Abstract
Background:
It remains controversial whether patients with Stage II colorectal cancer would benefit from adjuvant chemotherapy after radical resection. The aim of this study was to establish two mathematical models to identify the suitable patients for adjuvant chemotherapy.
Methods:
The current study comprised of two steps. In the first step, 353 patients with Stage II colorectal cancer who underwent surgical procedures at the Third Affiliated Hospital of Sun Yat-sen University between June 2006 and December 2015 were entered and followed up for 6–120 months. Their clinical data were collected and enrolled into the database. We established two mathematical models by univariate and multivariate Cox regression analysis to identify the target patients; in the second step, 230 patients under the same standard between January 2012 and December 2016 were entered and followed up for 3–62 months to verify the two models’ validation.
Results:
In the first step, totally 340 surgical patients with Stage II colorectal cancer were finally enrolled in this study. Statistical analysis showed that tumor differentiation (TD) (P < 0.001), lymphovascular invasion (LVI) (P < 0.001), uncertain or positive margins (UPM) (P < 0.001), and fewer lymph nodes (LNs) (<12) retrieved (P < 0.001) were correlated with the overall survival (OS) and disease free survival (DFS). We obtained two models: (1) OS risk score = 1.116 × TD + 2.202 × LVI + 3.676 × UPM + 1.438 × LN − 0.493; (2) DFS risk score = 0.789 × TD + 2.074 × LVI + 3.183 × UPM + 1.329 × LN − 0.432. According to the models and cutoff points [(0.07, 1.33) and (−0.04, 1.30), respectively], patients can be divided into three groups: low-risk, moderate-risk, and high-risk. Moreover, the high-risk group patients could benefit from adjuvant chemotherapy. In the second step, totally 221 patients were finally used to verify the models’ validation. The results proved that the models were accurate and feasible (P < 0.05).
Conclusions:
According to the predictive models, patients with Stage II colorectal cancer in the high-risk group are strongly recommended for adjuvant chemotherapy, thus facilitating the individualized and precise treatment.
Keywords: Adjuvant Chemotherapy, Risk Factors, Mathematical Models, Colorectal Cancer
INTRODUCTION
Colorectal cancer is the third leading cause of cancer deaths worldwide and in the USA.[1,2,3,4] In China, colorectal cancer is the fourth most common cancer and the fifth cause of cancer death.[5] An estimated 331,300 new cases of colorectal cancer and an estimated 159,300 deaths were reported in 2012.[5]
Chemotherapy is the most important adjuvant treatment for colorectal cancer after surgery. A majority of Stage III/IV patients can benefit from adjuvant chemotherapy.[6,7,8] However, it is controversial when it comes to patients with Stage II colorectal cancer due to the difficulty in distinguishing the target high-risk patients.[8,9] According to the National Comprehensive Cancer Network (NCCN) Guidelines, such clinicopathologic characteristics are considered high-risk Stage II patients for adjuvant treatment: fewer lymph nodes (LNs) retrieved (<12) during surgery,[10,11] T4 staging, poorly differentiated tumor, lymphovascular or perineural invasion,[9,12] obstructing or perforating cancers[8,13,14] and positive margins. However, the current clinical trials and guidelines lack quantification of each factor's impact on recurrence or poor prognosis, and there were no detailed recommendations for adjuvant chemotherapy.[15] The chemotherapy criterion remains undefined. In the current retrospective study, we analyzed the correlation between high-risk factors and prognosis of high-risk Stage II colorectal cancer patients, aimed to quantify each factor's impact, and established two mathematical models to identify the suitable patients for adjuvant chemotherapy.
METHODS
Ethical approval
As a retrospective study and data analysis were performed anonymously, this study was exempt from the ethical approval. All patients involved in the study were informed in the process of the follow-up and consented to participate in the study. All patient records and information were anonymized and de-identified before analysis.
Patients enrollment
In the first step, patients with Stage II colorectal cancer undergoing surgical procedures at the Department of Gastrointestinal Surgery, the Third Affiliated Hospital of Sun Yat-sen University between June 2006 and December 2015, were entered into the current retrospective study. Clinical data were collected and enrolled into the database. Overall 353 patients met the criteria and all of them underwent D2 or D3 lymphadenectomy with negative resection margins (R0). In the second step, patients under the same standard between January 2012 and December 2016 were entered for models verification. Overall 230 patients met the criteria.
The inclusion criteria were as follows: (1) pathological diagnosis of colorectal adenocarcinoma; (2) postoperative clinical pathological Stage II cancer; (3) surgical evaluation for R0 resection; (4) a complete medical record, and (5) D2 or D3 lymphadenectomy. The exclusion criteria were: (1) severe basic disease or American Society of Anesthesiology scoring above Grade III; (2) severe postoperative complications or perioperative mortality (<30 days postoperatively); (3) previous or accompanying other cancer; and (4) loss to follow-up.
Clinical, pathological stage and risk factor assessment
The clinical, pathological stage was evaluated by the American Joint Committee on Cancer TNM classification (the 7th edition) of colorectal cancer. According to the NCCN Guidelines (version 2, 2016) for colon and rectal cancer,[16,17] Stage II disease was defined as tumor invading through the muscularis propria into the pericolorectal tissues (T3), directly penetrating to the surface of the visceral peritoneum (T4a), or directly invading or adherent to other organs or structures (T4b), and without LN or distant organ metastases (N0 and M0).
Patients with high-risk Stage II disease were defined as those with poor prognostic features, including: (1) T4 tumors (IIB/IIC), (2) poorly tumor differentiation (TD), (3) lymphovascular invasion (LVI), (4) peripheral nerve invasion, (5) bowel obstruction or lesions with localized perforation, (6) uncertain or positive margins (UPM), and (7) inadequately sampled LNs (<12). The status of each risk factor was evaluated and recorded in a database. Bowel obstruction or perforation were determined by the clinical manifestation and imaging data. Considering similar hazard ratio of recurrence in these two kinds of patients[18] and the few cases of perforation, we combined patients with perforation and obstruction. Pathological evaluation was performed by two pathologists independently, and the final diagnosis was determined by discussion if the two pathologists disagreed with the primary result. The LNs were retrieved by the surgeon as soon as possible after the operation. According to NCCN Guidelines’ recommendation, a minimum of 12 LNs were required to get an accurate N stage.[14,19,20] Hence, it was considered inadequately sampled nodes if <12 LNs were harvested. Moreover, the M staging was evaluated by imaging data, including computed tomography (CT) scan of the abdomen, X-ray of chest and positron emission tomography/computed tomography (PET/CT) if necessary.
Other clinical characteristics were also obtained including age, gender, the extent of tumor resection, site of the tumor and the use of chemotherapy.
Follow-up
In the first step, all the patients were followed up for 6–120 months. Moreover, the median follow-up periods were 37 months. In the second step, all the patients were followed up for 3–62 months with a median follow-up period of 30 months. The examinations included CT scans and blood tests every 3 months during the first 2 years, and every 6 months from 3rd to 5th year after surgery, and every 12 months after 5th year; fiber colonoscopy every year, bone scan, and other diagnostic tests if necessary during the follow-up period. Tumor recurrence was defined as in situ recurrence or metastases of liver, peritoneum, bone, lung and brain, and no other types of the tumor were found. All patients with recurrence were diagnosed clinically or radiographically. Moreover, the recurrence type, recurrence date, date of death, causes of death, and other factors were collected and recorded into the database. Survival time was calculated from the date of surgery to the date of death or censoring.
Statistical analysis
Cox regression model was used to obtain the model formula. Univariate analysis and Cox's proportional hazard model in the multivariate analysis were estimated. The rates of overall survival (OS) and disease-free survival (DFS) were obtained using Kaplan-Meier estimation [Figures 1 and 2]. The log-rank test was applied to select the optimum cutoff score according to the maximum Chi-squared value. The log-rank test was also used to verify the models and cutoff score. A value of P < 0.05 was considered statistically significant. All statistical calculations were performed with R software, version 3.2.4 (Microsoft Company, Redmond city, Washington state, USA).
Figure 1.
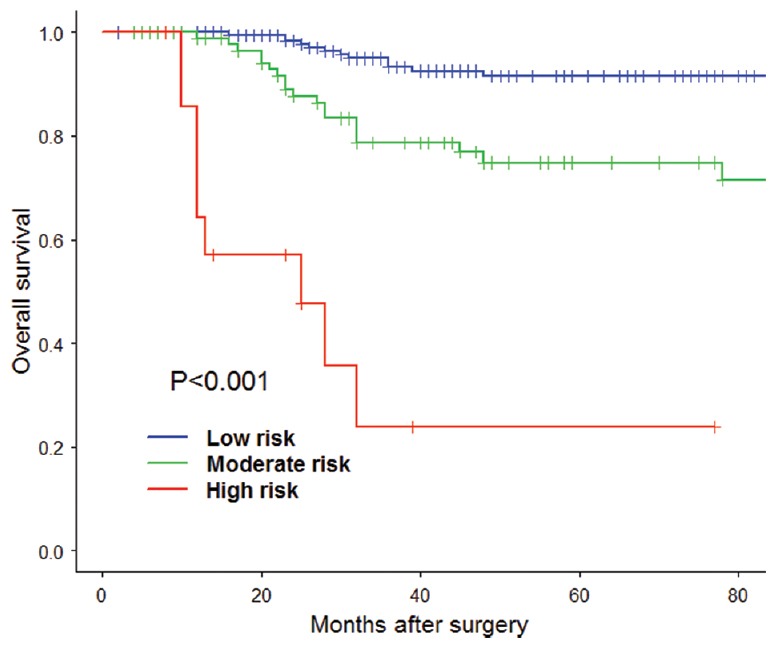
The overall survival risk score model Kaplan-Meier estimation of each group according to the two cutoff points.
Figure 2.
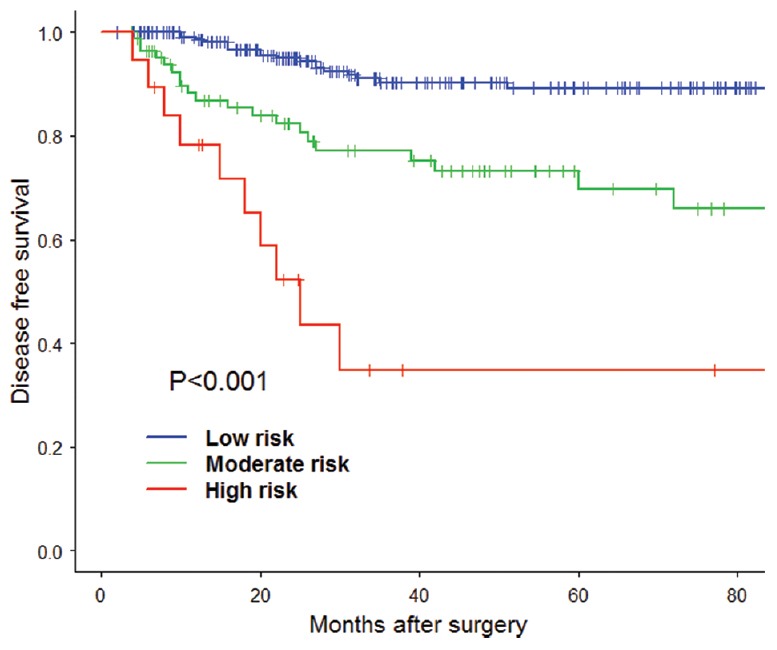
The disease free survival risk score model Kaplan-Meier estimation of each group according to the two cutoff points.
RESULTS
Clinicopathologic features and the univariate analysis of prognostic factors
In step one, totally 340 patients with Stage II colorectal cancer were enrolled in the study. The clinicopathologic features and the univariate analysis of high-risk factors are listed in Table 1. According to the results, the following factors were correlated with OS: TD (P < 0.001), LVI (P < 0.001), UPM (P < 0.001) and fewer than 12 LNs retrieved (P < 0.001). However, gender (P = 0.471), age (P = 0.699), tumor position (P = 0.555), T staging (P = 0.308), perineural invasion (P = 0.892) and obstructing or perforating cancers (P = 0.810) were identified as nonsignificantly correlated factors with OS. Since the chemotherapeutic indication was not strictly controlled in the early period, chemotherapy had no correlation with the prognosis in this group of data (P = 0.250). Besides, the following factors were correlated with DFS: TD (P < 0.01), LVI (P < 0.001), UPM (P < 0.001) and fewer than 12 LNs retrieved (P < 0.001). Similarly, gender (P = 0.371), age (P = 0.534), tumor position (P = 0.885), T staging (P = 0.591), perineural invasion (P = 0.376), obstructing, or perforating cancers (P = 0.579) and chemotherapy (P = 0.749) were identified as nonsignificantly correlated factors with DFS.
Table 1.
Univariate analysis of clinicopathologic factors for patients with Stage II colorectal cancer (n=340)
| Parameters | n | χ2 | P |
|---|---|---|---|
| Gender | |||
| Male | 183 | 0.520 | 0.471 |
| Female | 157 | ||
| Age | |||
| ≥65 years | 211 | 0.150 | 0.699 |
| <65 years | 129 | ||
| Tumor position | |||
| Right hemicolon | 111 | 1.179 | 0.555 |
| Left hemicolon | 115 | ||
| Rectum | 114 | ||
| T staging | |||
| T3 | 253 | 1.038 | 0.308 |
| T4 | 57 | ||
| TD | |||
| Poorly-differentiated | 31 | 12.675 | <0.001 |
| Well or maderat-differentiated | 309 | ||
| LVI | |||
| Present | 9 | 12.041 | <0.001 |
| Absent | 331 | ||
| Perineural invasion | |||
| Present | 9 | 0.018 | 0.892 |
| Absent | 331 | ||
| Obstructing or perforating cancers | |||
| Present | 22 | 0.058 | 0.810 |
| Absent | 218 | ||
| UPM | |||
| Present | 3 | 40.096 | <0.001 |
| Absent | 337 | ||
| LNs retrieved | |||
| <12 LNs | 71 | 22.134 | <0.001 |
| ≥12 LNs | 269 | ||
| Chemotherapy | |||
| Yes | 159 | 1.321 | 0.250 |
| No | 181 |
LNs: Lymph nodes; UPM: Uncertain or positive margins; TD: Tumor differentiation; LVI: Lymphovascular invasion.
Multivariate Cox regression analysis of prognostic factors for patients with Stage II colorectal cancer
To evaluate the impact of each high-risk factor, the multivariate Cox regression analysis was performed. Final results showed each of the four correlated prognostic factors had regression coefficients in OS risk score model as shown in Table 2: TD (1.116), LVI (2.202), UPM (3.676) and <12 LNs retrieved (1.438). And each of the four correlated prognostic factors had regression coefficients in DFS risk score model as shown in Table 3: TD (0.789), LVI (2.074), UPM (3.183), and <12 LNs retrieved (1.329). Moreover, the regression coefficients represent the weight of each factor.
Table 2.
Multivariate Cox regression analysis of the prognostic factors of OS in patients with Stage II colorectal cancer
| Parameters | Regression coefficient | SE | HR | Z | P | 95% CI |
|---|---|---|---|---|---|---|
| TD | 1.116 | 0.375 | 3.052 | 2.980 | 0.003 | 1.464–6.366 |
| LVI | 2.202 | 0.637 | 9.041 | 3.460 | 0.001 | 2.595–31.517 |
| UPM | 3.676 | 0.790 | 39.486 | 4.660 | <0.0001 | 8.395–185.750 |
| LNs retrieved | 1.438 | 0.326 | 4.214 | 4.410 | <0.0001 | 2.223–7.980 |
SE: Standard error; HR: Hazard ratio; CI: Confidence interval; LNs: Lymph nodes; UPM: Uncertain or positive margins; TD: Tumor differentiation; LVI: Lymphovascular invasion; OS: Overall survival.
Table 3.
Multivariate Cox regression analysis of the predictive factors of DFS in patients with Stage II colorectal cancer
| Parameters | Regression coefficient | SE | HR | Z | P | 95% CI |
|---|---|---|---|---|---|---|
| TD | 0.789 | 0.370 | 2.200 | 2.133 | 0.033 | 1.066–4.542 |
| LVI | 2.074 | 0.557 | 7.953 | 3.722 | 0.000 | 2.668–23.706 |
| UPM | 3.183 | 0.754 | 24.129 | 4.220 | <0.0001 | 5.501–105.829 |
| LNs retrieved | 1.329 | 0.296 | 3.777 | 4.493 | <0.0001 | 2.115–6.743 |
SE: Standard error; HR: Hazard ratio; CI: Confidence interval; LNs: Lymph nodes; TD: Tumor differentiation; LVI: Lymphovascular invasion; UPM: Uncertain or positive margins; DFS: Disease free survival.
Establishment of the predictive mathematical models
To evaluate the necessity of adjuvant chemotherapy, predictive mathematical models were established to systematically evaluate the influence of each factor on the patients’ OS and DFS [Tables 2 and 3]. According to the univariate and multivariate Cox regression analysis of prognostic factors, we found that TD, LVI, UPM and fewer LNs retrieved (<12) were related influential factors of the poor prognosis with respective weights.
The weight of the 4 variables in the OS model were TD (1.116), LVI (2.202), UPM (3.676), and <12 LNs retrieved (1.428), respectively, and that in the DFS model were TD (0.789), LVI (2.074), UPM (3.183) and <12 LNs retrieved (1.329), respectively. Assignments of the variables were TD (poorly-differentiated = 1, well or moderately differentiated = 0), LVI (present = 1, absent = 0), UPM (present = 1, absent = 0), and LNs retrieved (<12 LNs = 1, ≥12 LNs = 0).
The score was a summation of multiplicative products of assignments and regression coefficients of each correlated risk factor. According to the models, we calculated the risk score for each patient according to the individual status of prognostic factors. Lastly, we conducted every score for these patients through log-rank test to give the optimal cutoff points for the risk score. The scores with the maximum Chi-square values (or minimum P values) were the optimal cutoff points. The results showed two cutoff points (0.07 and 1.33) in OS risk score model, and the corresponding Chi-square were 30.9 and 38.8. Similarly, we got two cutoff points (−0.04 and 1.30) in DFS risk score model and the corresponding Chi-square were 33.6 and 38.6.
Thus, the OS risk score was divided into a low-risk group with a score below 0.07, a moderate-risk group with a score from 0.07 to 1.33, and a high-risk group with a score above 1.33 [Table 4]. Moreover, the Kaplan-Meier survival curve for low-, moderate-, and high-risk groups was demonstrated in Figure 1 with significant difference (P < 0.001). The value of the risk score was to indicate the high risk of poor prognosis and give the recommendation for adjuvant chemotherapy. And the groups divided by the DFS risk score was similar to the above shown in Table 5 and Figure 2. According to the results, the suggestions were as follow: no recommendation of adjuvant chemotherapy for low-risk group, the conditional recommendation of adjuvant chemotherapy for the moderate-risk group, and strong recommendation of adjuvant chemotherapy for the high-risk group.
Table 4.
The OS predictive model of patients with Stage II colorectal cancer
| Predictive model | Regression coefficient |
|---|---|
| Equation | Score = 1.116 × TD + 2.202 × LVI + 3.676 × UPM + 1.438 × LN − 0.493 |
| Variable | TD (poorly-differentiated = 1, well or moderate differentiated = 0) |
| LVI (present = 1, absent = 0) | |
| UPM (present = 1, absent = 0) | |
| LNs retrieved (<12 LNs = 1, ≥12 LNs = 0) | |
| Risk category | Low risk: Below 0.07 (no recommendation of adjuvant chemotherapy) |
| Moderate risk: Between 0.07 and 1.33 (conditional recommendation of adjuvant chemotherapy) | |
| High risk: Above 1.33 (strong recommendation of adjuvant chemotherapy) |
TD: Tumor differentiation; LVI: Lymphovascular invasion; UPM: Uncertain or positive margins; LNs: Lymph nodes; OS: Overall survival.
Table 5.
The DFS predictive model of patients with Stage II colorectal cancer
| Predictive model | Regression coefficient |
|---|---|
| Equation | Score = 0.789 × TD + 2.074 × LVI + 3.183 × UPM + 1.329 × LN − 0.432 |
| Variable | TD (poorly-differentiated = 1, well or moderate differentiated = 0) |
| LVI (present = 1, absent = 0) | |
| UPM (present = 1, absent = 0) | |
| LNs retrieved (<12 LNs = 1, ≥12 LNs = 0) | |
| Risk category | Low risk: Below −0.04 (no recommendation of adjuvant chemotherapy) |
| Moderate risk: Between −0.04 and 1.30 (conditional recommendation of adjuvant chemotherapy) | |
| High risk: Above 1.30 (strong recommendation of adjuvant chemotherapy) |
TD: Tumor differentiation; LVI: Lymphovascular invasion; UPM: Uncertain or positive margins; LNs: Lymph nodes; DFS: Disease free survival.
Validation of the predictive mathematical models
In step two, 221 patients were enrolled in the study. We calculated all the patients’ risk score based on the model in step one and divided them into three groups, including low-risk group, moderate-risk group, and high-risk group [Tables 4 and 5]. And we carried out Kaplan–Meier estimation and log-rank test to verify the accuracy of the models. The result of validation showed the OS of patients in the low-risk group, moderate-risk group and the high-risk group had significant difference [Figure 3 and Table 6]. The same result was found in disease free survival of patients in three groups [Figure 4 and Table 7]. The above results showed the model was accurate and feasible.
Figure 3.
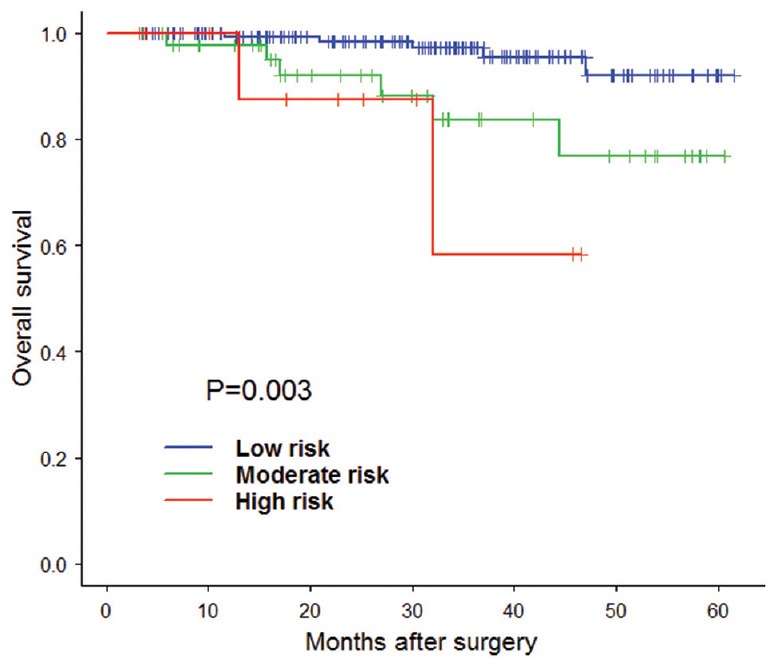
The validation of overall survival risk score model Kaplan-Meier estimation.
Table 6.
Validation the OS risk score model of patients with Stage II colorectal cancer
| Groups | n | Deaths | χ2 | υ | P |
|---|---|---|---|---|---|
| Low risk | 163 | 5 | 11.7 | 2 | <0.05 |
| Moderate risk | 47 | 6 | |||
| High risk | 11 | 2 | |||
| Total | 221 | 13 | |||
OS: Overall survival.
Figure 4.
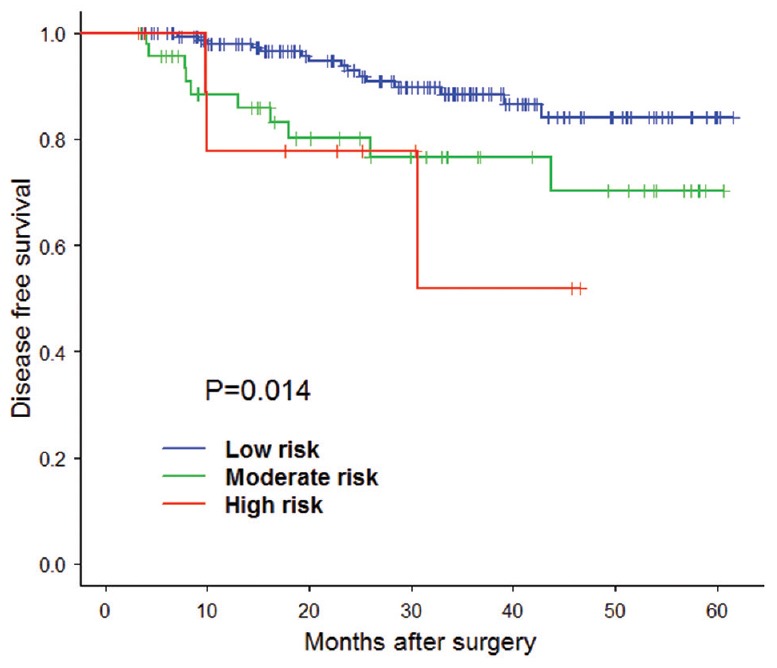
The validation of disease free survival risk score model Kaplan-Meier estimation.
Table 7.
Validation the DFS risk score model of Stage II colorectal cancer patients
| Groups | n | Recurrence | χ2 | υ | P |
|---|---|---|---|---|---|
| Low risk | 163 | 15 | 8.6 | 2 | <0.05 |
| Moderate risk | 47 | 10 | |||
| High risk | 11 | 3 | |||
| Total | 221 | 28 | |||
DFS: Disease free survival.
Correlation analysis on types of recurrence and risk factors
Recurrence or metastasis occurred in 46 of 340 patients in the first cohort of patients, and the median recurrent time was 16 months (4–72 months). The types of recurrence included local recurrence in 5 cases, peritoneal metastasis in 9 cases, organ metastases in 25 cases (liver metastasis in 19 cases, lung metastasis in 5 cases, and bone metastasis in 1 case), and LN metastasis in 7 cases. To investigate the correlation between the types of recurrence or metastasis and high-risk factors, the canonical correlation analysis and contingency table correlation analysis were performed respectively.
Canonical correlation analysis showed there were correlation between UPM, <12 LNs retrieved and local recurrence (canonical correlation coefficient 0.391, P < 0.0001); LVI or <12 LNs retrieved and organ or LN metastasis (canonical correlation coefficient 0.654, P < 0.0001); T4 staging or obstructing/perforating cancers and peritoneal metastasis (canonical correlation coefficient 0.793, P < 0.0099) [Table 8].
Table 8.
Canonical correlation analysis on types of recurrence or metastasis and risk factors
| High-risk factors | Types of recurrence or metastasis | Canonical correlation coefficient | P |
|---|---|---|---|
| UPM and <12 LNs retrieved | Local recurrence | 0.391 | <0.0001 |
| LVI and <12 LNs retrieved | Organ or LN metastasis | 0.654 | <0.0001 |
| T4 staging and obstructing or perforating cancers | Peritoneal metastasis | 0.793 | 0.0099 |
| LVI | Organ or LN metastasis | 0.334 | 0.0668 |
LNs: Lymph nodes; LVI: Lymphovascular invasion; UPM: Uncertain or positive margins.
DISCUSSION
Colorectal cancer is the third leading cause of cancer deaths worldwide. Almost a quarter of colorectal cancer cases are classified as Stage II once diagnosed. Operation remains the mainstay of treatment, with a postoperative 5-year survival rate of 80%.[1] For Stage I patients, surgical resection is adequate, and Stage III/IV patients can benefit from chemotherapy. However, adjuvant chemotherapy in Stage II disease still remains controversial. Some studies showed no benefit with adjuvant therapy in Stage II disease, including a meta-analysis from the IMPACT B2 investigators,[21] a pooled analysis of seven trials with 3302 patients,[8] and an analysis of the SEER database of 3151 Stage II patients,[4] etc., However, other studies gave the positive results for adjuvant therapy, such as the Quick and Simple and Reliable study, which showed 4% absolute benefit despite a 20% proportional reduction in the risk of recurrence and death.[22] The major concerns are: (1) surgery alone gives excellent outcomes; (2) adjuvant chemotherapy provides only a small benefit; and (3) furthermore chemotherapy has its toxicities, inconvenience, cost and psychological distress. The justification for adjuvant chemotherapy creates one of the most challenging decisions in the management of patients with Stage II colorectal cancer.
According to the NCCN Guidelines, clinicopathologic characteristics, including fewer than 12 LNs retrieved, T4 staging, poorly differentiated tumor, lymphovascular or perineural invasion, obstructing or perforating cancers and positive margins, are considered in selecting high-risk Stage II patients for adjuvant chemotherapy. For the patient selected as high-risk Stage II disease, the recommendation of adjuvant chemotherapy may be given by clinicians. However, the previous clinical trials have not quantified each high-risk factor's impact on the recurrence or poor prognosis risk. Hence, we presume adjuvant chemotherapy should not be given to high-risk Stage II patients without discrimination. Moreover, quantization of the high-risk factors may be more objective and precise for the selection of individualized treatment.
We took several steps to evaluate the impact in OS of each risk factor. First, the univariate analysis of prognostic factors showed TD, LVI, UPM and <12 LNs retrieved were correlated with OS. Second, multivariate Cox regression analysis was performed, and among the 4 correlated prognostic factors, UPM has the highest regression coefficient (3.676), followed by LVI (2.202), <12 LNs retrieved (1.428) and TD (1.116). Then, according to the above results, we established a predictive mathematical model to systematically evaluate the influence of each factor on the patients’ OS. The OS risk score = 1.116 × TD + 2.202 × LVI + 3.676 × UPM + 1.438 × LN − 0.493, with assignment for each factor.
Besides, we also obtained the impact of the 4 risk factors in DFS, as TD (0.789), LVI (2.074), UPM (3.183) and <12 LNs retrieved (1.329). The DFS risk score = 0.789 × TD + 2.074 × LVI + 3.183 × UPM + 1.329 × LN − 0.432.
So, we could obtain the risk score according to the clinicopathologic characteristics. Finally, we gave two groups of optimal cutoff points (0.07 and 1.33) and (−0.04 and 1.30), and divided the risk score into three groups as low-, moderate-, and high-risk group. The significance of the three cutoff points is to give appropriate recommendation for the patients to receive chemotherapy.
To obtain the optimal cutoff points, we conducted a log-rank test of every score for these patients. And, the corresponding risk score of maximum Chi-square values (or minimum P values) was the optimal cutoff point. In another word, the difference of survival curve is most remarkable at the cutoff point. So, according to our results, adjuvant chemotherapy is not recommended if the patient falls into the low-risk group, adjuvant chemotherapy is conditionally recommended for the patients in a moderate risk group, and adjuvant chemotherapy is strongly recommended for patients in high-risk group.
Hereafter, we used the subsequent follow-up of 221 patients to validate the accuracy and feasibility of the previous mathematical models. The results showed that log-rank test is statistically significant (P < 0.05). The models were accurate and feasible.
In addition, we conducted canonical correlation analysis to find the correlation between the types of recurrence or metastasis and high-risk factor. The results showed that local recurrence was correlated to UPM and <12 LNs retrieved. The phenomenon may be explained as the biological residual of cancer cells in the local area. If LVI occurred, cancer cells may be transported to distant organ or LN along a blood vessel or lymphangion. Thus, organ or LN metastasis is likely to arise. Moreover, if the LNs dissection is not sufficient, the residual cancer cells in lymphatic system may cause the metastasis of distant organ or LN. Similarly, peritoneal metastasis is likely to appear in the patients with T4 staging or obstructing/perforating cancers because of the shading of the cancer cells.
It is worth mentioning that our study has limitations. First, the retrospective study of the current equation only involves 340 cases; the limited sample may affect the results, especially the veracity of the model. Second, the indication of adjuvant chemotherapy was executed not very strictly, especially in the early period. Thus the results of survival rate may be influenced by the possible bias. And Finally, the data of single center may be less authentic. So, we are planning to conduct multicenter, prospective studies with large sample sizes to adjust and test our predictive model in the future.
Financial support and sponsorship
The study was supported by grants from National Natural Science Foundation of China (No. 81472825), Natural Science Foundation of Guangdong Province, China (No. 2014A030313078), Science and Technology Planning Project of Guangdong Province, China (No. 2013B021800078, No. 2014B090901066, No. 2017A010103009), Science and Technology Planning Project of Guangzhou City, China (No. 2014Y2-00503), and the Fundamental Research Funds for the Central Universities (No. 16ykjc23).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5. doi: 10.1093/jnci/djh275. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 2.Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: Results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–83. doi: 10.1016/S1470-2045(07)70245-0. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975– 2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 5.Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AndréT Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. doi: 10.1016/j.ctrv.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC intergroup trial 40983): A randomised controlled trial. Lancet. 2008;371:1007–16. doi: 10.1016/S0140-6736(08)60455-9. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol. 2004;22:1797–806. doi: 10.1200/JCO.2004.09.059. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 9.Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20:3999–4005. doi: 10.1200/JCO.2002.11.084. doi: 10.1200/JCO.2002.11.084. [DOI] [PubMed] [Google Scholar]
- 10.Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: An analysis of 50,042 patients. Ann Surg. 2002;236:416–21. doi: 10.1097/00000658-200210000-00003. doi: 10.1097/01.SLA.0000029243.59219.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–19. doi: 10.1200/JCO.2004.05.063. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 13.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. Apooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–7. doi: 10.1056/NEJMoa010957. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 14.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–94. doi: 10.5858/2000-124-0979-PFICC. doi: 10.1043/0003-9985(2000)124<0979: PFICC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Benson AB, 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 3. 2014. J Natl Compr Canc Netw. 2014;12:1028–59. doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 16.Benson AB, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Colon Cancer. Version 2. 2016. [Last accessed on 2016 Oct 20]. Available from: http://www.NCCN.org .
- 17.Benson AB, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Rectal Cancer. Version 2. 2016. [Last accessed on 2016 Oct 20]. Available from: http://www.NCCN.org .
- 18.Touchefeu Y, Provost-Dewitte M, Lecomte T, Morel A, Valo I, Mosnier JF, et al. Clinical, histological, and molecular risk factors for cancer recurrence in patients with stage II colon cancer. Eur J Gastroenterol Hepatol. 2016;28:1394–9. doi: 10.1097/MEG.0000000000000725. doi: 10.1097/MEG.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 19.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 20.Sobin LH. TNM classification: Clarification of number of regional lymph nodes for pN0. Br J Cancer. 2001;85:780. doi: 10.1054/bjoc.2001.1996. doi: 10.1054/bjoc.2001.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. international multicentre pooled analysis of B2 colon cancer trials (IMPACT B2) investigators. J Clin Oncol. 1999;17:1356–63. [PubMed] [Google Scholar]
- 22.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]


