Abstract
Background:
Screening on multidrug-resistant tuberculosis (MDR-TB) has been limited to the serious TB subpopulations excluding the new TB patients. This study aimed to examine MDR-TB burden among the new TB patients.
Methods:
We conducted a study in Zhejiang Province during 2009–2013 to screen for MDR-TB patients among the low MDR-TB risk patients and five subpopulations of high MDR-TB risk patients. The number, prevalence, and trend of MDR-TB were compared while the logistic regression model was used to examine risk factors related to MDR-TB.
Results:
A total of 200 and 791 MDR-TB cases were, respectively, identified from the 9830 new TB cases and 2372 high-risk suspects who took MDR-TB screening from 2009 to 2013. The MDR-TB rates went down in both of the new TB patients and five MDR-TB high-risk groups over the study time, but the percentage of MDR-TB patients identified from the new TB patients in all diagnosed MDR-TB cases kept stable from 28.3% in 2011 to 27.0% in 2012 to 26.0% in 2013.
Conclusions:
The study indicated that MDR-TB burden among new TB patients was high, thus screening for MDR-TB among the new TB patients should be recommended in China as well as in the similar situation worldwide.
Keywords: Drug Resistance, Multidrug-resistant Tuberculosis Screening, Tuberculosis
INTRODUCTION
China has the second highest multidrug-resistant tuberculosis (MDR-TB) burden worldwide, next to India, based on the estimated absolute number of diagnosed MDR-TB cases, although the officially reported number has been far below to the WHO's estimate.[1] The huge gap was mainly caused by low drug susceptible testing (DST) coverage in China, 19% in new TB patients and 54% in re-treatment patients.[1] There is very limited and affordable technique to rapidly diagnose or access drug-resistant TB test due to inadequate resources across the globe.[2] Poor accessibility for MDR-TB diagnosis has become the barrier for early detection of MDR-TB cases. Also, the absence of conducting DST for all TB patients at enrollment has also put a threat on the detection and control of MDR-TB.[3,4] This may lead to more primary MDR-TB patients through transmission.[5,6]
At the World Health Assembly, May 2009, most WHO member states have committed to achieving universal access to diagnosis and treatment of MDR-TB by the year 2015.[7] However, due to inadequate resources, screening on MDR-TB was limited to the serious TB patients including (1) new cases persisting sputum culture or smear positive at the end of 2nd month after treatment, (2) cases of initial treatment failure, (3) re-treatment cases of treatment failure, (4) cases with poor treatment compliance, and (5) recurrence cases.[7] The situation was similar in China, TB drug sensitivity testing has never been initiated among the new smear-sputum TB patients in most areas in China until their sputum were still present positive at the end of the 2nd month after treatment.[8,9]
In 2009, Global Fund intended to conduct a program in Zhejiang Province to estimate MDR-TB burden. Zhejiang Province is among the most developed provinces in China. Between 2001 and 2004, the TB case notification rate increased from 16 to 26/100,000 populations and has since remained approximately stable.[10] A MDR-TB program was initiated in six cities during the years 2009–2013. The work started in Hangzhou, Shaoxing, Huzhou, and Lishui in 2009, in Quzhou in 2010, and in Jiaxing in 2013. The present study was conducted to investigate the rate of MDR-TB among the new sputum smear-positive patients as well as among the serious TB patients.[11]
METHODS
Ethical issues
This study focused on the number and prevalence of MDR-TB in different groups and aimed to provide evidence relevant to TB control in China. During the study, there was no access to individual patient information in this study; therefore ethics committee approval was not pursued.
Setting and study design
To estimate the MDR-TB burden in the new TB patients, we conducted a study in six cities (Hangzhou, Huzhou, Shaoxing, Jiaxing, Quzhou, and Lishui) in Zhejiang Provinces from January 1, 2009, to December 31, 2013.
TB cases, collected in the study, were grouped into (1) new cases persisting sputum culture or smear positive at the end of 2nd month after treatment, (2) new cases of initial treatment failure, (3) re-treatment cases of treatment failure, (4) cases with poor treatment compliance (i.e., patients receiving treatment with interruption and retreatment again), (5) recurrence cases, and (6) the new cases which were defined as patients who had never received any anti-TB treatment or had <30 days of anti-TB treatment. Cases of subgroups 1–5 were usually regarded serious patients with a high risk of MDR-TB and generally defined as MDR-TB high-risk-group (HRG), while cases of subgroup 6 were regarded as MDR-TB low-risk-group (LRG) in the study.
A “new TB case” refers to the patient who received no previous treatment or <1 month of treatment previously, a “re-treatment case” refers to the case who had been treated for more than one month previously, and a “recurrence case” is reported to be cured but diagnosed as TB again. MDR-TB is defined as a resistance to at least isoniazid and rifampicin.[12] All sputum smear-positive cases were asked to conduct MDR-TB test in HRG and LRG.
The primary outcome was the number and rate of MDR-TB diagnosed from HRG and LRG. Time trend of MDR-TB was also identified while the risk factors related with MDR-TB were examined.
Screening procedure
The detailed process of TB diagnosis has been reported in our previous study.[10,13] The sputum-positive patients were sent to the provincial reference laboratory for drug sensitivity testing (isoniazid, rifampicin), which was performed by the proportion method.[13] The provincial reference laboratory is evaluated annually by the national reference laboratory, and needs to be recertified for drug sensitivity testing each year. Drug sensitivity tests were carried out in comparison with results from standard resistant strains. A questionnaire was completed on medical and medication history.
Statistical analysis
The rate of MDR-TB was calculated by the number of MDR-TB cases divided by the number of screening cases. We compared the rate of LRG and HRG directly as well as for the numbers of MDR-TB cases in both groups.
The Cochran-Armitage test was used to test for time trends in the rate of MDR-TB among the new TB patients. Logistic regression models were used to investigate factors associated with MDR-TB. The factors included age (age group 1: 0–29 years, age group 2: 30–60 years, and age group 3: >60 years), gender (0: female; 1: male), and treatment history (initial treatment = 0, re-treatment = 1). Relative risks were calculated as odds ratios (ORs) and 95% confidence interval (CI) was also provided.
The total number of MDR-TB patients was estimated by calculating the sum of products of registered TB cases and average MDR-TB rate in each group, namely LRG and all subgroups of HRG (1–5).
The trend of multidrug resistance and proportion of new MDR-TB over time was analyzed using the Cochran-Armitage test. The factors associated with MDR-TB were examined by a multivariate logistic regression model, and ORs as well as 95% CI were calculated for each factor. By convention, we took P < 0.05 to indicate statistical significance. All statistical analyses were done with SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
During the study period, a total of 12,202 smear-positive pulmonary TB patients took MDR-TB screening, 200 and 791 MDR-TB cases were diagnosed from 9830 cases in LRG and 2372 cases in HRG. In MDR-TB HRG, 59% of patients were recurrent TB patients, 14.3% were initial treatment failure TB patients, and 11.5% were retreatment failure TB patients who showed the highest MDR-TB rate in the study duration [Table 1].
Table 1.
MDR-TB cases among the new TB patients and five MDR-TB high-risk suspect group, % (MDR number/screening number)*
| Items | New patients | MDR-TB high-risk suspect groups | Total | ||||
|---|---|---|---|---|---|---|---|
| Group 1* | Group 2* | Group 3* | Group 4* | Group 5* | |||
| Total | 2.1 (200/9530) | 10.0 (18/180) | 46.1 (149/323) | 83.8 (218/260) | 23.6 (41/174) | 28.6 (365/1277) | 8.4 (991/11744) |
| Age (years) | |||||||
| 0–29 | 2.5 (58/2305) | 21.1 (8/38) | 61.4 (54/88) | 61.4 (37/41) | 19.2 (5/26) | 100 (75/156) | 48.1 (237/2654) |
| 30–60 | 2.5 (94/3777) | 11.3 (8/71) | 48.2 (68/141) | 90.3 (130/144) | 34.9 (29/83) | 36.5 (205/580) | 11.1 (534/4796) |
| >60 | 1.4 (48/3448) | 2.8 (2/71) | 28.7 (27/94) | 68.0 (51/75) | 10.8 (7/65) | 13.6 (85/541) | 5.1 (220/4294) |
| Sex | |||||||
| Male | 2 (134/6740) | 8.5 (12/141) | 43.7 (104/238) | 83.7 (180/215) | 21.3 (30/141) | 25.8 (253/982) | 8.4 (713/8457) |
| Female | 2.4 (66/2790) | 15.4 (6/39) | 52.9 (45/85) | 84.4 (38/45) | 33.3 (11/33) | 38.0 (112/295) | 8.5 (278/3287) |
| Areas | |||||||
| Hangzhou | 2.9 (102/3501) | 14.2 (16/113) | 50.4 (125/248) | 82.1 (151/184) | 30.4 (34/112) | 32.5 (209/644) | 13.3 (637/4802) |
| Huzhou | 1.6 (17/1044) | 25.0 (1/4) | 36.4 (8/22) | 100 (37/37) | 4.3 (1/23) | 16.4 (21/128) | 6.8 (85/1258) |
| Shaoxing | 2.2 (44/1959) | 0 (0/7) | 44.4 (4/9) | 75.0 (9/12) | 0 (0/9) | 29.3 (65/222) | 5.5 (122/2218) |
| Jiaxing | 1.8 (8/433) | 2.6 (1/39) | 20.0 (2/10) | 66.7 (2/3) | 0 (0/2) | 27.9 (12/43) | 4.7 (25/530) |
| Quzhou | 0.8 (12/1584) | 0 (0/14) | 12.0 (3/25) | 58.3 (7/12) | 6.3 (1/16) | 19.8 (33/167) | 3.1 (56/1818) |
| Lishui | 1.7 (17/1009) | 0 (0/3) | 77.8 (7/9) | 100 (12/12) | 41.7 (5/12) | 34.2 (25/73) | 5.9 (66/1118) |
| Year | |||||||
| 2009 | 66.7 (2/3) | 0 (0/0) | 100 (31/31) | 100 (38/38) | 100 (10/10) | 100 (33/33) | 99.1 (114/115) |
| 2010 | 7.3 (11/150) | 44.4 (4/9) | 100 (31/31) | 96.9 (63/65) | 100 (5/5) | 89.0 (73/82) | 54.7 (187/342) |
| 2011 | 2.3 (64/2729) | 14.5 (9/62) | 30.4 (28/92) | 67.2 (39/58) | 17.5 (10/57) | 20.9 (76/363) | 6.7 (226/3361) |
| 2012 | 1.8 (60/3318) | 7.3 (3/41) | 29.1 (25/86) | 68.3 (28/41) | 11.7 (7/60) | 24.5 (99/404) | 5.6 (222/3950) |
| 2013 | 1.9 (63/3330) | 2.9 (2/68) | 41.0 (34/83) | 86.2 (50/58) | 21.4 (9/42) | 21.3 (84/395) | 6.1 (242/3976) |
*Group 1: New cases persisting sputum culture or smear positive at the end of the 2nd month after treatment; Group 2: New cases of initial treatment failure; Group 3: Re-treatment cases of treatment failure; Group 4: Cases with poor treatment compliance: patients receiving treatment and interruption and re-treatment again; Group 5: Recurrence cases. MDR: Multidrug resistant; TB: Tuberculosis.
Four hundred and fifty-eight nontuberculosis mycobacteria (NTM) isolates were found from 12,202 smear-positive sputum specimens, accounting for only 3.8% of all positive strains. Among patients with NTM, 65.5% (300/458) were new patients. Recurrent TB patients had the highest NTM rate (8.1%, 113/1390) among six MDR-TB HRGs [Table 2]. The patients with NTM lung diseases had an older age compared with TB patients (61.9 ± 17.4 years vs. 51.0 ± 20.3 years, P < 0.001, respectively), and in patients aged under 30 years, there were no patients with NTM in HRG 1, 2, and 4. The patients with NTM lung diseases were also more likely of female gender (34.5% vs. 24.0%, P < 0.005).
Table 2.
NTMs among the new TB patients and five MDR-TB high-risk suspect group, % (NTMs number/screening number)*
| Items | New patients | MDR-TB high-risk suspect groups | Total | ||||
|---|---|---|---|---|---|---|---|
| Group 1* | Group 2* | Group 3* | Group 4* | Group 5* | |||
| Total | 3.1 (300/9830) | 3.7 (7/187) | 4.4 (15/338) | 4.8 (13/273) | 5.4 (10/184) | 8.1 (113/1390) | 3.8 (458/12202) |
| Age (years) | |||||||
| 0–29 | 1.4 (33/2338) | 0 (0/38) | 1.1 (1/89) | 0 (0/41) | 0 (0/26) | 2.5 (4/160) | 1.4 (38/2692) |
| 30–60 | 2.3 (88/3865) | 2.7 (2/73) | 4.7 (7/148) | 4.0 (6/150) | 4.6 (4/87) | 6.5 (40/620) | 3.0 (147/4943) |
| >60 | 4.9 (179/3627) | 6.6 (5/76) | 6.9 (7/101) | 8.5 (7/82) | 8.5 (6/71) | 11.3 (69/610) | 6.0 (273/4567) |
| Sex | |||||||
| Male | 2.8 (195/6935) | 3.4 (5/146) | 3.6 (9/247) | 3.2 (7/222) | 5.4 (8/149) | 7.2 (76/1058) | 3.4 (300/8757) |
| Female | 3.6 (105/2895) | 4.9 (2/41) | 6.6 (6/91) | 11.8 (6/51) | 5.7 (2/35) | 11.1 (37/332) | 4.6 (158/3445) |
| Area | |||||||
| Hangzhou | 4.0 (146/3647) | 3.4 (4/117) | 3.1 (8/256) | 4.7 (9/193) | 6.7 (8/120) | 10.3 (74/718) | 4.9 (249/5051) |
| Huzhou | 1.8 (19/1063) | 0 (0/4) | 4.3 (1/23) | 2.6 (1/38) | 8.0 (2/25) | 5.2 (7/135) | 2.3 (30/1288) |
| Shaoxing | 3.4 (69/2028) | 0 (0/7) | 25 (3/12) | 7.7 (1/13) | 0 (0/9) | 6.7 (16/238) | 3.9 (89/2307) |
| Jiaxing | 9.8 (47/480) | 4.9 (2/41) | 16.7 (2/12) | 0 (0/3) | 0 (0/2) | 14.0 (7/50) | 9.9 (58/588) |
| Quzhou | 1.2 (19/1603) | 6.7 (1/15) | 3.8 (1/26) | 14.3 (2/14) | 0 (0/16) | 5.1 (9/176) | 1.7 (32/1850) |
| Lishui | 0 (0/1009) | 0 (0/3) | 0 (0/9) | 0 (0/12) | 0 (0/12) | 0 (0/73) | 0 (0/1118) |
| Year | |||||||
| 2009 | 0 (0/3) | 0 (0/0) | 0 (0/31) | 0 (0/38) | 0 (0/10) | 0 (0/33) | 0 (0/115) |
| 2010 | 0 (0/150) | 0 (0/9) | 0 (0/31) | 0 (0/65) | 0 (0/5) | 0 (0/82) | 0 (0/342) |
| 2011 | 2.0 (55/2784) | 3.1 (2/64) | 5.2 (5/97) | 10.8 (7/65) | 5.0 (3/60) | 6.2 (24/387) | 2.8 (96/3457) |
| 2012 | 3.3 (115/3433) | 6.8 (3/44) | 5.5 (5/91) | 4.7 (2/43) | 9.1 (6/66) | 9.0 (40/444) | 4.1 (171/4121) |
| 2013 | 3.8 (130/3460) | 2.9 (2/70) | 5.7 (5/88) | 6.5 (4/62) | 2.3 (1/43) | 11.0 (49/444) | 4.6 (191/4167) |
*Group 1: New cases persisting sputum culture or smear positive at the end of the 2nd month after treatment; Group 2: New cases of initial treatment failure; Group 3: Re-treatment cases of treatment failure; Group 4: Cases with poor treatment compliance: patients receiving treatment and interruption and re-treatment again; Group 5: Recurrence cases. MDR: Multidrug resistant; TB: Tuberculosis; NTMs: Nontuberculosis mycobacteria.
Regarding few TB patients took MDR-TB screening in two groups in 2009 and 2010, we did time trend analysis since 2011, and the trends of MDR-TB rate were not significant both among LRG (Z = −1.3, P = 0.1.) and HRG [Z = 0.55, P = 0.29, Figure 1a]. Furthermore, the number of MDR-TB cases diagnosed from LRG had taken up similar proportions in the number of all diagnosed MDR-TB cases since 2011 [Figure 1b]. The screening proportion for MDR-TB increased sharply in both LRG and HRG, achieving 74.9% and 65.7% in 2013, respectively. However, the screening proportion for new cases persisting sputum culture or smear positive at the end of 2nd month after treatment (HRG 1) was still low at only 21.0% in 2013 [Figure 1b].
Figure 1.
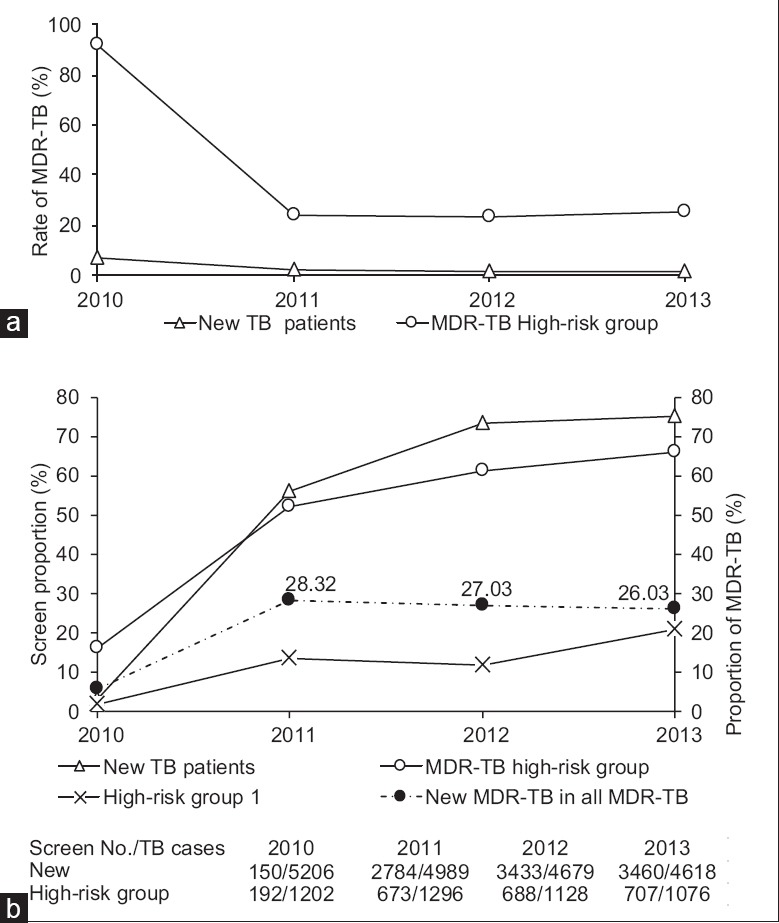
(a) Multidrug-resistant tuberculosis cases in the new tuberculosis patients and high-risk group. (b) Screen proportion in the new tuberculosis patients and high-risk group, the red line shows the percentage of multidrug-resistant tuberculosis cases diagnosed from new tuberculosis case in all diagnosed multidrug-resistant tuberculosis cases. Group 1 refers to the group of new cases persisting sputum culture or smear positive at the end of the 2nd month after treatment.
No significant difference of MDR-TB was found among different gender groups, while patients aged 0–30 and 30–60 years were both more likely to have MDR-TB compared with patients older than 60 years (OR = 1.79, 95% CI: 1.21–2.65; OR = 1.80, 95% CI: 1.27–2.57, respectively) in MDR-TB LRG [Table 3]. Furthermore, in MDR-TB HRG, our analysis indicated that patients aged 0–30 and 30–60 years were also both more likely to have MDR-TB in comparison with patients older than 60 years (OR = 4.28, 95% CI: 3.24–5.67; OR = 2.96, 95% CI: 2.40–3.67, respectively). Re-treatment was a strong predictor for MDR-TB and increased the risk of MDR-TB more than six times (OR = 6.16, 95% CI: 3.82–10.54).
Table 3.
Risk factors associated with MDR-TB*
| Items | New TB cases | MDR-TB high-risk group | ||
|---|---|---|---|---|
| OR (95% CI)* | P | OR (95% CI)† | P | |
| Age (years) | ||||
| 0–29 | 1.79 (1.21–2.65) | <0.01 | 4.28 (3.24–5.67) | <0.01 |
| 30–60 | 1.80 (1.27–2.57) | <0.01 | 2.96 (2.40–3.67) | <0.01 |
| >60 | Reference | Reference | Reference | Reference |
| Sex | ||||
| Male | Reference | Reference | Reference | Reference |
| Female | 1.14 (0.84–1.53) | 0.42 | 1.23 (0.99–1.52) | 0.06 |
| Treatment history | ||||
| Initial treatment | - | - | Reference | Reference |
| Re-treatment | - | - | 6.16 (3.82–10.54) | <0.01 |
*Adjusted for age, sex; †Adjusted for age, sex, and treatment history. CI: Confidence interval; OR: Odds ratio; MDR: Multidrug resistant; TB: Tuberculosis.
Estimate of multidrug-resistant tuberculosis cases under 100% screening
We estimated the number of MDR-TB patients using the average MDR-TB rate multiplied by the number of registered TB patients in each screening group. Moreover, if we had screened for MDR-TB in all TB patients, we could have found 1966 MDR-TB patients, nearly twice the number of MDR-TB patients we have found now [Table 4].
Table 4.
Estimate of MDR-TB cases under 100% screening situation during 2009–2013*
| Items | TB cases (n) | MDR-TB screening number (n) | MDR-TB cases (n) | Average MDR-TB rate (%) | Total MDR-TB cases estimated* (n) | Potential MDR-TB cases estimated (%) |
|---|---|---|---|---|---|---|
| New TB patients | 24,639 | 9830 | 200 | 2.0 | 501 | 301 (30.8) |
| MDR-TB high-risk groups | ||||||
| Group 1 | 2123 | 187 | 18 | 9.6 | 204 | 186 (19.1) |
| Group 2 | 443 | 338 | 149 | 44.1 | 195 | 46 (4.8) |
| Group 3 | 324 | 273 | 218 | 79.9 | 259 | 41 (4.2) |
| Group 4 | 320 | 184 | 41 | 22.3 | 71 | 30 (3.1) |
| Group 5 | 2800 | 1390 | 365 | 26.3 | 735 | 370 (38.0) |
| Sub-total | 6010 | 2372 | 791 | 33.4 | 1465 | 674 (69.2) |
| Total | 30,649 | 12,202 | 991 | 8.1 | 1966 | 975 |
*Using the average MDR-TB rate among 2009–2013 to calculate the estimated MDR-TB cases under 100% screening situation. Group 1: New cases persisting sputum culture or smear positive at the end of the 2nd month after treatment; Group 2: New cases of initial treatment failure; Group 3: Re-treatment cases of treatment failure; Group 4: Cases with poor treatment compliance: Patients receiving treatment and interruption and re-treatment again; Group 5: Recurrence cases. Estimated MDR-TB number = TB cases × average MDR-TB rate. MDR: Multidrug resistant; TB: Tuberculosis.
Among the potential 975 MDR-TB patients we would found under 100% screening situation, recurrent patients (HRG 5) would account for the largest proportion (38.0%), then came the new patients (LRG, 30.8%), and then came the new cases persisting sputum culture or smear positive at the end of 2nd month after treatment (HRG 1, 19.1%). Furthermore, the number of estimated MDR-TB patients detected in LRG patients accounted for 25.5% (501/1966) of the total number of estimated MDR-TB cases, if all TB patients were screened for MDR-TB [Table 4].
DISCUSSION
The increasing incidence of MDR-TB is a major concern for TB control worldwide.[5,14] Early diagnosis of MDR-TB is crucial to reduce the risk of household and community transmission.[15] In this study, the MDR-TB prevalence was only 2.1% (200/9530) in the MDR-TB LRG patients, while 35.7% (791/2214) among the MDR-TB HRG patients. However, the number of MDR-TB patients detected in LRG patients accounted for 20.18% (200/991) of the total number of estimated MDR-TB cases. This may be because that, at a given point of time, the total number of low-MDR-risk TB patients was more than four times the number of high-MDR-risk TB patients, and this is not only in our study, but also the case across China. The proportion of MDR cases diagnosed from LRG of all TB patients in our study was similar to the national level finding (20.96%), and the proportion was stable in Zhejiang Province from 2011 to 2013. This result reminds us that more than a quarter of MDR-TB patients would not be detected and treated properly in Zhejiang Province if new TB patients were not screened for MDR-TB. We need to pay attention to the MDR screening among new TB cases even after the Global Fund MDR-TB project, which could be a challenge in MDR-TB control.
The results from multivariate logistic analyses showed that, both in the low MDR-TB risk group and high MDR-TB risk group, patients aged under 30 and 30–60 years had higher MDR rate than patients older than 60 years, which was similar to many other studies.[16,17] This may because younger people have higher mobility during work, leading to irregular medication. Younger patients may also have higher labor intensity and longer treatment delay time which may increase their risk in MDR-TB infection. We found that, in MDR-TB HRG, previous treatment history was a strong predictor for MDR-TB, and this is consistent with many studies.[18,19] It has been widely recognized that previous use of anti-TB drugs could induce multidrug resistance of Mycobacterium tuberculosis,[20] and patients can be at a higher risk if they received an improper and inadequate treatment.
Due to financial constraints, the screening of MDR-TB was not usually performed for each patient in China. Our study financed by the Global Fund MDR-TB project increased the screening proportion dramatically both in LRG and HRG during the study period in Zhejiang Province. However, the screening proportion was still far below the 100% screening target. MDR-TB patients who do not conduct DST before treatment may be under the risk of using ineffective treatment regimen, thus inducing advanced resistance to anti-TB drugs and having longer course of smear-positive TB. Those MDR-TB patients can put a threat on the society through transmission of MDR M. tuberculosis. We also found that screening proportion among patients in Group 1, regarding new cases persisting sputum culture or smear positive at the end of 2nd month after treatment, was less than one-third of that in HRG. This was mainly because many new TB patients were screened when they were registered, and many of them did not conduct DST at the end of the 2nd month after treatment though their sputum culture or smear shown positive. We ought to improve screening proportion for those patients, since they were at nearly five times higher the risk of having MDR-TB compared to new patients.
There are some limitations of this study. First, the Global Fund MDR-TB Project screened only a few TB patients in 2009 and 2010 when the project was initially started, and patients who had higher risk of MDR-TB were more likely to be screened. Therefore, we overestimated the MDR-TB rate in the first two years. Second, due to financial constraints, the screening of MDR-TB was not performed for all TB patients in the study regions. This may have biased our results. Third, the information about education, socioeconomic status, and living conditions was not well described and recorded in the medical records, so we failed to show the relationship between these factors and the epidemic of drug-resistant TB, impeding us to a further design of related strategies.
The findings from this study highlight that MDR-TB patients were mainly from new TB cases, though the MDR rate was lower among new TB patients than MDR-TB high-risk patients, but the number of new TB cases was large, so the new TB patients should be the target of the most intensive screening efforts.
In conclusion, the study indicated that MDR-TB burden among new TB patients was high in Zhejiang Province, China. Screening for MDR-TB among the new TB patients should be recommended in China as well as in the similar situation worldwide.
Please refer to the Comments “Promising of MDR-TB screening among new TB patients in China” on this article in this issue.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China (No. U1611264, 71640019, and 61571001), Zhejiang Provincial Science and Technology Major Project (2014C03034), Zhejiang-National Committee of Health and Family Planning Co-Sponsored Project (WKJ-ZJ-07), Zhejiang Provincial Medical Research project (2016KYB055), and Beijing Municipal Science and Technology Plan (No. D141100000314002).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2.Pang Y, Dong H, Tan Y, Deng Y, Cai X, Jing H, et al. Rapid diagnosis of MDR and XDR tuberculosis with the MeltPro TB assay in China. Sci Rep. 2016;6:25330. doi: 10.1038/srep25330. doi: 10.1038/srep25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Deun A, Wright A, Zignol M, Weyer K, Rieder HL. Drug susceptibility testing proficiency in the network of supranational tuberculosis reference laboratories. Int J Tuberc Lung Dis. 2011;15:116–24. [PubMed] [Google Scholar]
- 4.Jaramillo E. Guidelines for the Programmatic Management of Drug-resistant Tuberculosis. Geneva: World Health Organization; 2008. [PubMed] [Google Scholar]
- 5.Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet. 2010;375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 6.Agerton T, Valway S, Gore B, Pozsik C, Plikaytis B, Woodley C, et al. Transmission of a highly drug-resistant strain (strain W1) of Mycobacterium tuberculosis. Community outbreak and nosocomial transmission via a contaminated bronchoscope. JAMA. 1997;278:1073–7. doi: 10.1001/jama.1997.03550130047035. [PubMed] [Google Scholar]
- 7.Falzon D, Jaramillo E, Wares F, Zignol M, Floyd K, Raviglione MC. Universal access to care for multidrug-resistant tuberculosis: An analysis of surveillance data. Lancet Infect Dis. 2013;13:690–7. doi: 10.1016/S1473-3099(13)70130-0. doi: 10.1016/S1473-3099(13)70130-0. [DOI] [PubMed] [Google Scholar]
- 8.Fan LH, Li GG, Zhang JL, Zhang JL, Dong B, Li JJ. Screening strategy of multi-drug resistant tuberculosis patient. J Med Pest Control. 2013;29:1409–10. doi: 10.7629/yxdwfz201312040. [Google Scholar]
- 9.Xu CH, Li RZ, Cheng MT, Wang LX, Lan R. Strategy for multi-drug resistant pulmonary tuberculosis case finding and treatment. Chin J Public Health. 2011;27:391–3. [Google Scholar]
- 10.Zhejiang Bureau of Statistical and Zhejiang Group of National Bureau of Statistics of China. Zhejiang Statistical Yearbook 2009. China Statistics Press; 2010. [Google Scholar]
- 11.Bowser D, Sparkes SP, Mitchell A, Bossert TJ, Bärnighausen T, Gedik G, et al. Global Fund investments in human resources for health: Innovation and missed opportunities for health systems strengthening. Health Policy Plan. 2014;29:986–97. doi: 10.1093/heapol/czt080. doi: 10.1093/heapol/czt080. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Definitions and Reporting Framework for Tuberculosis 2013 Revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 13.Wang X, Fu Q, Li Z, Chen S, Liu Z, Nelson H, et al. Drug-resistant tuberculosis in Zhejiang Province, China 1999-2008. Emerg Infect Dis. 2012;18:496–8. doi: 10.3201/eid1803.110760. doi: 10.3201/eid1803.110760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abubakar I, Zignol M, Falzon D, Raviglione M, Ditiu L, Masham S, et al. Drug-resistant tuberculosis: Time for visionary political leadership. Lancet Infect Dis. 2013;13:529–39. doi: 10.1016/S1473-3099(13)70030-6. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 15.Cox H, van Cutsem G. Household screening and multidrug-resistant tuberculosis. Lancet. 2011;377:103–4. doi: 10.1016/S0140-6736(10)61390-6. doi: 10.1016/S0140-6736(10)61390-6. [DOI] [PubMed] [Google Scholar]
- 16.Lange C, Abubakar I, Alffenaar JW, Bothamley G, Caminero JA, Carvalho AC, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: A TBNET consensus statement. Eur Respir J. 2014;44:23–63. doi: 10.1183/09031936.00188313. doi: 10.1183/09031936.00188313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rifat M, Milton AH, Hall J, Oldmeadow C, Islam MA, Husain A, et al. Development of multidrug resistant tuberculosis in Bangladesh: A case-control study on risk factors. PLoS One. 2014;9:e105214. doi: 10.1371/journal.pone.0105214. doi: 10.1371/journal.pone.0105214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammad O, Okab A, Zaki M. Situation of multidrug-resistant pulmonary tuberculosis in Alexandria governorate from July 2008 to December 2012. Egypt J Bronchology. 2016;10:64–8. doi: 10.4103/1687-8426.176792. [Google Scholar]
- 19.Gaude GP, Hattiholli J. Drug resistance patterns among pulmonary tuberculosis patients in a tertiary care hospital in northern Karnataka. J Med Trop. 2015;17:81–6. doi: 10.4103/0300-1652.137194. doi: 10.4103/2276-7096.161510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931–6. doi: 10.1056/NEJMra1205429. doi: 10.1056/NEJMra1205429. [DOI] [PubMed] [Google Scholar]


