Abstract
Recombinant adeno-associated viral (rAAV) vectors for human gene therapy require efficient and economical production methods to keep pace with the rapidly increasing clinical demand. In addition, the manufacturing process must ensure high vector quality and biological safety. The OneBac system offers easily scalable rAAV vector production in insect Sf9-derived AAV rep/cap-expressing producer cell lines infected with a single baculovirus that carries the rAAV backbone. For most AAV serotypes high burst sizes per cell were achieved, combined with high infectivity rates. OneBac 2.0 represents a 2-fold advancement: First, enhanced VP1 proportions in AAV5 capsids lead to vastly increased per-particle infectivity rates. Second, collateral packaging of foreign DNA is suppressed by removal of the Rep-binding element (RBE). In this study we show that this advancement of AAV5 packaging can be translated to OneBac 2.0-derived packaging systems for alternative AAV serotypes. By removal of the RBE, collateral packaging of nonvector DNA was drastically reduced in all newly tested serotypes (AAV1, AAV2, and AAV8). However, the splicing-based strategy to enhance VP1 expression in order to increase AAV5 infectivity hardly improved infectivity rates of AAV-1, -2, or -8 compared with the original OneBac cell lines. Our results emphasize that OneBac 2.0 represents an advancement for scalable, high-titer production of various AAV serotypes, leading to AAV particles with minimal packaging of foreign DNA.
Keywords: : AAV, baculovirus, OneBac, Rep-binding element (RBE), packaging
Introduction
Recombinant adeno-associated viral (rAAV) vectors have matured to become highly successful tools for human gene therapy. In clinical trials for various applications AAV vectors have shown impressive therapeutic long-term successes. Many of the current trials rely on AAV serotype 1, 2, or 8. The availability of a series of AAV serotypes with alternative cell tropism is being exploited to efficiently target specific cell types in various tissues. The first commercial human gene therapy product certified by the European Medicines Agency (EMA) is based on AAV1 vectors injected intramuscularly for the treatment of lipoprotein lipase deficiency.1 Other successful clinical trials aim at the treatment of hemophilia B, where factor IX expression in the liver is achieved by intravenous application of AAV8 vectors.2 Various gene therapies targeting the retina continue to use AAV vectors of serotype 2.3,4
For rAAV production the therapeutic transgene is flanked by the AAV inverted terminal repeats (ITRs). The AAV genes rep and cap are expressed in trans in the chosen cell system for vector packaging. In addition, helper genes from an unrelated helper virus are coexpressed for AAV production. Vectors pseudotyped with the various AAV serotypes can be manufactured simply by exchanging the required AAV capsid gene during vector production. The most widely used technology for the generation of AAV vectors at laboratory scale involves plasmid cotransfection of HEK-293 cells, where adenoviral helper genes are coexpressed to package rAAVs.5,6 For scale-up production, baculovirus-infected insect (Sf9) cell systems are increasingly used. The initially developed system requires coinfection with three baculoviruses that carry AAV rep, AAV cap, and the AAV vector backbone to achieve AAV vector production in Sf9 cells.7
Combination of AAV rep and cap on one baculovirus reduced the number of baculovirus constructs to be coinfected in order to facilitate and enhance rAAV production.8 The reduction of the number of required baculovirus has proven advantageous because the genetic instability of recombinant baculovirus limit their propagation over multiple successive passages.
We have developed the OneBac system, which is based on stable Sf9 cell lines that express integrated AAV rep and cap of the respective serotype. Infection with a single baculovirus that carries the AAV vector backbone is sufficient to induce AAV production.9,10 This system allows the production of vectors of various AAV serotypes at high burst sizes per cell.
In the OneBac system the AAV capsid protein VP1 is translated from an unspliced mRNA starting off a noncanonical start codon (ACG). This strategy was necessary to achieve the prototypic near 1:1:10 ratio of VP1, VP2, and VP3, because AAV splicing proved to be inefficient in insect cells.7 For many AAV serotypes this strategy translates to highly infectious genomic particles with ratios of capsid proteins close to 1:1:10. However, certain AAV serotypes showed significantly less VP1 expression, resulting in significantly reduced transduction efficiencies of the vectors.10 This led to the development of OneBac 2.0,11 where VP1 expression is increased by insertion of an artificial intron into the cap gene. For proof of concept we chose AAV5, in which VP1 was barely detectable, leading to low infectivity rates. The artificial intron fully restored VP1 expression levels and AAV5 infectivity rates. In OneBac 2.0 we also solved a second major issue that concerns several AAV production systems: The percentage of collateral packaging of foreign, nonvector DNA sequences derived mostly from transduced helper genes. In the case of OneBac, collateral packaging of rep and cap sequences was shown to be dependent on the presence of a Rep-binding element (RBE) in the rep/cap expression constructs. The presence of the RBE had initially been postulated to be essential for efficient AAV packaging in Sf9 cells. We found that the RBE could be deleted from the AAV5 cell lines without affecting vector yield or infectivity. Moreover, deletion of the RBE drastically reduced collateral packaging of foreign DNA into AAV5 capsids.11
Here we show that omitting the RBE from the rep/cap expression constructs for packaging of rAAV1, rAAV2, or rAAV8 reduces collateral packaging of nonvector DNA, as demonstrated by novel OneBac 2.0 cell lines for their production.
Materials and Methods
Plasmids
The cap coding sequences of AAV1, AAV2, and AAV8 were amplified by PCR. An artificial intron of 230 bp containing the baculovirus polyhedrin (polh) promoter12 was inserted into the cap genes at the authentic major splice acceptor site of the respective AAV serotype (Fig. 1) (AAV1 [Accession No. AF063497]: between nucleotides 2247 and 2248; AAV2 [Accession No. AF043303]: between nucleotides 2227 and 2228; and AAV8 [Accession No. AF513852]: between nucleotides 2145 and 2146). The AAV5 cap gene of pIR-AAV5-VP1-intron-hr2 was replaced by the intron-containing cap gene of AAV1, AAV2, or AAV8. Plasmid pIR-rep78-hr2 was described previously.11
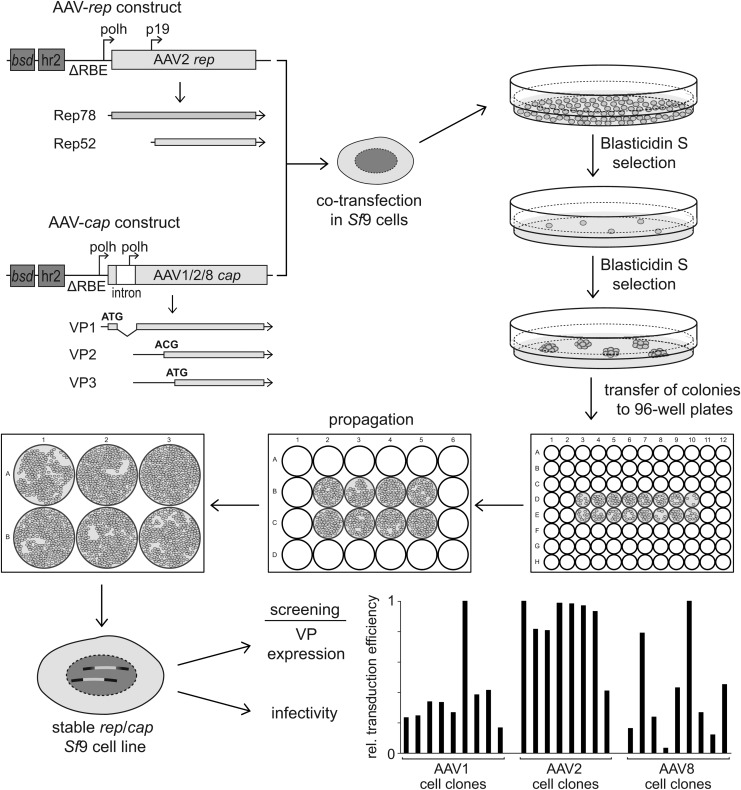
Figure 1.
Generation of OneBac 2.0 cell lines. Plasmid pIR-AAV2-rep-hr2 and plasmid pIR-VP1-intronAAV-cap-hr2 (for AAV serotypes 1, 2, and 8) are depicted. The plasmids are cotransfected in Sf9 cells at a molar ratio of 1:2.5 and the cells are selected with blasticidin S. Only cells containing at least one of the transfected constructs grow and eventually form colonies. These are transferred to multiwell plates and are expanded individually. The individual cell clones were screened for rep/cap expression and for the infectivity of the produced AAV vectors. Exemplary results from various cell clones are shown, measured as relative transduction efficiencies of cleared freeze–thaw supernatant-derived AAV vectors as determined in HeLa C12 cells. Transduction efficiency of rAAV vectors prepared in 293 cells was arbitrarily set to 1.0, and those of rAAV vectors derived from Sf9 cells are displayed as percentages thereof. bsd, blasticidin S deaminase; hr2, homologous region 2; polh, baculovirus polyhedrin promoter; RBE, Rep-binding element (AAV2).
Cell culture and construction of stable Sf9 cell lines
Sf9 cells, cell lines derived thereof, and HEK-293- or HeLa-derived C12 cells were cultivated as described.10 Sf9-derived cell lines for rAAV1, -2, and -8 production were generated, serially passaged over five to eight passages (Fig. 1), and screened for rep/cap expression, and rAAV production was initiated by infection with Bac-rAAV-GFP as described.11
rAAV production, purification, and vector analysis
rAAV vectors generated by pDG plasmid transfection in HEK-293 cells as described.5 For vector production in insect cells, Sf9-derived AAV rep/cap-expressing cell lines were held in suspension culture within the logarithmic growth phase before infection with the recombinant baculovirus Bac-rAAV-GFP (multiplicity of infection [MOI], 5). Infected cells were incubated for 72 hr and AAV vectors were purified by high-performance liquid chromatography (HPLC) from Benzonase-treated cell extracts by one-step AVB Sepharose (GE Healthcare Life Sciences, Pittsburgh, PA) affinity chromatography as described.13 By Western blot analysis the relative ratios of VP1, VP2, and VP3 expression in 293 or in Sf9 cells were visualized with monoclonal antibody (mAb) B1. For accurate determination, quantitative Western blots were analyzed on an Odyssey imager (Li-Cor, Lincoln, NE) with secondary antibodies emitting near-infrared fluorescence.
Analysis of rAAV transduction efficiency
Evaluation of the infectivity of rAAVs derived from various production systems were performed as described.10 Briefly, HeLa C12 cells were transduced with rAAV-GFP at an MOI of 1000 (genomic titer) and superinfected with adenovirus type 2 at an MOI of 10 (infectious titer). Cells were harvested 48 hr posttransduction, washed, and gently resuspended in phosphate-buffered saline (PBS). By fluorescence-activated cell-sorting (FACS) analysis (FACSCalibur; BD Biosciences, San Jose, CA) the proportion of green fluorescent protein (GFP)-positive cells was determined (100,000 cells per sample).
Quantification of rAAV vector preparations
Highly purified rAAV vector preparations or rAAV-containing freeze–thaw supernatants were digested with proteinase K (Carl Roth, Karlsruhe, Germany) to release the packaged genomes from the capsids. Aliquots of the vector preparation were incubated for 2 hr at 56°C in buffer containing 25 mM Tris-HCl (pH 8.5), 10 mM ETDA (pH 8.0), 1% N-lauroyl sarcosinate (w/v), 40 μg of proteinase K, and 1 μg of a carrier plasmid. DNA was purified by extractions with phenol and chloroform and precipitated with ethanol. DNAs were analyzed at various dilutions by quantitative LightCycler PCR, using a FastStart DNA master SYBR green kit (Roche, Indianapolis, IN). Genomic DNA titers of purified AAV particles and percentages of collateral packaging were analyzed with specific primers (Table 1).
Table 1.
Primers for quantitative analysis of rAAV vector preparations
| Primer | 5′-Sequence-3′ |
|---|---|
| Rep-Fwd | 5′-AGAAGGAATGGGAGTTGCCG-3′ |
| Rep-Rev | 5′-TCTGACTCAGGAAACGTCCC-3′ |
| Cap1-Fwd | 5′-GAGTGGTGGGACTTGAAACC-3′ |
| Cap1-Rev | 5′-TGGTTATACCGCAGGTACGG-3′ |
| Cap2-Fwd | 5′-GAGGACACTCTCTCTGAAGG-3′ |
| Cap2-Rev | 5′-CGTAGGCTTTGTCGTGCTCG-3′ |
| Cap8-Fwd | 5′-CGAGGACAACCTCTCTGAGG-3′ |
| Cap8-Rev | 5′-CCGCAGGTACGGATTGTCAC-3′ |
| Bsd-Fwd | 5′-AAGACTACAGCGTCGCCAGC-3′ |
| Bsd-Rev | 5′-CCAGGATGCAGATCGAGAAG-3′ |
| Bga-1 | 5′-CTAGAGCTCGCTGATCAGCC-3′ |
| Bga-2 | 5′-TGTCTTCCCAATCCTCCCCC-3′ |
| GmR-Fwd | 5′-TTGTATAGAGAGCCACTGCG-3′ |
| GmR-Rev | 5′-AAGACATTCATCGCGCTTGC-3′ |
| AmpR-Fwd | 5′-TCTTACTGTCATGCCATCCG-3′ |
| AmpR-Rev | 5′-TGCTGAAGATCAGTTGGGTG-3′ |
Bac titration
Baculovirus stocks were quantified by plaque assays on monolayer Sf9 cells. For that purpose Sf9 cells grown in suspension culture were seeded in 6-cm-diameter dishes (approximately 3 × 106 cells). After cell attachment the medium was removed, cells were infected with serial dilutions of 10−3 to 10−7 of the baculovirus stock solution, and incubated for 1 hr with occasional tilting of the dishes. After removal of the inoculum, cells were overlaid with 6 ml of 1% agarose in Sf9 medium (Gibco Sf-900 [1.3 × ]; Thermo Fisher Scientific, Waltham, MA), 5% fetal calf serum (FCS), streptomycin (50 μg/ml), penicillin (50 U/ml), and amphotericin B (125 ng/ml) (Invitrogen/Thermo Fisher Scientific, Carlsbad, CA). Solidified agarose was overlaid with additional 2 ml of liquid Spodopan medium (PAN-Biotech, Aidenbach, Germany). Cells were incubated for 4 days at 27°C and were then stained by adding 3 ml of 0.033% neutral red in 1 × PBS for 2–4 hr at 27°C. Staining solution was removed and the dishes were kept upside down in the dark for at least 3 hr to visualize the plaques.
Results
The OneBac 2.0 system was developed to improve infectivity of rAAV5 vectors. Modifications of the original OneBac-based rAAV5 production system were necessary because of relatively low VP1 expression levels in comparison with VP2 and VP3, leading to low per-particle transduction efficiencies. VP1 expression levels were enhanced by restoration of the authentic ATG start codon. The simultaneous introduction of an artificial intron near the 5′ end of the VP1 open reading frame, comprising a second polh promoter, led to enhanced VP1 expression and to the expected 1:1:10 protein ratios in relation to VP2 and VP3. Furthermore, the problem of collateral packaging of foreign DNA was solved by deleting the RBE from the rep/cap constructs needed for the generation of Sf9 cell lines. This led to a drastic reduction of copackaged nonvector DNA sequences.11
AAV5 represents the evolutionarily most distant AAV serotype. To investigate whether the improvements for AAV5 production in Sf9 cells can be translated to the prototype AAV2 and to other serotypes, Sf9 cell lines for the production of the AAV1, -2, or -8 vector were constructed (Fig. 1). The newly made cap expression constructs and the described AAV2 rep expression construct were cotransfected in Sf9 cells and selected with blasticidin. Individual cell clones were propagated and screened, as described previously.10 In brief, up to 30 single-cell clones were analyzed after baculovirus (Bac-rAAV-GFP) infection to determine VP expression, vector yields, and infectivity rates.
With the initially described OneBac system AAV vector yields greater than 1 × 105 genome-containing particles per cell (gp/cell) could be achieved for each AAV serotype, and the rep/cap gene cassette integrated in the Sf9 cell clones was proven to remain stable over more than 10 passages.10 Meanwhile, several cell lines have been passaged numerous additional times without loss of productivity (M. Mietzsch and M. Agbandje-McKenna, unpublished data). Similarly, AAV vector yields greater than 1 × 105 gp/cell could be achieved in the newly described stable cell lines for AAV serotypes 1, 2, and 8. At low cell concentrations of 1–2 × 106 cells/ml more than 1014 AAV genomes could be produced in 1-liter cell suspension. Because most of the generated cell lines showed comparable AAV genome-containing particle yields the focus of the cell line screening was mainly on VP1 expression levels and AAV infectivity. Highly efficient cell clones were chosen for further analysis.
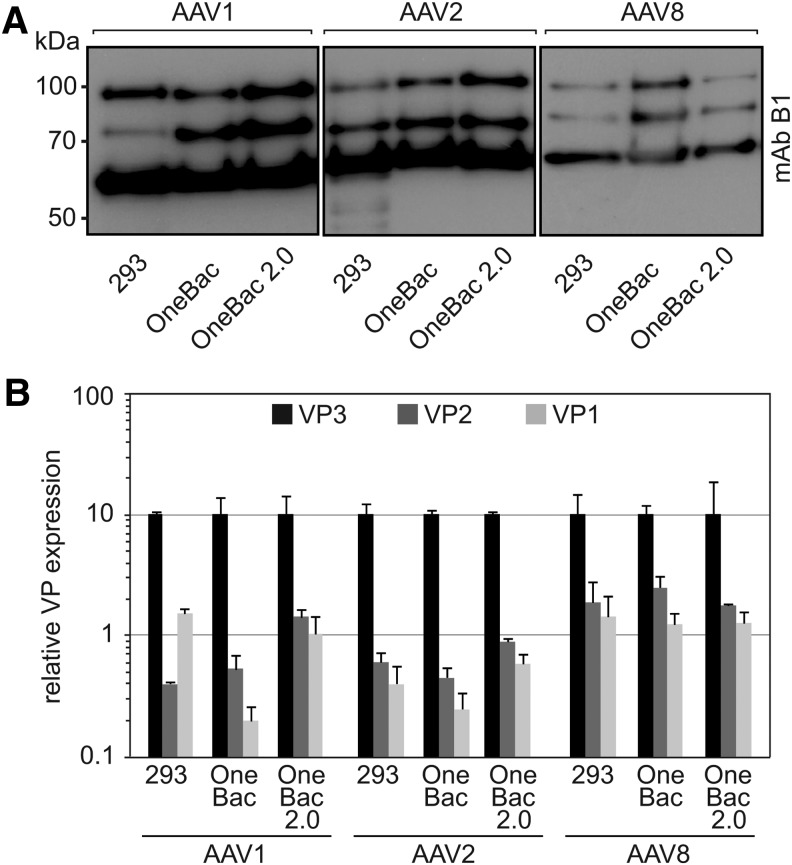
In a side-by-side analysis the VP expression ratios of the same AAV serotype were determined for the newly generated OneBac 2.0 cell lines, for the previously described producer cell lines (OneBac), and for plasmid- transfected HEK-293 cells. In contrast to AAV5, the AAV serotypes derived from the newly developed OneBac 2.0 cell lines hardly showed enhanced proportions of VP1 compared with the previously described OneBac cell lines (Fig. 2A and B). AAV vectors were highly purified by HPLC from Benzonase-treated cell extracts, using AVB columns, and the purity and VP composition of the AAV stocks were verified on silver gels (Fig. 3A). Although we have not performed iodixanol or CsCl gradients to quantify the percentage of empty to full capsids the equal VP pattern for 293-, OneBac-, or OneBac 2.0-derived AAV vectors demonstrated that the percentage of full to empty capsids does not vary between the platforms (Fig. 3A).
Figure 2.
Analysis of cap expression of the OneBac 2.0 cell lines. (A) AAV Cap expression was analyzed by Western blot analysis of cell extracts harvested after rAAV production. Cell extracts from 293 cells were generated 72 hr posttransfection, and cell extracts from rep/cap-expressing Sf9 cells were prepared 72 hr postinfection. The VP proteins were detected with mAb B1. (B) Quantitative Western blots generated as in (A) for comparison of the relative ratios of VP1, VP2, and VP3 expression in 293 cells compared with Sf9 cells, respectively. Expression levels of VP3 were arbitrarily set to 10, and expression levels of VP2 and VP1 were calculated as percentages thereof. The results of three experiments are given as means and SD. Please note the logarithmic scale of the y axis.
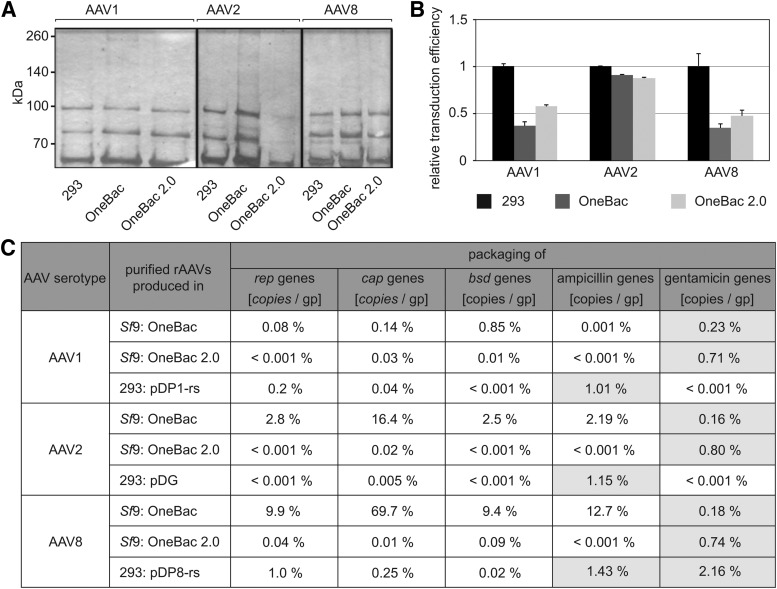
Figure 3.
Analysis of purified AAV vectors. (A) Silver-staining gel analysis of purified rAAV preparations separated on 8% SDS–polyacrylamide gels with 1010 genome-containing particles (gp) loaded per lane. (B) Analysis of the transduction efficiencies of highly purified rAAV preparation as determined in HeLa C12 cells (MOI, 1000). Transduction efficiencies of rAAVs prepared in 293 cells were arbitrarily set to 1.0, and those of rAAV vectors derived from Sf9 cells are displayed as percentages thereof. The experiments were performed in triplicate and are displayed as means and SD. (C) Analysis of copackaged rep, cap, bsd, ampicillin, and gentamicin gene sequences in AAV capsids during vector production. Aliquots from AVB Sepharose-purified AAV vector preparations were digested with proteinase K to release packaged DNA from the capsids. The samples were analyzed by qPCR with primers specific for the vector genome or the indicated genes. The titers of rAAV particles containing the vector genome are shown as absolute values whereas the portions of rep, cap, and bsd gene-containing particles are displayed as percentages thereof.
Transduction efficiencies of AAV1, AAV2, and AAV8 were compared among vectors produced in HEK-293 cells, the previously published OneBac producer cell lines, and the new OneBac 2.0 producer cell lines. Identical amounts of AVB-purified, enhanced GFP (EGFP) transgene-containing AAV vectors (genomic particles) were used to infect AAV rep/cap-expressing HeLa C12 cells as described.10 The percentage of GFP-positive cells was determined by FACS analysis. Because the absolute transduction of the selected target cell varied among the different AAV serotypes the transduction efficiency of 293-derived vectors for each AAV serotype was used as a reference and was arbitrarily set to 1.0. In the case of AAV1 and AAV8 the vectors produced in the new cell lines were slightly more infectious (Fig. 3B). In the new AAV2 cell line transduction efficiency was not increased.
The OneBac 2.0-derived AAV5 producer cells stand out with a second modification, even more relevant for the applicability of the system for clinical use. Collateral packaging of foreign DNA was drastically reduced by removal of the RBE from the transduced plasmids with rep and cap helper genes.11 Comparative analysis of AAV5 packaging cell lines with and without RBEs showed that the presence of the RBE in cis was responsible for packaging of adjacent DNA sequences into AAV5 capsids. Removal of the RBE only moderately affected template amplification, but not AAV packaging and vector yields. To test whether this strategy can be translated to the packaging of other AAV serotypes, the RBEs were removed from the AAV1, -2, and -8 rep and cap expression plasmids and Sf9 cell lines were generated for Bac-dependent production of AAV vectors. The percentages of collateral packaging of the rep, cap, ampicillin (bla), and blasticidin (bsd) genes into the capsids of rAAV1, rAAV2, and rAAV8 vectors were quantified by qPCR, using highly purified vectors from AVB columns, produced in parallel with the OneBac or OneBac 2.0 system, respectively. The results showed that AAV vectors produced in any of the new OneBac 2.0 cell lines contained up to 10,000 fold reduced levels of copackaged rep, cap, bla, and bsd genes compared with the original OneBac cell lines (Fig. 3C). The percentage of collateral packaging in OneBac 2.0-derived AAVs was in the range of or lower than in AAV vectors produced in plasmid-transfected HEK-293 cells. Furthermore, the percentage of copackaged antibiotic resistance genes derived from reverse packaging was determined. Reverse packaging described the unfavored encapsidation of DNA sequences flanking the AAV ITR transgene cassette. In the case of AAV vectors derived from plasmid transfected HEK-293 cells these sequences frequently contain antibiotic resistance genes (e.g., ampicillin) of the plasmid backbone. For the analyzed AAV1, AAV2, and AAV8 vectors ampicillin resistance gene sequences were found in about 1–1.5% of the AAV particles (Fig. 3C). In the case of Sf9 cell-derived AAV vectors reverse packaging is initiated from the AAV ITR transgene cassette inserted into the recombinant baculovirus genome. For the generation of the recombinant baculovirus an antibiotic resistance gene, preferably gentamicin, is inserted close to the ITR cassette. In the analyzed AAV1, AAV2, and AAV8 vectors gentamicin resistance gene sequences were found in about 0.2–0.8% of the AAV particles, with a slightly higher percentage in the OneBac 2.0 cell lines (Fig. 3C).
Discussion
Unintended encapsidation of foreign DNA represents a prime issue for the manufacturing of AAV vectors for clinical applications. In this study we advanced the previously described OneBac system for AAV vectors of serotypes 1, 2, and 8. In OneBac 2.0 the encapsidation of foreign DNA could be reduced by several orders of magnitude for all tested serotypes.
Importance of VP1 for AAV infectivity
Although the AAV capsid protein VP3 was shown to be sufficient to form stable AAV capsids when coexpressed with VP2 and/or the assembly-activating protein (AAP),14 the additional presence of VP1 is essential for AAV infectivity. The N terminus of VP1 contains a phospholipase domain15 that is required for endosomal escape of the virus after cell entry.16 The 1:1:10 ratio of the AAV capsid proteins, VP1, VP2, and VP3, was first described for AAV217 and assumed to be similar for other AAV serotypes. We have shown that AAV5 capsids with limited amounts of VP1 display significantly reduced infectivity rates, as measured by per-particle transgene expression in transduced target cells.10 Increasing VP1 transcription by means of an alternative promoter led to increased VP1 expression levels during AAV5 vector production and restored AAV5 infectivity.10
The same strategy, however, hardly increased VP1 expression for AAV1 and AAV2 and even lowered VP1 produced from AAV8 cell lines of OneBac 2.0 compared with the original OneBac system. It is therefore not surprising that the transduction efficiencies of the derived AAV vectors remained largely unchanged. We interpret these results as indicating that the insect polh promoter is sufficiently active to drive VP expression of most AAVs, except AAV5, where the split promoter strategy solved the problem.10 Although the relative proportion of VP1 protein in AAV capsids was previously shown to represent the critical parameter for AAV infectivity,18 our data in Sf9 cells further suggest that when a critical threshold of VP1 expression is reached, further increases will not further enhance AAV infectivity.
Minimal cross-packaging of nonvector DNA in AAV capsids in the absence of RBEs
The initial Sf9-based packaging cell lines for AAV2 employed the AAV2 Rep-binding element (RBE) as cis component of the rep/cap expression constructs. This element was thought to be required for Rep-dependent excision and replication of the RBE-carrying episomes, thereby ensuring high expression levels of Rep and VP proteins in Sf9 producer cells.9 This strategy was subsequently transferred to the OneBac system to construct the full repertoire of AAV1–AAV12 packaging cell lines.10 We have found that in the case of AAV5, removal of the RBE affected neither AAV5 vector yield nor infectivity.11 Although the copy numbers of the replicating rep/cap-carrying episomes were reduced in the absence of the RBE, baculovirus IE1- and hr2.0-dependent episomal replication induced sufficient Rep and VP expression. It seems clear that the AAV2 RBE present in the original cell lines not only promoted episomal DNA replication but also acted as an AAV packaging signal for adjacent gene sequences. Significant proportions of the resulting AAV5 particles were found to contain rep, cap, or different plasmid-derived DNA sequences.11 Similar results were obtained here for additional AAV serotypes. With the OneBac 2.0 approach to remove the RBE, collateral packaging was dramatically reduced by several orders of magnitude for AAV serotypes 1, 2, and 8 without affecting burst sizes or infectivity. Furthermore, OneBac 2.0 generally leads to collateral packaging proportions lower than those found in AAV preparations produced by plasmid cotransfection in HEK-293 cells. Total numbers of encapsidated rep, cap, or bsd gene sequences for OneBac 2.0 were down by 1000-fold, often below the qPCR detection limit, and irrespective of the AAV serotype analyzed. The low proportions of collateral packaging in AAV5 particles produced by OneBac 2.0 were fully reproducible.11 We therefore anticipate that the strategy to minimize cross-packaging by removal of the RBE will similarly work for other AAV serotypes.
The proportions of copackaged foreign sequences were reported in AAV2 vector lots purified for clinical application. The proportion of copackaged AAV2 cap genes of 0.02% reported here is equally low as those of rAAV2 factor IX vector lots for hemophilia B trials manufactured in HEK-293 cells.19 Other studies reported higher percentages of copackaged rep and cap gene sequences in AAV2 vector preparations.20
An analysis of DNA molecules in HEK-293 cell-derived rAAV8 vector preparations by next-generation sequencing (NGS) has been published.21 Apparently, the choice of AAV purification method also has an impact on the amount of DNA contaminants. In their AVB-purified AAV8 vector preparation about 0.01% (qPCR) or 0.06% (NGS) of helper plasmid-related DNA sequences was found. These values are comparable to those of AAV8 vectors prepared with the OneBac 2.0 system.
Using qPCR limits the determination of DNA contaminants to known DNA sequences that stem from plasmids for the AAV vector backbone or helper plasmids needed for AAV production. However, DNA contaminations could also be derived from the host cell genome. It has been shown that functional genomic RBE homologs may lead to collateral packaging of chromosomal DNA.22 An NGS analysis of cross-packaged human DNA did not, however, show increased packaging of the Rep binding site (RBS)-containing AAVS1 site of human chromosome 19.21 It will be interesting to analyze by NGS whether cross-packaged genomic DNA of the baculovirus or the Sf9 host cell genome can be detected when the complete genome will be released. At present, only the Spodoptera frugiperda draft genome has been published.23 In the absence of the full genome a BLAST search using the Spodobase database was performed. In none of the sequencing contigs was the full 16-bp RBE sequence of the AAV2 ITR or the AAVS1 site of human chromosome 19 detected. Only DNA sequences with 15- or 14-bp similarities were present, further reducing the probability of cross-packaged insect cell DNA.
Taken together, our data show that the improvements of OneBac 2.0 allow production of AAV vectors of high infectivity combined with significantly reduced encapsidation of foreign DNA, compared with the initial OneBac system. The consistent, minimal impurities of copackaged foreign DNA sequences in OneBac 2.0-derived AAV1, -2, -5, and -8 vector lots make this system particularly suitable as a scalable production platform for safe, clinically applicable AAV vectors of diverse serotypes
Acknowledgments
The authors thank Catrin Stutika of the Heilbronn laboratory for helpful discussions and critical reading of the manuscript. Generous financial support was provided to R.H. and M.M. by the German Academic Exchange Service DAAD (PROMOS and PPP).
Author Disclosure
R.H. and S.Z. are inventors of patents related to rAAV technology. S.Z. is inventor in a patent on the inducible insect cell-based system for highly efficient production of recombinant AAV vectors (U.S. 20120100606). R.H. owns equity in a company that is commercializing AAV for gene therapy.
References
- 1.Scott LJ. Alipogene tiparvovec: a review of its use in adults with familial lipoprotein lipase deficiency. Drugs 2015;75:175–182 [DOI] [PubMed] [Google Scholar]
- 2.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology 2013;120:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 2012;4:120ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimm D, Kay MA, Kleinschmidt JA. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol Ther 2003;7:839–850 [DOI] [PubMed] [Google Scholar]
- 6.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998;72:2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther 2002;13:1935–1943 [DOI] [PubMed] [Google Scholar]
- 8.Smith RH, Levy JR, Kotin RM. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol Ther 2009;17:1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslanidi G, Lamb K, Zolotukhin S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc Natl Acad Sci U S A 2009;106:5059–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mietzsch M, Grasse S, Zurawski C, et al. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1–12 vectors for gene therapy. Hum Gene Ther 2014;25:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mietzsch M, Casteleyn V, Weger S, et al. OneBac 2.0: Sf9 cell lines for production of AAV5 vectors with enhanced infectivity and minimal encapsidation of foreign DNA. Hum Gene Ther 2015;26:688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H. Intron splicing-mediated expression of AAV Rep and Cap genes and production of AAV vectors in insect cells. Mol Ther 2008;16:924–930 [DOI] [PubMed] [Google Scholar]
- 13.Mietzsch M, Broecker F, Reinhardt A, et al. Differential adeno-associated virus serotype-specific interaction patterns with synthetic heparins and other glycans. J Virol 2014;88:2991–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci U S A 2010;107:10220–10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girod A, Wobus CE, Zadori Z, et al. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J Gen Virol 2002;83:973–978 [DOI] [PubMed] [Google Scholar]
- 16.Nonnenmacher M, Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther 2012;19:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becerra SP, Koczot F, Fabisch P, et al. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J Virol 1988;62:2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlbrenner E, Aslanidi G, Nash K, et al. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol Ther 2005;12:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauck B, Murphy SL, Smith PH, et al. Undetectable transcription of cap in a clinical AAV vector: implications for preformed capsid in immune responses. Mol Ther 2009;17:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin J, Frederick A, Luo Y, et al. Generation and characterization of adeno-associated virus producer cell lines for research and preclinical vector production. Hum Gene Ther Methods 2013;24:253–269 [DOI] [PubMed] [Google Scholar]
- 21.Lecomte E, Tournaire B, Cogne B, et al. Advanced characterization of DNA molecules in rAAV vector preparations by single-stranded virus next-generation sequencing. Mol Ther Nucleic Acids 2015;4:e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hüser D, Weger S, Heilbronn R. Packaging of human chromosome 19-specific adeno-associated virus (AAV) integration sites in AAV virions during AAV wild-type and recombinant AAV vector production. J Virol 2003;77:4881–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakumani PK, Malhotra P, Mukherjee SK, et al. A draft genome assembly of the army worm, Spodoptera frugiperda. Genomics 2014;104:134–143 [DOI] [PubMed] [Google Scholar]