Abstract
Background:
Increased proinflammatory cytokines and chemokines might contribute to infiltration of inflammatory cells and remodeling in airways of asthma. Although these molecules may be associated with asthma, there is lack of systemic evidence showing which and how important these events are in the disease. We aimed to analyze the concentrations of these molecules in the airways and relationships with disease severity and with airway infiltration of inflammatory cells in a large cohort of asthmatics (n = 70, including 37 mild and 33 moderate/severe asthmatics) compared with controls (n = 30).
Methods:
Meso scale discovery system and commercial ELISA kits were used to measure the concentrations of proinflammatory cytokines interleukin (IL)-1β; tumor necrosis factor-alpha (TNF-α); IL-6; and IL-17 and CC and CXC chemokines CCL2, CCL4, CCL11, CCL13, CCL17, CCL22, and CCL26 and CXCL8, CXCL9, CXCL10, and CXCL11 in bronchoalveolar lavage fluid of asthmatics and controls.
Results:
The concentrations of IL-1, TNF-α, IL-6, CXCL8 and CXCL10, and CCL4, CCL11, CCL17, and CCL22 were significantly elevated in asthmatics compared with controls (P < 0.05). The concentrations of TNF-α and CXCL8, but not others, were negatively correlated with severity of disease (lung function forced expiratory volume in 1 s) (TNF-α vs. total: r = −0.359, P = 0.002 vs. moderate/severe: r = −0.541, P = 0.001; CXCL8 vs. total: r = −0.327, P = 0.006 vs. moderate/severe: r = −0.625, P = 0.0001, respectively). In addition, concentrations of these two molecules were also correlated with the absolute numbers of infiltrating eosinophils and neutrophils in asthmatic airways.
Conclusions:
Increased concentrations of TNF-α and CXCL8 are associated with pathogenesis of asthma. Targeting these molecules might provide an alternative therapeutic for this disease.
Keywords: Asthma, Bronchoalveolar Lavage, Chemokine, Proinflammatory Cytokines
INTRODUCTION
Asthma is a chronic airway disease characterized by reversible airflow obstruction and airway hyperresponsiveness, increased expression of numerous cytokines, chemokines, and other inflammatory mediators which possibly contribute to infiltrating inflammatory cells and remodeling in the airways.[1,2] Although increased evidence have implicated that asthma is a Th2-type inflammatory disorder,[1,2] other mediators including proinflammatory cytokines interleukin-1 (IL-1), IL-6, IL-17, and tumor necrosis factor-alpha (TNF-α) and various chemokines such as CXC and CC chemokines CXCL8, CXCL10, CCL2, CCL4, CCL11, and others may also participate in the pathogenesis of asthma, through acting directly or indirectly on the target cells.[1,2,3,4] Increased expression of these proinflammatory cytokines and various chemokines, at mRNA or protein levels, is observed in different clinical samples of asthmatics compared with controls, such as peripheral blood, sputum, bronchoalveolar lavage fluid (BALF), and bronchial biopsies.[5,6,7,8] However, these observations vary from individual studies and sometimes are even contradictory, possibly because of the limitation of numbers of subjects, different kinds of samples, and noncorrespondence between transcription and translation of certain molecules. In this case, little is known about the systemic evaluation of whether these proinflammatory cytokines and chemokines are all equally important and what is the associated status of other biomarkers in human asthmatic airways.
To address this, we have now measured the concentrations of proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-17 and 11 CXC and CC chemokines in bronchial alveolar lavage fluid from a large cohort of 100 subjects (asthmatics = 70, including 37 mild and 33 moderate/severe asthmatics, and controls = 30). Chemokines whose concentrations were measured in the present study included CXCL8 (IL-8), CXCL9 (monokine induced by gamma interferon), CXCL10 (interferon-gamma-induced protein 10 [IP-10]), CXCL11 (interferon-inducible T-cell alpha chemoattractant), CCL4 (macrophage inflammatory protein 1β [MIP-1β]), CCL11 (eotaxin-1), CCL26 (eotaxin-3), CCL17 (thymus and activation-regulated chemokine [TARC]), CCL22 (macrophage-derived chemokine [MDC]), CCL2 (monocyte chemoattractant protein 1 [MCP-1]), and CCL13 (MCP-4). We hypothesized that the elevated expression of these cytokines and chemokines is associated with a degree which correlates with the accumulation of inflammatory cells, as well as lung function.
METHODS
Ethical approval
The protocol for this study was approved by the Ethics Committee of King's College London Guy's Hospital (LREC Reference Number: 07/H0804/77). Written informed consent was obtained from the parents of all infants before their inclusion in the study.
Subjects
One hundred subjects (70 asthmatics, including 37 patients with mild and 33 patients with moderate and/or severe asthma, and 30 normal controls) were defined according to the Global INitiative for Asthma (GINA) guidelines[9] and recruited in the Department of Asthma, Allergy and Respiratory Science, King's College London School of Medicine, UK. Asthmatics had a clear history of relevant symptoms, documented reversible airway obstruction (20% improvement in forced expiratory volume in 1 s (FEV1) either spontaneously or after administration of inhaled β2 agonist), and/or histamine provocation concentration causing a 20% fall in FEV1 (<8 mg/ml) measured within 2 weeks before BAL. None of them had ever smoked, and there was no history of other respiratory diseases. All subjects were clinically free of respiratory infection and systemic glucocorticoid therapy for at least 1 month before the study. Atopy was defined as a positive skin prick test (wheal at 15 min >3 mm in diameter in the presence of positive histamine and negative diluent controls) to one or more extracts of common local aeroallergens. Of the asthmatics, 63 of 70 were atopic. Of the controls, 4 of 30 were atopic. Normal controls were healthy, lifelong, nonsmoking volunteers who had no history of lung disease.
Fiberoptic bronchoscopy and bronchoalveolar lavage
The fiberoptic bronchoscopy procedure was performed as described previously.[10,11] The volume of fluid returned from BAL was measured. The BALF was passed through sterile gauze to remove any mucus. Supernatants were stored at −80°C until used. Cytospins were prepared from the cell pellets, and differential cell counts were performed with hematoxylin and eosin staining. The absolute numbers and percentages of eosinophils, neutrophils, lymphocytes, and monocyte/macrophages were quantified.
Analysis of proinflammatory cytokines and chemokines
Meso scale discovery (MSD) system (MSD, Rockville, MD, USA)[12] was used to measure the concentrations of proinflammatory cytokines and chemokines for IL-1β, IL-6, IL-17, IL-18, TNF-α, CXCL8, CXCL10, CCL2, CCL4, CCL11, CCL13, CCL17, CCL22, and CCL26 using a commercial kit (K15054G-2). The concentrations of CXCL9 and CXCL11 in BALF were determined using commercial ELISA kits (R&D System, Inc., Minneapolis, MN, USA).[10]
Statistical analysis
Data were analyzed with the aid of a commercially available statistical package (Minitab for Windows Release 9.2; Minitab Inc., Coventry, UK). The Kruskal–Wallis analysis of variance and the Mann–Whitney U-test (with Bonferroni's correction) were used for between- and within-group comparisons, respectively. Correlation coefficients were obtained by Spearman's rank-order method with correction for tied values. For all tests, a P < 0.05 was considered significant.
RESULTS
Clinical data
The median FEV1 (percent predicted) of the asthmatics (median: 69.57%, range: 31.54–132.00) was significantly lower than that of the normal controls (median: 106.4%, 83.0–131.2%) (P < 0.0001). The clinical details are summarized in Table 1.
Table 1.
Clinical information and cell counts
| Asthma | Controls | ||
|---|---|---|---|
| Mild | Moderate/severe | ||
| Gender (female:male) | 22:15 | 6:27 | 11:19 |
| Age (years) | 28 (21–57) | 55 (29–73) | 26 (19–69) |
| FEV1 (percent predicted)* | 100 (81.6–133.0) | 58.7 (31.5–69.76)‡ | 106.4 (80.0–131.2) |
| Total cell number (×106/ml) | 1.8 (0.9–5.8) | 2.3 (2.1–2.3) | 1.6 (0.8–3.1) |
| Neutrophils* (×106/ml) | 0.072 (0.0015–0.16) | 0.0667‡ (0.017–0.135) | 0.0147 (0.00–0.069) |
| Eosinophils* (×106/ml) | 0.0589† (0.0–0.112) | 0.0637† (0.0247–0.165) | 0.00 (0.00–0.20) |
| Lymphocytes (×106/ml) | 0.09 (0.03–1.11) | 0.0923 (0.0305–0.254) | 0.10 (0.0288–0.495) |
| Macrophage (×106/ml) | 1.606 (0.797–5.46) | 2.072 (0.757–3.029) | 1.438 (0.74–2.83) |
Data are expressed as the median (range). *Kruskal–Wallis test P<0.05 between asthma and control; †P<0.0001 versus controls; ‡Mann–Whitney U-test and P<0.0001 (vs. mild and controls). FEV1: Forced expiratory volume in 1 s.
Inflammatory cellular infiltration
Although total cellular numbers in BALF were not significantly different between asthma and controls or between mild and moderate/severe asthmatics, the total numbers of neutrophils and eosinophils were significantly higher in asthma than those in controls (P < 0.05). Furthermore, total numbers of neutrophils in BALF from moderate/severe asthma were higher than those in mild asthma and/or those in control (P < 0.0001) [Table 1]. In addition, both total numbers of neutrophils and eosinophils in BALF from asthmatics correlated inversely with FEV1 (either of total asthmatics or of moderate/severe asthmatics: Total asthmatics – r = −0.56, P = 0.0001 for neutrophils, r = −0.258, P = 0.031 for eosinophils; moderate/severe asthmatics – r = −0.361, P = 0.04 for neutrophils, r = −0.43, P = 0.012 for eosinophils) (data not shown).
Concentrations of proinflammatory cytokines interleukin-1, interleukin-6, tumor necrosis factor-α, and interleukin-17 in bronchoalveolar lavage fluid
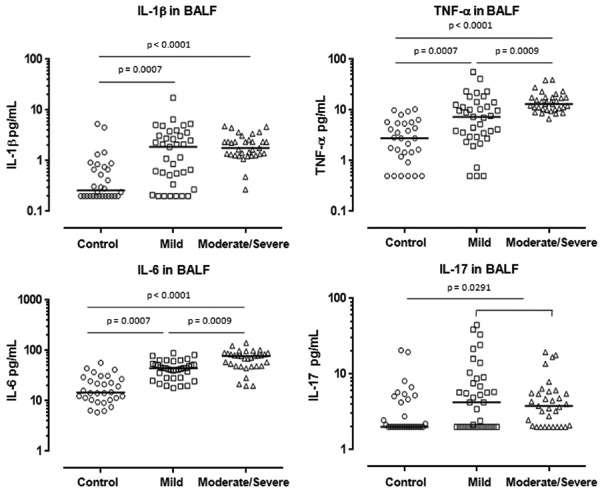
The median concentrations of IL-1 were significantly elevated in BALF from subjects with asthma compared with controls (P < 0.0001). Furthermore, the concentrations of IL-1 were significantly higher in mild and moderate/severe asthma than those of controls (P = 0.0007, P < 0.0001, respectively), although there was no significant difference in the median concentrations of IL-1 between mild and moderate/severe asthmatics [Figure 1]. The median concentrations of TNF-α significantly increased in the BALF of mild and moderate/severe asthmatics compared with those of controls (P = 0.0007, P < 0.0001, respectively) [Figure 1]. Furthermore, the median concentrations of TNF-α were significantly greater in BALF from moderate/severe asthma than those of mild asthma (P = 0.0009) [Figure 1]. Similar patterns were also observed in the concentrations of IL-6 in BALF. The median concentrations of IL-6 were significantly elevated in BALF of mild and moderate/severe asthma compared with those in controls (P = 0.0007, P < 0.0001, respectively) [Figure 1]. Again, the median concentrations of IL-6 were greater in BALF of moderate/severe asthma than those in mild asthma (P = 0.0009) [Figure 1].
Figure 1.
Concentrations of proinflammatory cytokines IL-1, TNF-α, IL-6, and IL-17 in BAL fluid from asthmatics (mild: n = 37, moderate/severe: n = 33) and controls (n = 30). Mann–Whitney U-test. IL-1: Interleukin-1; TNF-α: Tumor necrosis factor-α; BAL: Bronchoalveolar lavage.
In contrast to these proinflammatory cytokines, there were no significant differences in the medians concentrations of IL-17, either between controls and mild or moderate/severe asthma or between asthmatic subgroups [Figure 1], although the median concentrations of IL-17 were significantly greater in BALF of total asthmatics compared with controls (P = 0.0291).
Concentrations of CXC chemokines CXCL8, CXCL9, CXCL10, and CXCL11 in bronchoalveolar lavage fluid
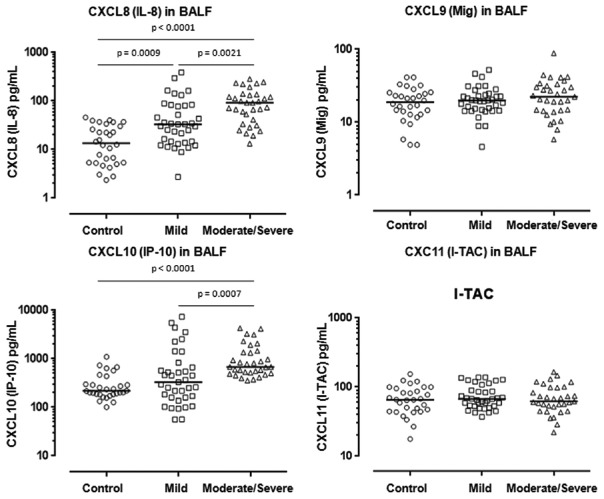
The median concentrations of CXCL8 (IL-8) were significantly higher in BALF of mild and moderate/severe asthma compared with those of controls (P = 0.0009, P < 0.0001, respectively) [Figure 2]. In addition, the median concentrations of CXCL8 were significantly greater in BALF of moderate/severe asthma than those of mild asthma (P = 0.0021) [Figure 1]. The median concentrations of CXCL10 (IP-10) were also significantly elevated in BALF of moderate/severe asthma compared with those of controls (P < 0.0001) and with those of mild asthma (P = 0.0007) [Figure 2]. However, there was no significant difference in the median concentration of CXCL10 in BALF between controls and mild asthma [Figure 1].
Figure 2.
Concentrations of CXC motif chemokines CXCL8 (IL-8), CXCL9 (Mig), CXCL10 (IP-10), and CXCL11 (I-TAC) in BAL fluid from asthmatics (mild: n = 37, moderate/severe: n = 33) and controls (n = 30). Mann–Whitney U-test. IL-8: Interleukin-8; Mig: Monokine induced by gamma interferon; IP-10: Interferon-gamma-induced protein 10; I-TAC: Interferon-inducible T-cell alpha chemoattractant.
In contrast to CXCL8 and CXCL10, there were no significant differences in the median concentrations of CXCL9 and CXCL11, either between asthma and controls or between the subgroups of asthma [Figure 2].
Concentrations of CC chemokines CCL2, CCL4, CCL11, CCL13, CCL17, CCL22, and CCL26 in bronchoalveolar lavage fluid
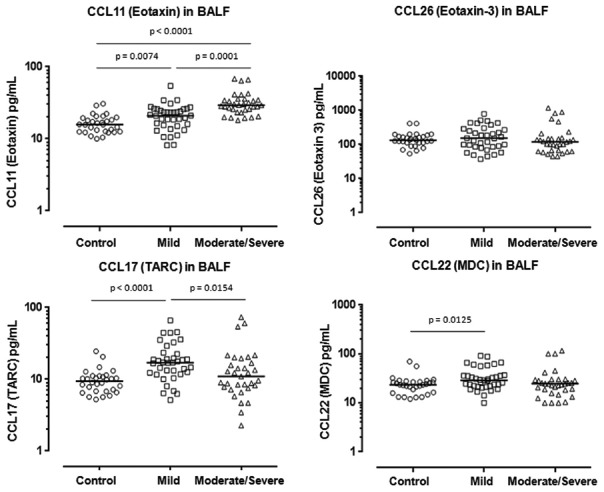
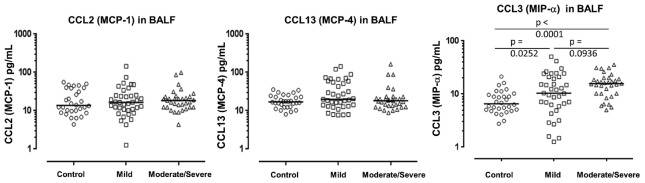
The median concentrations CCL4 (MIP-1β), CCL11 (eotaxin-1), and CCL17 (TARC), but not CCL2 (MCP-1), CCL13 (MCP-4), CCL22 (MDC), and CCL26 (eotaxin-3), were significantly greater in BALF from total asthmatics compared with controls (CCL4: P = 0.0001; CCL11: P = 0.0001; CCL17: P = 0.0008), while the median concentrations of CCL11, CCL17, CCL22, and CCL4 were significantly elevated in the BALF of mild asthma compared with those of controls (CCL11: P = 0.0074; CCL17: P = 0.0001; CCL22: P = 0.0125, CCL4: P = 0.0252, respectively) [Figures 3 and 4]. Furthermore, the median concentrations of CCL11 were significantly higher in BALF of moderate/severe asthma than those of mild asthma (CCL11: P = 0.0001) [Figures 3 and 4].
Figure 3.
Concentrations of CC motif chemokines CCL11 (eotaxin), CCL26 (eotaxin-3), CCL17 (TARC), and CCL22 (MDC) in BAL fluid from asthmatics (mild: n = 37, moderate/severe: n = 33) and controls (n = 30). Mann–Whitney U-test. TARC: Thymus and activation-regulated chemokine; MDC: Macrophage-derived chemokine; BAL: Bronchoalveolar lavage.
Figure 4.
Concentrations of CC motif chemokines CCL2 (MCP-1), CCL13 (MCP-4), and CCL4 (MIP-1) in BAL fluid from asthmatics (mild: n = 37, moderate/severe: n = 33) and controls (n = 30). Mann–Whitney U-test. MCP-1: Monocyte chemoattractant protein 1; MIP-1: Macrophage inflammatory protein 1; BAL: Bronchoalveolar lavage.
In contrast, there were no any significant differences in concentrations of CCL26, CCL2, and CCL13, either between total asthmatics and controls or between subgroups of asthma [Figures 3 and 4].
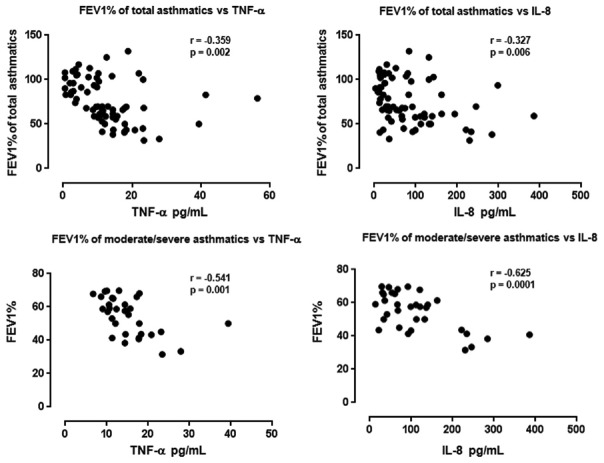
Correlations of tumor necrosis factor-α and CXCL8 with lung function (forced expiratory volume in 1 s) and infiltrating inflammatory cells in the airways of asthma
Concentrations of TNF-α and CXCL8, but not other proinflammatory cytokines or chemokines, correlated inversely with FEV1 of total asthmatics (TNF-α: r = −0.359, P = 0.002; CXCL8: r = −0.327, P = 0.006) [Figure 5]. Furthermore, concentrations of TNF-α and CXCL8 also correlated inversely with lung function (FEV1) of moderate/severe asthmatics (TNF-α: r = −0.541, P = 0.001; CXCL8: r = −0.625, P = 0.0001) [Figure 5]. Additionally, the concentrations of TNF-α and CXCL8 also positively correlated with the absolute numbers of neutrophils and eosinophils of total asthmatics and of moderate/severe asthmatics (total TNF-α vs. the numbers of neutrophils of total asthmatics: r = 0.243, P = 0.042; total TNF-α vs. the numbers of eosinophils of total asthmatics: r = 0.311, P = 0.009; TNF-α vs. the numbers of neutrophils of moderate/severe asthma: r = 0.379, P = 0.03; TNF-α vs. the numbers of eosinophils of moderate/severe asthma: r = 0.565, P = 0.001; CXCL8 vs. the numbers of neutrophils of total asthmatics: r = 0.398, P = 0.001; CXCL8 vs. the numbers of eosinophils of total asthmatics: r = 0.331, P = 0.05; CXCL8 vs. the numbers of neutrophils of moderate/severe asthma: r = 0.499, P = 0.003; CXCL8 vs. the numbers of eosinophils of moderate/severe asthma: r = 0.565, P = 0.001, respectively) (data not shown). Concentrations of IL-1g (or IL-1F4, IL-18) were undetectable in BALF from all subjects.
Figure 5.
Correlations between FEV1 of total asthmatics and of moderate/severe asthmatics and concentrations of TNF-α and CXCL8 (IL-8). Correlation coefficients were obtained by Spearman's rank-order method with correction for tied values. FEV1: Forced expiratory volume in 1 s; TNF-α: Tumor necrosis factor-α; IL-8: Interleukin-8.
DISCUSSION
Although it has been known that proinflammatory cytokines and chemokines also play an important role in the pathogenesis of asthma,[5,6,7,8] there is still a lack of systemic comparison and evaluation of these molecules in the airways of asthma, possibly because of the investigators’ interesting and limitation of various sources of samples. For example, levels of these molecules in the peripheral blood might not always reflect the status of such targets in local airways,[13] while transcriptional results might not always equate to the status of translation. Here, we used BALF as a window to evaluate the concentrations of these proinflammatory cytokines and chemokines in a relatively large cohort of subjects with asthma and controls. We also tried to clarify the relationships of these molecules with the degree of disease severity and with infiltration of inflammatory cells in airways. Our data showed that the concentrations of proinflammatory cytokine TNF-α and CXC motif CXCL8, but not others, were not only significantly elevated in BALF of asthmatics compared with controls, but also are inversely associated with lung function and positively correlated with local infiltration of neutrophils and eosinophils, suggesting the importance of these two molecules in the pathogenesis of asthma.
We had previously shown that TNF-α mRNA-positive cells were significantly elevated in the BALF of asthmatics compared with controls,[14] which was a first report regarding TNF-α in asthma. Since that observation, numerous studies have shown that the concentrations of TNF-α increase in BALF from subjects with asthma, including severe asthma.[15,16,17] Our data here also showed that the concentrations of TNF-α were greatly elevated in the BALF of asthmatics, accompanying with the association of lung function and airway inflammation, further confirming the findings of ours and others. Although the details of the roles of TNF-α in the pathogenesis of asthma are still incompletely explored, it has been shown that this inflammatory cytokine might contribute to local recruitment of neutrophils and eosinophils, promoting the activation of T-cells, inducing chronic airway remodeling and mediating steroid resistance.[18,19] Taken together, we conclude from this evidence that TNF-α can be used as a therapeutic target in severe or steroid-dependent asthma.[19,20]
Similar to TNF-α, our data also showed that the concentrations of CXCL8, another name of IL-8, increased in the BALF of asthmatics compared to controls and corresponded to the lung function and infiltration of neutrophils and eosinophils. It is worth to note that neutrophilia and increased concentration of IL-8 have been considered as biomarkers in elderly and severe asthma.[13] Although IL-8 is a relatively nonspecific chemokine for eosinophils, it has been shown that in the absence of eosinophil chemoattractants, neutrophils stimulated by IL-8 augment eosinophil trans-basement membrane migration by releasing superoxide anion, matrix metalloproteinase, leukotriene B4, and platelet-activating factor.[21] On the other hand, repeated recruitment of activated neutrophils might also contribute to allergic sensitization and airways inflammation.[22] These observations suggest that IL-8-stimulated neutrophils could lead eosinophils to accumulate in airways of asthmatics. We have currently showed that the concentration of IL-8/CXCL8 in the BALF might be useful to distinguish uncontrolled asthma from total asthmatics.[23] It has recently shown that the oral administration of SCH 527123, a dual CXCR1/CXCR2 antagonist, reduces neutrophil levels in the circulation and airways through inhibition of migration.[24] This may provide an alternative therapeutic for asthma, particularly for neutrophilic asthma.
Our data showed that another two proinflammatory cytokines IL-1 and IL-6 also significantly increased in the BALF of asthmatics compared with those of controls. Some studies implicate that the cytokines TNF-α and IL-8 together might be involved in Th17-cell polarization or induce IL-17 production.[25] It has been shown that IL-17 might be relevant to severe asthma and steroid resistance of asthma; however, the concentration of IL-17 in BALF appeared not to correspond to lung function or infiltration of neutrophils in the present study, although it was slightly higher in total asthmatics compared to the controls. Thus, importance of IL-1 and IL-6 in the pathogenesis of asthma remains to be explored,[26] at least when BALF is used as a window.
It has also been shown that CXC and CC chemokines may be involved in the pathogenesis of many diseases, including asthma, possibly through participating in the development and maintenance of innate and adaptive immunity, as well as in wound healing and angiogenesis.[27,28] We and others have previously reported that the expression of some CXC and CC cheomokines and their receptors are elevated in airways of asthma in a limited numbers of subjects.[10,29,30,31] Thus, in the present study, we also analyzed a number of CXC and CC motif chemokines in the BALF of asthmatics and controls. Our data showed that the median concentrations of CXCL10 (IP-10), CCL11 (eotaxin), CCL17 (TARC), and CCL4 (MIP-1) were significantly elevated in the BALF of asthmatics compared with controls, with increases accompanying disease severity. However, such increased concentrations of these chemokines did not associate with lung function or with airway inflammation, suggesting that the increased expression of chemokines might not directly contribute to either impaired lung function or infiltration of inflammatory cells in the airways. In addition, the increased expression of these chemokines did not associate with the concentrations of any proinflammatory cytokines measured in the study, although these molecules might induce the expression of chemokines by airway structural cells or inflammatory cells in vitro or in vivo in animal models.[27,28]
In summary, correlations of proinflammatory cytokines TNF-α and CXCL8 (IL-8) and lung functions and infiltration of inflammatory cells including neutrophils and eosinophils further support that these molecules might be potential biomarkers and that targeting these molecules or their receptors might benefit asthmatic treatment, such as precision therapy.
Financial support and sponsorship
The study was supported from the National Natural Science Foundation of China (81373177, 81471594, L1422025), the Scientific Research Common Program of the Beijing Municipal Commission of Education (KM201410025006), the Ministry of Science and Technology (2016YFC0206502), National Key R&D Program of China (2016YFC0206502, 2016YFC1303900), and the Key Projects in the National Science and Technology Pillar Program during the Twelfth 5-year Plan Period (2012BAI05B02).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Drs Matt Edwards and Betty Shamji at Respiratory Disease Area, Novartis Institute of Biomedical Research, Horsham, UK, for their technical support. The authors also acknowledge the financial support from the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to King's College London and the Guy's and St. Thomas’ and King's College Hospitals NHS Foundation Trusts.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–83. doi: 10.1038/nm.2731. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 2.Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 3.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor ß, and TNF-a: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010. doi: 10.1016/j.jaci.2016.06.033. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Page C, O’Shaughnessy B, Barnes P. Pathogenesis of COPD and asthma. Handb Exp Pharmacol. 2017;237:1–21. doi: 10.1007/164_2016_61. doi: 10.1007/164_2016_61. [DOI] [PubMed] [Google Scholar]
- 5.Koczulla AR, Vogelmeier CF, Garn H, Renz H. New concepts in asthma: Clinical phenotypes and pathophysiological mechanisms. Drug Discov Today. 2017;22:388–96. doi: 10.1016/j.drudis.2016.11.008. doi: 10.1016/j.drudis.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Tomankova T, Kriegova E, Liu M. Chemokine receptors and their therapeutic opportunities in diseased lung: Far beyond leukocyte trafficking. Am J Physiol Lung Cell Mol Physiol. 2015;308:L603–18. doi: 10.1152/ajplung.00203.2014. doi: 10.1152/ajplung.00203.2014. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi VD, Vliagoftis H. Airway epithelium interactions with aeroallergens: Role of secreted cytokines and chemokines in innate immunity. Front Immunol. 2015;6:147. doi: 10.3389/fimmu.2015.00147. doi: 10.3389/fimmu.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik SR, Wala SM. Inflammation, allergy and asthma, complex immune origin diseases: Mechanisms and therapeutic agents. Recent Pat Inflamm Allergy Drug Discov. 2013;7:62–95. [PubMed] [Google Scholar]
- 9.Global Initiative for Asthma. 2014. Available from: http://www.ginasthma.com .
- 10.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–8. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 11.Ying S, Khan LN, Meng Q, Barnes NC, Kay AB. Cyclosporin A, apoptosis of BAL T-cells and expression of Bcl-2 in asthmatics. Eur Respir J. 2003;22:207–12. doi: 10.1183/09031936.03.00098902. [DOI] [PubMed] [Google Scholar]
- 12.Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: Comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J Immunol Methods. 2011;372:71–7. doi: 10.1016/j.jim.2011.06.033. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergeron C, Tulic MK, Hamid Q. Tools used to measure airway remodelling in research. Eur Respir J. 2007;29:596–604. doi: 10.1183/09031936.00019906. doi: 10.1183/09031936.00019906. [DOI] [PubMed] [Google Scholar]
- 14.Ying S, Robinson DS, Varney V, Meng Q, Tsicopoulos A, Moqbel R, et al. TNF alpha mRNA expression in allergic inflammation. Clin Exp Allergy. 1991;21:745–50. doi: 10.1111/j.1365-2222.1991.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 15.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60:1012–8. doi: 10.1136/thx.2005.045260. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 17.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–8. doi: 10.1136/thx.2006.061358. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durrani SR, Viswanathan RK, Busse WW. What effect does asthma treatment have on airway remodeling? Current perspectives. J Allergy Clin Immunol. 2011;128:439–48. doi: 10.1016/j.jaci.2011.06.002. doi: 10.1016/j.jaci.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Holgate ST, Noonan M, Chanez P, Busse W, Dupont L, Pavord I, et al. Efficacy and safety of etanercept in moderate-to-severe asthma: A randomised, controlled trial. Eur Respir J. 2011;37:1352–9. doi: 10.1183/09031936.00063510. doi: 10.1183/09031936.00063510. [DOI] [PubMed] [Google Scholar]
- 20.Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16:70. doi: 10.1007/s11882-016-0650-5. doi: 10.1007/s11882-016-0650-5. [DOI] [PubMed] [Google Scholar]
- 21.Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol. 2012;158(Suppl 1):96–102. doi: 10.1159/000337801. doi: 10.1159/000337801. [DOI] [PubMed] [Google Scholar]
- 22.Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. 2016;16:45–50. doi: 10.1097/ACI.0000000000000231. doi: 10.1097/ACI.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoki K, Ying S, Corrigan C, Qi H, Kurosky A, Jennings K, et al. Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One. 2015;10:e0126035. doi: 10.1371/journal.pone.0126035. doi: 10.1371/journal.pone.0126035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todd CM, Salter BM, Murphy DM, Watson RM, Howie KJ, Milot J, et al. The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulm Pharmacol Ther. 2016;41:34–9. doi: 10.1016/j.pupt.2016.09.005. doi: 10.1016/j.pupt.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094–101. doi: 10.1164/rccm.201405-0859PP. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 26.Chi CH, Liao JP, Zhao YN, Li XY, Wang GF. Effect of inhaled budesonide on interleukin-4 and interleukin-6 in exhaled breath condensate of asthmatic patients. Chin Med J (Engl) 2016;129:819–23. doi: 10.4103/0366-6999.178962. doi: 10.4103/0366-6999.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317:575–89. doi: 10.1016/j.yexcr.2011.01.005. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall S, Agrawal DK. Key mediators in the immunopathogenesis of allergic asthma. Int Immunopharmacol. 2014;23:316–29. doi: 10.1016/j.intimp.2014.05.034. doi: 10.1016/j.intimp.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 30.Pillai P, Corrigan CJ, Ying S. Airway epithelium in atopic and nonatopic asthma: Similarities and differences. ISRN Allergy 2011. 2011:195846. doi: 10.5402/2011/195846. doi: 10.5402/2011/195846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, Zhou J, Bi H, Li L, Gao W, Huang M, et al. CCL11 as a potential diagnostic marker for asthma? J Asthma. 2014;51:847–54. doi: 10.3109/02770903.2014.917659. doi: 10.3109/02770903.2014.917659. [DOI] [PubMed] [Google Scholar]