Abstract
Background:
Treatment of adrenal metastasis from lung carcinoma may prolong survival in the selected patients. However, not all patients can undergo surgery; thus, minimally invasive ablation procedures such as radiofrequency ablation (RFA) and microwave ablation (MWA) have gained acceptance as alternative treatment methods. This study summarized a 5-year single-center experience regarding the evaluation of safety and efficacy of computed tomography (CT)-guided thermal ablation in the management of adrenal metastasis originating from non-small cell lung cancer (NSCLC).
Methods:
The data of NSCLC patients ablated for adrenal metastasis at the Department of Diagnostic Imaging and Interventional Radiology, General Hospital Sotiria, were retrospectively analyzed. Patients were divided into two groups: RFA group and MWA group according to the therapeutic approaches. Preprocedural blood tests included measurement of international normalized ratio, partial thromboplastin time, and platelet enumeration. A dual-phase contrast-enhanced spiral CT was performed immediately after the procedure to assess the immediate response after ablation and to screen for related complications. Follow-up was performed with CT or magnetic resonance imaging at 1, 3, 6 months and 1 year after ablation and every 6 months thereafter.
Results:
A total of 99 ablation sessions in 71 patients with adrenal metastasis originating from NSCLC were included in the final analysis. Self-limited, postablation syndrome occurred in 16/99 (16.1%) of ablation sessions. All procedures were technically successful. Immediate postablation imaging showed no contrast enhancement of the ablated tumor in all patients. Follow-up imaging at 3 months revealed local tumor progression in 8 (22.8%) patients of the RFA group and 7 (19.4%) patients of MWA group, all of them underwent a second session successfully. The 1-year assessment revealed local recurrence of the ablated tumor in six patients (17.1%) of RFA group and seven patients (19.4%) of MWA group. Among these 71 patients, those with tumor size >3.5 cm had a higher local recurrence rate (65.2%, 15/23) than those with tumors ≤3.5 cm (16.7%, 8/48; P = 0.012). There was no significant difference in the median survival time between RFA (14.0 months) and MWA (14.6 months) groups (P > 0.05).
Conclusions:
RFA and MWA showed comparable efficacy and safety in adrenal metastasis treatment.
Keywords: Adrenal Metastasis, Microwave, Nonsmall Cell Lung Cancer, Radiofrequency Ablation
INTRODUCTION
The adrenal glands can be a site for metastatic disease, originating mostly from tumors of the lung, breast, or colon, though practically any primary neoplasm may produce distant metastases to involve the adrenal glands.[1,2] It has been reported that surgical treatment of adrenal metastasis from lung, renal, and colorectal carcinoma may prolong survival in the selected patients with solitary, resectable lesions.[3,4] However, not all patients can undergo surgery and the associated morbidity and cost may be high, whereas chemotherapy and radiation therapy are not considered as effective options for adrenal metastasis.[4,5]
Alternatively, minimally invasive ablation procedures have gained acceptance as alternative methods to treat patients not eligible for surgical resection. Radiofrequency ablation (RFA), microwave ablation (MWA), cryotherapy, and laser ablation have been the most commonly performed techniques.[6,7] In RFA, alternating current is used to generate heat and produce tissue necrosis. Tissue temperatures in excess of 100°C are achieved, with cell death due to coagulative necrosis occurring approximately at 50°C. RFA electrodes are placed into the lesion under computed tomography (CT) guidance, with the patient placed in an appropriate position to target the adrenal mass. As opposed to RFA, MWA uses electromagnetic energy, causing ionic polarization and converting kinetic energy into heat. Thus, fast and homogeneous tissue heating and subsequently coagulation necrosis occur.[8,9] This study summarized a 5-year single-center experience regarding the performance of RFA and MWA to treat adrenal metastases, originating from NSCLC.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Boards of the General Hospital Sotiria. Informed written consent was obtained from all patients before their enrollment in the study.
Patients
The data of NSCLC patients ablated for adrenal metastasis at the Department of Diagnostic Imaging and Interventional Radiology, General Hospital Sotiria, were retrospectively analyzed. All patients included in the study met the following criteria: all patients were not surgical candidates for excision of adrenal metastasis, and had undergone surgery for NSCLC in the past, all adrenal metastases were metachronous and histologically verified with biopsy, all included lesions were <6 cm and secondary metastases in other organs were excluded, patients were in good performance status, according to Eastern Cooperative Oncology Group scale (<2). Patients were divided into two groups: RFA group and MWA group according to the therapeutic approaches.
Preprocedural blood tests included measurement of international normalized ratio (INR), partial thromboplastin time, and platelet enumeration. Exclusion criteria were coagulopathy (INR was >1.5) or platelet count <60,000/mm3. Three milligrams of bromazepam orally and 50 mg pethidine hydrochloride intramuscularly were administered 1 h before the procedure. Spiral CT imaging (Somatom Emotion Duo System; Siemens, Erlangen, Germany) was used to guide the percutaneous access. Patients were positioned in lateral, supine, or prone position according to lesion's localization and the appropriate insertion route was chosen by an experienced interventional radiologist that performed all studies. During the procedure, pulses, breaths, oxygen saturation, and blood pressure were carefully monitored.
RFA was performed with AMICA-PROBE electrosurgical generator (AMICA-PROBE, NH Hospital Service, Rome, Italy) and a straight monopolar electrode, 17-G diameter. The exposed tips from 2.0 cm up to 3.5 cm were used according to the volume of the ablated tumor. A grounding pad was placed on the thigh to complete the electrical circuit. MWA was performed with AMICA-GEN microwave generator (AMICA-GEN, NH Hospital Service, Rome, Italy), output up to 140 W continuous wave at 2450 MHz connected to a 16-G coaxial antenna. The latter had a sleeve choke endowed, aiding to minimize back heating and optimize the sphericity of the ablation zone.
Pulse RFA energy at 90–110 W was applied for 8–10 min (with median time of 9.4 min), depending on lesion size, vascularity, and location. The maximum tissue temperature achieved varied from 90°C to 110°C. The ablation was designed to induce coagulation necrosis of the surrounding tissue in a radial of 1 cm beyond its borders. At electrode retrieval, all tracts were ablated. Regarding MWA, maximum tissue temperature exceeded 110°C and the procedural time was 3–6 min (median 4.4 min).
Technically successful ablation requires that tumor is treated according to the protocol and the lesion is entirely covered, as it is confirmed by the immediate postablation imaging. To assess the immediate response after ablation and to screen for related complications, a dual-phase contrast-enhanced spiral CT was performed immediately after the procedure. The treated tumors were considered as “completely ablated” when no sign of enhancement was present at the site of the ablated lesions. For magnetic resonance imaging (MRI), the term “total necrosis” refers to a lesion with a high signal on T2-weighted images, low signal in T1-weighted images, and no enhancement after administration of gadolinium.
Postablation complications were classified as major (hypertensive crisis, hematoma, and pneumothorax) or minor (pain, postablation syndrome, and skin irritation). All patients were hospitalized and monitored for complications and were discharged within 24 h if no complication occurred. Follow-up was performed with CT or MRI at 1, 3, and 6 months and 1 year after ablation and every 6 months thereafter. Follow-up time was 18 months except death.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics software, version 22.0 (IBM, Armonk, NY, USA). Data were expressed as number and percentages for categorical variables and continuous data were presented as median (range). Intergroup difference was compared using Chi-square test. The comparison of pre- and post-therapy data was performed using one-way analysis of variance for repeated measures. Statistical significance was set at P < 0.05.
RESULTS
Seventy-one patients with adrenal metastasis originating from NSCLC (including 33 males and 38 females) were included in the final analysis. The median age of these patients was 70 years (range: 46–82 years). Clinical characteristics and treatment of these patients are summarized in Tables 1 and 2. A total of 99 ablation sessions were performed in 71 patients with adrenal metastasis originating from NSCLC during 5-year period. Self-limited, postablation syndrome occurred in 16/99 (16.1%) of ablation sessions, most patients return to preprocedural levels of activity within 7 days after ablation. No major complication occurred. No procedural mortality was noted. Thirty-five patients were treated with RFA and 36 patients with MWA.
Table 1.
Clinical characteristics and treatment of 35 non-small cell lung cancer patients treated with radiofrequency ablation
| Serial Number | Gender | Lesion location | Lesion size in diameter (cm) | Ablation time (min) | Recurrence at 3 months | Recurrence after 1 year | Numbers of sessions | Death during follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | Right | 2.5 | 8 | No | No | 1 | No |
| 2 | Female | Right | 3.5 | 10 | No | Yes | 2 | No |
| 3 | Female | Right | 4.0 | 10 | Yes | No | 2 | Yes (3 months later) |
| 4 | Female | Right | 2.5 | 8 | Yes | No | 2 | Yes (4 months later) |
| 5 | Female | Right | 4.5 | 10 | No | Yes | 2 | Yes (12 months later) |
| 6 | Female | Right | 2.7 | 8 | No | No | 1 | No |
| 7 | Female | Right | 5.0 | 10 | Yes | Yes | 3 | Yes (12 months later) |
| 8 | Female | Right | 3.2 | 10 | No | No | 1 | No |
| 9 | Male | Right | 3.8 | 10 | No | No | 1 | No |
| 10 | Male | Right | 3.0 | 10 | No | No | 1 | No |
| 11 | Male | Right | 3.5 | 8 | Yes | No | 2 | Yes (4 months later) |
| 12 | Male | Right | 3.0 | 10 | No | No | 1 | No |
| 13 | Male | Right | 4.6 | 10 | No | No | 1 | No |
| 14 | Female | Right | 1.5 | 8 | No | No | 1 | No |
| 15 | Female | Right | 4.0 | 10 | Yes | Yes | 3 | Yes (12 months later) |
| 16 | Female | Right | 5.2 | 10 | No | Yes | 2 | Yes (13 months later) |
| 17 | Female | Left | 3.0 | 10 | No | No | 1 | No |
| 18 | Female | Left | 3.2 | 10 | No | No | 1 | Yes (3 months later) |
| 19 | Female | Left | 2.0 | 8 | No | No | 1 | No |
| 20 | Female | Left | 3.0 | 10 | No | No | 1 | No |
| 21 | Female | Left | 4.0 | 10 | No | Yes | 2 | Yes (13 months later) |
| 22 | Female | Left | 2.2 | 8 | No | No | 1 | No |
| 23 | Female | Left | 3.0 | 10 | Yes | No | 2 | Yes (4 months later) |
| 24 | Female | Left | 3.0 | 9 | No | No | 1 | No |
| 25 | Female | Left | 4.9 | 9 | No | No | 1 | No |
| 26 | Female | Left | 3.3 | 10 | Yes | No | 2 | Yes (4 months later) |
| 27 | Male | Left | 2.0 | 8 | No | No | 1 | No |
| 28 | Male | Left | 3.0 | 10 | No | No | 1 | No |
| 29 | Male | Left | 2.5 | 9 | No | No | 1 | No |
| 30 | Male | Left | 3.0 | 10 | No | No | 1 | No |
| 31 | Male | Left | 4.0 | 10 | Yes | No | 2 | Yes (4 months later) |
| 32 | Male | Left | 2.5 | 8 | No | No | 1 | No |
| 33 | Male | Left | 3.0 | 8 | No | No | 1 | No |
| 34 | Male | Left | 3.8 | 8 | No | No | 1 | Yes (13 months later) |
| 35 | Male | Left | 3.5 | 9 | No | No | 2 | No |
Table 2.
Clinical characteristics and treatment of 36 non-small cell lung cancer patients treated with microwave ablation
| Serial Number | Gender | Location | Size (cm) | Ablation time (min) | Recurrence at 3 months | Recurrence after 1 year | Numbers of sessions | Death during follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | Right | 3.0 | 3 | No | No | 1 | No |
| 2 | Female | Right | 3.5 | 4 | No | No | 1 | Yes (4 months later) |
| 3 | Female | Right | 4.0 | 5 | Yes | No | 2 | Yes (5 months later) |
| 4 | Female | Right | 3.5 | 4 | No | No | 1 | No |
| 5 | Female | Right | 5.0 | 6 | No | Yes | 2 | Yes (13 months later) |
| 6 | Female | Right | 2.5 | 3 | No | No | 1 | No |
| 7 | Female | Right | 6.0 | 6 | Yes | Yes | 3 | Yes (12 months later) |
| 8 | Female | Right | 3.5 | 4 | No | No | 1 | No |
| 9 | Female | Right | 3.8 | 5 | No | No | 1 | No |
| 10 | Female | Right | 3.0 | 5 | No | No | 1 | No |
| 11 | Female | Right | 3.5 | 4 | No | No | 1 | No |
| 12 | Male | Right | 3.0 | 4 | Yes | No | 2 | Yes (6 months later) |
| 13 | Male | Right | 3.0 | 4 | No | No | 1 | No |
| 14 | Male | Right | 1.8 | 3 | No | No | 1 | No |
| 15 | Male | Right | 4.0 | 5 | No | No | 1 | No |
| 16 | Male | Right | 4.8 | 6 | No | Yes | 2 | Yes (14 months later) |
| 17 | Male | Right | 3.0 | 4 | No | No | 1 | No |
| 18 | Male | Right | 2.5 | 4 | No | No | 1 | Yes (4 months later) |
| 19 | Male | Right | 2.0 | 3 | No | No | 1 | No |
| 20 | Male | Right | 3.1 | 4 | No | No | 1 | No |
| 21 | Male | Right | 5.0 | 6 | Yes | No | 2 | Yes (4 months later) |
| 22 | Female | Left | 2.5 | 3 | No | No | 1 | No |
| 23 | Female | Left | 3.2 | 4 | No | Yes | 2 | Yes (13 months later) |
| 24 | Female | Left | 3.5 | 5 | No | No | 1 | No |
| 25 | Female | Left | 4.5 | 6 | Yes | Yes | 3 | Yes (14 months later) |
| 26 | Female | Left | 3.2 | 4 | No | No | 1 | No |
| 27 | Female | Left | 4.2 | 6 | Yes | No | 2 | Yes (4 months later) |
| 28 | Male | Left | 4.0 | 5 | No | Yes | 2 | No |
| 29 | Male | Left | 4.8 | 6 | No | No | 1 | Yes (5 months later) |
| 30 | Male | Left | 2.0 | 3 | No | No | 1 | No |
| 31 | Male | Left | 3.0 | 4 | No | No | 1 | No |
| 32 | Male | Left | 2.1 | 3 | No | No | 1 | No |
| 33 | Male | Left | 3.0 | 4 | No | No | 1 | No |
| 34 | Male | Left | 3.2 | 4 | Yes | Yes | 3 | Yes (13 months later) |
| 35 | Male | Left | 4.0 | 6 | No | No | 1 | Yes (5 months later) |
| 36 | Male | Left | 2.0 | 4 | No | No | 1 | No |
The median lesion size for patients treated with RFA was 3.3 cm in diameter (range: 1.5–5.2 cm) and the median time of ablation was 9.4 min (range: 8–10 min). In MWA group, the median lesion size was 3.5 cm (range 1.8–6.0 cm) and the median ablation time 4.4 min (range: 3–6 min).
All procedures were technically successful. Immediate postablation imaging showed no contrast enhancement of the ablated tumor in all patients. Follow-up imaging 3 months after the RFA revealed local tumor progression in 8/35 (22.8%) patients and all of them underwent a second RFA session successfully. The 1-year assessment revealed local recurrence of the ablated tumor in 6/35 patients (17.1%), two of them had a second RFA at 3 months due to recurrence at that time. All of these patients had an additional ablation. Follow-up imaging 3 months after MWA showed local tumor progression in 7/36 (19.4%) patients and a second ablation was performed in these seven patients. The 1-year assessment revealed local recurrence of the ablated tumor in seven patients (19.4%), three of them received a second MWA at 3 months due to recurrence at that time and all patients had one more MWA successfully [Figures 1–3].
Figure 1.
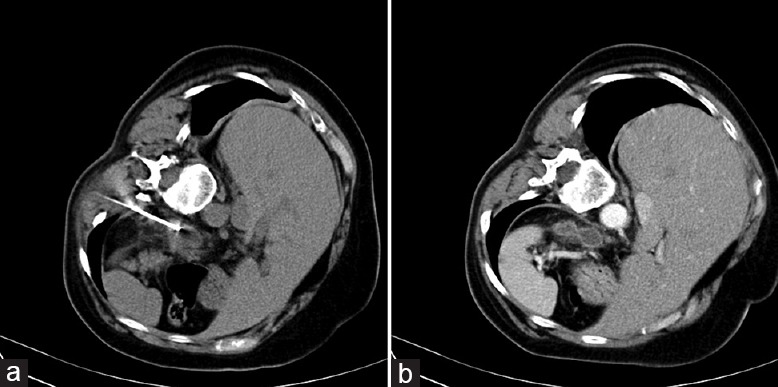
(a) Computed tomography imaging of a 56-year-old patient with metastasis in the left adrenal gland shows the right placement of radiofrequency ablation electrode; (b) computed tomography imaging 6 months after the radiofrequency ablation reveals no obvious residual contrast uptake at the adrenal ablation site, suggesting total necrosis.
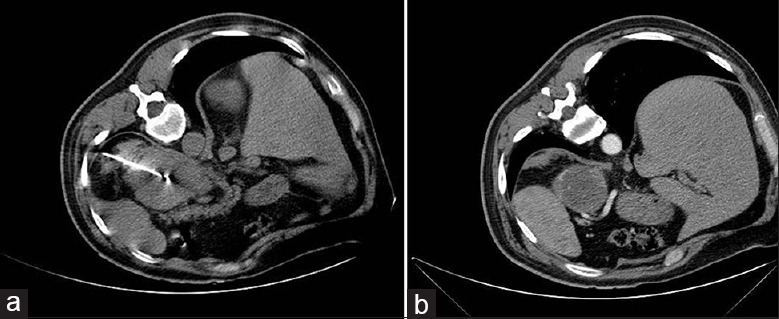
Figure 3.
(a) Computed tomography of a 67-year-old female with metastasis of the left adrenal gland, patient in lateral position and the microwave ablation electrode inside the lesion; (b) imaging 6 months after the procedure reveals tumor necrosis.
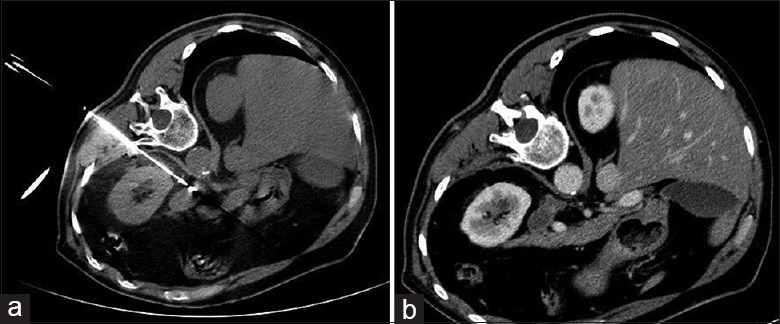
Figure 2.
(a) Computed tomography imaging of a 70-year-old male with metastasis in the left adrenal gland, patient in lateral position and the microwave ablation electrode inside the lesion; (b) computed tomography imaging 6 months after the microwave ablation procedure reveals tumor necrosis.
Among these 71 patients, those with tumor size >3.5 cm had a higher local recurrence rate (65.2%, 15/23) than those with tumors ≤3.5 cm (16.7%, 8/48; P = 0.012). During the 18 months of follow-up range, 27 patients (38.0%), including 13 patients in the RFA group and 14 patients in the MWA group, died due to NSCLC recurrence. Median overall survival time of all 71 patients was 14.0 months (range: 3–18 months); there was no significant difference in the survival time between RFA (median: 14.0 months) and MWA (median: 14.6 months) groups (P > 0.05).
DISCUSSION
This study suggested that thermal ablation is feasible and effective in patients with adrenal metastatic disease from NSCLC, with zero mortality and negligible morbidity, limited to a low rate of postablation syndrome. Our observations were in line with previous studies on the topic, highlighting the use of thermal ablation in both primary and secondary neoplasms of the adrenal glands. Wood et al.[10] had treated eight cases with unresectable primary or metastatic adrenocortical carcinoma with RFA and demonstrated efficacy in tumors smaller than 5 cm. Mayo-Smith and Dupuy[11] treated successfully 11 out of 13 adrenal masses with RFA. The authors assumed that the risk of residual tumor was higher for larger lesions.
In this study, the size of the lesions was also a significant parameter for the successful treatment of adrenal tumor masses. The lesions treated successfully with a single ablation were smaller than 3 cm in size, while patients who needed additional session had a tumor size larger than 3 cm.
Thermal ablation warranted significant attention to the most serious, though rare complications might accompany the procedure.[12] Complications consist of pneumothorax, hematoma, infection, tumor seeding, nontarget thermal damage, and grounding pad burns (exclusively for RFA). Moreover, the risks after adrenal ablation expand to include adrenal insufficiency, and hypoglycemia or hypertensive episode due to sudden release of insulin or catecholamines, respectively.[13] In this study, no patient experienced a hypertensive crisis. Postablation syndrome, characterized by low-degree fever (37.5°C–38.5°C), delayed pain, nausea, vomiting, malaise, and myalgia, occurred only in 16.1% of ablation sessions. These symptoms were self-limiting and most patients return to preprocedural levels of activity within 7 days after ablation. MWA advantages include ablation of larger ablation areas in faster ablation times, with fewer applicator insertions compared to RFA.[14] Furthermore, no grounding pads are needed avoiding the possibility of skin burns. Attenuated heat-sink effect makes MWA better in ablation of perivascular tumors.
To date, results concerning local tumor control rates of MWA and RFA are limited. Previous studies showed similar performance for both modalities. Two studies by Li et al.[15] and Wolf et al.[16] showed, in a limited number of patients with adrenal metastasis originating from lung cancer, lower rates of local recurrence after MWA, 10% compared to 15% after RFA. This study revealed no significant difference between the two methods as the rates of local recurrence at 3-month and 1-year follow-up were estimated to both 19.4% in MWA group and 22.8% and 17.1% in RFA group.
The current literature requires the lack of lesion's enhancement after ablation as criterion for complete pathologic tumor necrosis and has also been used as a good response to local ablation by our team for this study. Nevertheless, in a recent study, residual tumor on tissue histology may be present even in the absence of CT enhancement. As Wood et al.[10] suggested, the impact of short-term tumor shrinkage and lack of enhancement in long-term follow-up should further be evaluated to be correlated to successful local tumor control.
Given the fact that RFA and MWA are associated with high success rate in the treatment of small adrenal neoplasms and limited major complications, they have a potential of various and wide applications in adrenal gland disease. While surgery is considered to be the first-line approach for solitary adrenal metastasis, the extent of survival benefit is yet to be defined. Small studies have implied a benefit for specific patients, namely, those with favorable tumor biology, specific histologies, or long disease-free intervals.[4,5,15] On the other hand, many studies reported superiority of thermal ablation compared to surgical management with low morbidity, short recovery on an outpatient basis, and expand patient options to include nonsurgical candidates. Yang et al.[17] compared laparoscopic adrenalectomy (LA) to RFA and concluded that treatment success rate reached 100% in the RFA group versus 94.4% in the LA group during 6-month follow-up. RFA group demonstrated less postoperative pain (visual analog scale: 2.00 ± 1.16 vs. 4.22 ± 1.44, P < 0.001) and shorter operative time (105 ± 34 min vs. 194 ± 58 min, P < 0.001) compared with LA group.[17,18]
In conclusion, new therapeutic techniques have improved treatment options for oncologic patients with metastatic disease. Interventional radiology, specifically RFA and MWA, may contribute to the treatment of adrenal gland metastasis originating from NSCLC in a safe and efficient way. MWA outmatches RFA in terms of ablation volume and procedural time. Lesions with size of 5 cm or larger would have not been targeted with a single RFA; multiple or overlapping RFAs would have increased procedural time and morbidity. Although MWA has certain advantages, there is no documented proof of superiority of this method as far as it concerns local tumor control rate compared to RFA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Iacconi P, Donatini G, Iacconi C, De Bartolomeis C, Cucinotta M, Puccini M, et al. Unexpected histological findings of lesions diagnosed in the adrenal region in a series of 420 patients submitted to adrenal surgery. Review of our experience. J Endocrinol Invest. 2008;31:873–6. doi: 10.1007/BF03346434. doi: 10.1007/BF03346434. [DOI] [PubMed] [Google Scholar]
- 2.Ctvrtlík F, Herman M, Student V, Tichá V, Minarík J. Differential diagnosis of incidentally detected adrenal masses revealed on routine abdominal CT. Eur J Radiol. 2009;69:243–52. doi: 10.1016/j.ejrad.2007.11.041. doi: 10.1016/j.ejrad.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Muth A, Persson F, Jansson S, Johanson V, Ahlman H, Wängberg B. Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol. 2010;36:699–704. doi: 10.1016/j.ejso.2010.04.002. doi: 10.1016/j.ejso.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Lenert JT, Barnett CC, Jr, Kudelka AP, Sellin RV, Gagel RF, Prieto VG, et al. Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery. 2001;130:1060–7. doi: 10.1067/msy.2001.118369. doi: 10.1067/msy.2001.118369. [DOI] [PubMed] [Google Scholar]
- 5.Gunjur A, Duong C, Ball D, Siva S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’– A systematic review. Cancer Treat Rev. 2014;40:838–46. doi: 10.1016/j.ctrv.2014.04.001. doi: 10.1016/j.ctrv.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Welch BT, Atwell TD, Nichols DA, Wass CT, Callstrom MR, Leibovich BC, et al. Percutaneous image-guided adrenal cryoablation: Procedural considerations and technical success. Radiology. 2011;258:301–7. doi: 10.1148/radiol.10100631. doi: 10.1148/radiol.10100631. [DOI] [PubMed] [Google Scholar]
- 7.Yamakado K. Image-guided ablation of adrenal lesions. Semin Intervent Radiol. 2014;31:149–56. doi: 10.1055/s-0034-1373797. doi: 10.1055/s-0034-1373797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics. 2005;25(Suppl 1):S69–83. doi: 10.1148/rg.25si055501. doi: 10.1148/rg25si055501. [DOI] [PubMed] [Google Scholar]
- 9.Mayo-Smith WW, Boland GW, Noto RB, Lee MJ. State-of-the-art adrenal imaging. Radiographics. 2001;21:995–1012. doi: 10.1148/radiographics.21.4.g01jl21995. doi.org/10.1148/radiographics.21.4.g01jl21995. [DOI] [PubMed] [Google Scholar]
- 10.Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97:554–60. doi: 10.1002/cncr.11084. doi: 10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation – Preliminary results. Radiology. 2004;231:225–30. doi: 10.1148/radiol.2311031007. doi: 10.1148/radiol.2311031007. [DOI] [PubMed] [Google Scholar]
- 12.Carrafiello G, Laganà D, Ianniello A, Dionigi G, Novario R, Recaldini C, et al. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: One centre experience and review of published works. Australas Radiol. 2007;51:550–4. doi: 10.1111/j.1440-1673.2007.01871.x. doi: 10.1007/s00270-008-9337-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamakado K, Takaki H, Yamada T, Yamanaka T, Uraki J, Kashima M, et al. Incidence and cause of hypertension during adrenal radiofrequency ablation. Cardiovasc Intervent Radiol. 2012;35:1422–7. doi: 10.1007/s00270-012-0348-6. doi: 10.1007/s00270-012-0348-6. [DOI] [PubMed] [Google Scholar]
- 14.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr Microwave tumor ablation: Mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192–203. doi: 10.1016/j.jvir.2010.04.007. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Fan W, Zhang L, Zhao M, Huang Z, Li W, et al. CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: Preliminary results. Cancer. 2011;117:5182–8. doi: 10.1002/cncr.26128. doi: 10.1002/cncr.26128. [DOI] [PubMed] [Google Scholar]
- 16.Wolf FJ, Dupuy DE, Machan JT, Mayo-Smith WW. Adrenal neoplasms: Effectiveness and safety of CT-guided ablation of 23 tumors in 22 patients. Eur J Radiol. 2012;81:1717–23. doi: 10.1016/j.ejrad.2011.04.054. doi: 10.1016/j.ejrad.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Yang MH, Tyan YS, Huang YH, Wang SC, Chen SL, et al. Comparison of radiofrequency ablation versus laparoscopic adrenalectomy for benign aldosterone-producing adenoma. Radiol Med. 2016;121:811–9. doi: 10.1007/s11547-016-0662-1. doi: 10.1007/s11547-016-0662-1. [DOI] [PubMed] [Google Scholar]
- 18.Gao XL, Zhang KW, Tang MB, Zhang KJ, Fang LN, Liu W. Pooled analysis for surgical treatment for isolated adrenal metastasis and non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2017;24:1–7. doi: 10.1093/icvts/ivw321. doi: 10.1093/icvts/ivw321. [DOI] [PubMed] [Google Scholar]


