statistical software SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed using mean (standard deviation) and median (interquartile range [IQR]). We divided the study cohort into groups based on gender, presence/absence of abdominal pain, and finally presence/absence of hyponatremia at presentation. Differences between means of continuous variables were compared using the unpaired Student's t-test and that of categorical variables with the Fisher's exact test. Level of significance was expressed as P value. P < 0.05 was considered statistically significant.
RESULTS
This was a retrospective, observational study undertaken in a large tertiary care hospital at North India. We analyzed the case records of 15 AIP patients. Study cohort constituted of eight female and seven male patients. Mean age at presentation was 34.33 ± 15.86 years (range = 15–61 years). A history of precipitating factors could be elicited in six patients. A history of intake of potentially porphyrinogenic medications was noted in three patients. Infection was the precipitating factor in other three patients. Four patients had a past history of acute attacks.
Abdominal pain was noted as the presenting symptom in 11 (73.33%) patients. Diffuse, poorly localized abdominal pain was most common, seen in eight patients. Other gastrointestinal manifestations in the form of vomiting were noticed in seven patients, and three patients had constipation. Seven (46.67%)patients had seizure episodes either at presentation or during hospital stay. All except one had generalized tonic–clonic seizure. One patient had past history of seizure as well. They were managed with levetiracetam (five), gabapentin (four), and benzodiazepines (three). Fourteen (93.30%) patients had history of motor weakness. Thirteen patients (86.67%) had acute flaccid paralysis (AFP). All of them had documented peripheral neuropathy. One patient had weakness secondary to osmotic demyelination syndrome. These patients concomitantly had abdominal pain, seizure, encephalopathy, autonomic hyperactivity, history of passage of dark urine, and electrolyte abnormality (especially hyponatremia) in various combinations. Screening for urinary PBG was prompted in these patients by concurrent presence of these clinical features and laboratory abnormality. Nine patients (60%) had respiratory muscle weakness. Sensory symptoms in the form of paresthesia were seen in six (40%) patients. Five patients (33.33%) had passage of dark-colored urine. Vital parameters at presentation essentially revealed tachycardia and higher blood pressure, suggesting sympathetic nervous system hyperactivity. Further details of sociodemographic and clinical variables including vital parameters at presentation are elaborated in Table 1. Mean total leukocyte count and erythrocyte sedimentation rate were higher at presentation. Hyponatremia was noted in five patients. Two patients each had moderate and severe degree of hyponatremia. Further details of various hematological and biochemical parameters at presentation are documented in Table 1.
Table 1.
Details of demographic data, clinical, laboratory parameters at baseline and hospital outcome of 15 acute intermittent porphyria patients
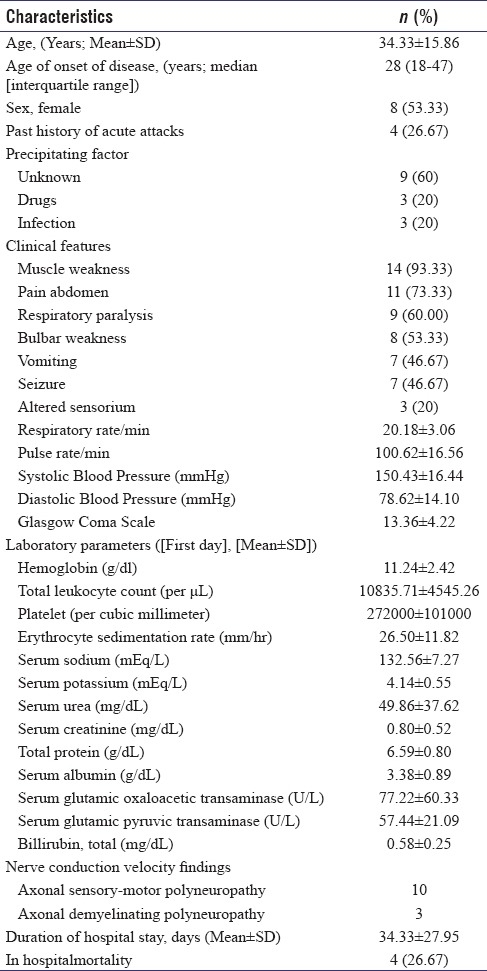
Among radiology investigations, USG abdomen showed cholelithiasis in one patient. MRI brain was done in two patients. It showed T2 flair hyperintensities in caudate nucleus and globus pallidus in one and showed changes of pontine/extrapontine myelinolysis in another patient. The first patient presented with abdominal pain and one episode of generalized tonic–clonic seizure. There was a history of progressive weakness of lower and upper limbs as well. Other patient had complaints of intermittent abdominal pain of 5 months duration. She had two episodes of seizures, followed by alteration of sensorium. Later, physical examination showed findings of spastic quadriplegia suggesting osmotic demyelination syndrome which was corroborated on MRI of the brain.
Nerve conduction study was undertaken in 13 patients. Ten patients showed changes suggestive of axonal motor-sensory polyneuropathy, while three patients showed changes of axonal and demyelinating polyneuropathy. Urine for PBG was done in all patients on three consecutive days and was positive in all of them. Three patients also underwent PBG/ALA quantitation in urine sample which showed raised levels. Median duration of hospital stay was 15 days (IQR = 11–34 days). Eleven patients had partial recovery and rest four (26.67%) had in-hospital mortality. Among those 11 survivors, seven patients with abdominal pain had complete resolution of pain with administration of high-dose dextrose and opioid analgesics. Three patients with seizure showed subsidence of further episodes. Ten patients had motor weakness. Out of them, six patients showed partial improvement in motor symptoms, while in remaining four, motor weakness did not progress further. All of 11 patients had shown improvement in autonomic reactivity.
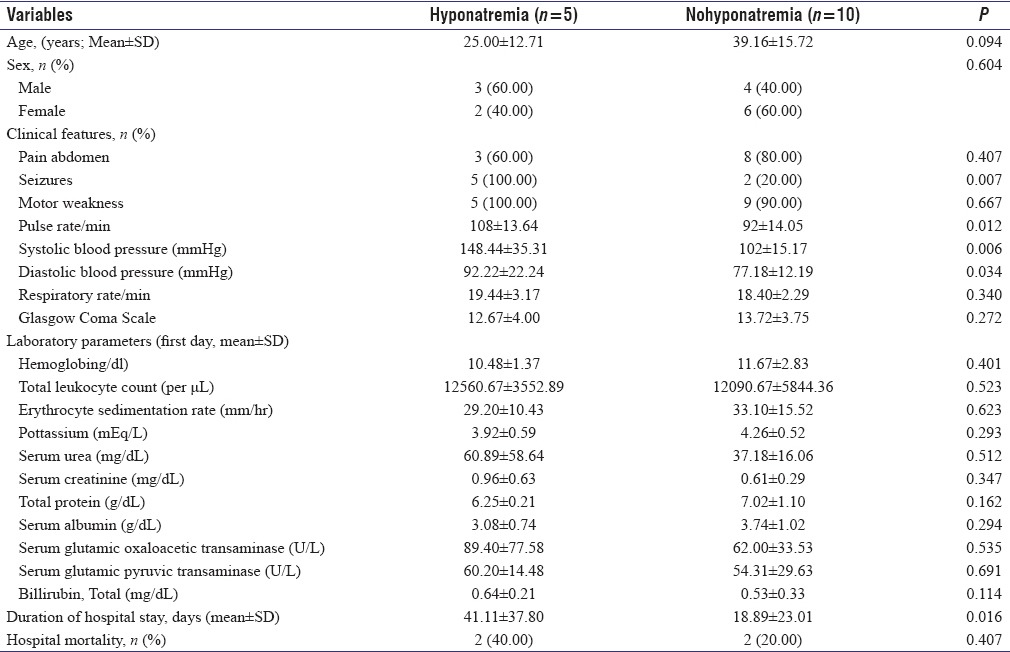
We did univariate analysis after dividing the study cohort into various subgroups based on gender, presence/absence of abdominal pain, and finally presence/absence of hyponatremia at presentation. There was no difference among various sociodemographic and clinical parameters between male and female patients. Four patients in the index study did not have abdominal pain at presentation. Patients with atypical presentation in the form of absent abdominal pain were older ([43.67 ± 20.95 vs. 26.68 ± 12.25 years]; P = 0.016) and had significantly prolonged hospital stay ([60.33 ± 37.83 vs. 16.57 ± 17.59 days]; P = 0.011). Five patients in the index study had hyponatremia at presentation. Significantly higher percentage of patients had seizures at presentation or during hospital stay in hyponatremic group (5/5 [100.00%] vs. 2/10 [20.00%]; P = 0.007). These patients had evidence of autonomic hyperactivity in the form of significantly higher pulse rate ([108 ± 13.64 vs. 92 ± 14.05/min]; P = 0.012), systolic blood pressure ([148.44 ± 35.31 vs. 102 ± 15.17 mmHg]; P = 0.006) and diastolic blood pressure ([92.22 ± 22.24 vs. 77.18 ± 12.19 mmHg]; P = 0.034) at presentation. These patients also had prolonged duration of hospital stay ([41.11 ± 37.80 vs. 18.89 ± 23.01 days]; P = 0.016). Detailed comparison of various clinical, laboratory variables, length of hospital stay, and in-hospital mortality among these subgroups has been provided in Tables 2 and 3. We had previously reported 13 patients of AIP with acute attacks admitted from January 1996 to March 2008 from our tertiary care center.[8] We pooled the data of these 28 patients. Six patients, four in index and two in past study, did not have abdominal pain at presentation. Nine patients, five in index and four in past study, had hyponatremia at presentation. Univariate analysis after dividing the study cohort into various subgroups based on sex, presence/absence of abdominal pain, and presence/absence of hyponatremia yielded similar results as noted above.
Table 2.
Comparison of various clinical, laboratory variables, length of hospital stay and in-hospital mortality among 15 acute intermittent porphyria patients with or without pain abdomen
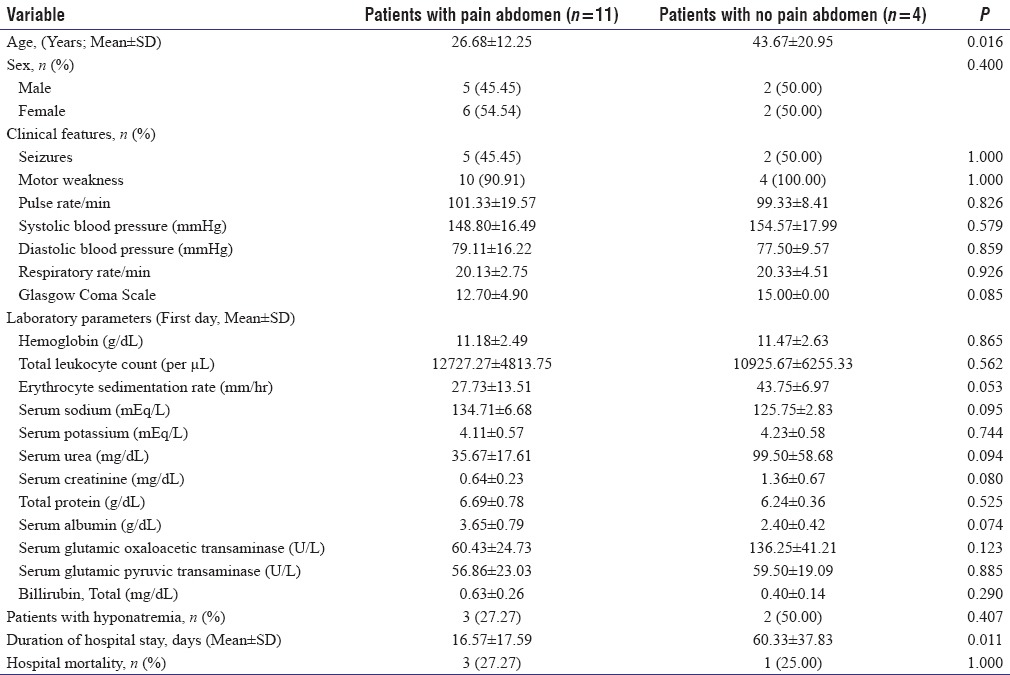
Table 3.
Comparison of various clinical, laboratory variables, length of hospital stay and in-hospital mortality among 15 acute intermittent porphyria patients with or without hyponatremia at presentation
DISCUSSION
This was a retrospective, observational study involving 15 AIP patients presenting during acute porphyric attack and admitted in a tertiary care referral center in North India. We found that acute attacks in majority occurred after puberty. There was preponderance of female patients. Patients had severe neurological involvement manifesting as AFP and seizure episodes. Patients with absent abdominal pain were older and also had prolonged hospital stay. Those with hyponatremia at presentation had more severe clinical manifestations.
AIP is characterized by acute neurovisceral attacks, which may be recurrent in some patients. These attacks most commonly occur between the ages of 15 and 45 years and are rare before puberty.[1,2,3,4,5,6] Attacks in the index study in majority occurred after puberty. Females predominated our study group. Previous studies have also reported porphyric attacks to be 4–5 times more common in females. Sex hormones especially progesterone is a potent inducer of 5-ALA synthase (ALAS), the rate-limiting enzyme in heme biosynthetic pathway.[9,10] Other potential precipitating factors include drugs, infection, starvation, stress, alcohol, and surgery.[1,2,3,4,5,6] In our study, drugs and infections were major triggering factors.
Abdominal pain was one of the common presenting symptoms in the index study. This finding is in consonance with various other studies reported in literature.[1,2,3,4,5,6] The pathophysiologic basis for abdominal pain has been postulated to be autonomic hyperactivity, leading to vasospasm and resultant intestinal ischemia. Intestinal dysmotility secondary to dysautonomia might also contribute to abdominal pain.[11] Atypical presentation in the form of absence of abdominal pain initially was noted in four of our patients. Absence or disappearance of abdominal pain in patients, especially with evolving neurological manifestation in the past, had been reported.[12,13,14] Clinicians should be aware of this fact. In the index study, these patients with atypical presentation had motor weakness of limbs and seizure. Two of them had reported passage of dark urine. They also had physical signs suggesting autonomic hyperactivity and laboratory abnormality in the form of hyponatremia. These symptoms/signs complex and laboratory abnormality prompted us to consider AIP as a diagnostic possibility. These patients were older and had prolonged hospital stay. Due to its rarity and relatively nonspecific initial clinical manifestations, AIP patients are likely to be diagnosed late in the course of their disease process. Presentation with minimal or absent abdominal pain results in further delay in the definitive diagnosis. In the index study, these patients were diagnosed late when they had developed florid neurological complications resulting in prolonged hospital stay.
Neurological manifestations dominated the clinical presentation in the index study. Fourteen patients had motor weakness and seven patients had seizure episodes. In a descriptive study from Finland, patients with acute porphyric attacks from 1967 to 1989 were analyzed. Twenty-one (18%) of them had motor weakness concomitantly.[15] Eight patients (57%) developed peripheral neuropathy in an observational study reported from South Africa. Two patients presented in acute quadriparesis while remaining had mild weakness of the extremities manifesting as wrist-drop and foot-drop.[16] In another study, 55 (38%) out of 143 AIP patients had one or more acute porphyric attacks. Motor weakness was documented in 13 patients before diagnosis of AIP. A variety of endogenous and exogenous precipitating factors contributed to paresis in this study.[13] In our study, predominantly, exogenous factors were associated with motor weakness. In a retrospective observational study, clinical characteristics of 13 patients of AIP were analyzed. Presentation in the form of acute quadriparesis/plegia was reported in seven patients (53.8%) patients. Peripheral neuropathy was noted in five of them.[8] In a prospective, observational study reported from North India, 133 consecutive adult patients presenting with weakness of duration <4 weeks over 12 months period were analyzed. Neuroparalytic snake envenomation (51.9%) was the most common etiology. AIP as cause of AFP was noted in 4.5% patients.[17] In a descriptive observational study, among 108 patients presenting with features of acute polyneuropathy or encephalopathy associated with pain and/or dysautonomia, porphyrin metabolites were screened. AIP was diagnosed in 12 patients.[14] We also as a routine screen for AIP in every patient presenting with features of AFP along with any combination of symptoms in the form of encephalopathy/seizures/dysautonomia and pain involving abdomen/back/muscles with electrolyte abnormality in the form of hyponatremia. We therefore recommend that in patients with these constellations of symptoms/signs/laboratory abnormality, urine screening for PBG/ALA should be undertaken.
Seizures are reported to occur in 3%–20% of patients of AIP.[18,19] In a descriptive study reported from Sweden, out of 268 patients of AIP queried, 10 (3.7%) had reported seizures. Seizure prevalence was 2.2% in all those with known AIP and 5.1% in all those with manifest AIP.[19] In our study, six patients had seizure as one of clinical manifestation during present acute attack. In a study from the Indian subcontinent, four out of six patients had gastrointestinal symptoms and seizures at the time of presentation.[20] A descriptive observational study from South Africa reported seizures in five AIP attacks.[13] Clinical, biochemical, and genetic features of ninety AIP patients were reported in an observational study from the USA. Seizure was noted in 9% of patients.[21] Twelve AIP patients were analyzed in a retrospective study. Out of nine patients with central nervous system involvement, seizures were reported in four patients.[22] Management of seizure episodes is especially challenging in AIP patients. Many of conventional antiepileptic medications are potentially porphyrinogenic and thus contraindicated. Inadvertent use of them has been reported to precipitate severe acute porphyric attacks manifesting as AFP. Anti-epileptic medications documented to be safe during porphyric attacks are benzodiazepine, gabapentin, and levetiracetam.[23,24,25] For uncontrolled status epilepticus, propofol infusion has been reported to be safe.[26]
The pathophysiologic basis of CNS and peripheral nervous system involvement in AIP is not entirely clear. Heme-containing proteins are required for many life-sustaining functions such as oxygen transport, electron transport, and cytochrome P450 system.[3] Deficient heme production thus results in disruption in axonal transport resulting in axonal degeneration. Decreased enzyme activity also leads to excess production of ALA and PBG which are potentially neurotoxic agents. There are structural similarities between ALA and gamma-amino butyric acid (GABA), resulting in ALA interfering and interacting with GABA receptors. This may be responsible for occurrence of seizures during acute porphyric attacks. Deficient heme production results in lower mitochondrial cytochrome levels and impairs ATP production in neurons. 5-Hydroxytryptophan and serotonin in the nervous system are produced in excess secondary to heme deficiency.[1,2,3,4,5,6,27,28] It might contribute to some of psychiatric symptoms as well as peripheral neuropathies. Axonal form of neuropathy is the predominant porphyric neuropathy. Our study also showed axonal neuropathy on electrodiagnostic study in 13 patients with motor weakness.
Electrolyte abnormality in the form of hyponatremia has been described in patients with acute attacks.[1,2,3,4,5,6] In the index study, five patients had hyponatremia at presentation. We found patients with hyponatremia at presentation were younger. These patients had more severe presentation indicating it as a severity marker of primary disease process. Various mechanisms have been postulated for this electrolyte abnormality. Dehydration, syndrome of inappropriate antidiuretic hormone, and primary tubular defect with salt wasting are predominant among them.[1,2,3,4,5,6,13] High dose of dextrose can contribute as well. In the index study, this is not the case as we documented the sodium levels at presentation. Hift and Meissner reported hyponatremia in one-third of the total 112 attacks in 25 patients (15 with AIP and 10 with VP).[16] In this study as well, hyponatremic patients had more severe attacks. In another study, Morales Ortega et al. reported hyponatremia in 53% of the acute attacks.[29]
Comprehensive approach to management of acute attacks essentially includes relief of symptoms including visceral pain, vomiting, sympathetic overactivity, and any electrolyte abnormality. It is imperative to discontinue all potentially porphyrinogenic medications. The aim of treatment is to reduce the activity of ALAS 1 (hepatic). Heme arginate is the definitive medication and very effective if given early in disease course. Recommended dose is 3–5 mg hematin/kg bodyweight once daily for 3–5 days. Patient also should receive at least 300 g/day of carbohydrate daily.[1,2,3,4,5,6] In our study, all patients received high dose dextrose; however, as heme arginate is not available, it could not be given to any patient. Potentially harmful drugs were discontinued in all patients as well.
Four patients (26.67%) died during the index admission. In studies by Morales Ortega et al. and De Siervi et al., 15% of patients died during the acute attack.[29,30] In a study by Hift and Meissner, three patients died during acute attack.[16]
There are many reasons behind severe neurological presentation in our study. Ours was a highly selected cohort of patients with severe symptoms presenting in a tertiary care referral hospital. The symptom complex of this disorder is also so variable and misleading that this condition is often misdiagnosed. Importantly, lack of awareness among physicians leads to delay in diagnosis and initiation of appropriate treatment, resulting in the development of multiple complications. Use of potentially porphyrinogenic drugs during acute attacks because of aforementioned reasons may have led to worsening of symptoms. Other important cause is unavailability of the specific therapy; heme arginate most of the time in this part of world and also due to prohibitive cost is out of reach of most of people if it is imported.
CONCLUSION
Gastrointestinal and neurological manifestations predominated our study cohort. Patients had severe neurological involvement manifesting as motor weakness and seizure episodes. We recommend screening for AIP in patients presenting with features of AFP along with any combination of clinical/laboratory manifestations such as abdominal pain, seizure, encephalopathy, autonomic hyperactivity, history of passage of dark urine, and hyponatremia. Patients with absent abdominal pain were older and also had prolonged hospital stay. Those with hyponatremia at presentation had more severe clinical manifestations, indicating it as a severity marker of underlying primary disease process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Puy H, Gouya L, Deybach JC. The Porphyrias. Lancet. 2010;375:924–37. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- 2.Arora S, Young S, Kodali S, Singal AK. Hepatic porphyria: A narrative review. Indian J Gastroenterol. 2016;35:405–18. doi: 10.1007/s12664-016-0698-0. [DOI] [PubMed] [Google Scholar]
- 3.Stein PE, Badminton MN, Rees DC. Update review of the acute porphyrias. Br J Haematol. 2017;176:527–38. doi: 10.1111/bjh.14459. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142:439–50. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Ramanujam VM, Anderson KE. Porphyria diagnostics-part 1: A brief overview of the porphyrias. Curr Protoc Hum Genet. 2015;86:17.20–1.26. doi: 10.1002/0471142905.hg1720s86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201–14. doi: 10.2147/TACG.S48605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elder G, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis. 2013;36:849–57. doi: 10.1007/s10545-012-9544-4. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Sharma N, Modi M, Sharma A, Mahi S, Varma S. Spectrum of emergency department presentation in patients of acute intermittent porphyria: Experience from a North Indian tertiary care center. Neurol India. 2010;58:95–8. doi: 10.4103/0028-3886.60410. [DOI] [PubMed] [Google Scholar]
- 9.Andersson C, Innala E, Bäckström T. Acute intermittent porphyria in women: Clinical expression, use and experience of exogenous sex hormones. A population-based study in Northern Sweden. J Intern Med. 2003;254:176–83. doi: 10.1046/j.1365-2796.2003.01172.x. [DOI] [PubMed] [Google Scholar]
- 10.Schuurmans MM, Schneider-Yin X, Rüfenacht UB, Schnyder C, Minder CE, Puy H, et al. Influence of age and gender on the clinical expression of acute intermittent porphyria based on molecular study of porphobilinogen deaminase gene among Swiss patients. Mol Med. 2001;7:535–42. [PMC free article] [PubMed] [Google Scholar]
- 11.Lithner F. Could attacks of abdominal pain in cases of acute intermittent porphyria be due to intestinal angina? J Intern Med. 2000;247:407–9. doi: 10.1046/j.1365-2796.2000.00653.x. [DOI] [PubMed] [Google Scholar]
- 12.Andersson C, Nilsson A, Bäckström T. Atypical attack of acute intermittent porphyria – Paresis but no abdominal pain. J Intern Med. 2002;252:265–70. doi: 10.1046/j.1365-2796.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 13.von Und Zu Fraunberg M, Pischik E, Udd L, Kauppinen R. Clinical and biochemical characteristics and genotype-phenotype correlation in 143 Finnish and Russian patients with acute intermittent porphyria. Medicine (Baltimore) 2005;84:35–47. doi: 10.1097/01.md.0000152455.38510.af. [DOI] [PubMed] [Google Scholar]
- 14.Pischik E, Kazakov V, Kauppinen R. Is screening for urinary porphobilinogen useful among patients with acute polyneuropathy or encephalopathy? J Neurol. 2008;255:974–9. doi: 10.1007/s00415-008-0779-9. [DOI] [PubMed] [Google Scholar]
- 15.Kauppinen R, Mustajoki P. Prognosis of acute porphyria: Occurrence of acute attacks, precipitating factors, and associated diseases. Medicine (Baltimore) 1992;71:1–13. [PubMed] [Google Scholar]
- 16.Hift RJ, Meissner PN. An analysis of 112 acute porphyric attacks in Cape Town, South Africa: Evidence that acute intermittent porphyria and variegate porphyria differ in susceptibility and severity. Medicine (Baltimore) 2005;84:48–60. doi: 10.1097/01.md.0000152454.56435.f3. [DOI] [PubMed] [Google Scholar]
- 17.Kaushik R, Kharbanda PS, Bhalla A, Rajan R, Prabhakar S. Acute flaccid paralysis in adults: Our experience. J Emerg Trauma Shock. 2014;7:149–54. doi: 10.4103/0974-2700.136847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonkowsky HL, Schady W. Neurologic manifestations of acute intermittent porphyria. Semin Liver Dis. 1982;2:108–24. doi: 10.1055/s-2008-1040701. [DOI] [PubMed] [Google Scholar]
- 19.Bylesjö I, Forsgren L, Lithner F, Boman K. Epidemiology and clinical characteristics of seizures in patients with acute intermittent porphyria. Epilepsia. 1996;37:230–5. doi: 10.1111/j.1528-1157.1996.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 20.Kochar DK, Pal M, Kochar SK, Vyas A, Kochar A, Bindal D, et al. Acute intermittent porphyria presenting with neurological emergency: Review of six cases. Neurol India. 2007;55:413–5. doi: 10.4103/0028-3886.33303. [DOI] [PubMed] [Google Scholar]
- 21.Bonkovsky HL, Maddukuri VC, Yazici C, Anderson KE, Bissell DM, Bloomer JR, et al. Acute porphyrias in the USA: Features of 108 subjects from porphyrias consortium. Am J Med. 2014;127:1233–41. doi: 10.1016/j.amjmed.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo HC, Huang CC, Chu CC, Lee MJ, Chuang WL, Wu CL, et al. Neurological complications of acute intermittent porphyria. Eur Neurol. 2011;66:247–52. doi: 10.1159/000330683. [DOI] [PubMed] [Google Scholar]
- 23.Zadra M, Grandi R, Erli LC, Mirabile D, Brambilla A. Treatment of seizures in acute intermittent porphyria: Safety and efficacy of gabapentin. Seizure. 1998;7:415–6. doi: 10.1016/s1059-1311(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 24.Zaatreh MM. Levetiracetam in porphyric status epilepticus: A case report. Clin Neuropharmacol. 2005;28:243–4. doi: 10.1097/01.wnf.0000185828.80561.ad. [DOI] [PubMed] [Google Scholar]
- 25.Bilo L, Meo R, Fulvia de Leva M. Levetiracetam in idiopathic generalised epilepsy and porphyria cutanea tarda. Clin Drug Investig. 2006;26:357–9. doi: 10.2165/00044011-200626060-00007. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia R, Vibha D, Srivastava MV, Prasad K, Tripathi M, Bhushan Singh M. Use of propofol anesthesia and adjunctive treatment with levetiracetam and gabapentin in managing status epilepticus in a patient of acute intermittent porphyria. Epilepsia. 2008;49:934–6. doi: 10.1111/j.1528-1167.2007.01518_2.x. [DOI] [PubMed] [Google Scholar]
- 27.Solis C, Martinez-Bermejo A, Naidich TP, Kaufmann WE, Astrin KH, Bishop DF, et al. Acute intermittent porphyria: Studies of the severe homozygous dominant disease provides insights into the neurologic attacks in acute porphyrias. Arch Neurol. 2004;61:1764–70. doi: 10.1001/archneur.61.11.1764. [DOI] [PubMed] [Google Scholar]
- 28.Meyer UA, Schuurmans MM, Lindberg RL. Acute porphyrias: Pathogenesis of neurological manifestations. Semin Liver Dis. 1998;18:43–52. doi: 10.1055/s-2007-1007139. [DOI] [PubMed] [Google Scholar]
- 29.Morales Ortega X, Wolff Fernández C, Leal Ibarra T, Montaña Navarro N, Armas-Merino R. Porphyric crisis: Experience of 30 episodes. Medicina (B Aires) 1999;59:23–7. [PubMed] [Google Scholar]
- 30.De Siervi A, Rossetti MV, Parera VE, Mendez M, Varela LS, del C Batlle AM. Acute intermittent porphyria: Biochemical and clinical analysis in the Argentinean population. Clin Chim Acta. 1999;288:63–71. doi: 10.1016/s0009-8981(99)00139-4. [DOI] [PubMed] [Google Scholar]


