Abstract
This study examined whether peripheral vision reaction time (PVRT) in patients with mild traumatic brain injury (mTBI) correlated with white matter abnormalities in centroaxial structures and impairments in neuropsychological testing. Within 24 h after mTBI, crossed reaction times (CRT), uncrossed reaction times (URT), and crossed–uncrossed difference (CUD) were measured in 23 patients using a laptop computer that displayed visual stimuli predominantly to either the left or the right visual field of the retina. The CUD is a surrogate marker of the interhemispheric transfer time (ITT). Within 7 days after the injury, patients received a diffusion tensor-MRI (DTI) scan and a battery of neuropsychological tests. Nine uninjured control subjects received similar testing. Patients 18–50 years of age were included if they had a post-resuscitation Glasgow Coma Scale >13 and an injury mechanism compatible with mTBI. Healthy controls were either age- and gender-matched family members of the TBI patients or healthy volunteers. CUD deficits >2 standard deviations (SD) were seen in 40.9% of patients. The CUD of injured patients correlated with mean diffusivity (MD) (p < 0.001, ρ = −0.811) in the posterior corpus callosum. Patients could be stratified on the basis of CUD on the Stroop 1, Controlled Oral Word Association Test (COWAT), and the obsessive-compulsive component of the Basic Symptom Inventory tests. These studies suggest that the PVRT indirectly measures white matter integrity in the posterior corpus callosum, a brain region frequently damaged by mTBI.
Keywords: : diagnosis, mTBI, reaction time, peripheral vision, reaction time, neuropsychiatric tests
Introduction
Mild traumatic brain injury (mTBI) represents a challenge to clinicians, as symptoms and signs are protean, nonspecific, and overlap with other neurological or psychological diseases. Further, although most patients with mTBI recover fully, a significant minority do not, and early identification of those patients at risk of developing persistent symptoms is critical in order to develop effective therapies.1,2 mTBI commonly damages centroaxial white matter tracts such as the corpus callosum.3,4 The corpus callosum transfers information between the two cerebral hemispheres, and the peripheral vision reaction time (PVRT) is an assessment of interhemispheric transfer of information (ITT).3,4 The PVRT measures the interval between the appearance of a visual stimulus and the pressing of a computer key with the patient's dominant hand. Responses are recorded in terms of a crossed reaction time (CRT) or uncrossed reaction time (URT) depending upon the visual field in which the stimulus was presented. The difference between the CRT and URT is known as the crossed–uncrossed difference (CUD), which is a surrogate marker for ITT. The CUD is known to increase after moderate to severe TBI; less is known about whether CUD changes following mTBI.5,6
Diffusion tensor-MRI (DTI) is a sensitive brain imaging method that can measure white matter abnormalities following mTBI.7–10 mTBI commonly damages the corpus callosum and other centroaxial structures. We tested the hypothesis that a slowing of the CUD correlated with DTI abnormalities in the corpus callosum and other centroaxial white matter.
Methods
Twenty-three traumatically injured patients and nine controls were recruited from the Emergency Department and Neurological Surgery service at Parkland Hospital, Dallas Texas between February 2011 and January 2014. All the injured patients were 18–50 years old with a Glasgow Coma Score ≥13 (Table 1).
Table 1.
Characteristics of Control and Injured Cohorts
| Control | Injured (all) | Injured with CUD z < 2 | Injured with CUD z > 2 | Injured with 2 < CUD z < 10 | Injured with CUD z > 10 | |
|---|---|---|---|---|---|---|
| n | 10 | 24a | 14 | 8 | 4 | 4 |
| % Left handed | 11.1 | 18.2 | 21.4 | 12.5 | 0 | 25 |
| % Female | 33.3 | 22.7 | 21.4 | 25 | 50 | 0 |
| Glasgow Coma Score on arrival | 14.9 ± 0.07 | 14.9 ± 0.10 | 14.9 ± 0.13 | 14.75 ± 0.25 | 15 ± 0 | |
| Years of education | 16.0 ± 0.6 | 12.4 ± 0.5 | 13.3 ± 0.6 | 11.1 ± 0.9 | 12.6 ± 0.7 | 11.8 ± 0.6 |
| Initial CT findings n (%) | ||||||
| Any intracranial abnormality | 18 (75) | 11 (78.6) | 6 (75) | 2 (50) | 4 (100) | |
| Traumatic subarachnoid hemorrhage | 10 (41.7) | 8 (57.1) | 4 (50) | 2 (50) | 2 (50) | |
| Contusion | 9 (37.5) | 5 (35.7) | 4 (50) | 3 (75) | 1 (25) | |
| Subdural hemorrhage | 4 (16.7) | 5 (35.7) | 2 (25) | 0 (0) | 2 (50) | |
| Extradural hemorrhage | 3 (12.5) | 2 (14.29) | 1 (12.5) | 0 (0) | 1 (25) | |
| Other | 3 (12.5) | 1 (7.14)e | 2 (25)f | 0 (0) | 2 (50)f | |
| Skull fracture(s) | 10 (41.7) | 7 (50.0) | 3 (37.5) | 0 (0) | 3 (75) | |
| Facial/sinus fracture(s) | 10 (41.7)b | 4 (28.57) | 4 (50) | 2 (50) | 2 (50) | |
| Mechanism of injury | ||||||
| Fall | 8 | 6 | 2 | 1 | 1 | |
| Aggravated assault | 6c | 2 | 3 | 2 | 1 | |
| Automobile collision | 5d | 2 | 2 | 1 | 1 | |
| Motorcycle /moped collision | 4 | 3 | 1 | 0 | 1 | |
| Automobile pedestrian collision | 1 | 1 | 0 | 0 | 0 | |
The control and injured groups were matched for age, handedness, and sex, but not for education. Differences in the educational level were controlled in the neuropsychological testing.
Two injured subjects did not receive the PVRT.
Two had facial / sinus fractures with no intracranial abnormalities.
One who had experienced aggravated assault did not get IHT testing.
One who had been in an automobile collision did not get IHT testing.
Refers to punctate hemorrhagic foci of the bilateral hemispheres compatible with shear injury.
Refers to 1) Focal hyperdensity along the left lateral aspect of the medulla, of uncertain etiology and 2) fracture through the petrous temporal bone crossing the horizontal portion of the petrous segment of the right internal carotid artery.
CUD, crossed–uncrossed difference.
Patients with mTBI were given a peripheral visual reaction test within 24 h after injury, and controls were given the test upon giving consent (Fig. 1A and B). The PVRT was administered to subjects using a laptop computer running tachistoscopic software. This software measures the time elapsed between exposure to a plus sign 31.6 degrees on the left or the right from the central point, and the striking of a computer space bar with the subject's dominant hand. The stimulus remained on the screen until the key press. The test consisted of 50 trials that were randomized to side and inter-trial interval. This test yielded three outcomes, the CRT in which the stimulus was ipsilateral to the patient's dominant hand, the URT in which the stimulus was contralateral to the dominant hand, and the CUD that was the difference between the CRT and the URT. Both the mTBI and control subjects showed a similar decrease in the URT or CRT throughout the 50 trials (in msec/trial): URT, control: −2.90 ± 1.27; injured: −4.92 ± 2.86, t29 = −0.45, p > 0.5; CRT, control: −0.38 ± 0.95; injured, −2.15 ± 2.56, t29 = −0.43, p > 0.5. These data suggest that injured and control patients had a similar ability to improve performance on the task regardless of the size of their CRT or URT impairment. As a result, the CRT and URT were computed from the average of the final five trials on each side. Within 7 days after injury, the same cohort of mTBI patients received MRI scans including a DTI sequence using a 3 tesla magnet MRI at the Advanced Imaging Research Center at University of Texas Southwestern Medical Center. Controls also received MRI scans.
FIG. 1.
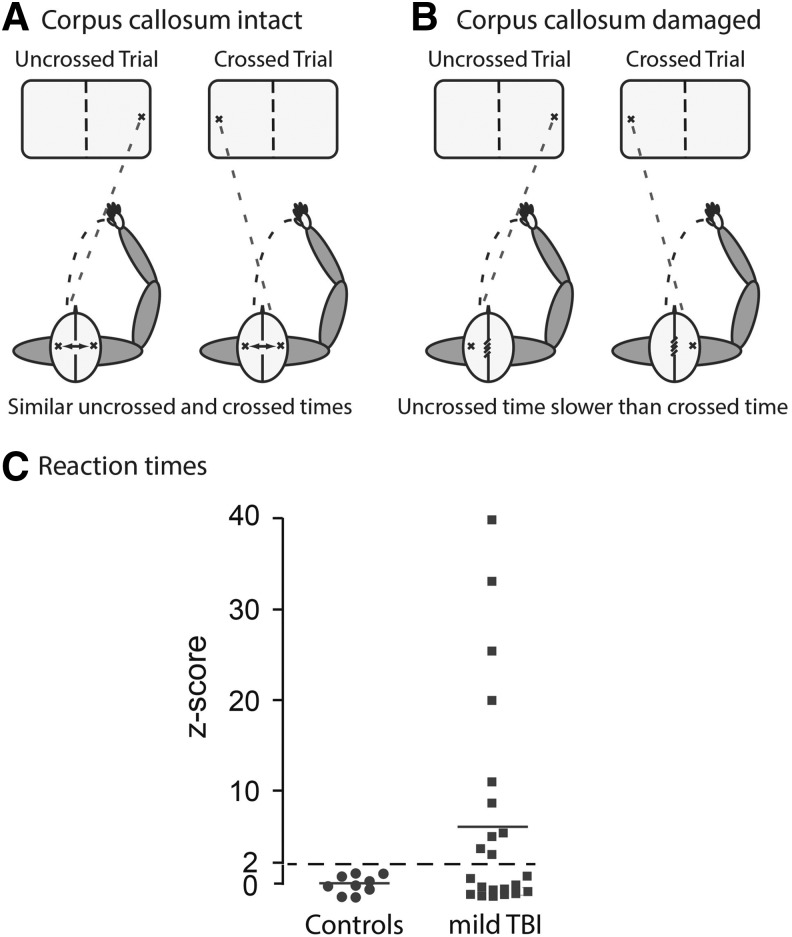
Mild traumatic brain injury (mTBI) patients have deficits in the peripheral reaction time test. Panel A: The peripheral reaction time tests measure an uncrossed reaction time (URT) with a peripheral stimulus initially placed in the cerebral hemisphere ipsilateral to the motor cortex controlling the dominant hand, and a crossed reaction time (CRT) when the peripheral stimulus is initially placed in the cerebral cortex contralateral to the motor cortex controlling the dominant hand. Because control patients have efficient interhemispheric information transfer, the crossed–uncrossed difference (CUD) between the CRT and URT is anticipated to be small. Panel B: Inefficient interhemispheric information transfer is anticipated to increase the CUD of injured patients. Panel C: Measurements of CUD in control and mTBI patients within 24 h of injury. CUD z-scores are presented because of the large scatter in the mTBI group. The percentage of patients is indicated with CUD z-scores >2.
MRI scan acquisition and preprocessing
DTI data were acquired with an acquisition matrix of 128 × 128, field of view of 224 × 224 mm, slice thickness of 2 mm, and an inter-slice gap of 1 mm. The DTI protocol employed 30 gradient directions with a b-value of 1000 sec/mm2 along with one b0 image and was processed using FSL 5.0.8 (www.fmrib.ox.ac.uk/fsl/). First, the data underwent correction for motion and eddy current distortions, followed by skull stripping.11,12 Tensors were estimated using the DTIFIT module and fractional anisotropy (FA) data were processed using the tract-based spatial statistics (TBSS) pipeline that co-registers scans into Montreal Neurological Institute (MNI) space and creates a skeletonized representation of central regions of white matter from the FA map. FA values were projected onto the skeleton after thresholding the mean FA map at 0.2. This processing was also applied to mean diffusivity (MD) maps.13 Region of interest (ROI) masks for tracks of interest were from an in-house atlas compiled from 28 healthy, normal, young adults who underwent DTI imaging as part of a study on normal aging. Deterministic DTI tractography was performed in native space using MedINRIA, and the occupied voxels were exported for each tract. These tract representations were transformed into MNI space, combined across subjects, and thresholded to retain only voxels present in a majority of subjects.
Procedures
Twenty-three civilian patients with mTBI were recruited from Parkland Memorial Hospital, Dallas, TX after a review of the injury and their first post-resuscitation Glasgow Coma Scale. Consent was obtained according to the local institutional review board (IRB) regulations from the patient or, in one case, from a legally authorized representative. Included patients were alert, oriented, and able to follow commands by a nurse. The experimenter performing the computer testing further confirmed the patient's level of awareness and orientation. Healthy controls were age- and gender-matched family members of the TBI patients or other healthy volunteers who agreed to participate in the study.
Patients were included if they were 18–50 years of age with an injury compatible with TBI and a post-resuscitation Glasgow Coma Score ≥13 within 24 h of consent. They were excluded if they had a history of pre-existing neurological disease, including a previous hospitalization for TBI >1 day, were pregnant, or were contraindicated for MRI. Controls were included if they were in good health, had normal brain anatomy on MRI, were not cognitively impaired, and could provide written consent. Controls were excluded if they had a neurological disease, including head trauma with loss of consciousness, or were contraindicated for MRI. All patients received the PVRT test within 24 h after injury. Of the 23 patients consenting, 14 injured patients received MRI scans within 1 week of injury. Of the remainder, six of these patients left the hospital after consenting and receiving the peripheral vision reaction time test, but did not return for their scheduled appointment for scanning and could not be contacted. One patient voluntarily left the study and one could not be scanned because the MRI scanner was not working properly. All nine controls received the PVRT test and then received a MRI scan that included a DTI sequence.
Cranial CT scans were performed on admission to the emergency department on all patients. CT scans were read by a neuroradiologist, and clinical reports were generated. Findings noted in the reports were abstracted to identify trauma-related abnormalities.
Patients received the following neuropsychological tests on the day of MRI scanning in an outpatient setting within 7 days of injury: Digit Symbol, Symbol Search, Digit Span, Controlled Oral Word Test, Trails A and B, Brief Symptom Inventory, and California Verbal Learning Test. Controls received the same testing. This study was formally reviewed and approved by the IRBs of the State University of New York-Downstate Medical Center and the University of Texas-Southwestern
Statistical analysis
The primary hypothesis of this study was that aberrant FA or MD in selected white matter regions correlated with CUD deficits. TBSS-derived skeletonized DTI data were analyzed using two methods. 1) Tract level analyses were obtained by isolating regions of the skeletonized data with ROI masks representing 1 of 20 white matter tracts. The voxels within each ROI were then averaged for each tract. The 20 tracts consisted of 4 midline tracts (forceps minor, anterior corpus callosum, posterior corpus callosum, and the forceps major) and the left and right representations of 8 lateralized tracts (corticospinal tract, frontal aslant tract, frontal occipital fasciculus, inferior longitudinal fasciculus, cingulum bundle, perforant pathway, superior longitudinal fasciculus, and uncinate fasciculus). Mean tract values were used for between-group comparisons and correlations.14,15 2) Voxelwise between-group comparisons and correlations were run using the Randomise tool in FSL, which is a permutation-based Monte Carlo technique.16 We used 5000 permutations, threshold-free cluster enhancement, and correction for multiple comparisons using a familywise error rate of p < 0.05.
Analyses of variance were analyzed for groups of three or more. Subsequent pairwise comparisons were made using the Student–Neumann–Keuls post-hoc test. Groups of two were analyzed by Student's t test. Chi-square analysis analyzed the percent of left-handed subjects in the control and experimental groups. Nonparametric Spearman correlation analyses were initially performed with Prism Version 6.0f (GraphPad Software, Inc., La Jolla, CA). The Benjamini–Hochberg procedure was applied to the Spearman correlations to control for the false discovery rate of multiple comparison testing.17 In Figure 2, a log transformation of negative z-scores of FA and MD was avoided by converting z-scores into t-scores. In all tests, statistical significance was set at 0.05.
FIG. 2.
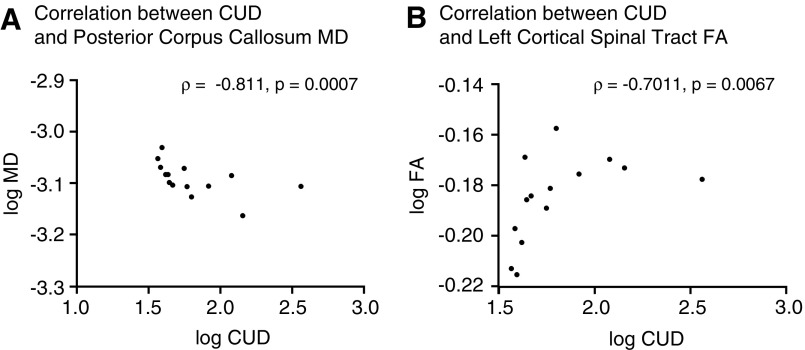
Correlations between the crossed–uncrossed difference (CUD) and white matter abnormalities. Scatter plots comparing the t-score of the CUD with the log mean diffusivity (MD) of the posterior corpus callosum (Panel A) or the log fractional anisotropy (FA) of the left corticospinal trace (Panel B). Plots include the coefficient correlation and the statistical significance of each comparison.
Results
There was no difference in age or gender between mTBI subjects and uninjured controls (Table 1). The control subjects had significantly higher education. Despite having Glasgow Coma scores >14; 18 out of 24 injured patients had trauma-related intracranial abnormalities noted on the CT scan. In most cases, these were minor traumatic subarachoid hemorrhages (tSAH) or small contusions (Table 1). Using similar inclusion criteria, Sours and coworkers classified this population as having “complicated mTBI.”6
The CUD has been used as a surrogate marker for the time needed to transfer information between the two hemispheres.11 The CUD of the control and mTBI groups did not significantly differ (in msec: control, 10.2 ± 4.2; mTBI, 13.7 ± 10.6, t29 = 0.72, p > 0.5) (Fig. 1C). There was, however, substantial scatter in the CUD of the mTBI patients, with 40.9% having slower CUD times, with z-scores >2 SDs greater than controls (Fig. 1C). A smaller percentage (18.2%) of mTBI patients had very slow CUD times, with z-scores >10 SDs greater than controls (Fig. 1C). In contrast, no control subjects had a CUD time with a z-score >2. These data suggest that a percentage, but not all, of putative mTBI patients had significant slowing of the CUD. Patients with slower CUD scores were hypothesized to have white matter injury, in particular in the corpus callosum.
We then tested for whether the slowing of the CUD correlated with the presence of white matter abnormalities in the mTBI patients. DTI tractography assessed 20 white matter tracts of interest for changes in FA and MD. The CUD of each individual patient was then correlated with the FA and MD values of the 20 white matter tracts and corrected with a false discovery rate of 0.05. The CUD significantly correlated with lower MD of the posterior corpus callosum (ρ = ‐0.811, p = 0.0007) (Fig. 2A). CUD values also had a significant positive correlation to increased FA of the left corticospinal tract (ρ = 0.715, p = 0.007, Fig 2B) and the right arcuate fasciculus (ρ = −0.578, p = 0.03). The high correlation with the posterior corpus callosum provides strong evidence that CUD is a surrogate marker for information transfer across the corpus callosum. In addition, mTBI frequently damages the posterior corpus callosum.18 White matter tracts other than those that cross the midline also are likely contributors to CUD slowing, because slower CUDs were also significantly correlated with damage to a longitudinal tract (arcuate fasciculus) and to a descending tract (corticospinal). These data suggest that injury to a variety of white matter tracts correlates with a slowing of the CUD. A correlation of CUD slowing with DTI abnormalities in multiple white matter tracts increases its potential utility, because mTBI is heterogeneous, and patients differ in the patterns of white matter injury.19
On neuropsychological testing, mTBI patients significantly differed from controls on the processing speed index as well as its individual components of Digit Symbol and Digit Span forward and backward, Trails A and B, and the California Verbal Learning Test (Table 2). On these tests, the mTBI patients with a CUD z-score >2 did not different from those with a CUD <2 SD. mTBI patients with a CUD >2 SD, however, did significantly differ from those with a CUD <2 SD on the Stroop 1, the overall and the phonemic component of the Controlled Oral Word Association Test (COWAT), the obsessive-compulsive components, and the number of overall positive symptoms portion of the Brief Symptom Inventory. These data suggest that the CUD test stratified patients with performance on processing speed, Stroop 1, and COWAT. On the remaining neuropsychological tests, controls and mTBI patients had similar scores.
Table 2.
Summary of Neuropsychological Testing
| Test | Controls | mTBI | t test comparison of controls and mTBI | CUD z score <2 SD | CUD z score >2SD | Post-hoc ANOVA statistic comparing CUD z-score <2 vs. >2 |
|---|---|---|---|---|---|---|
| Processing Speed Index | 108 ± 4.0 | 88.4 ± 3.2 | t16 = 3.7 p < 0.002 |
85.3 ± 0.7 | 90.2 ± 5.1 | NS |
| Digit Symbol | 11.3 ± 0.7 | 6.4 ± 0.5 | NS | 6.8 ± 0.7 | 5.7 ± 0.3 | NS |
| Digit Span | 11.0 ± 0.6 | 7.9 ± 0.9 | t16 = 3.4 p < 0.005 |
9.0 ± 0.9 | 6.0 ± 1.0 | NS |
| Controlled Oral Word Association Test | 50 ± 2.8 | 26.6 ± 5.5 | t16 = 2.3 p < 0.05 |
13.3 ± 6.3 | 34.6 ± 5.3 |
F2,16 = 15.95, p < 0.001; p < 0.02 |
| Trail Marking Test A | 50.2 ± 3.0 | 41.9 ± 3.7 | NS | 37.7 ± 5.7 | 44.4 ± 4.9 | NS |
| Trail Marking Test B | 46.8 ± 6.6 | 94.0 ± 15.0 | NS | 85.6 ± 21.4 | 108.0 ± 20.0 | NS |
| Stroop 1 | 38.6 ± 1.6 | 55.9 ± 7.9 | t15 = −2.3 p < 0.05 |
43.8 ± 4.4 | 76.0 ± 14.0 |
F2,16 = 11.62 p < 0.0001; p < 0.002 |
| Stroop 2 | 83.2 ± 6.1 | 125.5 ± 17.1 | t15 = −2.4 p < 0.05 |
110.0 ± 20.8 | 151.3 ± 27.7 | NS |
| Stroop Interference | 44.7 ± 5.8 | 69.6 ± 12.6 | NS | 66.2 ± 19.6 | 75.3 ± 13.7 | NS |
| Basic Symptom Inventory- Obsessive Compulsive | 60.6 ± 1.8 | 54.0 ± 6.1 | NS | 65.8 ± 6.1 | 38.3 ± 0.3 |
F2,15 = 17.96 p < 0.001; p < 0.001 |
| Basic Symptom Inventory- Positive Symptoms Total | 54.6 ± 2.2 | 51.1 ± 5.8 | NS | 39.0 ± 9.0 | 60.3 ± 3.6 |
F2,15 = 5.47 p < 0.02; p < 0.02 |
| Basic Symptom Inventory- Positive Symptoms Distress Index | 51.8 ± 1.5 | 53.3 ± 3.0 | NS | 56.0 ± 2.0 | 49.7 ± 6.7 | NS |
| Basic Symptom Inventory- Global Severity Index | 54.2 ± 2.3 | 52.9 ± 5.5 | NS | 60.8 ± 3.6 | 42.3 ± 9.3 | NS |
| California Verbal Learning Test Total Learning Slope, Trials 1–5 | 0.4 ± 0.2 | −0.9 ± 0.5 | t14 = 3.1 p 0.01 |
0.63 ± 0.3 | −1.3 ± 0.7 | NS |
Control and mTBI patients differed significantly on processing speed index, digit span, COWAT, Stroop 1, Stroop 2 and California Verbal Learning Test. mTBI patients with a CUD <2SD differed significantly from patients >2SD on COWAT, Stroop 1, and Basic Symptom Inventory obsessive compulsive. These data suggest that impairments in select neuropsychology tests can be stratified based on CUD scores.
mTBI, mild traumatic brain injury; CUD, crossed–uncrossed difference; COWAT, Controlled Oral Word Association Test.
Discussion
The major conclusions of this study are that: 1) large CUD deficits occur in a substantial subset of mTBI patients, 2) CUD times negatively correlate with MD in the posterior corpus callosum, when imaged within 1 week post-injury, 3) CUD times significantly correlated with FA of the left corticospinal tract and the arcuate fasiculus, and 4) CUD times could stratify performance on multiple neurosychological tests.
CUD time appears to be an indirect measure of the normal functioning of the corpus callosum. The corpus callosum, and particularly the posterior corpus callosum, has been implicated by multiple studies as an important white matter tract for CUD.3,5,20–25 CUD deficits have been reported in moderate to severe TBI.5,21 These deficits have been studied less in mTBI. In moderate to severe TBI patients, a slowing of the CUD correlated with a reduced volume of the corpus callosum or with increased DTI abnormalities.5,21 The present study compared CUD with DTI measurements in 20 white matter regions (Table 3). The CUD had the strongest correlation with the MD of posterior corpus callosum but did not significantly correlate with the remaining 19 white matter tracts (Fig. 2A).
Table 3.
FA and MD in 20 White Matter Tracts of Control Subjects and mTBI Patients
| Brain region | Control FA | mTBI FA | Control MD (×10−4) | mTBI MD (×10−4) |
|---|---|---|---|---|
| Anterior corpus callosum | 0.635 ± 0.016 | 0.643 ± 0.015 | 8.616 ± 0.182 | 8.668 ± 0.201 |
| Posterior corpus callosum | 0.709 ± 0.017 | 0.719 ± 0.012 | 8.191 ± 0.124 | 8.113 ± 0.160 |
| Cingulum left | 0.507 ± 0.009 | 0.514 ± 0.11 | 6.627 ± 0.008 | 6.693 ± 0.008 |
| Cingulum right | 0.490 ± 0.012 | 0.493 ± 0.012 | 6.589 ± 0.007 | 6.654 ± 0.007 |
| Forceps major | 0.568 ± 0.008 | 0.568 ± 0.008 | 7.188 ± 0.004 | 7.185 ± 0.004 |
| Forceps minor | 0.506 ± 0.010 | 0.513 ± 0.007 | 7.237 ± 0.009 | 7.255 ± 0.007 |
| Cortical-spinal left | 0.663 ± 0.008 | 0.654 ± 0.007 | 6.430 ± 0.005 | 6.440 ± 0.005 |
| Cortical-spinal right | 0.648 ± 0.008 | 0.640 ± 0.007 | 6.485 ± 0.007 | 6.592 ± 0.005 |
| Frontal aslant path left | 0.463 ± 0.009 | 0.464 ± 0.005 | 6.616 ± 0.005 | 6.632 ± 0.006 |
| Frontal aslant path right | 0.459 ± 0.009 | 0.452 ± 0.005 | 6.686 ± 0.006 | 6.774 ± 0.006 |
| Fronto-occipital fasciculus left | 0.538 ± 0.008 | 0.537 ± 0.007 | 7.108 ± 0.007 | 7.121 ± 0.007 |
| Fronto-occipital fasciculus right | 0.498 ± 0.009 | 0.494 ± 0.007 | 7.042 ± 0.009 | 7.107 ± 0.006 |
| Inferior longitudinal fasciculus left | 0.517 ± 0.007 | 0.513 ± 0.007 | 6.966 ± 0.008 | 6.970 ± 0.008 |
| Inferior longitudinal fasciculus right | 0.521 ± 0.010 | 0.514 ± 0.008 | 6.821 ± 0.009 | 6.899 ± 0.12 |
| Superior longitudinal fasciculus left | 0.522 ± 0.010 | 0.524 ± 0.007 | 6.678 ± 0.004 | 6.736 ± 0.008 |
| Superior longitudinal fasciculus right | 0.524 ± 0.013 | 0.519 ± 0.011 | 6.873 ± 0.008 | 6.934 ± 0.005 |
| Perforant path left | 0.467 ± 0.010 | 0.461 ± 0.012 | 7.101 ± 0.009 | 6.980 ± 0.010 |
| Perforant path right | 0.487 ± 0.011 | 0.482 ± 0.010 | 6.570 ± 0.007 | 6.566 ± 0.007 |
| Uncinate fasciculus left | 0.447 ± 0.006 | 0.439 ± 0.008 | 6.990 ± 0.004 | 7.047 ± 0.006 |
| Uncinate fasciculus right | 0.467 ± 0.009 | 0.465 ± 0.009 | 7.027 ± 0.007 | 7.123 ± 0.006 |
mTBI patients were scanned within 1 week of injury. Neither FA nor MD was significantly different between the control and the mTBI groups.
FA, fractional anisotropy; MD, mean diffusivity; mTBI, mild traumatic brain injury.
MD consists of two components that are combined: radial diffusivity and axial diffusivity [MD = (2RD+AD)/3]. Reduced axial diffusivity was more responsible than radial diffusivity for the correlation of ITT with decreased MD. This finding differs from previous studies that imaged subjects several weeks to months after injury or with moderate to severe TBI.5 Time after injury or injury severity can alter the changes in FA and MD. Elevated FA has been seen in studies that image mTBI patients within 1 week.19 Bazarian and coworkers and Wilde and coworkers reported increased FA and lowered MD in the corpus callosum 1–6 days after injury, a finding consistent with our results.26,27 Therefore, mTBI patients when imaged early have MD and FA findings that are in the opposite direction of what is typically reported in more severe injuries and in those imaged later. The reduction of MD in the corpus callosum may represent restricted diffusion that may be associated with cytotoxic edema seen with acute neuronal damage. Studies in mice have shown that DTI can distinguish axonal damage from demyelination. After demyelination by cuprizone or by retinal ischemia, acute axonal pathology resulted in decreased axial diffusivity that was only later followed by increased radial diffusivity during development of secondary demyelination.28,29 Therefore, the negative correlation of the CUD with the MD of posterior corpus callosum likely reflects impaired function of the corpus callosum that leads to a slowing of the CUD.19–26
This study agrees with previous studies that impairments on neuropsychological testing are common after mild TBI.30 The stratification of patients based on CUD, however, revealed neuropsychological testing deficits not seen without stratification (Table 2). On the Stroop 1 test, patients with CUD z-scores >2 had significantly larger deficits than those with CUD z-scores <2 (Table 2). Stratification of patients based on CUD was not seen with the cognitively more difficult Stroop 2 test or with Stroop interference. The greater impairment in Stroop 1 in patients with a CUD z-score > than 2 suggests a slower processing speed than in patients with a CUD z-score <2.31
Mildly injured patients with CUD z-scores >2 reported significantly more symptoms on the Obsessive-Compulsive subtest in the Basic Symptoms Inventory than controls or injured patients with CUD z-scores >2. This result agrees with a previous study showing that mildly injured patients report the most symptoms on the Obsessive-Compulsive subtest in the Basic Symptoms Inventory.32 The Obsessive-Compulsive subtest examines psychological issues that are common complaints after TBI: trouble recalling, feeling blocked, difficulty making decisions, trouble concentrating, and needing to re-check one's actions.32
Patients with mTBI have reaction time deficits using stimuli peripheral to a central fixation point.33,34 Deficits noted in previous reports have not been as large as those seen in this study. In uninjured patients, ITT increases as the distance between the central fixation point and the visual stimulus increases. This suggests that the large (31.6 degree) eccentricity between the central fixation point and the peripheral visual stimulus used in our study contributes to the large size of the reaction time deficits (Fig. 1B).35,36
The potential utility of the PVRT test is limited by the fact that it does not distinguish between controls and all the mildly injured patients (Fig. 1). It may provide an inexpensive and rapid method, however, for distinguishing between mildly injured patients who have controaxial white matter damage from those who do not. The PVRT test could also be potentially incorporated into future studies to test drugs that limit white matter damage after mTBI.
The study was limited because the sample size was modest, and different results may arise when larger cohorts are studied. Recruitment from the emergency department likely resulted in more patients with intracranial CT abnormalities than in patients recruited from other clinical settings. The presence of cranial abnormalities complicates the question of whether these patients had mTBI despite having a GCS >13.
There were also differences in the educational levels of the control and experimental groups. The study should be replicated in a larger group to confirm the utility of the test. Only a subset of the patients who took the PVRT test received MRI imaging. In addition, the mTBI group was heterogeneous in pathology that included the presence of SAH and tSAH.
Future studies should focus on mTBI patients with negative neuroimaging on CT. Long-term (≥3 months) functional outcomes were not assessed; therefore, the relationship among acute ITT prolongation, ITT evolution, and persistent neurocognitive deficits remains unknown. The potential utility of the peripheral vision reaction test is clear because, at present, mTBI is diagnosed with subjective clinical assessments. The peripheral vision reaction test is an objective, inexpensive, and rapid test that detects deficits in a large percentage of mTBI patients. Additional, larger studies are needed to assess the utility of ITT as a biomarker of centroaxial white matter injury.
Acknowledgments
This work was funded an award from the United States Army Medical Research and Materiel Command W81XWH1011061. L.C.T. and R.D.-A. were also supported by the Center for Neuroscience and Regenerative Medicine. The United States Army Medical Research and Materiel Command had no input into the design and conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Diaz-Arrastia R., Kochanek P.M., Bergold P., Kenney K., Marx C.E., Grimes C.J., Loh L.T., Adam L.T., Oskvig D., Curley K.C., and Salzer W. (2014). Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin H.S., and Diaz–Arrastia R.R. (2015). Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 14, 506–517 [DOI] [PubMed] [Google Scholar]
- 3.Schulte T., Sullivan E.V., Muller–Oehring E.M., Adalsteinsson E., and Pfefferbaum A. (2005). Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb. Cortex 15, 1384–1392 [DOI] [PubMed] [Google Scholar]
- 4.Bigler E.D. (2013). Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychol. Rev. 23, 169–209 [DOI] [PubMed] [Google Scholar]
- 5.Dennis E.L., Ellis M.U., Marion S.D., Jin Y., Moran L., Olsen A., Kernan C., Babikian T., Mink R., Babbitt C., Johnson J., Giza C.C., Thompson P.M., and Asarnow R.F. (2015). Callosal function in pediatric traumatic brain injury linked to disrupted white matter integrity. J. Neurosci. 35, 10,202–10,211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sours C., Rosenberg J., Kane R., Roys S., Zhuo J., Shanmuganathan K., and Gullapalli R.P. (2014). Associations between interhemispheric functional connectivity and the Automated Neuropsychological Assessment Metrics (ANAM) in civilian mild TBI. Brain Imaging Behav. 9, 190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mechtler L.L., Shastri K.K., and Crutchfield K.E. (2014). Advanced neuroimaging of mild traumatic brain injury. Neurol. Clin. 32, 31–58 [DOI] [PubMed] [Google Scholar]
- 8.Yuh E.L., Hawryluk G.W., and Manley G.T. (2014). Imaging concussion: a review. Neurosurgery 75, Suppl. 4, S50–63 [DOI] [PubMed] [Google Scholar]
- 9.Wang J.Y., Bakhadirov K., Abdi H., Devous M.D., Sr., Marquez de la Plata C.D., Moore C., Madden C.J., and Diaz–Arrastia R. (2011). Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology 77, 818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J.Y., Bakhadirov K., Devous M.D., Sr., Abdi H., McColl R., Moore C., Marquez de la Plata C.D., Ding K., Whittemore A., Babcock E., Rickbeil T., Dobervich J., Kroll D., Dao B., Mohindra N., Madden C.J., and Diaz–Arrastia R. (2008). Diffusion tensor tractography of traumatic diffuse axonal injury. Arch. Neurol. 65, 619–626 [DOI] [PubMed] [Google Scholar]
- 11.Smith S.M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen–Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., and Matthews P.M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Suppl. 1, S208–219 [DOI] [PubMed] [Google Scholar]
- 13.Smith S.M., Jenkinson M., Johansen–Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., and Behrens T.E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 14.Strain J., Didehbani N., Cullum C.M., Mansinghani S., Conover H., Kraut M.A., Hart J., Jr., and Womack K.B. (2013). Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology 81, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H., Fan X., Weiner M., Martin–Cook K., Xiao G., Davis J., Devous M., Rosenberg R., and Diaz–Arrastia R. (2012). Distinctive disruption patterns of white matter tracts in Alzheimer's disease with full diffusion tensor characterization. Neurobiol. Aging 33, 2029–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., and Nichols T.E. (2014). Permutation inference for the general linear model. Neuroimage 92, 381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamini Y., and Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc.. Series B Stat. Methodol. 57, 289–300 [Google Scholar]
- 18.Aoki Y., Inokuchi R., Gunshin M., Yahagi N., and Suwa H. (2012). Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J. Neurol. Neurosurg. Psychiatry 83, 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eierud C., Craddock R.C., Fletcher S., Aulakh M., King–Casas B., Kuehl D., and LaConte S.M. (2014). Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 4, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerhausen R., Kreuder F., Woerner W., Huster R.J., Smit C.M., Schweiger E., and Wittling W. (2006). Interhemispheric transfer time and structural properties of the corpus callosum. Neurosci. Lett. 409, 140–145 [DOI] [PubMed] [Google Scholar]
- 21.Mathias J.L., Bigler E.D., Jones N.R., Bowden S.C., Barrett–Woodbridge M., Brown G.C., and Taylor D.J. (2004). Neuropsychological and information processing performance and its relationship to white matter changes following moderate and severe traumatic brain injury: a preliminary study. Appl. Neuropsychol. 11, 134–152 [DOI] [PubMed] [Google Scholar]
- 22.Benavidez D.A., Fletcher J.M., Hannay H.J., Bland S.T., Caudle S.E., Mendelsohn D.B., Yeakley J., Brunder D.G., Harward H., Song J., Perachio N.A., Bruce D., Scheibel R.S., Lilly M.A., Verger–Maestre K., and Levin H.S. (1999). Corpus callosum damage and interhemispheric transfer of information following closed head injury in children. Cortex 35, 315–336 [DOI] [PubMed] [Google Scholar]
- 23.Bendon R.W., Dungy–Poythress L., Miodovnik M., and Siddiqi T.A. (1996). Perinatal pathology of interhemispheric cyst with thinned posterior corpus callosum: four cases. Pediatr. Pathol. Lab. Med. 16, 299–317 [PubMed] [Google Scholar]
- 24.Fabri M., Del Pesce M., Paggi A., Polonara G., Bartolini M., Salvolini U., and Manzoni T. (2005). Contribution of posterior corpus callosum to the interhemispheric transfer of tactile information. Brain Res. Cogn. Brain Res. 24, 73–80 [DOI] [PubMed] [Google Scholar]
- 25.Fabri M., Polonara G., Del Pesce M., Quattrini A., Salvolini U., and Manzoni T. (2001). Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J. Cogn. Neurosci. 13, 1071–1079 [DOI] [PubMed] [Google Scholar]
- 26.Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., and Peterson D. (2007). Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma 24, 1447–1459 [DOI] [PubMed] [Google Scholar]
- 27.Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., and Levin H.S. (2008). Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955 [DOI] [PubMed] [Google Scholar]
- 28.Sun S.W., Liang H.F., Trinkaus K., Cross A.H., Armstrong R.C., and Song S.K. (2006). Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 55, 302–308 [DOI] [PubMed] [Google Scholar]
- 29.Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., and Neufeld A.H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20, 1714–1722 [DOI] [PubMed] [Google Scholar]
- 30.FitzGerald D.B., and Crosson B.A. (2011). Diffusion weighted imaging and neuropsychological correlates in adults with mild traumatic brain injury. Int. J. Psychophysiol. 82, 79–85 [DOI] [PubMed] [Google Scholar]
- 31.Ben–David B.M., Nguyen L.L., and van Lieshout P.H. (2011). Stroop effects in persons with traumatic brain injury: selective attention, speed of processing, or color-naming? A meta-analysis. J. Int. Neuropsychol. Soc. 17, 354–363 [DOI] [PubMed] [Google Scholar]
- 32.Slaughter J., Johnstone G., Petroski G. and Flax J. (1999). The usefulness of the Brief Symptom Inventory in the neuropsychological evaluation of traumatic brain injury. Brain Inj. 13, 125–130 [DOI] [PubMed] [Google Scholar]
- 33.Cifu D.X., Wares J.R., Hoke K.W., Wetzel P.A., Gitchel G., and Carne W. (2015). Differential eye movements in mild traumatic brain injury versus normal controls. J. Head Trauma Rehabil. 30, 21–28 [DOI] [PubMed] [Google Scholar]
- 34.Drew A.S., Langan J., Halterman C., Osternig L.R., Chou L.S., and van Donkelaar P. (2007). Attentional disengagement dysfunction following mTBI assessed with the gap saccade task. Neurosci. Lett. 417, 61–65 [DOI] [PubMed] [Google Scholar]
- 35.Berlucchi G., Heron W., Hyman R., Rizzolatti G., and Umilta C. (1971). Simple reaction times of ipsilateral and contralateral hand to lateralized visual stimuli. Brain 94, 419–430 [DOI] [PubMed] [Google Scholar]
- 36.Strasburger H., Rentschler I., and Jüttner M. (2011). Peripheral vision and pattern recognition: a review. J. Vis. 11, 13–13 [DOI] [PMC free article] [PubMed] [Google Scholar]