Abstract
ATP7A is a P-type ATPase essential for cellular copper (Cu) transport and homeostasis. Loss-of-function ATP7A mutations causing systemic Cu deficiency are associated with severe Menkes disease or its milder allelic variant, occipital horn syndrome. We previously identified two rare ATP7A missense mutations (P1386S and T994I) leading to a non-fatal form of motor neuron disorder, X-linked distal hereditary motor neuropathy (dHMNX), without overt signs of systemic Cu deficiency. Recent investigations using a tissue specific Atp7a knock out model has demonstrated that Cu has an essential role in motor neuron maintenance and function, however the underlying pathogenic mechanisms of ATP7A mutations causing axonal degeneration remain unknown. We have generated an Atp7a conditional knock in mouse model of dHMNX expressing Atp7aT985I, the orthologue of the human ATP7AT994I identified in dHMNX patients. Although a degenerative motor phenotype is not observed, the knock in Atp7aT985I/Y mice show altered Cu levels within the peripheral and central nervous systems, increased diameter of the muscle fibres and altered myogenin and myostatin gene expression. Atp7aT985I/Y mice have reduced Atp7a protein levels and recapitulate the defective trafficking and altered post-translational regulatory mechanisms observed in the human ATP7AT994I patient fibroblasts. Our model provides a unique opportunity to characterise the molecular phenotype of dHMNX and the time course of cellular events leading to the process of axonal degeneration in this disease.
Introduction
Copper (Cu) is a trace element essential for the normal growth and development of all living organisms. In particular, Cu plays a crucial role in the development and function of the central nervous system (CNS) with involvement in processes including neurodevelopment, synaptogenesis, axon extension 1, modulation of neurotransmitter receptor activity and synaptic transmission 2. Cu dysregulation constitutes a key pathological process in many neurodegenerative disorders, including those where length dependent axonal degeneration occurs such as motor neuron disease (MND) 3 and Parkinson’s disease 4, 5. Cu uptake, transport and utilisation are tightly regulated by an integrated network of proteins (reviewed in 6). Among these proteins is ATP7A, a Cu-transporting P-type ATPase with a dual role of Cu export across the plasma membrane to maintain Cu homeostasis and providing Cu to Cu-dependent enzymes at the trans-Golgi network (TGN). ATP7A executes this dual function through unique trafficking properties. Under normal Cu conditions ATP7A primarily localizes to the TGN. However, when cells are exposed to increased Cu levels, ATP7A traffics to the cell periphery to export the metal from the cell to maintain cellular Cu levels below toxic concentrations 7–9.
Mutations in the ATP7A gene cause Menkes disease (MD) 10–12 and a milder allelic variant occipital horn syndrome (OHS) 13. Both of these disorders have similar clinical and biochemical manifestations in which Cu metabolism is severely affected, although neither of the disorders is reported to be associated with motor neuron dysfunction. MD is caused by profound loss-of-function mutations 14–18 whereas OHS is associated with residual Cu transport, often via leaky splice-junction mutations 13, 19 (see review 20). We previously identified two missense mutations (T994I and P1386S) in different unrelated families with a form of X-linked hereditary distal motor neuropathy (dHMNX) 21. This non-fatal form of MND does not have overt signs of systemic Cu-dependent enzyme dysfunction 21. A mouse model in which the Atp7a gene has been selectively knocked out in motor neurons 22 has provided important evidence for the role of Atp7a and Cu in the maintenance and function of motor neurons. This model however, is unable to elucidate the subtle cellular pathomechanisms of the dHMNX point mutations that lead to axonal degeneration in motor neurons. To more closely mimic the natural disease progression observed in dHMNX patients, we generated an Atp7a conditional knock in mouse model of dHMNX (to be known as Atp7aT985I). Our model introduces the human ATP7AT994I dHMNX mutation into the mouse orthologue. Although a degenerative motor neuropathy phenotype was not observed, we have evidence for nervous system-specific Cu dysregulation, structural and gene expression changes in the muscles from the knock in mice and a molecular phenotype in Atp7aT985I primary cells that recapitulate pathogenic cellular events observed in dHMNX patient cells with the T994I mutation. This model represents a unique opportunity to further explore the molecular phenotype of the human disease by examining the time course of early cellular events leading to axonal degeneration in the dHMNX patients.
Materials and methods
Generation of Atp7aT985I knock in mice
The Atp7aT985I knock in mice were generated by Ozgene (Perth, Australia). The dHMNX ATP7AT994I mutation was introduced into the mouse Atp7a orthologue to generate Atp7aT985I using the “mini cDNA” strategy 23. C57BL/6J knock in mice were generated which expressed the wild type cDNA for Atp7a exons 15–23 (including a terminating stop codon) which was flanked by lox P sites. Targeted DNA containing the T985I knock in mutation was inserted immediately downstream of the floxed region. These mice were initially crossed with Flp deleter knock in mice for Flp recombinase mediated removal of the PGK-neo selection marker flanked by the frt sequences. Offspring generated minus the PGK-neo cassette were then crossed with OzCre mice (a Cre-deleter line developed by Ozgene) to produce offsprings in which the wild type cDNA is excised and Atp7a containing the T985I mutation is expressed from the time of conception. Experimental genotypes included knock in males (Atp7aT985I/Y), carrier females (Atp7aT985I/+), homozygous females (Atp7aT985I/T985I) and wild type males and females (Atp7a+/Y and Atp7a+/+, respectively). The following genotyping primers were designed for testing both the germline target construct transmission as well as genotyping the experimental animals after Cre-lox recombination: P1: CCTACTTTCCCGTAAGTGACTCAT; P2: AGTATGAAGGGAGAAACAGCTGAG; P3: AGGATCTCCTGTCATCTCACCTT. In the experiments involving mouse embryonic fibroblasts, the sex of the dissected embryos was determined using a previously designed PCR assay to co-amplify the X-chromosome and the Y-chromosome-specific genes, Jarid1c and Jarid1d respectively, 24 using the following primers: mSexF: CTGAAGCTTTTGGCTTTGAG and mSexR: CCACTGCCAAATTCTTTGG.
Human primary fibroblasts cultures
Individuals participating in this study provided written consent according to protocols approved by the Sydney Local Health District Human Ethics Review Committee, Concord Repatriation General Hospital, Sydney, Australia (HREC/11/CRGH/105). Primary fibroblasts were cultured from skin biopsies of 3 clinically normal subjects and a dHMNX patient harboring the ATP7AT994I mutation. Cultures were maintained in F-DMEM media consisting of DMEM (Gibco, Life technologies) supplemented with 10% (v/v) fetal bovine serum (SAFC Biosciences), 1% (v/v) Penicillin Streptomycin and 1% (v/v) L-glutamine (Gibco, Life technologies) at 37 °C and 5% CO2.
Mouse embryonic fibroblasts (MEF) cultures
MEF were derived from day 13.5 embryos (E13.5). Tissue from the embryos was washed with PBS and manually dissociated using a sterile scalpel blade. 1ml of trypsin (0.5% v/v) was added to the dissociated fetal tissue and the embryo suspension pipetted intensively to obtain a single cell suspension and incubated at 37°C for 20 min. The trypsin was neutralized by adding F-DMEM (6 ml) supplemented with 10% (v/v) MEM Non-Essential Amino Acid Solution (Gibco, Life technologies), mixed and each suspension transferred to a T25 flask and maintained at 37 °C and 5% CO2
Western blotting
Brain and spinal cord were dissected from 6 month old animals and snap frozen in liquid nitrogen. Tissues were homogenized in lysis buffer (10 mM Tris-HCl pH 7.4, 0.1% w/v SDS, 1% v/v Triton X-100, 1X cOmplete, Mini EDTA-free protease inhibitor). Cell lysates were obtained from confluent human fibroblasts (1X106 cells) and MEF (5X105 cells) using RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.1% w/v SDS, 1% v/v Triton X-100, 1% w/v Sodium deoxycholate, 1X cOmplete, Mini EDTA-free protease inhibitor). After protein determination (Pierce BCA Protein Assay Kit, ThermoScientific) 40 µg of tissue homogenates and 15 µg of cell lysates were subjected to SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were probed with a rabbit monoclonal ATP7A antibody (Auspep) raised specifically to amino acids of the carboxy-terminus of human ATP7A (1463-DKHSLLVGDFREDDDTAL-1480). This sequence is identical to the mouse sequence with the exception of the Ala1479 in human (Thr1491 in mouse) and was chosen based on previous studies showing an ATP7A antibody effectively reacting with the rodent Atp7a 25. Alpha tubulin (SIGMA Aldrich), β-actin (Cell Signaling) and GAPDH (Abcam) antibodies were used as loading controls. Anti-rabbit (SIGMA Aldrich) and anti-mouse (Abcam) horseradish peroxidase (HRP) conjugated secondary antibodies were used and signal detected by adding a chemiluminescent substrate (Merck).
Behavioral tests
Rotarod
Mice were trained using a trial run during which the rotarod (LE8200 Accelerating Rota-Rod; SDR Scientific) accelerated from 4 to 40 rpm over 300 s. After this acclimation, mice were timed for three runs on their ability to successfully continue running on the rod and the best performance was used to generate mean scores for each age group and genotype. A fall, a complete rotation, or completion of 300 s ended the run. Breaks (20 min) were given between each run. Data was collected using the Sedacom software provided with the instrument.
Hind limb clasping
Global motor function of the mice was tested with the hind limb clasping function. Weekly, mice were suspended by the tail and the presence of reflexive splaying of the hind limbs and digits in response to elevation was observed and documented.
Histology
Nerve semi-thin plastic sectioning
Mice (18 months) were perfused with freshly made 4% (m/v) paraformaldehyde (PFA). Dissected sciatic nerves were post-fixed in 2% (v/v) formaldehyde and 2.5% (v/v) glutaraldehyde overnight at 4°C. Samples were stained in 1% (v/v) osmium tetroxide for 2 h, dehydrated, and then hard-plastic polymer embedded using a mixture of Poly/Bed 812, Araldite 502, dodecenylsuccinic anhydride, and dimethylbenzylamine. After hardening overnight at 80–85°C, samples were sliced on a microtome into sections (1 µm) and stained with toluidine blue.
Muscle histology
Mouse tibialis anterior muscles (24 months) were fixed in 4% (m/v) PFA overnight and embedded in paraffin wax. 5 µm sections were cut and mounted onto glass slides before being dewaxed and stained with hematoxylin and eosin (H&E). Sections were observed with transmission light microscopy on an Olympus BX53 at 20X magnification. Image J analysis suite software was used to quantify total and centralized nuclei, as well as fibre diameter.
Myogenin and myostatin mRNA expression
RNA was extracted from the soleus muscles of the mice (24 months) in Trizol reagent (Life Technologies) with a Kinematica Polytron homogenizer as previously described 26. RNA was reverse transcribed using SuperScript III (Life Technologies). The gene expression of myogenin and myostatin in the soleus muscle was quantified by real time quantitative PCR, using TaqMan® fluorogenic probes and a Sequence Detection System 7000 (Applied Biosystems™/Thermo Fisher Scientific). The ΔΔCt method 27 was used to measure the relative quantitation expression of myogenin and myostatin and mouse GAPDH was used as the housekeeping gene. mRNA from the wild type Atp7a+/Y mice was chosen as the calibrator sample (i.e. target expression = 1).
Immunofluorescence
After the indicated treatments outlined in the Results, human fibroblasts or MEF were fixed using 4% (m/v) PFA, for 12 min at room temperature (RT), permeabilized (0.3% (v/v) Triton X-100, 10 min) and blocked (5% (v/v) bovine serum albumin for human cells or 5% (v/v) normal goat serum for MEF, 1 h RT). Cells were incubated with primary antibodies overnight at 4°C and with Alexa Fluor-labeled secondary antibodies (Molecular Probes-Invitrogen, Paisley, UK) at RT for 2 h. Nuclei were counterstained with 300 nM 4,6-diamidino-2- phenylindole (DAPI, Molecular Probes) and mounted using Prolong Gold antifade reagent (Invitrogen). Cells were visualized using a Leica SPE-II confocal microscope and images acquired at 63X magnification. Primary antibodies used include: rabbit monoclonal ATP7A 1:250 (Antibody Solutions) and mouse monoclonal golgin 97 1:500 (CDF4) (Santa Cruz).
Trafficking assay
Cells were treated with 200 µM CuCl2 in culture medium for 2 h at 37°C, with the final 30 min in the presence of 50 µg/ml cycloheximide, CHX (SIGMA Aldrich). For the Cu washout, cells were treated with CuCl2 as described above, washed once with culture medium, and incubated with culture medium containing 50 µg/ml CHX and 200 µM of the Cu-chelating agent bathocuproinedisulfonic acid, BCS (SIGMA Aldrich), for 4 h at 37°C. Addition of the protein synthesis inhibitor CHX ensured only internalized ATP7A/Atp7a was observed after the Cu washout, and not new protein synthesised during the time course of the experiment 28. Cells were then processed for immunofluorescence as described above.
Quantification of ATP7A/Atp7a present at the TGN
For quantification of the ATP7A/Atp7a present at the TGN subsequent to the treatments outlined in the Results, cells were stained with a rabbit monoclonal ATP7A antibody (Antibody Solutions) and a mouse anti-Golgi97 antibody followed by incubation with an anti-rabbit ALEXA-488 and anti-mouse ALEXA-555 secondary antibodies respectively. Using a Leica SPE-II confocal microscope, settings for optimal visualization of ATP7A/Atp7a at the TGN in control samples and basal conditions were determined and used for the acquisition of all subsequent samples in the experiment. At least 20 images were acquired at 63X magnification for each condition. To precisely define the region on each image corresponding to the TGN and specifically quantify the presence of ATP7A/Atp7a at that location, a region of interest (ROI) was defined using the image processing package Fiji 29. Briefly, a threshold was applied to the channel containing the TGN information to set appropriate signal-to-noise ratios. By using the “Analyze Particles” option and selecting an appropriate size for the organelle (5–10 µm), the region corresponding to the TGN was outlined in every channel. Using the multi measure option of the ROI master tool, the mean intensity of the signal corresponding to ATP7A/Atp7a was calculated and averaged for every cell.
Cytotoxicity assays
Cell viability in MEF following a 16 h exposure to increasing concentrations of CuCl2 was determined with the CCK8 assay kit (SIGMA Aldrich). Briefly, 48 h before the experiment 1.5X104 cells per well were plated in 96 well plates. Prior to the experiment (16 h) the F-DMEM was replaced with fresh media containing the indicated CuCl2 concentration. Prior to the cytotoxicity assay, cells were washed with DMEM and incubated with CCK8 diluted 1:10 (v/v) in DMEM for 90 min at 37°C. The absorbance at 450nm was measured with an EnSpire Multimode Plate Reader (Perkin Elmer). Data was obtained from 3 independent experiments. The total number of cell lines tested was n = 8 embryos per genotype.
Intracellular metal analysis
Total intracellular metal content was measured in the Atp7aT985I tissues (6 months and 12 months) dissolved in 70% nitric acid as previously described 30. Metal content was determined in MEF (Atp7a+/Y and Atp7aT985I/Y) subsequent to the treatments outlined in the Results as previously shown 31. Briefly, 1X105 cells were seeded in 6 well plates. Cells were grown till confluent and then treated with 200 µM CuCl2 in culture medium for 16 h at 37°C. For the wash out time points, cells were treated with 200 µM CuCl2 then media was removed, cells washed with fresh medium and incubated with Cu-free medium for 6 h. After the treatments, cells were harvested and intracellular metal concentration measured using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7700, Varian). The mean value with standard deviations (± SD) determined in triplicate for each test condition was used for comparison.
Neuromuscular junction (NMJ) analysis
Preparation and dissection of the distal hind limb lumbrical muscles was performed as described 32. Briefly, 24 month old Atp7a+/Y and Atp7aT985I/Y mice were sacrificed, dissected and hind limb lumbrical muscles fixed for 10 min by placing them into a drop of PFA 4% (m/v) at room temperature. All subsequent steps (permeabilization and antibody incubation) were carried out by transferring the muscles through consecutive wells of a clear bottomed 96 well plate at 4°C with agitation. Overnight incubation with the primary antibodies using a 1:100 chicken polyclonal to 68 kDa neurofilament (NF) antibody (Abcam) and a 1:100 mouse monoclonal synaptic vesicle protein 2 (SV2) antibody (DSHB) was performed. Secondary antibodies were incubated for 2 h using 1:250 goat anti-chicken ALEXA Fluor 647 (Abcam) and 1:250 goat anti-mouse ALEXA Fluor 488 (Molecular Probes) antibodies at 2mg/ml respectively in the dark. To stain postsynaptic acetylcholine receptors (AchR) a conjugated 1:250 α-bungarotoxin peptide-Alexa Fluor-555 was used (Molecular Probes). Lumbrical muscles were mounted using ProLong Gold antifade. Images were analysed as previously described 33 and NMJ occupancy was scored as previously defined 34.
Statistical analysis
For the statistical analysis, 3 independent experiments under the same conditions were performed and a Student’s t test used to assess the significance of the results. The data are expressed as mean ± SEM. The following statistical thresholds have been applied throughout the study: * p < 0.05; ** p < 0.01; *** p < 0.001.
Results
Atp7aT985I mouse generation
To create a mouse model that would closely mimic the natural disease progression observed in dHMNX patients harboring the ATP7AT994I mutation we engineered an Atp7aT985I conditional knock in mouse using the “mini cDNA” strategy as previously described 23. The human ATP7A and mouse Atp7a genes have 89% amino acid identity overall and 100% identity in the TM6 domain where the T985 residue is located 35. The targeting strategy for generation of the knock in Atp7aT985I mouse model is shown in Fig. 1. A targeting construct (Atp7aT985I targeted allele w/neo) was designed to introduce the T985I mutation into exon 15 of the wild type murine Atp7a gene (Fig. 1A). By using this strategy, the T985I mutation can be conditionally expressed after removal of the wild type Atp7a cDNA following Cre-Lox recombination (knock in Atp7aT985I). Germline transmission of the knock in T985I allele was confirmed by a multiplex PCR genotyping assay that co-amplified differential sized DNA fragments detecting the wild type X chromosome and mutant X chromosome containing the T985I mutation (Fig. 1B top panel). The gender of embryos for functional studies were confirmed using the PCR assay designed to co-amplify the X-chromosome and the Y-chromosome-specific homologue genes, Jarid1c and Jarid1d (Fig. 1B bottom panel).
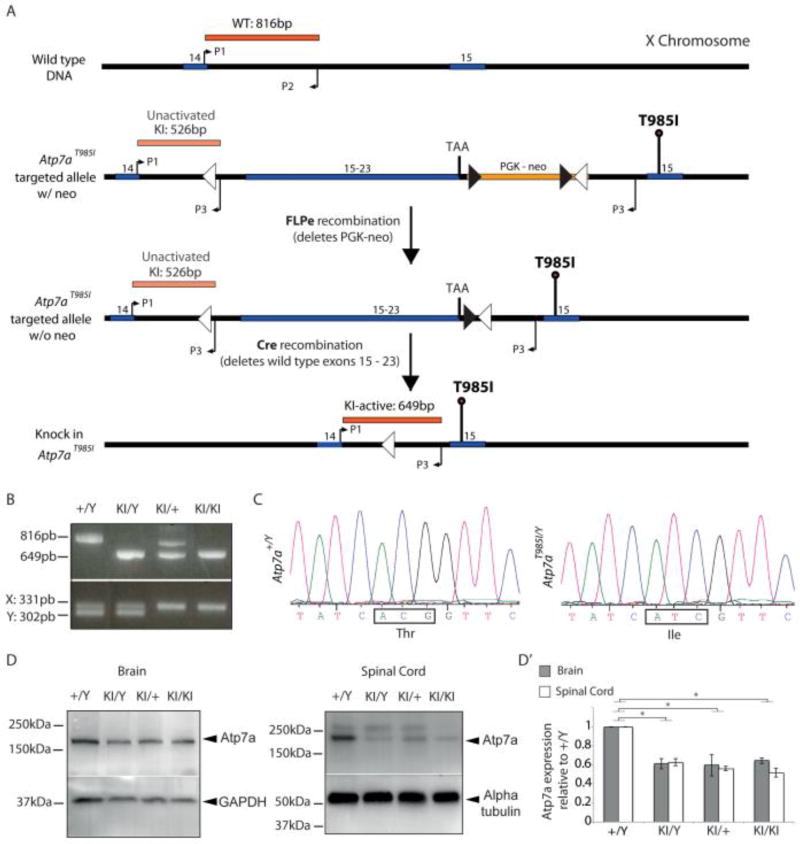
Figure 1.
Gene targeting strategy and genotype analysis for Atp7aT985I knock in mouse model. (A) Diagram illustrates gene targeting strategy using the mini cDNA approach to produce the Atp7aT985I knock in mice. The targeted allele introduces a partial cDNA consisting of the exon to be mutated (exon 15) and all coding downstream exons (16 to 23). The mutated exon 15 (T985I) is inserted downstream of the mini cDNA and the selection marker (PGK-neo). Lox P sites and frt sites are represented by open and closed triangles respectively. FLPe recombination removes the PGK-neo selection marker flanked by the frt sites. Cre-mediated recombination excises the mini cDNA, leaving the T985I mutation and an intronic lox site. P1, P2 and P3 represent primers used for genotyping both the germline target construct transmission as well as genotyping experimental animals after Cre-lox recombination. (B) PCR amplicons (top panel) showing all possible genotypes of the knock in Atp7aT985I model: (+/Y) wild type male; (KI/Y) knock in male; (KI/+) carrier females and (KI/KI) homozygous females in E13.5 embryos. The lower panel shows the gender of the embryos based on primers that co-amplify the X chromosome (331 pb) and the Y chromosome-specific (302 pb) genes, Jarid1c and Jarid1d respectively (C) Partial DNA sequence chromatograms of Atp7aT985I reverse transcribed template prepared from brain mRNA obtained from 1 month old mice showing wild type sequence (Atp7a+/Y) and the mutant sequence (Atp7aT985I/Y). (D) Representative western blots showing reduced Atp7a protein levels in brain and spinal cord (40µg total protein loaded) from 6 month old Atp7aT985I mice. Alpha tubulin and GAPDH have been used as the loading control. Quantification of Atp7a protein levels is shown (D’) and represented as the levels of protein for each genotype relative to the wild type male (+/Y).
Reverse transcribed template prepared from brain mRNA was sequenced and confirmed inheritance of the knock in point mutation in the Atp7aT985I/Y males (Fig 1C). Real time quantitative PCR studies did not detect significant differences in Atp7a mRNA expression in the brain, spinal cord, sciatic nerve, dorsal root ganglia, liver and skeletal muscle harvested from 6 month old Atp7a+/Y and Atp7aT985I/Y animals (data not shown). However, western blot analysis of tissues from this age group (Fig. 1D) revealed reduced Atp7a protein levels in the brain and spinal cord of Atp7aT985I/Y, Atp7aT985I/+ and Atp7aT985I/T985I animals when compared to the age matched wild type mice (Fig. 1D’).
Behavioral studies and nerve/muscle histology in Atp7aT985I aging mice
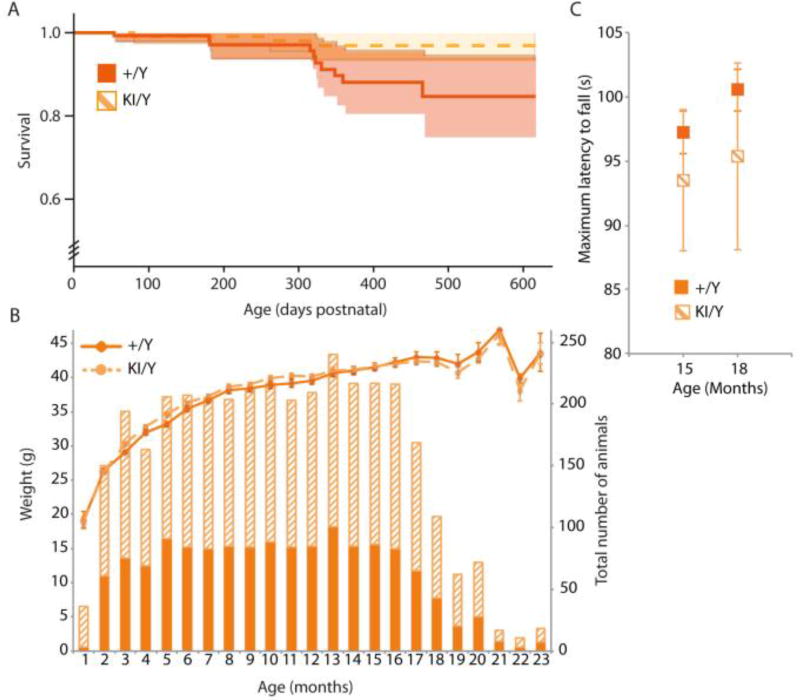
Atp7aT985I mice were physically examined on a weekly basis and tested behaviorally for motor abnormalities starting at 4 weeks of age (Fig. 2). The lifespan and the body weight over the course of 2 years were the same in Atp7aT985I/Y and Atp7a+/Y males (Fig. 2A and 2B, respectively) as well as the Atp7aT985I/+, Atp7aT985I/T985I and Atp7a+/+ females (data not shown). Animals representing all genotypes up to 24 months of age were tested and showed no physical manifestations of peripheral neuropathy as assessed by limb clasping when suspended by the tail (data not shown). The motor abilities of Atp7aT985I/Y and Atp7a+/Y mice tested by rotarod performance, showed no statistically significant differences during the timeline of the study (Fig. 2C).
Figure 2.
Characterisation of the Atp7aT985I mouse phenotype shows no global motor abnormalities. (A) Kaplan Meir survival curves for wild type (+/Y) and knock in (KI/Y) males revealed no significant differences in survival rate between the two groups. (B) Body weight (g) and the total number of animals studied for each age point (months) are shown. (C) Rotarod test at ages 15 (n=35/genotype) and 18 months old (n=16/genotype). The best performance out of three runs for their ability to successfully continue running on an accelerated program (from 4 to 40 rpm over 300 s) was recorded and the mean value for each genotype is shown as the maximum latency to fall.
In humans, dHMNX causes degeneration of distal portions of peripheral nerves. To examine evidence for axonal degeneration, semi-thin sections of sciatic nerves from 18 month old Atp7aT985I/Y and Atp7a+/Y mice were analysed. We did not observe histological manifestations of acute or chronic neuropathy with light microscopy. Schwann cell changes, missing axons, accumulated membranous debris and clusters of regenerating axons were absent in the nerve preparations. Qualitative analysis of nerves in Atp7aT985I/Y mice did not show pathogenic changes in axonal width or myelin thickness when compared to Atp7a+/Y mice (Fig. 3A).
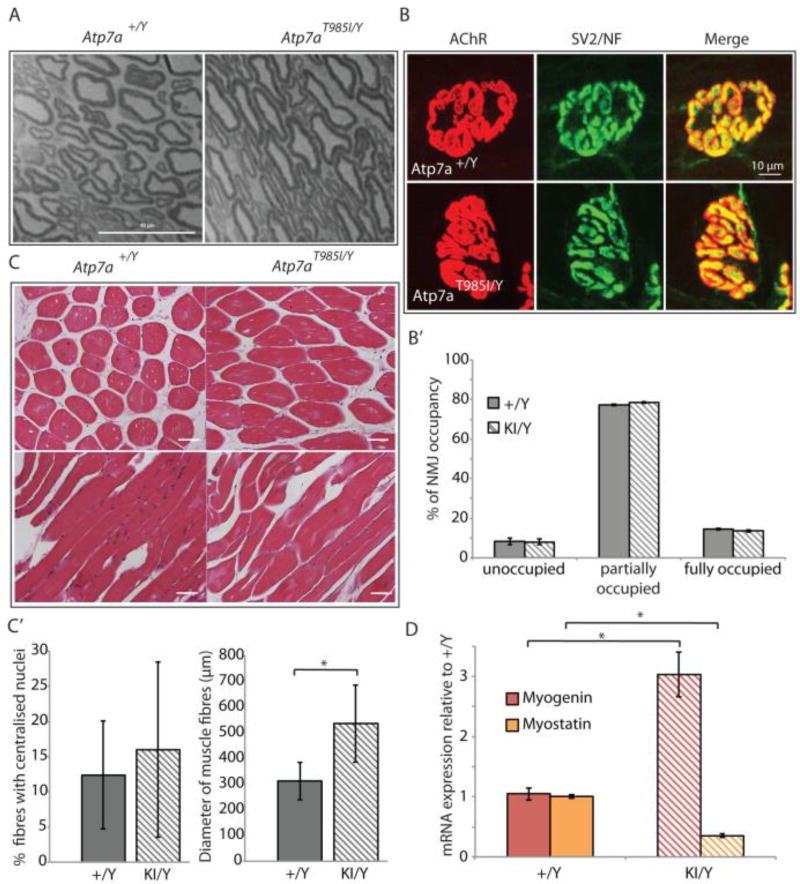
Figure 3.
Nerve/muscle histology and NMJ analysis in Atp7aT985I aging mice. (A) Microtome sections (1 µm) from 18 month old Atp7aT985I/Y and Atp7a+/Y sciatic nerves stained with toluidine blue. Scale bar is 40 µm. (B) NMJ from hind limb lumbrical muscles from 24 month old knock in Atp7aT985I/Y (KI/Y) and wild type Atp7a+/Y (+/Y) mice, stained for post-synaptic (AChR) and pre-synaptic (SV2/NF) structures. The merge panel was used to quantify the NMJ occupancy (B’). Previously defined categories of NMJ percentage occupancy included 34: unoccupied (0–33%), partially occupied (34–66%) and fully occupied (67–100%). (C) 5 µm tibialis anterior sections from 24 month old Atp7aT985I/Y and Atp7a+/Y mice stained with H&E (top panels: cross sections; bottom panels: transverse sections). Stained sections were observed under transmission light at a 20X magnification and quantified (C’) for total and centralized nuclei, as well as fibre diameter. (D) Expression analysis of myogenin and myostatin from reversed transcribed template of soleus muscle by real time quantitative PCR. Mouse GAPDH was used to normalize expression of myogenin and myostatin.
The neuromuscular junction (NMJ) is a specialised synapse formed between a lower motor neuron and a skeletal muscle fibre and is an early pathological target during axonal degeneration. We therefore investigated the occupancy of NMJ from the hind limb lumbrical muscles of both Atp7aT985I/Y and Atp7a+/Y mice. We stained axonal presynaptic buttons with antibodies against neurofilament (NF) and the synaptic vesicle protein 2 (SV2), as well as the nicotinic acetylcholine receptor (AChR) at the postsynaptic end plate with α-bungarotoxin peptide (Fig. 3B). Neuromuscular synapses were then scored for degenerative phenotypes by assessing the overlap between pre-synaptic SV2/NF and post-synaptic AChR staining, also termed NMJ occupancy (Fig. 3B’). We did not observe any differences in the NMJ occupancy at 24 months. Histological examination of cross sections of tibialis anterior muscles from 24 month old animals (Fig. 3C, top panel) revealed a statistically significant increase in the diameter of the Atp7aT985I/Y fibres when compared to Atp7a+/Y fibres (Fig. 3C’). This occurred without changes in the proportion of centralised nuclei between wild type and mutant genotypes (a process found to occur during muscle denervation and atrophy) (Fig. 3C’). Interestingly, transverse sections of the tibialis anterior muscles in the knock in mice showed disorganization of muscle fibres when compared to the wild type (Fig. 3C, bottom panel). Given these unexpected observations we assessed the soleus muscle, the antagonist muscle located in the posterior compartment of the hind limb, for expression of myogenin and myostatin, genes associated with myogenesis and muscle damage repair 36, 37. Gene expression analysis in Atp7aT985I/Y soleus muscles using quantitative real time PCR revealed a statistically significant increase of myogenin (a muscle-specific transcription factor associated with muscle growth) and reduced expression of myostatin (a myogenesis inhibitor) when compared to controls (Fig. 3D).
Atp7aT985I/Y mice exhibit nervous system Cu dysregulation
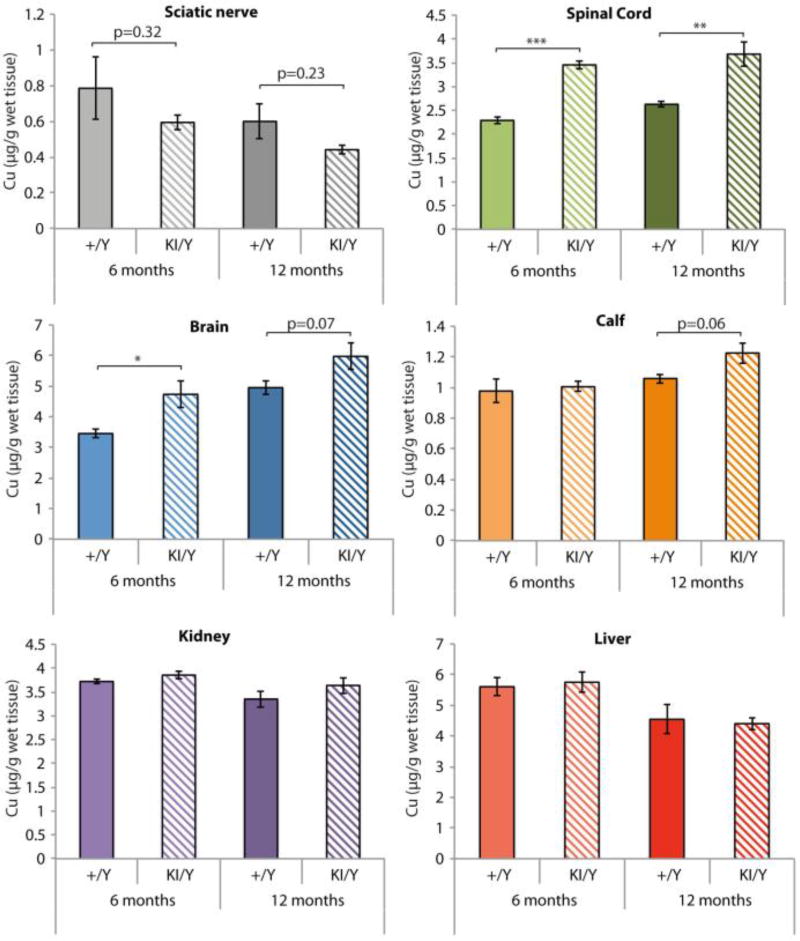
We measured Cu concentration in tissues from Atp7aT985I/Y and Atp7a+/Y animals at ages 6 and 12 months (Fig. 4). Our data showed that the brain and spinal cord Cu concentrations were increased in the knock in Atp7aT985/Y mice from the age of 6 months. No statistically significant differences in Cu levels were detected in calf muscle, kidney or liver. A non-significant trend suggested a reduction in the Cu content of the sciatic nerve from Atp7aT985I/Y mice when compared to Atp7a+/Y animals.
Figure 4.
Cu concentrations in wild type and knock in mouse tissues. Cu levels in tissues were determined using ICP-MS at ages 6 (light colors) and 12 months (dark colors) for wild type males (+/Y; solid) and knock in males (KI/Y; striped). The data is represented as the mean ± SEM (n=4 mice/genotype).
Atp7aT985I primary cells recapitulate molecular defects observed in dHMNX patient derived ATP7AT994I fibroblasts
Atp7a protein levels are reduced in MEFT985I/Y
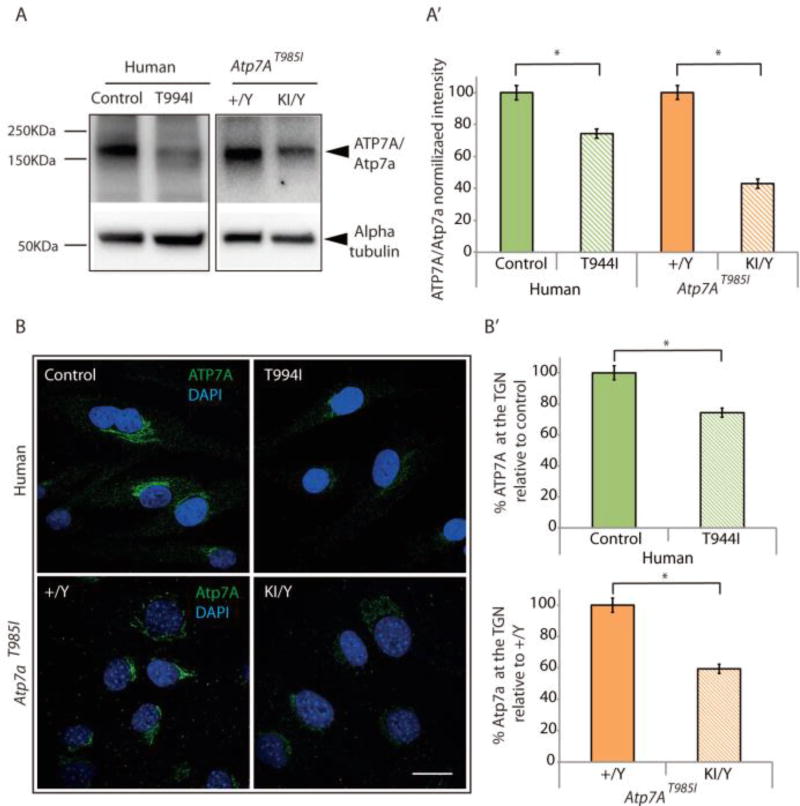
To further investigate the reduced Atp7a protein levels found in the knock in Atp7aT985I/Y tissues (Fig. 1D) we measured ATP7A/Atp7a protein levels in both ATP7AT994I patient fibroblasts and MEF isolated from Atp7aT985I mice (Fig. 5A). Significantly reduced levels of Atp7a in MEFT985I/Y compared to MEF +/Y (Fig. 5A’) was observed. A similar decrease in ATP7A protein was also detected in ATP7AT994I fibroblasts when compared to fibroblasts from clinically normal individuals (Fig. 5A’). These results were further confirmed by immunofluorescence analysis (Fig. 5B). In basal conditions ATP7A localizes at the TGN where it supplies Cu to newly synthesized Cu-dependent enzymes. Human control fibroblasts and MEF+/Y showed strong staining of the Cu transporter at this location. However, the intensity of the ATP7A/Atp7a staining was significantly decreased in both the ATP7AT994I fibroblasts (30% reduction) and Atp7aT985I/Y MEF (40% reduction) (Fig 5B’).
Figure 5.
Atp7aT985I MEF shows reduced Atp7a protein levels as observed in the patient ATP7AT994I fibroblasts. Human control fibroblasts (Control), ATP7AT994I fibroblasts (T994I) and MEF derived from wild type (+/Y) and knock in (KI/Y) mouse embryos were cultured and ATP7A/Atp7a protein levels determined with western blot and immunofluorescence analysis. (A) Western blot showing ATP7A/Atp7a protein levels in the human samples (20 µg) and MEF (15µg), respectively. Cell lysates probed with an anti-rabbit ATP7A antibody show a band at the expected molecular weight (~180 kDa). (A’) Alpha tubulin was used as a loading control for normalization and quantitation of the ATP7A/Atp7a protein levels. (B) Cellular ATP7A/Atp7a levels were determined by immunofluorescence using a primary rabbit anti-ATP7A antibody and an anti-rabbit ALEXA-488 secondary antibody (green). ATP7A/Atp7a levels were quantitated within the ROI as defined in the methods and are shown relative to the human fibroblasts (Controls) and wild type MEF (+/Y), respectively (B’). Data is obtained from 3 independent experiments (n=50 cells per experiment). Nuclei were stained with DAPI. Scale bar is 20 µm.
Atp7a trafficking is impaired in MEFT985I/Y
Exposing cells to elevated levels of Cu induces ATP7A to traffic out of the TGN to the cell surface to mediate exporting cellular Cu 7. This relocalisation is reversible and upon Cu washout ATP7A returns to the TGN 9, 28. Previous studies have suggested ATP7A trafficking abnormalities occurring in the dHMNX ATP7A mutants 21, 38. We therefore monitored ATP7A/Atp7a Cu dependent trafficking in patient ATP7AT994I fibroblasts and Atp7aT985I MEF, using immunofluorescence and confocal microscopy (Fig. 6A, B). In the presence of the Cu chelator BCS, control cells (human control fibroblasts and MEF+/Y) show ATP7A/Atp7a localizing at the TGN. In contrast, the dHMNX ATP7AT994I fibroblasts and MEFT985I/Y confirmed a ~30% reduction of protein staining at the TGN in these conditions as demonstrated above (Fig. 5). When the cells were exposed to 200 µM CuCl2 for 2 h only a residual proportion of the initial ATP7A was found at the TGN 7 in all the cell lines tested, indicating that the dHMNX mutation does not affect trafficking of ATP7A/Atp7a out of the TGN in response to Cu. Quantitation of the fluorescence signal of cells after the Cu wash out showed defective relocation of ATP7A/Atp7a back to the TGN in ATP7AT994I patient fibroblasts (~20% reduction, Fig. 6A’) and MEFT985I/Y (~50%, Fig. 6B’). Whether the ATP7A/Atp7a retrograde trafficking is affected by the dHMNX mutation or a defect in the mutant protein causes ATP7A/Atp7a to be retained at alternative intracellular locations after Cu exposure remains to be determined. However, this data confirms that the Atp7aT985I knock in mouse reproduces the molecular observations made in the ATP7AT994I patient fibroblasts.
Figure 6.
Defective relocation of ATP7A/Atp7a to the TGN is observed in both ATP7AT994I human fibroblasts and Atp7aT985I/Y MEF. (A) Human control (Control) and ATP7AT994I fibroblasts (T994I) and (B) MEF derived from wild type (+/Y) and knock in (KI/Y) mouse embryos were cultured to assess ATP7A/Atp7a intracellular localisation under the following conditions: BCS; cells treated with the Cu-chelating agent at 200 µM for 2 h. Cu; cells treated with 200 µM CuCl2 in culture medium for 2h and Cu washout (wo); cells treated with 200 µM CuCl2, then washed once and incubated with medium containing 50 µg/ml CHX and 200 µM BCS for 2 h. Levels within the ROI as described in the methods for ATP7A (A’) and Atp7a (B’) were quantified for each condition. The mean fluorescence intensity is shown relative to the BCS condition for each genotype. Data was obtained from 3 independent experiments with n = 50 cells per experiment. Nuclei were stained with DAPI. Scale bar is 20 µm.
Cu does not stabilize Atp7a protein in MEFT985I/Y
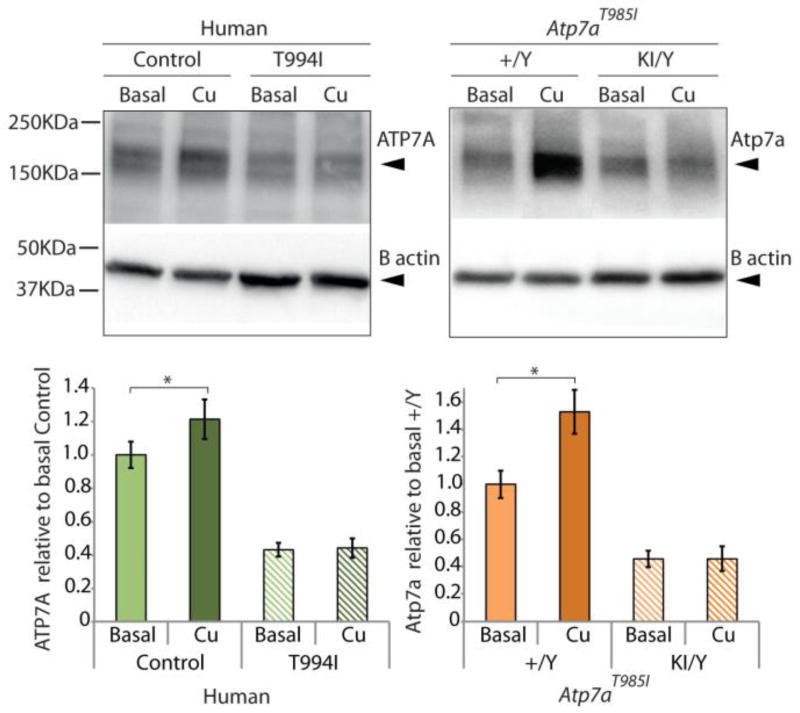
Recent investigations have shown a novel post-translational mechanism of Atp7a regulation through stabilisation of the protein by Cu 39. Evidence found using rat intestinal epithelial cells (IEC-6) suggested that intracellular Cu accumulation increased steady-state Atp7a protein levels through binding of Cu to one or more of the intracellular Cu-binding domains. We therefore designed experiments to assess the response of Cu loading on ATP7A/Atp7a protein levels in both ATP7AT994I fibroblasts and MEFT985I to determine the effect of the mutation on this process. Human ATP7AT994I and MEFT985I were treated with 200 µM CuCl2 for 16 h. Western blot analysis (Fig. 7) confirmed that exposure of the cells to Cu, increased ATP7A/Atp7a protein levels in control human fibroblasts (20%) and MEF+/Y cells (40%). Interestingly, this effect was absent in both the ATP7AT994I patient fibroblasts and the Atp7aT985I/Y MEF. In both cases the ATP7A/Atp7a levels were maintained after Cu loading. This result suggests the ATP7AT994I mutation abolishes ATP7A stabilisation in the presence of excess Cu and confirms that the Atp7aT985I knock in mouse recapitulates this cellular event observed in the patient cells.
Figure 7.
Cu loading increases ATP7A/Atp7a protein levels in control cells but has no effect on ATP7AT994I fibroblasts or Atp7aT985I/Y MEF. Human control fibroblasts (Control), ATP7AT994I patient fibroblasts (T994I) and MEF derived from wild type (+/Y) and knock in (KI/Y) mouse embryos were cultured to assess the effect of Cu loading on cellular ATP7A/Atp7a protein levels. Cells were incubated with 200 µM CuCl2 for 16 h. Cell lysates (20 µg protein per lane) probed with an anti-rabbit ATP7A antibody showed a band at the expected molecular weight (~180 kDa). β-actin was used as a loading control. ATP7A/Atp7a protein levels for each condition are represented relative to the protein levels detected for the human fibroblasts (Control) or the wild type MEF (+/Y) respectively under basal Cu conditions.
MEFT985I/Y accumulate intracellular Cu and are more sensitive to Cu-induced toxicity
Central to the role of ATP7A within cells is the capacity to traffic to the PM in response to Cu for export and to maintain intracellular levels below toxic concentrations. We propose that the post-translational mechanism for ATP7A regulation examined in our study may have a role in ensuring the cell can effectively restore Cu to physiological levels by increasing the half-life and enhancing the stability of the transporter after Cu insult. The absence of an increase in ATP7A/Atp7a protein levels in both the human and mouse mutant cell lines exposed to elevated Cu therefore suggests that maintenance of intracellular Cu homeostasis may be affected in the ATP7AT994I patient fibroblasts and MEFT985I/Y.
Using ICP-MS we determined intracellular Cu concentrations in Atp7aT985I MEF cultured in basal, Cu loading and Cu-free culture media after exposure to Cu (wash out). Both MEF+/Y and MEFT985I/Y cells showed increased intracellular Cu when exposed to 200 µM CuCl2 for 16 h. The MEFT985I/Y had a significantly higher cellular Cu accumulation after Cu loading than the wild type MEF+/Y (Fig. 8A). After restoration to a Cu free culture media (6 h), MEF+/Y were more efficient at reducing the intracellular Cu concentration than the MEFT985I/Y.
Figure 8.
Knock in Atp7aT985I/Y MEF accumulate higher Cu levels and show increased Cu-induced toxicity. (A) Wild type (+/Y) and knock in (KI/Y) MEF were treated with basal medium or 200 µM CuCl2 for 16 h at 37°C. For the wash out cells were treated with 200 µM CuCl2 for 16 h and then transferred to Cu-free medium for 6 h. Cu content of cell pellets was measured in triplicate by ICP-MS. Data are expressed as the mean ± SEM for three independent experiments (n=3 embryos/genotype). (B) CCK-8 toxicity assay to determine concentration-dependent Cu toxicity on Atp7aT985I MEF. Wild type (+/Y) and knock in (KI/Y) MEF were exposed to a range of CuCl2 concentrations from 0 µM to 1000 µM. After 16 h, CuCl2 containing media was removed and replaced with a solution of 1:10 CCK-8 reagent in fresh DMEM and incubated for 4 h. Absorbance readings at 450nm were recorded and cell survival curves represented relative to the data obtained in the absence of CuCl2. The data was obtained from 3 independent experiments and represented as the mean ± SEM (n=8 embryos/genotype).
These results suggest the dysregulation of Cu homeostasis may therefore be a pathological mechanism underlying the dHMNX ATP7AT994I mutation. To determine whether the Atp7aT985I mutation enhances the cytotoxic effect of Cu, we assessed Cu-induced toxicity for Atp7aT985I MEF, using a Cell Counting Kit-8 (CCK-8) assay to quantify viable cells. MEFT985I showed a dose dependent Cu toxic effect (Fig. 8B) at concentrations over 500 µM CuCl2. Above this concentration, the toxic effect of Cu in the mutant MEFT985I/Y was significantly higher when compared to wild type MEF+/Y. This suggests that the altered capacity of mutant cells to regulate intracellular Cu makes them more susceptible to Cu-induced toxicity.
Discussion and conclusions
The contribution of ATP7A and Cu to neurodevelopment and neurodegeneration has been extensively explored. However the early cellular events leading to axonal degeneration in the dHMNX patients remains poorly understood. We have characterised an Atp7a conditional knock in mouse model of dHMNX (Atp7aT985I) and demonstrated important cellular alterations related with the Atp7a and Cu biology which would help to investigate the early molecular and cellular pathogenic events that occur in the dHMNX patients.
We did not observe a global motor phenotype over the course of this study or demonstrate axonal degeneration through histologic examination of sciatic nerves and NMJ analysis. Our data however, consistently demonstrated the Atp7aT985I primary MEF reproduced the molecular observations made in the ATP7AT994I patient fibroblasts. The juvenile onset of distal muscular atrophy in the dHMNX patients harboring the T994I mutation 21 suggests this mutation produces attenuated effects that require years for patients to present with pathological consequences and this would provide a possible rationale for the absence of a global motor phenotype in the Atp7aT985I mice. The previously reported Atp7aMN/Y mouse model, in which Atp7a had been completely knocked out in the motor neurons, only exhibited behavioral abnormalities after 6 months of age 22. This suggests the possibility of compensatory processes occurring in mice that would mitigate the absence or pathogenic function of mutant Atp7a within the motor neurons.
In contrast to Menkes disease, where most ATP7A mutations lead to a complete loss of function of the transporter (see 20 for review), the dHMNX ATP7AT994I mutation produces a subtle reduction in ATP7A protein levels (30%) that had not been observed in previous studies and was detected in both the ATP7AT994I patient fibroblasts and in the mutant MEFT985I/Y. Our data also confirmed a previous observation that wild type Atp7a protein levels are increased in the presence of Cu 39. Interestingly the ATP7AT994I patient fibroblasts and MEFT985I/Y lacked this post-translational regulatory mechanism. We therefore proposed the absence of such stabilisation by Cu in the mutant ATP7A/Atp7a protein would affect the capacity of the cells to maintain intracellular Cu homeostasis. The reduced cell viability observed in the MEFT985I/Y in the presence of high concentrations of Cu suggests Cu accumulation as a possible pathogenic mechanism in dHMNX disease. The contribution of Cu toxicity as an underlying pathogenic mechanism for neurodegenerative disorders has been extensively studied and reviewed 40. The exact mechanism by which Cu triggers axonal degeneration in the dHMNX patient remains to be determined. Deficiency of Cu dependent enzymes as a result of the decreased presence of Atp7a in the TGN (Fig. 5) represents an alternative disease mechanism in dHMNX. Abnormal function of Cu dependent enzymes, including cytochrome c oxidase 41 and superoxide dismutase 1 42 have been associated with Charcot-Marie-Tooth and amyotrophic lateral sclerosis (ALS), respectively. In addition, Atp7a loss of function at the TGN is consistent with outcomes from the Atp7aMN/Y mice in which the degenerative motor neuropathy of the animals is associated with an overall decrease in Cu in the spinal cord 22. Our ICP-MS data showing a non-significant reduction in the levels of Cu in the sciatic nerve of the Atp7aT985I/Y mice will need further investigation. Interestingly, assessment of iron levels showed a significant increase in the Atp7aT985I/Y sciatic nerves, whereas no changes were found in any other tissues (Supplementary Fig. S1). Iron accumulation in the CNS is reported in both familial and sporadic forms of ALS 3 and the impact of iron mediated toxicity through disruption of mitochondrial iron homeostasis in an ALS mouse model has been described 43. The Atp7aAT985I knock in mouse provides a valuable tool to test the impact of iron on the pathology of dHMNX.
Although no signs of axonal degeneration were observed, the muscle histology revealed an increased diameter of the muscle fibres and signs of disorganized fibre alignment in the Atp7aT985I/Y tibialis anterior muscles. Furthermore, gene expression analysis showed significant concomitant increased myogenin and reduced myostatin gene expression in the Atp7aT985I/Y soleus when compared to controls. These observations in the different muscle tissues may reflect a pre-symptomatic stage of the disease we speculate to be associated with enhanced neuronal input occurring in the Atp7aT985I/Y muscles at the NMJ. Studies have established that Cu negatively regulates the activity of the NMDA receptors in hippocampal neurons 44 and the relevance of this regulatory mechanism in synaptic plasticity and excitotoxic cell death has been described 45. Schlief et al demonstrated that Cu chelation exacerbates NMDA-mediated excitotoxic cell death via an increased flux of Ca2+ into neurons. More recently, NMDA receptors have been found at the postsynaptic end plate of NMJs 46–48 suggesting a regulatory function of glutamate and NMDA receptors at the vertebrate NMJ. The release of Cu at the end of the nerves may negatively regulate the activity of the muscle in a similar way that has been proven for hippocampal neurons. The increased diameter of the tibialis anterior fibres we report in the Atp7aT985I/Y mice may be a sign of muscle hyperactivity due to impaired release of Cu at the NMJ synapse. Although no changes were observed in the nerve pathology (18 months) or NMJ (24 months) analysis in the knock in animals, the increased gene expression of myogenin we have observed might also suggest events of muscle repair due to excitotoxicity from muscle hyperactivity. It is plausible that both the reduction of Atp7a protein and trafficking deficits we have reported in the Atp7aT985I/Y mice could contribute to this process in the early stages of the disease. Whilst this hypothesis requires further studies in the Atp7aT985I knock in model, in humans the NMDA receptor-dependent release of Cu into the NMJ may protect both motor neurons and muscle fibres from Ca2+ induced cytotoxicity in clinically normal individuals.
Our study has characterized a knock in Atp7aT985I mouse model, which recapitulates at the cellular level important aspects found in the dHMNX patient cells. Our data suggests that the altered capacity of mutant MEFT985I/Y to regulate Cu intracellular levels make them more susceptible to Cu-induced toxicity. Alternative pathological mechanisms such as increased NMDA receptor-mediated excitotoxicity 45 in dHMNX patients, the occurrence of aberrant protein interactions of the mutant ATP7A 38 selectively affecting motor neurons or deficiency of cuproenzymes 41 with a critical impact on the peripheral nerves also may contribute to the disease pathomechanism. Further investigations using primary Atp7aT985I motor neurons will provide an ideal tool to investigate the proposed disease mechanisms in the relevant cell model for dHMNX.
Supplementary Material
Acknowledgments
The authors thank the families. This research was supported by the National Health and Medical Research Council Project Grant (APP1007705) awarded to M.L.K. and G.A.N. and USA Muscular Dystrophy Association Project Grant (MDA217729) awarded to M.L.K. and G.A.N. We dedicate this work to the memory of our colleague and friend Jim Garbern.
References
- 1.El Meskini R, Crabtree KL, Cline LB, Mains RE, Eipper BA, Ronnett GV. ATP7A (Menkes protein) functions in axonal targeting and synaptogenesis. Molecular and cellular neurosciences. 2007;34:409–421. doi: 10.1016/j.mcn.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Ambrosi N, Rossi L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochemistry international. 2015;90:36–45. doi: 10.1016/j.neuint.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Kasarskis EJ, Tandon L, Lovell MA, Ehmann WD. Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: a preliminary study. Journal of the neurological sciences. 1995;130:203–208. doi: 10.1016/0022-510x(95)00037-3. [DOI] [PubMed] [Google Scholar]
- 4.Larner F, Sampson B, Rehkamper M, Weiss DJ, Dainty JR, O'Riordan S, Panetta T, Bain PG. High precision isotope measurements reveal poor control of copper metabolism in parkinsonism. Metallomics : integrated biometal science. 2013;5:125–132. doi: 10.1039/c3mt20238k. [DOI] [PubMed] [Google Scholar]
- 5.Paik SR, Shin HJ, Lee JH, Chang CS, Kim J. Copper(II)-induced self-oligomerization of alpha-synuclein. The Biochemical journal. 1999;340(Pt 3):821–828. [PMC free article] [PubMed] [Google Scholar]
- 6.Scheiber IF, Mercer JF, Dringen R. Metabolism and functions of copper in brain. Progress in neurobiology. 2014;116:33–57. doi: 10.1016/j.pneurobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. The EMBO journal. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 8.Monty JF, Llanos RM, Mercer JF, Kramer DR. Copper exposure induces trafficking of the menkes protein in intestinal epithelium of ATP7A transgenic mice. The Journal of nutrition. 2005;135:2762–2766. doi: 10.1093/jn/135.12.2762. [DOI] [PubMed] [Google Scholar]
- 9.Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. American journal of physiology. Gastrointestinal and liver physiology. 2007;292:G1181–1194. doi: 10.1152/ajpgi.00472.2006. [DOI] [PubMed] [Google Scholar]
- 10.Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nature genetics. 1993;3:14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- 11.Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D, et al. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nature genetics. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- 12.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nature genetics. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 13.Kaler SG, Gallo LK, Proud VK, Percy AK, Mark Y, Segal NA, Goldstein DS, Holmes CS, Gahl WA. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nature genetics. 1994;8:195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Levinson B, Whitney S, Vulpe C, Packman S, Gitschier J. Diverse mutations in patients with Menkes disease often lead to exon skipping. American journal of human genetics. 1994;55:883–889. [PMC free article] [PubMed] [Google Scholar]
- 15.Moizard MP, Ronce N, Blesson S, Bieth E, Burglen L, Mignot C, Mortemousque I, Marmin N, Dessay B, Danesino C, Feillet F, Castelnau P, Toutain A, Moraine C, Raynaud M. Twenty-five novel mutations including duplications in the ATP7A gene. Clinical genetics. 2011;79:243–253. doi: 10.1111/j.1399-0004.2010.01461.x. [DOI] [PubMed] [Google Scholar]
- 16.Tumer Z, Lund C, Tolshave J, Vural B, Tonnesen T, Horn N. Identification of point mutations in 41 unrelated patients affected with Menkes disease. American journal of human genetics. 1997;60:63–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PC, McAndrew PE, Kaler SG. Rapid and robust screening of the Menkes disease/occipital horn syndrome gene. Genetic testing. 2002;6:255–260. doi: 10.1089/10906570260471778. [DOI] [PubMed] [Google Scholar]
- 18.Kaler SG. Metabolic and molecular bases of Menkes disease and occipital horn syndrome. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 1998;1:85–98. doi: 10.1007/s100249900011. [DOI] [PubMed] [Google Scholar]
- 19.Tang J, Robertson S, Lem KE, Godwin SC, Kaler SG. Functional copper transport explains neurologic sparing in occipital horn syndrome. Genetics in medicine : official journal of the American College of Medical Genetics. 2006;8:711–718. doi: 10.1097/01.gim.0000245578.94312.1e. [DOI] [PubMed] [Google Scholar]
- 20.Tumer Z. An overview and update of ATP7A mutations leading to Menkes disease and occipital horn syndrome. Human mutation. 2013;34:417–429. doi: 10.1002/humu.22266. [DOI] [PubMed] [Google Scholar]
- 21.Kennerson ML, Nicholson GA, Kaler SG, Kowalski B, Mercer JF, Tang J, Llanos RM, Chu S, Takata RI, Speck-Martins CE, Baets J, Almeida-Souza L, Fischer D, Timmerman V, Taylor PE, Scherer SS, Ferguson TA, Bird TD, De Jonghe P, Feely SM, Shy ME, Garbern JY. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. American journal of human genetics. 2010;86:343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgkinson VL, Dale JM, Garcia ML, Weisman GA, Lee J, Gitlin JD, Petris MJ. X-linked spinal muscular atrophy in mice caused by autonomous loss of ATP7A in the motor neuron. The Journal of pathology. 2015;236:241–250. doi: 10.1002/path.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skvorak K, Vissel B, Homanics GE. Production of conditional point mutant knockin mice. Genesis. 2006;44:345–353. doi: 10.1002/dvg.20222. [DOI] [PubMed] [Google Scholar]
- 24.Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. BioTechniques. 2005;38:702, 704, 706. doi: 10.2144/05385BM05. [DOI] [PubMed] [Google Scholar]
- 25.Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA. Menkes protein contributes to the function of peptidylglycine alpha-amidating monooxygenase. Endocrinology. 2003;144:188–200. doi: 10.1210/en.2002-220716. [DOI] [PubMed] [Google Scholar]
- 26.Brennan-Speranza TC, Henneicke H, Gasparini SJ, Blankenstein KI, Heinevetter U, Cogger VC, Svistounov D, Zhang Y, Cooney GJ, Buttgereit F, Dunstan CR, Gundberg C, Zhou H, Seibel MJ. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. The Journal of clinical investigation. 2012;122:4172–4189. doi: 10.1172/JCI63377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 28.Holloway ZG, Grabski R, Szul T, Styers ML, Coventry JA, Monaco AP, Sztul E. Activation of ADP-ribosylation factor regulates biogenesis of the ATP7A-containing trans-Golgi network compartment and its Cu-induced trafficking. American journal of physiology. Cell physiology. 2007;293:C1753–1767. doi: 10.1152/ajpcell.00253.2007. [DOI] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgkinson VL, Zhu S, Wang Y, Ladomersky E, Nickelson K, Weisman GA, Lee J, Gitlin JD, Petris MJ. Autonomous requirements of the Menkes disease protein in the nervous system. American journal of physiology. Cell physiology. 2015 doi: 10.1152/ajpcell.00130.2015. ajpcell 00130 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology. 2006;130:493–506. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 32.Sleigh JN, Burgess RW, Gillingwater TH, Cader MZ. Morphological analysis of neuromuscular junction development and degeneration in rodent lumbrical muscles. Journal of neuroscience methods. 2014;227:159–165. doi: 10.1016/j.jneumeth.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tse N, Morsch M, Ghazanfari N, Cole L, Visvanathan A, Leamey C, Phillips WD. The neuromuscular junction: measuring synapse size, fragmentation and changes in synaptic protein density using confocal fluorescence microscopy. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/52220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comley LH, Fuller HR, Wishart TM, Mutsaers CA, Thomson D, Wright AK, Ribchester RR, Morris GE, Parson SH, Horsburgh K, Gillingwater TH. ApoE isoform-specific regulation of regeneration in the peripheral nervous system. Human molecular genetics. 2011;20:2406–2421. doi: 10.1093/hmg/ddr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gourdon P, Liu XY, Skjorringe T, Morth JP, Moller LB, Pedersen BP, Nissen P. Crystal structure of a copper-transporting PIB-type ATPase. Nature. 2011;475:59–64. doi: 10.1038/nature10191. [DOI] [PubMed] [Google Scholar]
- 36.Meadows E, Cho JH, Flynn JM, Klein WH. Myogenin regulates a distinct genetic program in adult muscle stem cells. Developmental biology. 2008;322:406–414. doi: 10.1016/j.ydbio.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Carnac G, Vernus B, Bonnieu A. Myostatin in the pathophysiology of skeletal muscle. Current genomics. 2007;8:415–422. doi: 10.2174/138920207783591672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi L, Donsante A, Kennerson ML, Mercer JF, Garbern JY, Kaler SG. Altered intracellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy. Human molecular genetics. 2012;21:1794–1807. doi: 10.1093/hmg/ddr612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie L, Collins JF. Copper stabilizes the Menkes copper-transporting ATPase (Atp7a) protein expressed in rat intestinal epithelial cells. American journal of physiology. Cell physiology. 2013;304:C257–262. doi: 10.1152/ajpcell.00336.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiology of disease. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- 41.Tamiya G, Makino S, Hayashi M, Abe A, Numakura C, Ueki M, Tanaka A, Ito C, Toshimori K, Ogawa N, Terashima T, Maegawa H, Yanagisawa D, Tooyama I, Tada M, Onodera O, Hayasaka K. A mutation of COX6A1 causes a recessive axonal or mixed form of Charcot-Marie-Tooth disease. American journal of human genetics. 2014;95:294–300. doi: 10.1016/j.ajhg.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilton JB, White AR, Crouch PJ. Metal-deficient SOD1 in amyotrophic lateral sclerosis. J Mol Med (Berl) 2015;93:481–487. doi: 10.1007/s00109-015-1273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong SY, Rathore KI, Schulz K, Ponka P, Arosio P, David S. Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:610–619. doi: 10.1523/JNEUROSCI.5443-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlief ML, Craig AM, Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlief ML, West T, Craig AM, Holtzman DM, Gitlin JD. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14919–14924. doi: 10.1073/pnas.0605390103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mays TA, Sanford JL, Hanada T, Chishti AH, Rafael-Fortney JA. Glutamate receptors localize postsynaptically at neuromuscular junctions in mice. Muscle & nerve. 2009;39:343–349. doi: 10.1002/mus.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malomouzh AI, Nurullin LF, Arkhipova SS, Nikolsky EE. NMDA receptors at the endplate of rat skeletal muscles: precise postsynaptic localization. Muscle & nerve. 2011;44:987–989. doi: 10.1002/mus.22250. [DOI] [PubMed] [Google Scholar]
- 48.Walder KK, Ryan SB, Bzdega T, Olszewski RT, Neale JH, Lindgren CA. Immunohistological and electrophysiological evidence that N-acetylaspartylglutamate is a co-transmitter at the vertebrate neuromuscular junction. The European journal of neuroscience. 2013;37:118–129. doi: 10.1111/ejn.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.