Abstract
Explosive blast-induced traumatic brain injury (TBI) is the signature insult in modern combat casualty care and has been linked to post-traumatic stress disorder, memory loss, and chronic traumatic encephalopathy. In this article we report on blast-induced mild TBI (mTBI) characterized by fiber-tract degeneration and axonal injury revealed by cupric silver staining in adult male rats after head-only exposure to 35 psi in a helium-driven shock tube with head restraint. We now explore pathways of secondary injury and repair using biochemical/molecular strategies. Injury produced ∼25% mortality from apnea. Shams received identical anesthesia exposure. Rats were sacrificed at 2 or 24 h, and brain was sampled in the hippocampus and prefrontal cortex. Hippocampal samples were used to assess gene array (RatRef-12 Expression BeadChip; Illumina, Inc., San Diego, CA) and oxidative stress (OS; ascorbate, glutathione, low-molecular-weight thiols [LMWT], protein thiols, and 4-hydroxynonenal [HNE]). Cortical samples were used to assess neuroinflammation (cytokines, chemokines, and growth factors; Luminex Corporation, Austin, TX) and purines (adenosine triphosphate [ATP], adenosine diphosphate, adenosine, inosine, 2′-AMP [adenosine monophosphate], and 5′-AMP). Gene array revealed marked increases in astrocyte and neuroinflammatory markers at 24 h (glial fibrillary acidic protein, vimentin, and complement component 1) with expression patterns bioinformatically consistent with those noted in Alzheimer's disease and long-term potentiation. Ascorbate, LMWT, and protein thiols were reduced at 2 and 24 h; by 24 h, HNE was increased. At 2 h, multiple cytokines and chemokines (interleukin [IL]-1α, IL-6, IL-10, and macrophage inflammatory protein 1 alpha [MIP-1α]) were increased; by 24 h, only MIP-1α remained elevated. ATP was not depleted, and adenosine correlated with 2′-cyclic AMP (cAMP), and not 5′-cAMP. Our data reveal (1) gene-array alterations similar to disorders of memory processing and a marked astrocyte response, (2) OS, (3) neuroinflammation with a sustained chemokine response, and (4) adenosine production despite lack of energy failure—possibly resulting from metabolism of 2′-3′-cAMP. A robust biochemical/molecular response occurs after blast-induced mTBI, with the body protected from blast and the head constrained to limit motion.
Key words: adenosine, antioxidant, ascorbate, ATP, axonal injury, chemokine, combat casualty care, cyclic AMP, cytokine, gene array, glutathione, improvised explosive device, lipid peroxidation, multiplex, post-traumatic stress disorder, purine
Introduction
Explosive blast-induced traumatic brain injury (TBI) has taken on enormous importance in U.S. combat casualty care. Information on the pathobiology and neuropathology of blast TBI in humans—particularly cases of mild injury uncomplicated by previous history of head impact—is limited. MacDonald and colleagues,1 using diffusion tensor imaging (DTI), revealed axonal injury in deep white matter in soldiers with mild TBI (mTBI) within 3 months of exposure to an improvised explosive device blast associated with another blast-related event, such as a fall or a motor vehicle crash. Goldstein and colleagues2 reported on neuropathology of three military veterans exposed to blast TBI and a fourth who experienced multiple nonblast concussions. Perivascular tau was noted along with axonal degeneration. Based on parallel studies of blast (shock tube) exposure in rats, Goldstein and colleagues suggested a limited role for direct head blast or thoracic-mediated mechanisms, but implicated resulting rotational head acceleration in producing the injury. Other studies in blast TBI models have reported on axonal injury using a blast loading ranging from shock tubes to explosive blast in several species, including studies with and without head motion constraint.3–11
Studies in experimental models have, however, provided few clues as to underlying pathomechanisms mediating secondary damage and/or dysfunction after blast TBI. In addition, many soldiers with post-traumatic stress disorder (PTSD) have no abnormalities on conventional brain imaging,1,12 despite explosive blast exposure, and it has been suggested that, in some cases, pathological processes below the level of neuronal death or axonal injury, such as alterations in brain activation or cell signaling, could be important.13–15 An approach to screen mechanisms in a blast TBI model at the threshold of detectible neuropathology could provide useful insight into therapeutic targets. Clues as to possible secondary injury mechanisms after experimental blast TBI were first provided by Cernak and colleagues,10 who reported on a role for oxidative stress (OS) and neuroinflammation,16 but studies are limited.
The Defense Advanced Research Projects Agency (DARPA) of the U.S. Department of Defense (DoD) established the Preventing Violent Explosive Neurologic Trauma (PREVENT) program to link experts in blast physics and injury biomechanics, experimental and clinical TBI, neuropathology, and pharmacology to study explosive blast TBI across species and models, link explosive physics with biology, explore the biophysics and pathomechanisms of injury, and evaluate potential therapies and diagnostics. A component of the program includes studies using a rat shock tube model. In a report on the neuropathology in that model, Garman and colleagues11 showed that a head-only simulated free-field exposure with a peak pressure of ∼35 pounds per square inch (psi) and a positive phase duration of ∼4 ms, producing ∼25% mortality, in rats with total body shielding and head constraint, resulted in axonal injury in deep white-matter structures, including both the cerebellum and brainstem, consistent with the clinical report of MacDonald and colleagues1 and also with a more recent report showing that in uncomplicated blast injury in soldiers, DTI changes are noted in the deep white matter of the cerebellum.17 Consistent fiber tract injury in the hippocampus bilaterally, but rare neuronal death, was observed. It was clear that the lesions reported by Garman and colleagues11 were relatively mild, given that they were detectible only with highly sensitive cupric silver staining; other signatures of injury were limited.
A key question that remains is whether or not an uncomplicated blast exposure at mild injury levels produces substantive biochemical and/or molecular changes and if these are unique. One might posit that the secondary injury cascade in blast TBI resembles that noted in any type of TBI. However, reports from blast TBI victims suggest that there are unique findings across the injury spectrum, such as a strong link to PTSD after mild blast TBI, and rapid and malignant brain swelling and vasospasm after severe blast TBI.18,19 Thus, an approach to screening secondary injury and neuroprotectant cascades after a blast exposure could help in determining whether there are unique biochemical and/or molecular signatures. Assessment of these mechanisms at an injury level near the threshold of neuropathological damage could also provide insight as to whether primary uncomplicated blast exposure could produce biochemical and/or molecular alterations that might underlie important sequelae, such as PTSD or memory loss in the absence of neuronal death. We thus undertook a broad screening approach to secondary injury mechanisms in blast TBI in injured rat brain tissue that included (1) assessment of a gene array, (2) assessment of cytokines, chemokines, and growth factors using a multiplex-based array, (3) assessment of markers of OS, and (4) assessment of levels of adenosine triphosphate (ATP), purine metabolites, and the endogenous neuroprotectant, adenosine, in our established blast TBI model in rats. We screened all of these mechanisms in the same rats to address whether or not they occurred concomitantly. We hypothesized that mild-to-moderate uncomplicated primary blast exposure limited to the brain can induce a robust secondary injury response in rat brain.
Methods
Blast TBI model in rats
Male Sprague-Dawley rats (n=17; Charles River Laboratories, Wilmington, MA) were studied, with 11 exposed to blast and 6 shams. Rats were between 350 and 425 g in weight at the time of injury. Details of the insult were previously reported on.11 Briefly, using an Institutional Animal Care and Use Committee–approved protocol, the bodies of isoflurane-anesthetized rats (2% isoflurane in air) were shielded in a steel wedge that was supported by a rod positioned 7 feet (ft; 2130 mm) from the end of a 14-ft (4270 mm) helium-driven shock tube. A fully formed decay profile developed in the waveform at the test station (resulting from the reflected rarefaction from the driver), and there were no artifacts from either open-end rarefaction or contact surface in the blast simulation. Surface-flush PCB® (PCB Piezotronics, Inc., Depew, NY) gauges were used for shock measurements. A diaphragm of 3×0.004-inch (in) Mylar layers was used because it produced a faster, more complete rupture than a single 0.012-in Mylar sheet; a sharp shock front had fully formed by the test station. The driver gas was helium charged into an initial volume of ambient air; this helium/air mix ensures a negligible density discontinuity at the “contact surface” interface between the expanding driver gases and shocked air, although, in fact, that interface did not affect the test station. As a result of the inefficiencies of plastic diaphragm rupture, the driver was pressurized to well above the value expected from theory. This shock tube generated a well-defined, uniform, and highly reproducible shockwave with a peak pressure of ∼35 psi and a positive-phase duration of ∼4 ms, as previously reported.11 A steel wedge in the shock tube (“Mach Stem Device”) was designed to protect the thorax and abdomen of the rat, as well as to enhance the shock-wave intensity. Details of this device, along with the time-pressure profile that is produced, were recently published.11 The design ensured that the triple point of the resultant Mach stem passed well above the head and thereby imparted a uniform shock-wave exposure. The apex of the wedge pointed toward the incident shock front, and, at the apex, the incident shock front skimmed off the surface of the wedge, producing an amplified reflected wave—a Mach stem—that impinged on the left side of the rat's head. The rat's head was located 29.5 in (75 mm) along the ramp face from the leading edge—with the head being 7 ft (2130 mm) from the shock tube muzzle and 14 ft (4270 mm) from the Mylar diaphragm.
To isolate the effects of the blast shock and assure that any ensuing brain damage would have been the result of the shock only without significant effects from impact or acceleration, rats' heads were constrained against a compliant leather “sling” fitted between two short posts to ensure minimal whole-head motion. In our earlier report, the blast exposure in the shock tube produced an injury with ∼25% mortality from apnea.11
Surviving rats were allowed to recover from anesthesia and randomized to follow-up for either 2 or 24 h. Shams, subjected to an identical anesthetic exposure as injured, were allowed to recover in an identical manner and also followed for either 2 or 24 h. Injuries were performed at ORA laboratories (Fredericksburg, VA).
Overall approach to tissue sampling
The analyses and respective sites at which they were carried out included (1) gene array analysis at the University of Pittsburgh Genetics Core (Pittsburgh, PA), (2) multiplex analysis for cytokines, chemokines, and growth factors at the University of Pittsburgh Multiplex Core Facility/Pittsburgh Cancer Institute, (3) biochemical markers of OS and oxidative injury at the University of Pittsburgh Center for Free Radical and Antioxidant Health, and (4) analysis of levels of ATP, purine metabolites, and adenosine at the University of Pittsburgh Department of Pharmacology and Chemical Biology. Tissue-processing requirements for the various assays to be performed were different and highly specific for each laboratory. In addition, concerns over potential freeze-thaw artifacts related to partitioning samples and the need to supply each laboratory with adequate tissue sample volume were also important considerations. Thus, we chose to provide each laboratory with consistent samples by apportioning a specific brain structure to each mechanism/laboratory, namely, hippocampus ipsilateral to blast exposure for gene array, cortex ipsilateral to blast exposure for multiplex analysis, hippocampus contralateral to blast exposure for markers of OS, and cortex contralateral to blast exposure for ATP, purines, and adenosine. The rationale for this approach is also supported by our earlier description of the neuropathology in this model, which revealed similar degrees of axonal injury (the principle neuropathological finding) on the right and left sides of the brain.11 A diagram of the specific brain-tissue distribution plan is shown in Figure 1.
FIG. 1.
Overall study design for screening biochemical and molecular mechanisms of secondary injury and repair after experimental blast-induced TBI (helium-driven shock tube) in rats using a model previously characterized for neuropathology.11 Studies were carried out in rats exposed to blast TBI (35 psi, with body protection, and head restraint), with the left side of the head facing the shock-wave exposure. At 2 or 24 h after either blast or sham anesthesia exposure, rats were sacrificed and brain tissue samples were rapidly harvested and snap-frozen. Samples were obtained from left (L) and right hippocampus and prefrontal cortex. Each brain region was used for a separate molecular or biochemical assay as shown, at various expert core laboratory facilities at the University of Pittsburgh School of Medicine, also as shown. A total of 14 rats completed the protocol. Please see text for details. TBI, traumatic brain injury; psi, pounds per square inch. Color image is available online at www.liebertpub.com/neu
Brains were removed in whole at 2 and 24 h after injury, rapidly dissected to define and isolate hippocampus and frontal-parietal cortex both ipsilateral and contralateral to blast exposure. Samples were flash-frozen in liquid nitrogen for subsequent analysis. For these exploratory studies, rats were injured until a total of n=3 per group and n=4 per group survived until the predefined sacrificed time point of 2 or 24 h postinjury, respectively. Shams (n=3 per group) were also generated for each of the 2- and 24-h time points.
Gene array
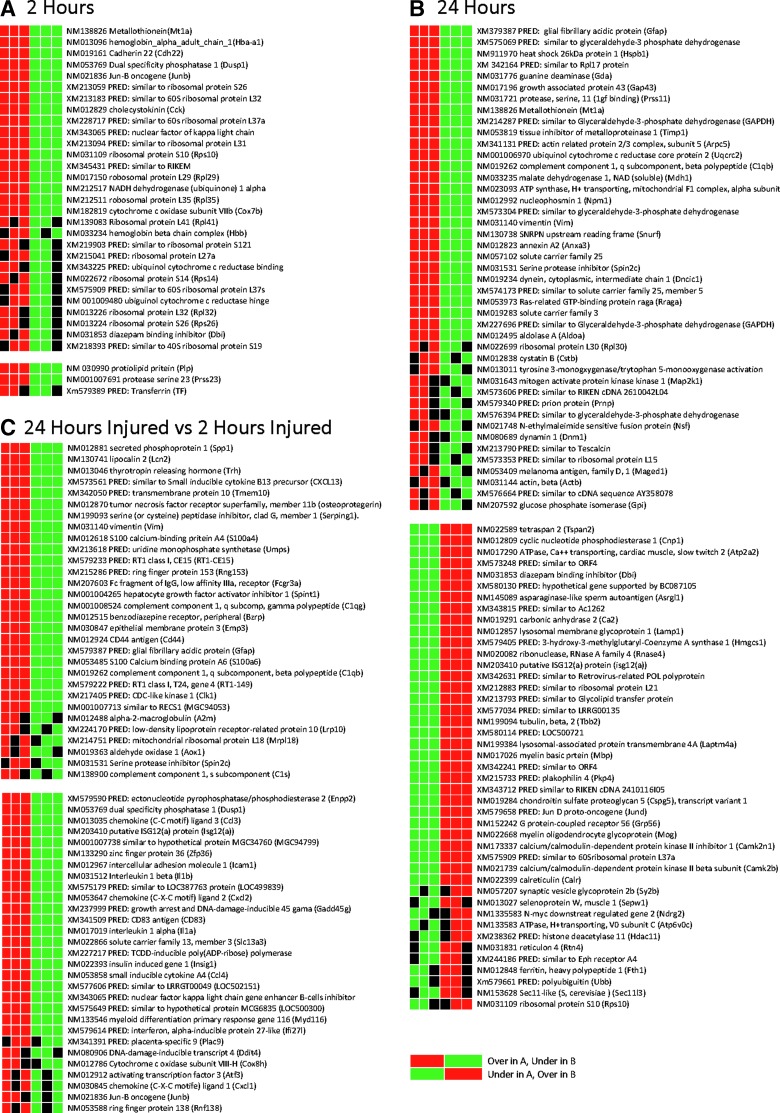
Total RNA was isolated using the Ambion RNAqueous®-Micro RNA isolation kit (Applied Biosystems, Austin, TX). RNA Amplification and conjugation were performed using the BD Biosciences Super SMART RNA amplification and labeling system (Clontech Laboratories, Inc., Mountain View, CA), per manufacturer instructions. Biotinilated complementary RNA was hybridized to an Illumina RatRef-12 Expression BeadChip for genome-wide expression analysis (Illumina, Inc., San Diego, CA). The BeadChip was processed and scanned per manufacturer instructions. Based on an efficiency analysis, performed to determine the analysis method and normalization/feature selection combination leading to the most internally consistent gene set,20 data were normalized with log2 and z-transformation, and the J5 test was used to identify differentially expressed genes.21 A pathway-level impact analysis was implemented, which was designed to provide both statistical and biological significance in indicating the pathways affected by observed gene expression changes.22 The results are summarized as impact scores and p-values. The Gene Expression Pattern Grids (GEPD)21 were also used to prioritize the genes considered to be differentially expressed in most samples for each group-wise comparison (Fig. 2). The GEPD allows the direct visualization of the status of the genes in each sample in the microarray data. In this study, we focused on GEPD groups that included genes whose expression distributions tend to be unambiguously nonoverlapping.
FIG. 2.
Expression grid pattern of differentially expressed genes in hippocampus comparing injured to sham rats at 2 (A) and 24 h (B), as well as injured rats at 24 h to injured rats at 2 h after blast injury (see Methods for details). Predicted genes are labeled “PRED,” in which the mRNA sequence has been identified, but the complete protein sequence is not verified. These genes were excluded from the analysis. When individual sample expression value is greater than the 95th percentile, compared to the other group, it is represented as a red box. If less than the 5th percentile, it is represented as a green box. If the expression value is within the 5th and 95th percentile, it is represented as a black box. The associated tables (Tables 1–3) demonstrate the J5 values for each significant gene and its associated category. mRNA, messenger RNA.
Multiplex assessment of cytokines, chemokines, and growth factors
The frozen frontal-parietal cortical tissue samples were weighed and placed in 5×volume of phosphate-buffered saline (PBS). Brain tissue was homogenized in PBS, sonicated, and centrifuged for 30 min at 14,000g for 30 min. Supernatant was collected and used for multiplex analysis.
Multiplex cytokine assays (Invitrogen, Carlsbad, CA) were processed according to the manufacturer's instructions. Briefly, bead stock was vortexed, sonicated, and diluted from the 10×solution with working wash solution. Standard and sample wells in a 96-well plate were prewet with 200 μL of working wash solution. Solution was aspirated from the bottom of the wells by a prepared suction apparatus. Capture bead solution (25 μL) was added to each well. Incubation buffer was added to all wells as directed. Standards and samples were prepared in the first two columns as directed. The 96-well plate was placed in a dark room to avoid photobleaching of the beads and was incubated for 2 h on an orbital shaker.
After incubation, each well was aspirated and washed with 200 μL of working wash solution. Biotinylated detector antibody (Ab; 100 μL) was added to each well and the plate was incubated for an additional hour covered, on an orbital shaker. Streptavadin/R-phycoerythrin solution was diluted according to instructions, and 100 μL were placed in each well. The plate was incubated for an additional 30 min. After an additional wash, the beads were resuspended in working wash solution and read on a Luminex 200 instrument (Luminex, Austin, TX). The multiplex assay was performed at the University of Pittsburgh Cancer Institute Core Multiplex Facility. Protein concentration in each sample was determined by bicinchoninic acid assay (Thermo Fisher Scientific, Rockford, IL). Cytokine concentrations, as determined by tmultiplex assay, were normalized to protein concentration and expressed as pg/mg protein. Twenty-four proteins were included in the platform, namely, interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17, IL-18, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), macrophage inflammatory protein 1 alpha (MIP-1α), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), growth-related oncogene KC (GRO/KC), monocyte chemotactic protein 1 (MCP-1), eotaxin, IFN-γ-induced protein 10 (IP-10), regulated upon activation, normal T-cell expressed (RANTES), leptin, and vascular endothelial growth factor (VEGF).
Markers of OS and oxidative damage
Hippocampi were thawed on ice and homogenized using a Tissue Tearor (BioCold Scientific, Fenton, MO) in lysis buffer (tissue weight/volume ratio, 1:20) consisting of 30 mM of Tris HCl, 150 mM of NaCl, and 1% Triton X-100 (Aldrich Chemicals, Milwaukee, WI). Homogenates were centrifuged at 1000g for 10 min, then divided into aliquots for analysis of protein, ascorbate, glutathione (GSH), and 4-hydroxynonenal (HNE). Metaphosphoric acid was added to a final concentration of 5% (v/v) to the aliquot for ascorbate measurements, protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) was added to the aliquot for HNE assay, and all samples were stored at −80°C until use.
Fluorescence assay of GSH, low-molecular-weight thiols (LMWTs), and protein sulfhydryls
Protein concentration in brain homogenates was determined using working reagent (Bio-Rad Laboratories, Hercules, CA) with a bovine serum albumin (BSA) standard (0.1 mg/mL). A standard curve was established by the addition of GSH (0.04–4.0 mM) to 50 mM of Na, Na-phosphate buffer (pH 7.4) containing 10 mM of ThioGloTM-1 (Calbiochem, San Diego, CA), a maleimid reagent that produces a highly fluorescent product upon its reaction with thiol groups.22 GSH concentrations were determined by the addition of GSH peroxidase and cumene hydro peroxide to brain homogenates with ThioGloTM-1 working solution, and the resultant fluorescence response was subtracted from the LMWT measured by the fluorescence response of the same specimens with only the addition of ThioGloTM-1 (Calbiochem). Levels of total protein sulfhydryls were determined as fluorescence response after adding 4 mM of sodium dodecyl sulfate (SDS) to each LMWT sample. A Packard Fusion alpha plate reader (PerkinElmer/Packard, Waltham, MA) was used to detect fluorescence at excitation and emission wavelengths of 388 and 500 nm, respectively. Samples were analyzed in triplicate.
Measurement of ascorbate levels
Hippocampal ascorbate levels were measured using a fluorescence assay, as described previously.24 Briefly, each sample was divided into two aliquots, each containing 0.1–0.6 mg/mL of protein. To ensure the selectivity of the assay toward ascorbate, one of the aliquots (50 μL) was treated with 1 U of ascorbate oxidase (AO) for 40 min at room temperature (RT). Samples were transferred onto a 96-well plate along with ascorbate standards (0–1.5 nmols). After this, 130-μL 4-((9-acridinecarbonyl)-amino)-2,2,6,6-tetramethylpiperidine 1-oxyl stock aliquots (23.1 μM in phosphate buffer) were added immediately, using a multi-channel pipette to each well, and samples were incubated for 40 min at RT in the dark and analyzed using a Packard “Fusion α” multifunctional plate reader (PerkinElmer Life Sciences, Boston, MA). Fluorescence was measured using a 390-±15-nm filter for excitation and 460-±35-nm filter for emission. Fluorescence readings from the AO-treated samples were subtracted from nontreated and normalized to protein. Samples were analyzed in triplicate.
Detection of HNE protein Michael adducts
After separation by SDS/polyacrylamide gel electrophoresis, proteins were transferred electrophoretically (190 V, 90 min) to nitrocellulose membranes (Thermo Scientific Pierce), which were blocked (18 h, 20°C) with 5% nonfat milk in 0.01 mol/L of PBS (pH 7.4), 150 mmol/L of NaCl, and 0.05% Tween 20 (PBS-T). For detection of HNE protein Michael adducts, blots were incubated overnight at 4°C with monoclonal mouse anti-HNE antibody (Ab; 1:2000 dilution in 1% BSA-T; R&D Systems, Minneapolis, MN). For loading control, blots were incubated overnight at 4°C with anti-actin Ab (1:10,000 dilution; Sigma-Aldrich). After four 15-min washes in PBS-T, immunocomplexed membranes were probed (1 h, 20°C) with an anti-rabbit (1:5000 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) horseradish-peroxidase–conjugated secondary Ab. Probed membranes were washed (10 min, PBS-T) four times, and immunoreactive proteins were detected using the Super Signal West Femto Kit (Thermo Scientific Pierce).
Measurement of purines
Brain tissue samples were placed in liquid nitrogen, quick-smashed with a tissue clamp device that was kept in liquid nitrogen, and quick-smashed tissues were placed immediately in ice-cold perchloric acid containing 10-13C-ATP (internal standard) or 1-propanol containing 10-13C-adenosine (internal standard). Tubes containing the ice-cold stop solutions were preweighted so that the weight of brain tissue could be obtained by weighing the tubes after the brain tissue had been placed in the stop solution. After extraction of purines (1 h), samples were centrifuged and the supernatants were taken to dryness under vacuum and then reconstituted in distilled water. Brain samples placed in 1-propanol were analyzed for 3′,5′-cAMP (cyclic adenosine monophosphate), 2′,3′-cAMP, 5′-AMP, 3′-AMP, 2′-AMP, adenosine, and inosine using our standard liquid chromatography/tandem mass spectrometry (LC-MS/MS) purine assay with selected reaction monitoring (SRM).25 Brain samples placed in perchloric acid were analyzed for ATP and adenosine diphosphate (ADP), also using LC-MS/MS, but with modifications of our standard purine assay. For ATP and ADP, the high-performance liquid chromatography reversed-phase column was a Bonus-RP (1×50 mm; 3.5 μm; Agilent Technology, Lexington, MA), and the mobile phase consisted of two eluents. Eluent A was 5 mM of dimethylhexylamine (pH 7), and Eluent B was acetonitrile/water (70:30). The gradient (A/B) was as follows: 0–1.2 min, 95%/5%; 5.5–7.0 min, 30%/70%; and 8.0–12.0 min 95%/5%. The flow rate was 80 μL/min, and the column temperature was kept at 20°C. The SRM mass transitions monitored were as follows: 506→408 for ATP with a collision energy of 18 volts; 516→418 for 13C-ATP with a collision energy of 18 volts; and 426→328 for ADP with a collision energy of 18 volts. All results were normalized to tissue sample weight.
Statistical analysis
Data are presented as mean and standard error of the mean or as dot plots with corresponding median value, as appropriate. Data analyses for the gene array studies are described above. For multiplex, OS, and purine biomarker data, one-way analysis of variance was used, followed by post-hoc comparison by Student-Newman-Keuls' test or Kruskal-Wallis' test for data that were not normally distributed. Linear correlation was also used for purine analyses.
Results
Blast TBI model in rats
It was necessary to generate 11 blast exposures to achieve the 8 surviving rats to the desired endpoints. Three of the eleven rats died of apnea immediately after exposure, producing a ∼27% mortality rate from apnea, which is nearly identical to the 25% mortality reported in our initial publication using this model at this injury level.11 There was no delayed death.
Gene array
The microarray data showed a complex set of changes affecting multiple categories of cellular function after injury. Three comparisons were made: (1) sham versus injured at 2 h after blast (Fig. 2A; Table 1); (2) sham versus injured at 24 h (Fig. 2B; Table 2); and (3) injured at 24 h versus injured at 2 h (Fig. 2C). In these three comparisons, 21, 58, and 39 genes were differentially expressed, respectively.
Table 1.
Overexpressed (Positive J5) and Underexpressed (Negative J5) Genes in the Ipsilateral Hippocampus 2 H after Blast Exposure
| Gene symbol | Gene name | Accession no. | J5 |
|---|---|---|---|
| Mt1a | Metallothionein | NM_138826.2 | 35.163 |
| Hbb | Hemoglobin beta-chain complex | NM_033234.1 | 28.035 |
| Mt3 | Metallothionein 3 | NM_053968.2 | 26.539 |
| 4 | 65102_hemoglobin_alpha_adult_chain_1_(Hba-a1) | NM_013096.1 | 26.186 |
| 5 | 64138_synaptic_vesicle_glycoprotein_2b_(Sv2b) | NM_057207.2 | 25.773 |
| 6 | 53794_transthyretin_(Ttr) | NM_012681.1 | −25.165 |
| 7 | 63461_PRED_similar_to_60S_ribosomal_protein_L37a_(LOC500547) | XM_575909.1 | 24.972 |
| 8 | 48559_PRED_ribosomal_protein_L37a_(pred)_(Rpl37a_pred) | XM_343587.1 | 24.033 |
| 9 | 56273_BCL2/adenovirus_E1B_19_kDa-interacting_protein_3_(Bnip3) | NM_053420.2 | 23.389 |
| 10 | 50959_cadherin_22_(Cdh22) | NM_019161.1 | 21.006 |
| 11 | 67501_PRED_ectonucleotide_pyrophosphatase/phosphodiesterase_2_(Enpp2) | XM_579590.1 | −20.004 |
| 12 | 50318_ribosomal_protein_S15a_(Rps15a) | NM_053982.1 | 19.914 |
| 13 | 68233_dual_specificity_phosphatase_1_(Dusp1) | NM_053769.2 | 19.546 |
| 14 | 59917_PRED_cytochrome_c_oxidase_subunit_VIa_polypeptide_1_(Cox6a1) | XM_341094.2 | 19.433 |
| 15 | 59596_ATP_synthase_H+_transporting_mitochondrial_F0_complex_subunit_e_(Atp5i) | NM_080481.1 | 19.269 |
| 16 | 67909_Jun-B_oncogene_(Junb) | NM_021836.2 | 18.922 |
| 17 | 57918_PRED_similar_to_Finkel-Biskis-Reilly_murine_sarcoma_virus_(FBR-MuSV)_ubiquitously_expressed_(fox_derived)_(LOC499305) | XM_574605.1 | 18.855 |
| 18 | 52798_PRED_Finkel-Biskis-Reilly_murine_sarcoma_virusubiquitously_expressed_(Fau) | XM_342000.2 | 18.422 |
| 19 | 56492_PRED_similar_to_ribosomal_protein_S26_(LOC298785) | XM_213058.2 | 18.041 |
| 20 | 60481_PRED_similar_to_60S_ribosomal_protein_L32_(LOC302445) | XM_213183.2 | 17.705 |
| 21 | 63177_insulin-like_growth_factor_2_(Igf2) | NM_031511.1 | −17.689 |
| 22 | 53083_PRED_similar_to_novel_cell_death-regulatory_protein_GRIM19_(LOC290671) | XM_214305.3 | 17.668 |
| 23 | 62109_ribosomal_protein_S14_(Rps14) | NM_022672.1 | 17.463 |
| 24 | 69225_cholecystokinin_(Cck) | NM_012829.1 | 17.254 |
| 25 | 60479_PRED_similar_to_60S_ribosomal_protein_L37a_(LOC302528) | XM_228717.1 | 17.23 |
| 26 | 57073_proteolipid_protein_(Plp) | NM_030990.1 | −17.12 |
| 27 | 70009_PRED_ribosomal_protein_L27a_(pred)_(Rpl27a_pred) | XM_215041.3 | 17.094 |
| 28 | 57081_ribosomal_protein_S21_(Rps21) | NM_031111.1 | 17.077 |
| 29 | 49934_PRED_nuclear_factor_of_kappa_light_chain_gene_enhancer_in_B-cells_inhibitor_alpha_(Nfkbia) | XM_343065.2 | 17.074 |
| 30 | 52466_PRED_ribosomal_protein_s25_(Rps25) | XM_579143.1 | 16.851 |
| 31 | 50109_PRED_similar_to_ribosomal_protein_L31_(LOC299935) | XM_213094.2 | 16.849 |
| 32 | 48223_PRED_similar_to_ribosomal_protein_S23_(LOC498360) | XM_573594.1 | 16.749 |
| 33 | 49821_ribosomal_protein_L32_(Rpl32) | NM_013226.1 | 16.667 |
| 34 | 60315_diazepam_binding_inhibitor_(Dbi) | NM_031853.3 | 16.525 |
| 35 | 65774_PRED_similar_to_ribosomal_protein_S19_(LOC503110) | XM_578630.1 | 16.385 |
| 36 | 49079_ribosomal_protein_L41_(Rpl41) | NM_139083.1 | 16.335 |
| 37 | 48590_ribosomal_protein_S10_(Rps10) | NM_031109.1 | 16.287 |
| 38 | 49912_PRED_similar_to_ribosomal_protein_S12_(LOC309408) | XM_219903.2 | 16.107 |
| 39 | 65073_PRED_transferrin_(Tf) | XM_579389.1 | −16.056 |
| 40 | 70369_PRED_similar_to_RIKEN_cDNA_3110001N18_(LOC366188) | XM_345431.1 | 15.984 |
| 41 | 62237_PRED_ubiquinol-cytochrome_c_reductase_binding_protein_(pred)_(Uqcrb_pred) | XM_343225.2 | 15.914 |
| 42 | 52471_protease_serine_23_(Prss23) | NM_001007691.1 | −15.899 |
| 43 | 63466_ribosomal_protein_L29_(Rpl29) | NM_017150.1 | 15.813 |
| 44 | 56800_NADH_dehydrogenase_(ubiquinone)_1_alpha_subcomplex_11_(Ndufa11) | NM_212517.1 | 15.787 |
| 45 | 65114_ubiquinol-cytochrome_c_reductase_hinge_protein_(Uqcrh) | NM_001009480.1 | 15.742 |
| 46 | 48172_ribosomal_protein_L35_(Rpl35) | NM_212511.1 | 15.702 |
| 47 | 63159_PRED_similar_to_ribosomal_protein_L34_(LOC307135) | XM_225596.1 | 15.569 |
| 48 | 50133_ribosomal_protein_S26_(Rps26) | NM_013224.1 | 15.53 |
| 49 | 58168_cytochrome_c_oxidase_subunit_VIIb_(Cox7b) | NM_182819.1 | 15.51 |
| 50 | 50093_PRED_NADH_dehydrogenase_(ubiquinone)_1_beta_subcomplex_2_(pred)_(Ndufb2_pred) | XM_342664.2 | 15.415 |
| 51 | 57561_PRED_similar_to_40S_ribosomal_protein_S19_(LOC502302) | XM_218303.1 | 15.264 |
Results are shown in order of decreasing J5 score.
Table 2.
Overexpressed (Positive J5) and Underexpressed (Negative J5) Genes in the Ipsilateral Hippocampus 24 H after Blast Exposure
| Rank | Name | J5 | Accession_no. |
|---|---|---|---|
| 1 | 68224_PRED_glial_fibrillary_acidic_protein_(Gfap) | 238.816 | XM_579387.1 |
| 2 | 64138_synaptic_vesicle_glycoprotein_2b_(Sv2b) | −178.53 | NM_057207.2 |
| 3 | 66032_PRED_similar_to_glyceraldehyde-3-phosphate_dehydrogenase_(LOC500506) | 104.238 | XM_575868.1 |
| 4 | 65073_PRED_transferrin_(Tf) | −93.134 | XM_579389.1 |
| 5 | 59800_PRED_similar_to_ORF4_(LOC361942) | −76.364 | XM_342241.2 |
| 6 | 53757_PRED_similar_to_Tubulin_alpha-2_chain_(alpha-tubulin_2)_(LOC500929) | −74.248 | XM_576339.1 |
| 7 | 64101_PRED_hypothetical_gene_supported_by_BC087105_(LOC500856) | −72.23 | XM_580130.1 |
| 8 | 66108_PRED_3-hydroxy-3-methylglutaryl-coenzyme_A_synthase_1_(Hmgcs1) | −70.16 | XM_579405.1 |
| 9 | 70349_heat_shock_27kDa_protein_1_(Hspb1) | 69.913 | NM_031970.1 |
| 10 | 53820_PRED_similar_to_Rpl17_protein_(LOC361869) | 67.616 | XM_342164.2 |
| 11 | 67056_PRED_similar_to_testin_(LOC503278) | 66.574 | XM_578812.1 |
| 12 | 56963_PRED_similar_to_ORF4_(LOC498048) | −65.987 | XM_573248.1 |
| 13 | 60315_diazepam_binding_inhibitor_(Dbi) | −65.766 | NM_031853.3 |
| 14 | 58313_PRED_similar_to_glyceraldehyde-3-phosphate_dehydrogenase_(LOC500983) | 64.087 | XM_576394.1 |
| 15 | 67501_PRED_ectonucleotide_pyrophosphatase/phosphodiesterase_2_(Enpp2) | −63.742 | XM_579590.1 |
| 16 | 68346_tubulin_alpha_1_(Tuba1) | −63.423 | NM_022298.1 |
| 17 | 56273_BCL2/adenovirus_E1B_19_kDa-interacting_protein_3_(Bnip3) | −63.352 | NM_053420.2 |
| 18 | 53205_PRED_similar_to_60S_ribosomal_protein_L17_(L23)_(amino_acid_starvation-induced_protein)_(ASI)_(LOC367398) | −61.607 | XM_576661.1 |
| 19 | 65816_guanine_deaminase_(Gda) | 61.181 | NM_031776.1 |
| 20 | 66022_PRED_plakophilin_4_(pred)_(Pkp4_pred) | −60.531 | XM_215733.3 |
| 21 | 50398_PRED_similar_to_RIKEN_cDNA_2410116I05_(LOC363377) | −59.531 | XM_343712.2 |
| 22 | 60643_serum/glucocorticoid_regulated_kinase_(Sgk) | 57.752 | NM_019232.1 |
| 23 | 65851_PRED_similar_to_LRRG00116_(LOC362543) | −57.401 | XM_342864.2 |
| 24 | 64861_cyclic_nucleotide_phosphodiesterase_1_(Cnp1) | −56.878 | NM_012809.1 |
| 25 | 66358_actin_beta_(Actb) | 55.115 | NM_031144.2 |
| 26 | 64690_growth_associated_protein_43_(Gap43) | 55.06 | NM_017195.1 |
| 27 | 64865_ribosomal_protein_L17_(Rpl17) | −54.338 | NM_201415.1 |
| 28 | 64748_N-myc_downstream_regulated_gene_2_(Ndrg2) | −53.958 | NM_133583.1 |
| 29 | 57328_PRED_similar_to_Ac1262_(LOC363492) | −53.4 | XM_343815.2 |
| 30 | 67903_carbonic_anhydrase_2_(Ca2) | −53.371 | NM_019291.1 |
| 31 | 57073_proteolipid_protein_(Plp) | −50.942 | NM_030990.1 |
| 32 | 64064_protease_serine_11_(Igf_binding)_(Prss11) | 49.761 | NM_031721.1 |
| 33 | 53575_metallothionein_(Mt1a) | 48.76 | NM_138826.2 |
| 34 | 66191_ribonuclease_RNase_A_family_4_(Rnase4) | −47.407 | NM_020082.2 |
| 35 | 65814_myelin_and_lymphocyte_protein_(Mal) | −47.3 | NM_012798.1 |
| 36 | 60399_selenoprotein_W_muscle_1_(Sepw1) | −46.179 | NM_013027.1 |
| 37 | 53801_ATPase_H+_transporting_V0_subunit_C_(Atp6v0c) | −46.147 | NM_130823.2 |
| 38 | 57715_PRED_similar_to_LRRG00135_(LOC501637) | −45.936 | XM_577034.1 |
| 39 | 51075_PRED_nel-like_2_homolog_(chicken)_(Nell2) | 45.847 | XM_579504.1 |
| 40 | 58188_glycoprotein_38_(Gp38) | 45.463 | NM_019358.1 |
| 41 | 51219_metallothionein_3_(Mt3) | −44.751 | NM_053968.2 |
| 42 | 56089_N-ethylmaleimide_sensitive_fusion_protein_(Nsf) | 44.528 | NM_021748.1 |
| 43 | 70319_PRED_Jun_D_proto-oncogene_(Jund) | −44.094 | XM_579658.1 |
| 44 | 69046_myelin_oligodendrocyte_glycoprotein_(Mog) | −43.883 | NM_022668.1 |
| 45 | 50141_calcium/calmodulin-dependent_protein_kinase_II_inhibitor_1_(Camk2n1) | −43.749 | NM_173337.1 |
| 46 | 63461_PRED_similar_to_60S_ribosomal_protein_L37a_(LOC500547) | −43.535 | XM_575909.1 |
| 47 | 67911_calreticulin_(Calr) | −42.917 | NM_022399.1 |
| 48 | 52581_protein_phosphatase_3_catalytic_subunit_alpha_isoform_(Ppp3ca) | −42.451 | NM_017041.1 |
| 49 | 64945_reticulon_4_(Rtn4) | −42.348 | NM_031831.1 |
| 50 | 60797_PRED_similar_to_Ac1147_(LOC310926) | 41.71 | XM_227769.2 |
| 51 | 61449_PRED_similar_to_glyceraldehyde-3-phosphate_dehydrogenase_(GAPDH)_(LOC290634) | 41.416 | XM_214287.3 |
| 52 | 65242_PRED_calmodulin_1_(Calm1) | −41.244 | XM_579543.1 |
| 53 | 57628_ARP2_actin-related_protein_2_homolog_(yeast)_(Actr2) | −40.818 | NM_001009268.1 |
| 54 | 55545_tyrosine_3-monooxygenase/tryptophan_5-monooxygenase_activation_protein_zeta_polypeptide_(Ywhaz) | 40.56 | NM_013011.1 |
| 55 | 55306_PRED_similar_to_RIKEN_cDNA_2610042L04_(LOC498371) | 39.677 | XM_573606.1 |
| 56 | 52278_PRED_prion_protein_(Prnp) | 38.656 | XM_579340.1 |
| 57 | 54246_ATPase_Na+/K+_transporting_alpha_2_polypeptide_(Atp1a2) | −38.12 | NM_012505.1 |
| 58 | 47800_fatty_acid_binding_protein_7_brain_(Fabp7) | −38.103 | NM_030832.1 |
| 59 | 62559_tissue_inhibitor_of_metalloproteinase_1_(Timp1) | 37.855 | NM_053819.1 |
| 60 | 56078_tumor_protein_translationally controlled_1_(Tpt1) | −37.607 | NM_053867.1 |
| 61 | 50209_PRED_actin_related_protein_2/3_complex_subunit_5_(pred)_(Arpc5_pred) | 37.341 | XM_341131.2 |
| 62 | 48559_PRED_ribosomal_protein_L37a_(pred)_(Rpl37a_pred) | −37.317 | XM_343587.1 |
| 63 | 65995_ATPase_H+_transporting_V1_subunit_B_isoform_2_(Atp6v1b2) | 37.104 | NM_057213.2 |
| 64 | 54015_PRED_similar_to_L-lactate_dehydrogenase_A_chain_(LDH-A)_(LDH_muscle_subunit)_(LDH-M)_(LOC500965) | 37.018 | XM_576374.1 |
| 65 | 52257_stearoyl-Coenzyme_A_desaturase_2_(Scd2) | −36.929 | NM_031841.1 |
| 66 | 67309_PRED_similar_to_retrovirus-related_POL_polyprotein_(LOC362315) | −36.921 | XM_342631.2 |
| 67 | 66374_LIM_domain_only_4_(Lmo4) | −36.828 | NM_001009708.1 |
| 68 | 58863_PRED_similar_to_Tescalcin_(LOC288689) | 35.721 | XM_213790.3 |
| 69 | 47777_PRED_similar_to_LRRG00135_(LOC501548) | −34.265 | XM_576950.1 |
| 70 | 64580_protein_phosphatase_3_regulatory_subunit_B_alpha_isoform_(calcineurin_B_type_I)_(Ppp3r1) | −33.858 | NM_017309.2 |
| 71 | 67723_tubulin_beta_2_(Tubb2) | −33.638 | NM_199094.1 |
| 72 | 54363_PRED_LOC500721_(LOC500721) | −33.432 | XM_580114.1 |
| 73 | 57098_lysosomal-associated_protein_transmembrane_4A_(Laptm4a) | −33.427 | NM_199384.1 |
| 74 | 48846_myelin_basic_protein_(Mbp) | −33.262 | NM_017026.1 |
| 75 | 48072_phosphatidylethanolamine_binding_protein_(Pbp) | 33.202 | NM_017236.1 |
| 76 | 63688_ubiquinol_cytochrome_c_reductase_core_protein_2_(Uqcrc2) | 33.148 | NM_001006970.1 |
| 77 | 55248_ribosomal_protein_L30_(Rpl30) | 32.804 | NM_022699.2 |
| 78 | 60306_chondroitin_sulfate_proteoglycan_5_(Cspg5)_transcript_variant_1 | −32.792 | NM_019284.1 |
| 79 | 61448_complement_component_1_q_subcomponent_beta_polypeptide_(C1qb) | 32.534 | NM_019262.1 |
| 80 | 55787_PRED_similar_to_RIKEN_cDNA_4930555G01_(LOC498375) | 32.514 | XM_573610.1 |
| 81 | 68428_fasciculation_and_elongation_protein_zeta_1_(zygin_I)_(Fez1) | −32.454 | NM_031066.1 |
| 82 | 61404_cystatin_C_(Cst3) | −32.403 | NM_012837.1 |
| 83 | 59957_calcium/calmodulin-dependent_protein_kinase_II_beta_subunit_(Camk2b) | −32.239 | NM_021739.1 |
| 84 | 55606_malate_dehydrogenase_1_NAD_(soluble)_(Mdh1) | 32.113 | NM_033235.1 |
| 85 | 54601_neurogranin_(Nrgn) | −32.094 | NM_024140.2 |
| 86 | 50017_PRED_similar_to_small_membrane_protein_1_(pred)_(LOC298552) | −31.812 | XM_216545.3 |
| 87 | 53344_ATP_synthase_H+_transporting_mitochondrial_F1_complex_alpha_subunit_isoform_1_(Atp5a1) | 31.591 | NM_023093.1 |
| 88 | 69434_similar_to_Nur77_downstream_protein_2_(MGC105647) | −31.347 | NM_001007008.1 |
| 89 | 61545_chemokine_(C-X3-C_motif)_ligand_1_(Cx3cl1) | −31.229 | NM_134455.1 |
| 90 | 59239_PRED_similar_to_T-complex_associated-testis-expressed_1-like_(Protein_91/23)_(LOC501521) | −31.201 | XM_576923.1 |
| 91 | 64944_nucleophosmin_1_(Npm1) | 30.119 | NM_012992.2 |
| 92 | 66984_PRED_similar_to_glyceraldehyde-3-phosphate_dehydrogenase_(LOC498099) | 29.995 | XM_573304.1 |
| 93 | 62489_PRED_histone_deacetylase_11_(pred)_(Hdac11_pred) | −29.814 | XM_238362.3 |
| 94 | 53421_aldolase_C_fructose-biphosphate_(Aldoc) | −29.776 | NM_012497.1 |
| 95 | 67589_PRED_similar_to_LRRG00116_(LOC500867) | −29.478 | XM_576265.1 |
| 96 | 52641_PRED_similar_to_Eph_receptor_A4_(LOC316539) | −29.462 | XM_244186.3 |
| 97 | 55111_PRED_polyubiquitin_(Ubb) | −28.934 | XM_579661.1 |
| 98 | 54242_vimentin_(Vim) | 28.92 | NM_031140.1 |
| 99 | 60033_tetraspan_2_(Tspan2) | −28.641 | NM_022589.1 |
| 100 | 52135_PRED_nucleoside_phosphorylase_(Np) | −28.542 | XM_214155.3 |
| 101 | 63348_ATPase_Ca++_transporting_cardiac_muscle_slow_twitch_2_(Atp2a2) | −28.174 | NM_017290.1 |
| 102 | 50549_PRED_similar_to_ribosomal_protein_L15_(LOC498143) | 28.158 | XM_573353.1 |
| 103 | 57629_SNRPN_upstream_reading_frame_(Snurf) | 28.101 | NM_130738.1 |
| 104 | 53861_PRED_cyclin_I_(pred)_(Ccni_pred) | −28.1 | XM_214007.3 |
| 105 | 54685_asparaginase-like_sperm_autoantigen_(Asrgl1) | −28.073 | NM_145089.2 |
| 106 | 57519_annexin_A3_(Anxa3) | 27.934 | NM_012823.1 |
| 107 | 55334_ribosomal_protein_L9_(Rpl9) | −27.874 | NM_001007598.2 |
| 108 | 63257_cystatin_B_(Cstb) | 27.841 | NM_012838.1 |
| 109 | 63580_lysosomal_membrane_glycoprotein_1_(Lamp1) | −27.467 | NM_012857.1 |
| 110 | 56027_heat_shock_70kD_protein_5_(Hspa5) | −27.204 | NM_013083.1 |
| 111 | 56464_Sec11-like_3_(S_cerevisiae)_(Sec11l3) | −27.158 | NM_153628.1 |
| 112 | 48685_solute_carrier_family_25_(mitochondrial_carrier;_adenine_nucleotide_translocator)_member_5_(Slc25a5) | 26.761 | NM_057102.1 |
| 113 | 66571_clusterin_(Clu) | 26.725 | NM_053021.2 |
| 114 | 50412_beta-2_microglobulin_(B2m) | 26.336 | NM_012512.1 |
| 115 | 59171_mitogen_activated_protein_kinase_kinase_1_(Map2k1) | 26.192 | NM_031643.3 |
| 116 | 48424_putative_ISG12(a)_protein_(isg12(a)) | −26.13 | NM_203410.1 |
| 117 | 61561_ferritin_heavy_polypeptide_1_(Fth1) | −26.123 | NM_012848.1 |
| 118 | 60484_serine_protease_inhibitor_(Spin2c) | 25.975 | NM_031531.1 |
| 119 | 52089_PRED_similar_to_ribosomal_protein_L21_(LOC293642) | −25.798 | XM_212883.3 |
| 120 | 60218_dynein_cytoplasmic_intermediate_chain_1_(Dncic1) | 25.396 | NM_019234.1 |
| 121 | 52149_PRED_similar_to_solute_carrier_family_25_member_5_(LOC498885) | 25.28 | XM_574173.1 |
| 122 | 60826_PRED_similar_to_glycolipid_transfer_protein_(LOC288707) | −25.017 | XM_213793.3 |
| 123 | 54968_crystallin_alpha_B_(Cryab) | 24.924 | NM_012935.2 |
| 124 | 58278_calmodulin_2_(Calm2) | −24.813 | NM_017326.1 |
| 125 | 48422_Ras-related_GTP-binding_protein_ragA_(Rraga) | 24.729 | NM_053973.1 |
| 126 | 62993_peroxiredoxin_6_(Prdx6) | −24.669 | NM_053576.1 |
| 127 | 66828_pregnancy_upregulated_nonubiquitously_expressed_CaM_kinase_(Pnck) | 24.604 | NM_017275.1 |
| 128 | 69562_dynamin_1_(Dnm1) | 24.573 | NM_080689.2 |
| 129 | 59917_PRED_cytochrome_c_oxidase_subunit_vIa_polypeptide_1_(Cox6a1) | −24.313 | XM_341094.2 |
| 130 | 69749_solute_carrier_family_3_(activators_of_dibasic_and_neutral_amino_acid_transport)_member_2_(Slc3a2) | 24.305 | NM_019283.1 |
| 131 | 65297_neurofilament_light_polypeptide_(Nfl) | 23.585 | NM_031783.1 |
| 132 | 51621_PRED_similar_to_glyceraldehyde-3-phosphate_dehydrogenase_(GAPDH)_(LOC295452) | 23.542 | XM_227696.3 |
| 133 | 69972_SPARC-like_1_(mast9_hevin)_(Sparcl1) | −23.518 | NM_012946.1 |
| 134 | 69307_melanoma_antigen_family_D_1_(Maged1) | 23.505 | NM_053409.1 |
| 135 | 53094_G_protein-coupled_receptor_56_(Gpr56) | −23.49 | NM_152242.1 |
| 136 | 67819_aldolase_A_(Aldoa) | 23.179 | NM_012495.1 |
| 137 | 52165_integral_membrane_protein_2B_(Itm2b) | 22.974 | NM_001006963.1 |
| 138 | 53919_PRED_similar_to_cDNA_sequence_AY358078_(LOC501245) | 22.889 | XM_576664.1 |
| 139 | 49011_macrophage_migration_inhibitory_factor_(Mif) | −22.813 | NM_031051.1 |
| 140 | 63888_glucose_phosphate_isomerase_(Gpi) | 22.548 | NM_207592.1 |
| 141 | 68784_thyrotropin_releasing_hormone_(Trh) | 22.292 | NM_013046.2 |
| 142 | 48590_ribosomal_protein_S10_(Rps10) | −22.227 | NM_031109.1 |
Results are shown in order of decreasing J5 score.
Comparison of 2 h after blast TBI versus sham
The first comparison showed that genes were mostly upregulated at 2 h after injury, compared to shams; specifically, there were 18 upregulated genes and three downregulated genes (Fig. 2A; Table 1). The most noticeable changes were increases in seven ribosomal proteins (Rps10, Rpl29, Rpl35, Rpl41, Rps14, Rpl32, and Rps26) in the hippocampus. Three genes involved in mitochondrial respiration and electron transport (Cox7b, Ndufa11, and Uqcrh) as well as hemoglobin alpha- and beta-chain genes (Hbb and Hba-a1) were also upregulated at 2 h.
In addition, pathway-level impact analysis of the gene array data at 2 h after blast TBI was most consistent and most highly significantly associated with a ribosomal pattern (Table 3), likely reflecting the aforementioned rapid increases in expression of a variety of ribosomal, presumably for translation of key gene products in the early post-TBI response. Three of the four other patterns that showed statistically significant associations with blast TBI at 2 h included Parkinson's (PD), Huntington's, and Alzheimer's diseases (AD), respectively, suggesting similarities in the early blast TBI response to those noted in important neurodegenerative diseases that have been linked to TBI.26
Table 3.
Impact Analysis: Ipsilateral Hippocampus 2 H after Blast Exposure
| Rank | Pathway name | Impact factor | No. of genes in pathway | No. of input genes in pathway | No. of pathway genes on array | Input genes in pathway (%) | Pathway genes in input (%) | p-value |
|---|---|---|---|---|---|---|---|---|
| 1 | Ribosome | 30.538 | 77 | 9 | 58 | 31.034 | 11.688 | 1.27E-13 |
| 2 | Cardiac muscle contraction | 5.23 | 81 | 2 | 49 | 6.897 | 2.469 | 0.01175 |
| 3 | Parkinson's disease | 4.813 | 157 | 2 | 61 | 6.897 | 1.274 | 0.017834 |
| 4 | Huntington's disease | 4.05 | 213 | 2 | 92 | 6.897 | 0.939 | 0.038257 |
| 5 | Alzheimer's disease | 3.828 | 213 | 2 | 104 | 6.897 | 0.939 | 0.047754 |
Comparison of 24 h after blast TBI versus sham
The second comparison showed that at 24 h after injury, hippocampal tissue had upregulation of 29 and downregulation of 29 genes, indicating an important overall shift in gene expression pattern. Genes involved in differentiation, neurogenesis, and growth (Gap43 and Prss11), as well as those involved in apoptosis and stress response (Hspb1, Mt1a, and Anxa3, among others) were upregulated versus sham. At 24 h, there was also increased expression of some genes involved in energy metabolism (Uqcrc2, Mdh1, Gpi, and Aldoa) and transport function (solute carrier family 25, solute carrier family 3, mitochondrial membrane ATP synthase, Dncic1, and Nsf). However, several other transport function-associated genes (Atp6v0c, Sv2b, Laptm4a, and Atp2a2) were downregulated, suggesting differential expression in this category. There was also a downregulation of calcium signaling-associated genes at 24 h (Camk2n1, Camk2b, and calreticulin) along with downregulation of several differentiation and growth-related genes (Ndrg2, Rtn4, and Cspg5).
Pathway-level impact analysis of the gene array data at 24 h after blast TBI (Table 4) indicated that the blast TBI gene expression profile was significantly associated with 28 established pathways. Blast TBI gene expression was most significantly associated with two profiles importantly linked to learning and memory, namely, AD and long-term potentiation. The next two pathways that were most significantly associated with blast TBI with regard to gene expression profiles at 24 h included calcium signaling and VEGF signaling, and PD was again significantly associated and in the top 10 most significant pathways at 24 h.
Table 4.
Impact Analysis: Ipsilateral Hippocampus 24 H after Blast Exposure
| Rank | Pathway name | Impact factor | No. of genes in pathway | No. of input genes in pathway | No. of pathway genes on array | Input genes in pathway (%) | Pathway genes in input (%) | p-value |
|---|---|---|---|---|---|---|---|---|
| 2 | Alzheimer's disease | 11.7 | 213 | 8 | 104 | 8 | 3.756 | 2.63E-05 |
| 3 | Long-term potentiation | 10.733 | 71 | 5 | 52 | 5 | 7.042 | 3.27E-04 |
| 4 | Calcium-signaling pathway | 9.011 | 189 | 7 | 131 | 7 | 3.704 | 8.10E-04 |
| 5 | VEGF-signaling pathway | 7.828 | 69 | 4 | 48 | 4 | 5.797 | 0.002279 |
| 7 | Cardiac muscle contraction | 7.163 | 81 | 4 | 49 | 4 | 4.938 | 0.00246 |
| 8 | Ribosome | 6.264 | 77 | 4 | 58 | 4 | 5.195 | 0.004548 |
| 9 | Parkinson's disease | 6.123 | 157 | 4 | 61 | 4 | 2.548 | 0.005447 |
| 11 | Antigen processing and presentation | 5.608 | 100 | 3 | 36 | 3 | 3.000 | 0.008293 |
| 10 | Glioma | 5.864 | 64 | 3 | 40 | 3 | 4.688 | 0.011098 |
| 13 | Protein export | 5.141 | 12 | 1 | 1 | 1 | 8.333 | 0.011651 |
| 20 | Amyotrophic lateral sclerosis | 4.744 | 65 | 3 | 50 | 3 | 4.615 | 0.020255 |
| 22 | Huntington''s disease | 4.548 | 213 | 4 | 92 | 4 | 1.878 | 0.02221 |
| 16 | PPAR-signaling pathway | 4.987 | 70 | 3 | 52 | 3 | 4.286 | 0.022462 |
| 19 | Melanogenesis | 4.814 | 96 | 3 | 54 | 3 | 3.125 | 0.024796 |
| 17 | Gap junction | 4.821 | 90 | 3 | 57 | 3 | 3.333 | 0.028532 |
| 14 | Natural killer cell-mediated cytotoxicity | 5.029 | 98 | 3 | 62 | 3 | 3.061 | 0.035385 |
| 25 | GnRH-signaling pathway | 4.366 | 91 | 3 | 62 | 3 | 3.297 | 0.035385 |
| 15 | Axon guidance | 5.019 | 123 | 3 | 66 | 3 | 2.439 | 0.041425 |
| 21 | T-cell receptor-signaling pathway | 4.626 | 110 | 3 | 71 | 3 | 2.727 | 0.049655 |
| 24 | Wnt-signaling pathway | 4.368 | 142 | 3 | 77 | 3 | 2.113 | 0.060501 |
| 23 | B-cell receptor-signaling pathway | 4.477 | 67 | 2 | 40 | 2 | 2.985 | 0.078789 |
| 27 | MAPK-signaling pathway | 3.973 | 253 | 4 | 170 | 4 | 1.581 | 0.136474 |
| 31 | Apoptosis | 3.346 | 87 | 2 | 59 | 2 | 2.299 | 0.150408 |
| 32 | ErbB-signaling pathway | 2.858 | 83 | 2 | 60 | 2 | 2.41 | 0.154472 |
| 33 | Biosynthesis of unsaturated fatty acids | 2.596 | 26 | 1 | 18 | 1 | 3.846 | 0.190354 |
| 34 | Thyroid cancer | 2.561 | 29 | 1 | 20 | 1 | 3.448 | 0.209151 |
| 38 | Bladder cancer | 2.366 | 36 | 1 | 25 | 1 | 2.778 | 0.254274 |
| 37 | Insulin-signaling pathway | 2.393 | 130 | 2 | 87 | 2 | 1.538 | 0.269282 |
VEGF, vascular endothelial growth factor; PPAR, peroxisome proliferator-activated receptor; GnRH, gonadotropin-releasing hormone MAPK, mitogen-activated protein kinase.
Comparison of 24 versus 2 h after blast TBI
The third comparison, between samples at 24 and 2 h after blast injury, showed that 39 genes are differentially expressed: increased expression of 20 genes and decreased expression of 19 genes in injured animals. The most noticeable alteration was in immune-related genes, which showed differential expression of 10 genes: Four genes (three complement-related genes and one immunoglobulin G [IgG]-related gene) were upregulated, and six genes (IL-1β, IL-1α, and chemokine motifs) were downregulated at 24 h, compared to 2 h, postinjury. Also, increases in genes for various secreted extracellular proteins occurred at 24 h, compared to 2 h, after blast injury, including proteins involved in iron trafficking (Lcn2), endocrine effects (Trh), and that function as protease inhibitors (A2m, Spin2c, and Serping1). Several membrane associated genes were also upregulated at 24 h versus 2 h, including those involved in receptor function (Bzrp), membrane adhesion (Cd44), and cell-cell interaction (Emp3).
Multiplex assessment of cytokines, chemokines, and growth factors
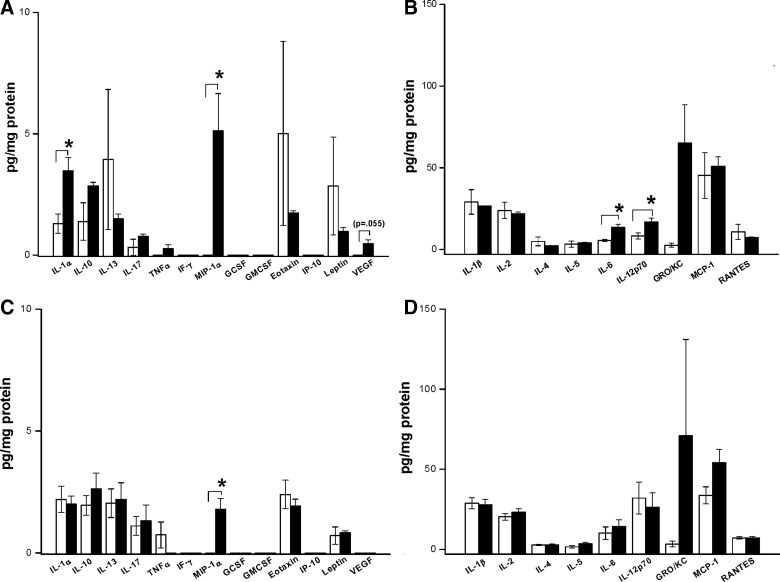
At 2 h after injury, three cytokines and one chemokine exhibited significant increases (Fig. 3A,B). Levels of IL-1α increased by ∼3-fold versus sham (1.3±0.4 versus 3.5±0.6 pg/mg protein; p<0.05). Levels of IL-6 also increased ∼3-fold versus sham (5.5±0.6 versus 13.4±2.0 pg/mg protein; p<0.05). Levels of IL-12p70 increased ∼2-fold versus sham (8.3±1.9 versus 16.9±2.2 pg/mg protein; p<0.05). The chemokine, MIP-1α, showed the greatest increase with undetectable levels in shams and 5.12±1.54 pg/mg protein in injured cortex (p<0.05). One additional cytokine, chemokine, and growth factor, IL-10 (p=0.078), GRO/KC (p=0.072), and VEGF (p=0.055) showed at least 2-fold greater levels after blast injury versus sham, but these did not reach statistical significance.
FIG. 3.
Cytokine, chemokine and growth-factor protein levels after blast TBI or sham exposure. (A) Thirteen proteins with levels in the range of ∼0–10 pg/mg protein at 2 h after injury are shown. Both the cytokine, interleukin-1 alpha (IL-1α), and the chemokine, macrophage inflammatory protein 1 alpha (MIP-1α), were increased versus sham (*p<0.05). The increase in MIP-1α was marked. A trend toward an increase in vascular endothelial growth factor (VEGF) was also noted, although it did not reach significance. (B) Nine proteins with levels in the range of ∼10–100 pg/mg protein at 2 h after injury are shown. Both IL-6 and IL-12p70 were increased versus sham (*p<0.05). The chemokine, growth-related oncogene KC (GRO/KC), showed a trend toward increase versus sham. (C) Thirteen proteins with levels in the range of ∼0–10 pg/mg protein at 24 h after injury are shown. Only the chemokine, MIP-1α, remained increased versus sham at 24 h (*p<0.05). (D) Nine proteins with levels in the range of ∼10–100 pg/mg protein at 24 h after injury are shown. A trend toward an increase in a second chemokine, GRO/KC, was also noted, although this did not reach significance. Data for IL-9 and IL-18 also did not differ between blast and sham groups at either 2 or 24 h (data not shown). Please see text for details. Interleukin=IL, tumor necrosis factor alpha (TNFα), interferon gamma (IFN-γ), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), monocyte chemotactic protein 1 (MCP-1), eotaxin, IFN-γ-induced protein 10 (IP-10), regulated upon activation, normal T cell (RANTES), leptin, and vascular endothelial growth factor (VEGF). TBI, traumatic brain injury.
At 24 h after blast injury, all cytokine levels (IL-1α, IL-6, and IL-12p70) returned to values that were not different from sham (Fig. 3C,D). However, the chemokine, MIP-1α, continued to demonstrate a significant increase versus respective sham (Fig. 3C).
Markers of OS and damage
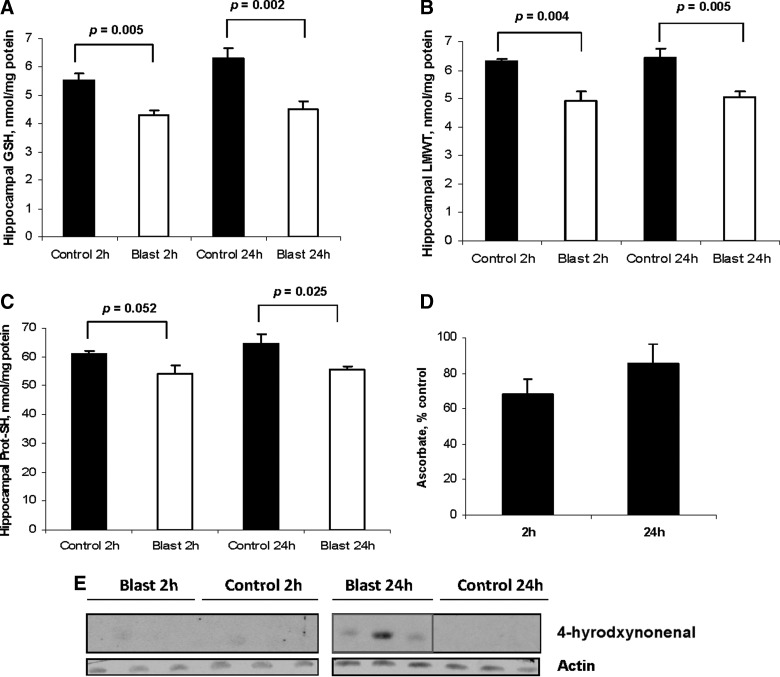
Blast TBI produced a significant decrease (∼30% reduction versus sham control) in GSH in the hippocampus ipsilateral to blast exposure at 2 and 24 h after insult (p<0.05; Fig. 4A). In addition, LMWTs were significantly reduced (∼15–20%) versus sham control at both 2 and 24 h after blast injury (p<0.05; Fig. 4B). Protein thiols were significantly reduced versus sham at 24 h after injury (Fig. 4C), although there was also a trend toward reduction at 2 h (p=0.052; Fig. 4C). Ascorbate levels in the hippocampus ipsilateral to blast exposure were not significantly decreased versus respective sham, although a trend toward reduction (∼30%) was again noted at 2 h (p=0.08; Fig. 4D). Screening for formation of HNE adducts as evidence of lipid peroxidation (LPO) on Western blot revealed that they were present at 24, but not 2, h after blast exposure (Fig. 4E).
FIG. 4.
Blast TBI induces OS in rat hippocampus at 2 and 24 h after injury. (A) Blast TBI produced a significant decrease in reduced glutathione (GSH) in the hippocampus ipsilateral to blast exposure at 2 and 24 h after insult (p=0.005 and 0.002 versus respective sham). (B) Low molecular weight thiols (LMWTs) were also significantly reduced at both 2 and 24 h after blast injury (p=0.004 and 0.005 versus respective sham). (C) Protein thiols were also significantly reduced, but only at 24 h after blast injury (p=0.025 versus respective sham), although a trend was noted at 2 h. (D) Ascorbate levels were not significantly decreased versus respective sham, although a trend toward reduction (∼30%) was noted at 2 h (p=0.08). (E) Screening for formation of 4-hydroxynoneanal adducts as evidence of lipid peroxidation on Western blot revealed that they were present at 24, but not 2, h after blast exposure.
ATP, purine metabolites, and adenosine
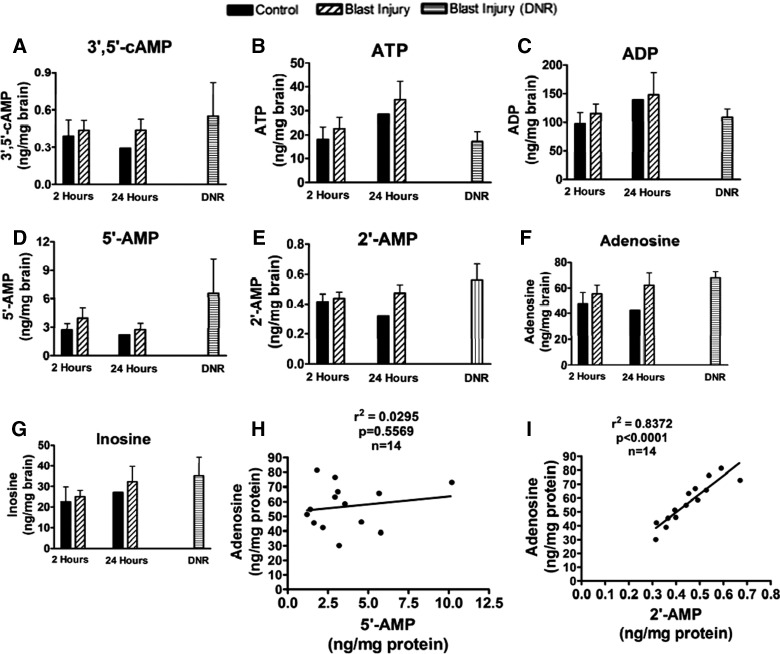
As shown in Figure 5A–G, no statistically significant changes in brain levels of ATP, ADP, 5′-AMP, 2′-AMP, 3′,5′-cAMP, adenosine, or inosine were observed. Neither 2′,3′-cAMP nor 3′-AMP were detected. When the control, 2-h injury, 24-h injury, and lethal exposures (rats that died from acute hypoxemia from impact apnea) were regressed, adenosine levels in brain tissue were not associated with 5′-AMP, but were highly correlated with 2′-AMP (Fig. 5H,I, respectively), suggesting that adenosine after blast TBI is derived from 2′-AMP.
FIG. 5.
Effect of blast TBI on purine metabolites in prefrontal cortex at 2 or 24 h after injury. LC-MS/MS assessment of (A) 3′,5′-cAMP, (B) ATP, (C) ADP, (D) 5′-AMP, (E) 2′-AMP, (F) adenosine, and (G) inosine using LC-MS/MS. There was no difference between sham and blast injury groups at either 2 or 24 h after injury for any of these metabolites, suggesting that energy failure and ATP depletion were not occurring. Levels of the endogenous neuroprotectant, adenosine, were not correlated with 5′-AMP (H), but were highly correlated with 2′-AMP (I), suggesting the possibility that mRNA breakdown after blast TBI was leading to the generation of adenosine from 2′-AMP through the 2′,3′-cAMP pathway. See text for details. LC-MS/MS, liquid chromatography tandem mass spectrometry; cAMP, cyclic adenosine monophosphate; ATP, adenosine triphosphate; ADP, adenosine diphosphate; mRNA, messenger RNA.
Discussion
Despite a blast exposure with body shielding and fixation of the head to minimize any acceleration, and using an injury level that mimicked the human condition reported in uncomplicated blast exposure (i.e., producing neurological damage largely restricted axonal injury in the deep white matter),1,27,28 we noted robust biochemical and molecular alterations of TBI-related secondary injury pathways in the hippocampus and cortex in both hemispheres. We have also examined brain sections from our earlier report11 in this model for tau staining after injury using AT8 and a pan-tau immunostaining and did not identify this protein up to 2 weeks postblast, indicating that this insult is likely below the threshold required for acute tau deposition (data not shown). Our findings, thus, support the hypothesis that mild to moderate uncomplicated blast exposure limited to the brain can induce robust biochemical and molecular features of the secondary injury response in rat brain.
Gene array
Changes in gene array reflected tissue alterations in the hippocampus ipsilateral to exposure. We reported, in this model, that neuronal death in CA1 was rare, but there were consistent alterations of dendrites assessed by cupric silver staining in multiple hippocampal subfields.11 These changes were similar both ipsi- and contralateral to blast exposure and were greatest between 24 and 72 h.
The most obvious changes in the gene array at 2 h were increases in seven ribosomal proteins (Rps10, Rpl29, Rpl35, Rpl41, Rps14, Rpl32, and Rps26). Similar increases were noted at 4 h after controlled cortical impact (CCI),29 a much more highly characterized rat model of TBI than blast exposure. Because ribosomal proteins regulate transcription and translation, the early increase may support acute upregulation of various proteins after injury.
We also noted that three genes involved in mitochondrial respiration and electron transport (cytochrome oxidase 7b [COX7b], Ndufa11, and Uqcrh) were upregulated. COX7b is a component of COX that may have a role in the assembly of the protein, whereas Ndufa 11 is likened to mitochondrial complex I.30 Sajja and colleagues31 reported decreases in succinate and other metabolites consistent with mitochondrial stress in the hippocampus of rats exposed to blast (shock tube, ∼17 psi). Hemoglobin alpha and beta chain (Hbb and Hba-a1) were also upregulated at 4 h. This mirrors the work of Natale and colleagues32 in fluid percussion injury (FPI) and may reflect local tissue hypoxia, because apnea accounted for 25–27% acute mortality in our model11 and is noted after FPI. However, downregulation of mitochondrial genes has been reported by others at 3–4 h after CCI,33,34 contrasting our findings. Thus, unlike the previous reports of downregulation of mitochondrial genes in CCI, we noted general upregulation of mitochondrial metabolic genes at 2 h. This could reflect our sampling time, differences in injury level, or a unique profile for blast.
At 24 h after blast injury, the greatest increases in gene expression were noted in two astrocyte proteins (glial fibrillary acidic protein [GFAP] and vimentin) and the inflammatory protein, complement component 1. Increase in gene expression of astrocyte markers at 24 h mirrors other reports using proteomics or immunohistochemistry.31,35,36 In the CCI model in rats, GFAP and vimentin, assessed by two-dimensional (2D) gel, also showed large increases in the hippocampus.37 Astrocytes play a neuroprotectant after CCI38; however, their role in blast TBI is undefined. Gao and colleagues39 reported increases in complement component 1 after TBI in human cerebrospinal fluid (CSF) by 2D gel, suggesting that it is highly expressed after injury. It signals leukocyte recruitment.
At 24 h, genes involved in differentiation, neurogenesis, growth (Gap43, Prss11), apoptosis, and the stress response (Hspb1, Mt1a, and Anxa3) were upregulated versus sham. Natale and colleagues32 reported similar increases in metallothionine (Mt) and another member of the heat shock protein family, Hsp70, at 4–24 h after FPI in rats. Increases in Mt and Hsp gene expression could also reflect a response to acute blood–brain barrier (BBB) injury and a stress response, respectively. Upregulation of Gap43 and Prss11 could reflect increased extracellular signaling in the hippocampus, possibly resulting from neurite outgrowth. We previously reported on cortical BBB injury in our model at 24 h; however, BBB alterations in the hippocampus were not noted.11
There was increased expression of genes involved in transport function (solute carrier family 3 and 25, mitochondrial membrane ATP synthase, Dncic1, and Nsf) at 24 h. Increased function of genes, such as Dncic1, would support dynein-associated organelle transport, and solute carrier family 25 and 3 would support transport of ATP and amino acids. These transport functions may serve processes, such as cellular signaling and remodeling, after injury. However, other transport-function–associated genes (Atp6v0c, Sv2b, Laptm4a, and Atp2a2) were downregulated at 24 h, suggesting a differential modulation of various transport functions.
There was also increased expression of genes involved in glycolysis and oxidative phorphorylation (Uqcrc2, Mdh1, Gpi, and Aldoa) at 24 h.
Calcium-signaling–associated genes were downregulated at 24 h (Camk2n1, Camk2b, and calreticulin). Downregulation of CaMKII has been reported after CCI.33 Calcium-dependent signaling may be beneficial or detrimental after injury.40,41
The most obvious change in gene expression at 24 versus 2 h was differential expression of 10 immune-response–related genes: Four genes (three complement-related genes and one IgG-related gene) were upregulated, and six genes (IL-1β, IL-1α, and chemokine motifs) were downregulated. Complement and IgG upregulation are integral to the acute-phase response. However, downregulation of IL-1β, IL-1α, and chemokines at 24 versus 2 h suggests recovery after an early inflammation. An early increase in IL-1α with recovery by 24 h corroborates our protein data (see Fig. 3). However, the delayed downregulation in chemokine gene expression contrasts our protein data, which showed sustained increases in cortex. The delayed decrease in chemokine gene expression may thus represent feedback inhibition.
Increases in genes for various secreted extracellular proteins were noted at 24 versus 2 h. These proteins regulate iron trafficking (Lcn2) and endocrine effects (Trh) and function as protease inhibitors (A2m, Spin2c, and Serping1). Thus, there is an increase in expression of genes for extracellularly secreted proteins at 24 versus 2 h. Several membrane-associated genes were also upregulated, including those involved in receptor function (Bzrp), membrane adhesion (Cd44), and cell-cell interaction (Emp3). Finally, downregulation of several differentiation and growth-related genes (Ndrg2, Rtn4, and Cspg5) was noted at 24 versus 2 h.
Multiplex analysis of cytokines, chemokines, and growth factors
Multiplex cytokine analysis has been applied to rat brain tissue in nonblast models with excellent correlation to gold-standard enzyme-linked immunosorbent assay.42 Previous work in central nervous system (CNS) injury has shown varied cytokine patterns, depending on the injury mechanism. Takamiya and colleagues43 applied a 27-plex over 240 h after stab wound and noted elevations of proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-12p40, IL-12p70, IL-18, and TNF-α), anti-inflammatory cytokines (IL-5 and IL-10), and chemokines (G-CSF, GRO/KC, and MIP-2) at various time points. Buttram and colleagues44 applied multiplex to CSF of TBI patients and noted increases of proinflammatory cytokines (IL-1β, IL-6, and IL-12p70), an anti-inflammatory cytokine (IL-10), and chemokines (IL-8 and MIP-1α). In adult human brain tissue from patients who died from TBI, Frugier and colleagues45 noted a rapid increase in IL-1β, IL-6, IL-8, and TNF-α. We found a somewhat unique brain tissue cytokine pattern after blast TBI, with rapid increases of the chemokine, MIP-1α, and proinflammatory cytokines IL-1α, IL-6, and IL-12p70 at 2 h, as well as a sustained elevation of MIP-1α at 24 h. Unlike other forms of TBI, anti-inflammatory cytokines were not increased. Our data mirror those of Kwon and colleagues35 who reported increases in IL-6 in prefrontal cortex and hippocampus of rats exposed to blast TBI (shock tube) with concurrent predator stress to mimic battlefield conditions.
MIP-1α is a β (or CC) chemokine46 stimulating macrophage infiltration at injury sites. It is produced in white-matter lesions in multiple sclerosis and is associated with microglial activation.47 MIP-1α is elevated in CSF at 24 h after clinical TBI.44 After olfactory bulb axotomy in mice, MIP-1α was markedly increased at 3 days and regulated dendritic cell migration.48 Fe2+-mediated free radical production and free ATP by purinergic P2X7 receptors initiate MIP-1α expression49—which could thus be relevant to blast TBI. No increase in MIP-1α was noted after a mouse stab wound, suggesting differences between blast and penetrating TBI.43
Surprisingly, we noted an increase of IL-1α at 2 h, but not IL-1β. This corroborates our gene array data. IL-1α and IL-1β are agonists for type 1 and 2 IL-1 receptors. In glia, IL-1-receptor activation by either agonist triggers activation of mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B.50 Lemke and colleagues51 showed that after lipopolysaccharide/interferon gamma (IFN-γ) injection, IL-1α and IL-1β are induced in perilesional microglia. IL-1β, but not IL-1α, also induces nerve growth factor,52 which can be neuroprotective. Though both cytokines can induce chemokines, only IL-1β induces IL-6 expression.53 IL-1α associates with nuclear chromatin and is released after necrotic cell death and incites inflammation.54 IL-1α, but not IL-1 ILβ, is released by platelets after vascular injury and initiates chemokine release by endothelium.55 Given the role of vascular injury in blast TBI, this theoretical mechanism bears further study and might contribute to vascular dysfunction after blast TBI. Despite the lack of an IL-1β elevation, we noted an increase of IL-6 after blast TBI. IL-6 is elevated in CSF after clinical TBI.56,57 It can act as a neuronal survival factor56 and/or as a chemokine.58 Further study is needed to determine whether IL-6 is neuroprotective in blast TBI or inciting further inflammation.
IL-12 stimulates CD4+ T cells to acquire a proinflammatory T-helper (Th)1 phenotype59 and is elevated in CSF after TBI in humans.44 However, injection of IL-12 into lesioned spinal cord causes neurogenesis and remeylination in mice.60 Given the degree of axonal injury in blast TBI, further study is needed to determine whether IL-12p70 facilitates myelin repair after axonal injury.
In light of the link between neuroinflammation and chronic traumatic encephalopathy (CTE),26 our gene array and multiplex data suggest that blast TBI, even at an injury level below that needed to yield appreciable cell death, amyloid precursor protein accumulation, or acute tau accumulation, initiates a neuroinflammatory response that includes acute cytokine and more-prolonged chemokine elevation. The long-term consequences of blast TBI at this injury level could be important with regard to neuroinflammation and CTE. It is certainly unclear whether one would anticipate immediate or more-delayed tau deposition after blast TBI. Further study is needed in this regard.
Markers of OS
Blast TBI produced a significant decrease in both reduced GSH and LMWT in the hippocampus ipsilateral to exposure to a degree similar to that noted in the CCI model of TBI in rats at a moderate injury level. A 31% reduction in GSH was noted at 24 h in CCI.61 This is surprising, given that neuronal death was rare in the hippocampus in this model.11 However, a consistent pattern of synapse or terminal degeneration was noted in the hippocampus in that report, assessed by silver staining. Cernak and colleagues10 reported cytoplasmic vacuoles and expanded perineural spaces on electron microscopic assessment in the hippocampus of rats exposed 24 h earlier to whole-body or local-chest blast TBI (shock tube; ∼49 psi) and an ∼50% increase in the LPO marker, malondialdehyde was also noted. Cernak and colleagues10 also noted a ∼30–40% reduction of GSH that recovered by 5 days. Our results build upon that work by showing that head-only blast exposure produces a similar level of OS in the hippocampus as either whole-body or body-only exposure. It is remarkable that these three divergent approaches each produced subtle hippocampal neuropathology and substantive evidence of OS.
Ascorbate levels in the hippocampus ipsilateral to blast exposure were not significantly decreased versus sham, and although a trend toward reduction (∼30%) was noted, it suggests that the OS in our blast TBI model is somewhat less in magnitude than that noted in CCI, where significant reductions in ascorbate were observed.61 Studies of CSF ascorbate levels in humans with severe TBI show marked, sustained depletion.62 However, the effect of mild TBI on ascorbate in human TBI remains undefined. Nevertheless, our data suggest that OS is an important therapeutic target, even in mild blast TBI. Our data also suggest that caution is in order with regard to the use of hyperbaric oxygen in blast TBI, particularly early after exposure. They also suggest the need to study therapies targeting OS.
ATP, purines, and adenosine
Blast injury did not alter brain tissue levels of ATP, ADP, or 5′-AMP, suggesting that our mild insult did not induce energy failure. This is consistent with the fact that we previously reported on axonal injury, rather than neuronal death, and argues against a major role for ischemia in the initial 24 h. Although 5′-AMP (produced from ATP/ADP metabolism) is considered the major precursor for adenosine, recent studies suggest that adenosine may also derive from the metabolism of 2′,3′-cAMP to 3′-AMP or 2-AMP, with subsequent metabolism to adenosine.25 Our current study reveals that brain tissue levels of adenosine are strongly associated with 2′-AMP, but not 5′-AMP, consistent with 2′-AMP as a source of adenosine in the injured brain. It has been shown that 2′,3′-cAMP is produced from the poly-A tails in the breakdown cellular messenger RNA (mRNA).25 Thus, adenosine levels after even relatively mild blast TBI are correlated with brain tissue levels of 2′-AMP, suggesting that mRNA breakdown occurs and may drive production of the neuroprotectant, adenosine. Additional studies are warranted to address this hypothesis.
Limitations
Our sample size was limited; however, this study was designed as a screening study to direct future definitive mechanistic investigation. Thus, we believe it is valuable, particularly given the expertise in blast physics and modeling that guided the work, and the comprehensive neuropathological evaluation we previously reported.11 Given the different methodological requirements for the many assays used in this study, and limitations of tissue sample volume, it could have compromised the assays to try to carry out all of them in each brain region. Also, in that these studies were carried out before the neuropathology had been defined—it was unclear where the most damage would be observed. Because our model results in global injury, our findings can direct future work. However, because axonal injury was greatest in the cerebellum and brainstem, these regions should be evaluated in the future. It will also be important to extend these studies to >24 h after injury. Our model is associated with ∼25% mortality from impact apnea, providing some insight into severity. However, characterization of behavioral deficits is needed to make appropriate correlations. We did not video record head movement. The head was the only part of the rat that was exposed and it was constrained to prevent acceleration. However, given recent findings,2 the role of head acceleration should be explored. We included anesthesia controls; additional controls, such as rats exposed to blast noise, could also be informative.63 We purposefully sought to eliminate extracerebral blast exposure using body protection, but this does not preclude effects of extracerebral blast on CNS injury. Finally, study of repeated blast TBI deserves attention in light of its importance.
Conclusions
Our mechanistic screening study strongly suggests a marked astrocytic response, neuroinflammation, including both cytokines and chemokines, and OS after blast-induced TBI in rats produced by a shock tube exposure of ∼35 psi. In recent studies of a large animal model as a part of PREVENT, a significant increase in GFAP-positive astrocyte expression has also been reported.6 These findings were observed in the setting of blast exposure (1) limited to the head, (2) with head constraint to limit acceleration, and (3) a relatively mild injury that is associated primarily with axonal and fiber tract injury noted only with cupric silver staining. Our data argue against a role for energy failure early after injury and suggest that the 2′,3′-cAMP pathway may be involved. Finally, pattern analysis on gene array suggests similarities in the response to those noted in both AD and long-term potentiation, conditions intimately linked to memory processing and/or disturbances in memory function. Our studies provide clues for further exploration of mechanistic alterations and therapeutic testing in experimental blast TBI.
Acknowledgments
This work was supported by DARPA PREVENT (N660001-10-C2124). The authors thank Keri Janesko-Feldman and Jeremy Henchir for their technical support. The authors thank Marci Provins and Fran Mistrick for their help with manuscript preparation. Microarray data were generated by the Genomics Core Laboratory, University of Pittsburgh, and statistical analyses were performed by the GPCL-Bioinformatics Analysis Core (GPCL-BAC), University of Pittsburgh. The authors thank Dr. James Lyons-Weiler for his valuable assistance. The views, opinions, and/or findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies either expressed or implied of DARPA or the DoD. This work has been approved for public release, distribution unlimited.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.MacDonald C.L. Johnson A.M. Cooper D. Nelson E.C. Werner N.J. Shimony J.S. Snyder A.Z. Raichle M.E. Witherow J.R. Fang R. Flaherty S.F. Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein L.E. Fisher A.M. Tagge C.A. Zhang X.L. Velisek L. Sullivan J.A. Upreti C. Kracht J.M. Ericsson M. Wojnarowicz M.W. Goletiani C.J. Maglakelidze G.M. Casey N. Moncaster J.A. Minaeva O. Moir R.D. Nowinski C.J. Stern R.A. Cantu R.C. Geiling J. Blusztajn J.K. Wolozin B.L. Ikezu T. Stein T.D. Budson A.E. Kowall N.W. Chargin D. Sharon A. Saman S. Hall G.F. Moss W.C. Cleveland R.O. Tanzi R.E. Stanton P.K. McKee A.C. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koliatsos V.E. Cernak I. Xu L. Song Y. Savonenko Al. Crain B.J. Eberhart C.G. Frangakis C.E. Melnikova T. Kim H. Lee D. A mouse model of blast injury to brain: Initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 2011;70:399–416. doi: 10.1097/NEN.0b013e3182189f06. [DOI] [PubMed] [Google Scholar]
- 4.Long J.B. Bentley T.L. Wessner K.A. Cerone C. Sweeney S. Bauman R.A. Blast overpressure in rats: recreating a battlefield injury in the laboratory. J. Neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- 5.Bauman R.A. Ling G. Tong L. Januszkiewicz A. Agoston d. Delanerolle N. Kim Y. Ritzel D. Bell R. Ecklund J. Armonda R. Bandak F. Parks S. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma. 2009;26:841–860. doi: 10.1089/neu.2008.0898. [DOI] [PubMed] [Google Scholar]
- 6.de Lanerolle N.C. Bandak F.A. Kang D. Li A.Y. Du F. Swauger P. Parks S. Ling G. Kim J.H. Characteristics of an explosive blast-induced brain injury in an experimental model. J. Neuropathol. Exp. Neurol. 2011;70:1046–1057. doi: 10.1097/NEN.0b013e318235bef2. [DOI] [PubMed] [Google Scholar]
- 7.Readnower R.D. Chavko M. Adeeb S. Conroy M.D. Pauly J.R. McCarron R.M. Sullivan P.G. Increase in blood brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast induced traumatic brain injury. J. Neurosci. Res. 2010;88:3530–3539. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svetlov S.I. Prima V. Kirk D.R. Gutierrez H. Curley K.C. Hayes R.L. Wang K.K.W. Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure. J. Trauma. 2010;69:795–804. doi: 10.1097/TA.0b013e3181bbd885. [DOI] [PubMed] [Google Scholar]
- 9.Lu J. Ng K.C. Ling G. Wu J. Poon D.J.F. Kan E.M. Tan M.H. Wu Y.J. Li P. Moochhala S. Yap E. Lee L.K.H. Teo M. Yeh I.B. Sergio D.M.B. Chua F. Kumar S.D. Ling E.A. Effect of exposure on the brain structure and cognition in Macaca fascicularis. J. Neurotrauma. 2012;29:1434–1454. doi: 10.1089/neu.2010.1591. [DOI] [PubMed] [Google Scholar]
- 10.Cernak I. Wang Z. Jiang J. Bian X. Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma. 2001;50:695–706. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Garman R.H. Jenkins L.W. Switzer R.C. Bauman R.A. Tong L.C. Swauger P.V. Parks S.A. Dixon C.E. Clark R.S.B. Bayır H. Kagan V. Jackson E.K. Kochanek P.M. Blast exposure injury in rats with body protection is characterized primarily by diffuse axonal injury. J. Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- 12.Levin H.S. Wilde E. Troyanskaya M. Peterson N.J. Scheibel R. Newsome M. Radaideh M. Wu T. Yallampalli R. Chu Z. Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- 13.Scheibel R.S. Newsome M.R. Troyanskaya M. Lin X. Steinberg J.L. Radaideh M. Levin H.S. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J. Int. Neuropsychol. Soc. 2012;18:89–100. doi: 10.1017/S1355617711001433. [DOI] [PubMed] [Google Scholar]
- 14.Risling M. Plantman S. Angeria M. Rostami E. Bellander B.-M. Kirkegaard M. Arborelius U. Davidsson J. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage. 2011;54:S89–S97. doi: 10.1016/j.neuroimage.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Elder G.A. Dorr N.P. De Gasperi R. Sosa M.A.G. Shaughness M.C. Maudlin-Jeronimo E. Hall A.A. McCarron R.M. Ahlers S.T. Blast exposure induces post traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma. 2012;29:2564–2575. doi: 10.1089/neu.2012.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front. Neurol. 2010;1:151. doi: 10.3389/fneur.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald C. Johnson A. Cooper D. Malone T. Sorrell J. Shimony J. Parsons M. Snyder A. Raichle M. Fang R. Flaherty S. Russell M. Brody D.L. Cerebellar white matter abnormalities following primary blast injury in US military personnell. PLoS One. 2013;8:e55823. doi: 10.1371/journal.pone.0055823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling G. Bandak F. Armonda R. Grant G. Ecklund J. Explosive blast neurotrauma. J. Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- 19.Armonda R.A. Bell R.S. Vo A.H. Ling G. DeGraba T.J. Crandall B. Ecklund J. Campbell W.W. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215–1225. doi: 10.1227/01.NEU.0000249190.46033.94. [DOI] [PubMed] [Google Scholar]
- 20.Jordan B.R. How consistent are expression chip platforms? Bioessays. 2004;26:1236–1242. doi: 10.1002/bies.20128. [DOI] [PubMed] [Google Scholar]
- 21.Patel S. Lyons-Weiler J. caGEDA: a web application for the integrated analysis of global gene expression patterns in cancer. Appl. Bioinformatics. 2004;3:49–62. doi: 10.2165/00822942-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 22.Draghici S. Khatri P. Tarca A.L. Amin K. Done A. Voichita C. Georgescu C. Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayır H. Tyurin V.A. Tyurina Y.Y. Viner R. Ritov V. Amoscato A.A. Zhao Q. Zhang X.J. Janesko-Feldman K.L. Alexander H. Basova L.V. Clark R.S. Kochanek P.M. Kagan V.E. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann. Neurol. 2007;62:154–169. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- 24.Belikova N.A. Glumac A.L. Kapralova V. Tyurina Y.Y. Kochanek P.M. Kagan V.E. Bayır H. A high-throughput screening assay of ascorbate in brain samples. J. Neurosci. Methods. 2011;201:185–190. doi: 10.1016/j.jneumeth.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson E.K. Ren J. Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J. Biol. Chem. 2009;284:33097–33106. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeKosky S.T. Ikonomovic M.D. Gandy S. Traumatic brain injury—football, warfare, and long-term effects. N. Engl. J. Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- 27.Morey R.A. Haswell C.C. Selgrade E.S. Massoglia D. Liu C. Weiner J. Marx C.E. Cernak I. McCarthy G. MIRECC Work Group. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum. Brain Mapp. 2012 2012 Jun 15; doi: 10.1002/hbm.22117. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazarian J.J. Donnelly K. Petersen D.R. Warner G.C. Zhu T. Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during operations Enduring Freedom and Iraqi Freedom: a diffusion tensor imaging study. J. Head Trauma Rehabil. 2013;28:1–12. doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- 29.Yoshiya K. Tanaka H. Kasai K. Irisawa T. Shiozaki T. Sugimoto H. Profile of gene expression in the subventricular zone after traumatic brain injury. J. Neurotrauma. 2003;20:1147–1162. doi: 10.1089/089771503770802844. [DOI] [PubMed] [Google Scholar]
- 30.Berger I. Hershovitz E. Shaag A. Edvardson S. Saada A. Elpeleg O. Mitochondrial complex I deficiency caused by a deleterious NDUFA11 mutation. Ann. Neurol. 2008;63:405–408. doi: 10.1002/ana.21332. [DOI] [PubMed] [Google Scholar]
- 31.Sajja V.S.S.S. Galloway M.P. Ghoddoussi F. Thiruthalinathan D. Kepsel A. Hay K. Bir C.A. VandeVord P.J. Blast-induced neurotrauma leads to neurochemical changes and neuronal degeneration in the rat hippocampus. NMR Biomed. 2012;25:1331–1339. doi: 10.1002/nbm.2805. [DOI] [PubMed] [Google Scholar]
- 32.Natale J.E. Ahmed F. Cernak I. Stoica B. Faden A.I. Gene expression profile changes are commonly modulated across models and species after traumatic brain injury. J. Neurotrauma. 2003;20:907–927. doi: 10.1089/089771503770195777. [DOI] [PubMed] [Google Scholar]
- 33.Matzilevich D.A. Rall J.M. Moore A.N. Grill R.J. Dash P.K. High-density microarray analysis of hippocampal gene expression following experimental brain injury. J. Neurosci. Res. 2002;67:646–663. doi: 10.1002/jnr.10157. [DOI] [PubMed] [Google Scholar]
- 34.Long Y. Zou L. Liu H. Lu H. Yuan X. Robertson C.S. Yang K. Altered expression of randomly selected genes in mouse hippocampus after traumatic brain injury. J. Neurosci. Res. 2003;71:710–720. doi: 10.1002/jnr.10524. [DOI] [PubMed] [Google Scholar]
- 35.Kwon S.-K.C. Koveski E. Gyorgy A.B. Wing D. Kamnaksh A. Walker J. Long J.B. Agoston D.V. Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front. Neurol. 2011;2:12. doi: 10.3389/fneur.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svetlov S.I. Prima V. Glushakova O. Svetlov A. Kirk D.R. Gutierrez H. Serebruany V.L. Curley K.C. Wang K.K.W. Hayes R.L. Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to “composite” blast. Front. Neurol. 2012;3:15. doi: 10.3389/fneur.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kochanek A.R. Kline A.E. Gao W.M. Chadha M. Lai Y. Clark R.S. Dixon C.E. Jenkins L.W. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev. Neurosci. 2006;28:410–419. doi: 10.1159/000094167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myer D.J. Gurkoff G.G. Lee S.M. Hovda D.A. Sofroniew M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 39.Gao W. Chadha M.S. Berger R.P. Omenn G. Allen D. Pisano M. Adelson P.D. Clark R.S.B. Jenkins L.W. Kochanek P.M. Biomarkers and diagnosis: a gel-based proteomic comparison of human cerebrospinal fluid between inflicted and non-inflicted pediatric traumatic brain injury. J. Neurotrauma. 2007;24:43–53. doi: 10.1089/neu.2006.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalewska T. Zablocka B. Domanska-Janik K. Changes of Ca2+/calmodulin-dependent protein kinase-II after transient ischemia in gerbil hippocampus. Acta Neurobiol. Exp. (Wars.) 1996;56:41–48. doi: 10.55782/ane-1996-1102. [DOI] [PubMed] [Google Scholar]
- 41.Waxham M.N. Grotta J.C. Silva A.J. Strong R. Aronowski J. Ischemia-induced neuronal damage: a role for calcium/calmodulin-dependent protein kinase II. J. Cereb. Blood Flow Metab. 1996;16:1–6. doi: 10.1097/00004647-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Hulse R.E. Kunkler P.E. Fedynyshyn J.P. Kraig R.P. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J. Neurosci. Methods. 2004;136:87–98. doi: 10.1016/j.jneumeth.2003.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takamiya M. Fujita S. Saigusa K. Aoki Y. Simultaneous detections of 27 cytokines during cerebral wound healing by multiplexed bead-based immunoassay for wound age estimation. J. Neurotrauma. 2007;24:1833–1844. doi: 10.1089/neu.2007.0336. [DOI] [PubMed] [Google Scholar]
- 44.Buttram S.D. Wisniewski S.R. Jackson E.K. Adelson P.D. Janesko-Feldman K. Bayır H. Berger R.P. Clark R.S.B. Kochanek P.M. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J. Neurotrauma. 2007;24:1707–1718. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- 45.Frugier T. Morganti-Kossmann M.C. O'Reilly D. McLean C.A. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic brain injury. J. Neurotrauma. 2010;27:497–507. doi: 10.1089/neu.2009.1120. [DOI] [PubMed] [Google Scholar]
- 46.DiPietro L.A. Burdick M. Low Q.E. Kunkel S.L. Strieter R.M. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J. Clin. Invest. 1998;101:1693–1698. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balashov K.E. Rottman J.B. Weiner H.L. Hancock W.W. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zozulya A.L. Reinke E. Baiu D.C. Karman J. Sandor M. Fabry Z. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1alpha chemokine and matrix metalloproteinases. J. Immunol. 2007;178:520–529. doi: 10.4049/jimmunol.178.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kataoka A. Tozaki-Saitoh H. Koga Y. Tsuda M. Inoue K. Activiation of P2X7 reeptors induces CCL3 production in microglial cells through transcription factor NFAT. J. Neurochem. 2009;108:115–125. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- 50.Andre R. Pinteaux E. Kimber I. Rothwell N.J. Differential actions of IL-1 alpha and IL-1 beta in glial cells share common Il-1 signalling pathways. Neuroreport. 2005;16:153–157. doi: 10.1097/00001756-200502080-00017. [DOI] [PubMed] [Google Scholar]
- 51.Lemke R. Hartlage-Rübsamen M. Schliebs R. Differential injury-dependent glial expression of interleukins-1 alpha, beta, and interleukin-6 in rat brain. Glia. 1999;27:75–87. [PubMed] [Google Scholar]
- 52.DeKosky S.T. Styren S.D. O'Malley M.E. Goss J.R. Kochanek P.M. Marion D. Evans C.H. Robbins P.D. Interleukin-1 receptor antagonist suppresses neurotrophin response in injured rat brain. Ann. Neurol. 1996;39:123–127. doi: 10.1002/ana.410390118. [DOI] [PubMed] [Google Scholar]
- 53.Tsaikir N. Kimber I. Rothwell N.J. Pinteaux E. Differential effects of interleukin-1 alpha and beta on interleukin-6 and chemokine synthesis in neurons. Mol. Cell. Neurosci. 2008;38:259–265. doi: 10.1016/j.mcn.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Cohen I. Rider P. Carmi Y. Braiman A. Dotan S. White M.R. Voronov E. Martin M.U. Dinarello C.A. Apte R.N. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thornton P. McColl B.W. Greenhalgh A. Denes A. Allan S.M. Rothwell N.J. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010;115:3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- 56.Kossmann T. Hans V. Imhof H.G. Trentz O. Morganti-Kossmann M.C. Interleukin-6 released in human cerebrospinal fluid following traumatic brain injury may trigger nerve growth factor production in astrocytes. Brain Res. 1996;713:143–152. doi: 10.1016/0006-8993(95)01501-9. [DOI] [PubMed] [Google Scholar]
- 57.Bell M. Kochanek P.M. Doughty L.A. Carcillo J.A. Adelson P.D. Clark R.S.B. Wisniewski S.R. Whalen M.J. DeKosky S.T. Interleukin-6 and Interleukin-10 in cerebrospinal fluid after traumatic brain injury in children. J. Neurotrauma. 1997;14:451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- 58.Clahsen T. Schaper F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J. Leukoc. Biol. 2008;84:1521–1529. doi: 10.1189/jlb.0308178. [DOI] [PubMed] [Google Scholar]
- 59.Trinchieri G. Interleukin-12: a pro-inflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 60.Yaguchi M. Ohta S. Toyama Y. Kawakami Y. Toda M. Functional recovery after spinal cord injury in mice through activation of microglia and dendritic cells after IL-12 administration. J. Neurosci. Res. 2008;86:1972–1980. doi: 10.1002/jnr.21658. [DOI] [PubMed] [Google Scholar]
- 61.Tyurin V.A. Tyurina Y.Y. Borisenko G.G. Sokolova T.V. Ritov V.B. Quinn P.J. Rose M. Kochanek P. Graham S.H. Kagan V.E. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J. Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- 62.Bayır H. Kagan V.E. Tyurina Y.Y. Tyurin V.A. Ruppel R.A. Adelson P.D. Graham S.H. Janesko K. Clark R.S.B. Kochanek P.M. Assessment of antioxidant reserve and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 2002;51:571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Kamnaksh A. Kovesdi E. Kwon S.K. Wingo d. Ahmed F. Grunberg N.E. Long J. Agoston D.V. Factors affecting blast traumatic brain injury. J. Neurotrauma. 2011;28:2145–2153. doi: 10.1089/neu.2011.1983. [DOI] [PubMed] [Google Scholar]