Abstract
Background:
The chimney/periscope technique has been used to address complex aortic pathologies. This study aimed to report the outcomes and experiences of chimney and/or periscope grafts (CPGs) used in the endovascular management of complex aortic pathologies.
Methods:
Twenty-two patients with complex aortic pathologies were retrospectively studied from January 2013 to August 2016 in two vascular centers of teaching hospitals. All patients were diagnosed using computed tomography angiography (CTA). The patients were followed up at postoperative 1, 3, 6, and 12 months and yearly thereafter with X-ray, ultrasound, and/or CTA.
Results:
Twenty-two cases (17 males; mean age 60.7 ± 16.3 years) with complex aortic pathologies were analyzed. Nineteen patients underwent CPGs only, and the other three cases underwent the simultaneous implantation of chimney/periscope and fenestrated/scallop grafts. Twenty-six arteries were managed with forty CPGs during the procedures. Complete angiographies revealed two Type I endoleaks, one Type III endoleak, and one Type IV endoleak. Other intraoperative complications included brachial thrombosis, external iliac artery rupture, and left renal stenosis. The 30-day mortality was 0. The mean follow-up was 26.1 ± 10.1 months with a range of 2–39 months. During the follow-up, two Type I endoleaks and one Type IV endoleak were observed. One right renal stent occlusion occurred in the 5th month and turned patent after reintervention. Three patients died during the follow-up, one due to an aneurysm rupture as a Type I endoleak, and two due to myocardial infarction. The instant technical success was 96%. The primary and secondary patencies were 92% and 96%, respectively. The overall survival rates were 95%, 84%, and 84% at 12, 24, and 36 months, respectively. Stent migration was not observed in any patient.
Conclusions:
Chimney/periscope techniques could be used to tackle complex aortic pathologies, but the indications must be strictly controlled, and additional experiences are required.
Keywords: Aneurysm, Chimney/Periscope Technique, Complex Aortic Pathologies, Dissection, Endovascular Aortic Repair
INTRODUCTION
Endovascular techniques in the treatment of aortic pathologies have progressed much in recent years, although open surgery remains the “gold standard” treatment for aortic pathologies according to several articles in the literature.[1,2] Endovascular techniques can provide less invasive procedures for patients for whom open surgery presents a high risk. There are several endovascular techniques to treat complex aortic pathologies including the fenestrated/branched, chimney/periscope, and sandwich techniques. The fenestrated technique has become a mature treatment paradigm for complex aortic aneurysm diseases.[3] Several retrospective studies[4,5] have demonstrated no statistical difference in the short-/mid-term results between fenestrated and chimney repairs. Whereas, a meta-analysis[6] recently reported that chimney stent repair is associated with a lower reintervention rate compared with fenestrated endovascular aneurysm repair when treating patients with complex aortic aneurysms. In this study, we aimed to evaluate the safety and feasibility of the chimney/periscope technique for complex aortic pathologies.
METHODS
Ethical approval
As a retrospective study and data analysis was performed anonymously, this study was exempt from the ethical approval and informed consent from patients.
Patients
Twenty-two patients (17 males; mean age 60.7 ± 16.3 years old, ranging from 38 to 83 years [Table 1]) with complex aortic pathologies were retrospectively studied from January 2013 to August 2016 in two vascular centers of teaching hospitals (Beijing Hospital and Peking Union Medical College Hospital). Nineteen patients underwent only chimney and/or periscope techniques, while the other three underwent the chimney/periscope technique and received fenestrated/scallop stents at the same time. The complex aortic pathologies in this study refer to aortic pathologies that required repair and were accompanied by reconstructions of supra-aortic branches or visceral vessels. These complex aortic pathologies included the following: multiple aortic ulcers (1 case), thoracic aorta pseudoaneurysm (1 case), reintervention for a prior endovascular aortic repair (EVAR, 3 cases), thoracoabdominal aortic aneurysm (3 cases), complex abdominal aneurysm (7 cases), and aortic dissection (Stanford type B, 7 cases).
Table 1.
Demographic characteristics of patients with complex aortic pathologies
| Characteristics | Values |
|---|---|
| Age (years), mean ± SD (range) | 60.7 ± 16.3 (38–83) |
| Gender (male:female) | 17:5 |
| Comorbidities, n | |
| Hypertension | 18 |
| Coronary artery disease | 5 |
| Diabetes mellitus | 3 |
| Cerebrovascular disease | 4 |
| Chronic renal insufficiency | 2 |
| Persistent atrial fibrillation | 1 |
| Nephritis of Schonlein–Henoch purpura | 1 |
| Chronic obstructive pulmonary disease | 1 |
| Bronchial asthma | 1 |
| ASA (Class III/IV), n | 12 |
| Vascular surgery history, n | 3 |
| Emergency, n | 4 |
| eGFR baseline (ml·min−1·1.73 m−2) | 75.3 ± 30.5 |
SD: Standard deviation; ASA: American Society of Anesthesiologists; eGFR: Estimated glomerular filtration rate.
Diagnosis and procedure
All patients were diagnosed by computed tomography angiography (CTA). All patients were assessed in detailed, and individual therapeutic schedules were created. All patients underwent general anesthesia. The upper limbs, neck, and hypogastrium were regularly sterilized. Aortic angiography was performed to confirm the diagnoses and to reevaluate the endovascular approaches. Femoral arterial access was obtained to place the main body device, and the brachial artery, right axillary artery, left subclavian artery (L-SA), or left common carotid artery provided choices for access to place the chimney/periscope grafts (CPGs). Complete angiographies were performed to ensure that the devices were implanted correctly, the endoleaks occurred, and the CPGs were patent.
Devices
The main body devices included the Excluder (Gore, USA), Hercules (Microport, China), Zenith (COOK, USA), Ankura (Lifetech Scientific, China), and Sinus (Optimed, Germany). The CPGs included the Viabahn (Gore, USA), Zilver (COOK, USA), Sinus (Optimed, Germany), Complete SE (Medtronic, USA), Scuba (Invatec, Italy), Palmaz (Cordis, USA), Josten (Abbott Vascular, USA), and Express LD (Boston Scientific, USA) [Table 2].
Table 2.
Complex aortic pathologies and intraoperative variables in 22 patients with complex aortic pathologies
| Diseases | n | Main body devices | Cuff | Target vessels/CPGs | Approach | Assisted surgical techniques |
|---|---|---|---|---|---|---|
| MAU | 1 | Excluder 45-200 | 0 | L-CA/1 | Femoral, L-BA, L-CA | None |
| TAPA | 1 | Hercules 36-32-160 | Hercules 38-80 | L-SA/1 | Femoral, L-BA | Thrombectomy in L-BA |
| Reintervention for a prior EVAR | 3 | Zenith 24-60 | 0 | R-RA/1, L-RA/1 | Femoral, L-BA, R-BA | None |
| Ankura 38-34-160 | 0 | L-CA/2 | Femoral, L-BA | None | ||
| Hercules 40-36-160 | 0 | L-SA/2 | Femoral, L-BA | None | ||
| TAAA | 3 | Zenith 34-34-152 | Zenith 32-40 | SMA/2 | Femoral, L-BA | Right iliac-femoral bypass |
| Excluder 31-14.5-13 | Zenith 37-100 | SMA/1, CT/1 | Femoral, L-BA | None | ||
| Excluder 37-200/42-200/45-150 | 0 | L-SA/2 | Femoral, L-BA | None | ||
| AD | 7 | Ankura 40-34-180 | 0 | L-SA/2 | Femoral, L-BA | None |
| Excluder TAG-37-200 | 0 | L-SA/1 | Femoral, L-BA | None | ||
| Hercules 28-80 | Hercules 26-80 | SMA/2 | Femoral, R-Axi | None | ||
| Sinus 38-30-200 | 0 | R-SA/5, L-CA/2 | Femoral, B-BA, L-CA, L-SA | None | ||
| Zenith 40-36-158/optimed 30-8 | 0 | L-CA/1 | Femoral, L-CA, L-SA | None | ||
| Gore 37-150 | 0 | L-SA/1 | Femoral, L-BA | None | ||
| Gore 34-150 | 0 | L-SA/2 | Femoral, L-BA | None | ||
| cAAA | 7 | Hercules HT 38-36-160/HBB 32-14-170 | 0 | L-RA/2 | Femoral, L-BA | None |
| Excluder 31-14-170 | 0 | R-RA/1 | Femoral, L-BA | None | ||
| Zenith 28-82 | 0 | L-RA/1 | Femoral, L-BA | None | ||
| Excluder 24-14-180 | Excluder 28-30 | L-RA/2 | Femoral, L-BA | None | ||
| Zenith 24-12-94 | Zenith 28-00 | L-RA/1, R-RA/1 | Femoral, L-BA | None | ||
| Hercules 30-14-170 | 0 | R-RA/1 | Femoral, R-BA | None | ||
| Medtronic endurant 36-26-145 | 0 | R-RA/1 | Femoral, L-BA | None |
MAU: Multiple aortic ulcer; TAPA: Thoracic aorta pseudoaneurysm; re-EVAR: Reintervention for a prior endovascular aortic repair; TAAA: Thoracoabdominal aortic aneurysm; cAAA: Complex abdominal aneurysm; AD: Aortic dissection; L-RA: Left renal artery; R-RA: Right renal artery; L-SA: Left subclavian artery; R-SA: Right subclavian artery; L-CA: Left common carotid artery; SMA: Superior mesenteric artery; CT: Celiac trunk; R-Axi: Right axillary artery; B-BA: Bilateral brachial artery; L-BA: Left brachial artery; R-BA: Right brachial artery; CPGs: Chimney and/or periscope grafts.
Follow-up
All patients were followed up at postoperative 1, 3, 6, and 12 months and yearly thereafter with X-ray, ultrasound, and/or CTA. The shapes of the stents, the patencies of the target vessels, the changes in the endoleaks, and other complications were recorded.
Definitions
The instant technical success was defined as the successful deployment of the stents without high flow Type I/III endoleaks, occlusion of the target vessels or technique-related mortality during the procedures. The definition of primary patency was related to the freedom from occlusion in the target vessels before reintervention. The secondary patency indicated no occlusion in the target vessels with assisted or secondary surgical procedures. The estimated glomerular filtration rate (eGFR) was estimated with the simplified Modification of Diet in Renal Disease formula.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) for Windows was used for the statistical analyses. Continuous variables are expressed as the mean ± standard deviation (SD). Categorical data are presented as the absolute values (n) and percentages (%). Graphpad Prism 6.0 (GraphPad Software, Inc., California, USA) was used to draw the survival curve.
RESULTS
Patient demographics
The demographics of the patients in this study are presented in Table 1. Twenty-two patients underwent endovascular management with the chimney/periscope techniques. The mean age at intervention was 60.7 ± 16.3 years old (range, 38–83 years old). The comorbidities included hypertension (n = 18), coronary arterial disease (n = 5), diabetes mellitus (n = 3), cerebrovascular disease (n = 4), persistent atrial fibrillation (n = 1), chronic renal insufficiency (n = 1), nephritis of Schonlein-Henoch purpura (n = 1), chronic obstructive pulmonary disease (n = 1), bronchial asthma (n = 1), smoking (n = 12), and alcohol use (n = 6). The preoperative mean eGFR was 75.3 ± 30.5 ml·min−1·1.73 m−2.
Intraoperative management and early complications (<30 days)
Twenty-two patients were implanted with twenty-six main body devices [presented in Table 2] including the Excluder (Gore, USA, n = 10), Hercules (Microport, China, n = 6), Zenith (COOK, USA, n = 5), Ankura (Lifetech Scientific, China, n = 3), Sinus (Optimed, Germany, n = 1), and Endurant (Medtronic, USA, n = 1). Six cuffs were also deployed and included the Excluder (Gore, USA, n = 3), Hercules (Microport, China, n = 2), and Zenith (COOK, USA, n = 1). Twenty-six target vessels were fitted with forty CPGs [presented in Table 3] that included the Viabahn (Gore, USA, n = 17), Zilver (COOK, USA, n = 7), Sinus (Optimed, Germany, n = 4), Complete SE (Medtronic, USA, n = 3), Scuba (Invatec, Italy, n = 3), Palmaz (Cordis, USA, n = 4), Josten (Abbott Vascular, USA, n = 1), and Express LD (Boston Scientific, USA, n = 1).
Table 3.
Vessels and stents in 22 patients with complex aortic pathologies who underwent chimney/periscope techniques (n)
| Target vessel | Total vessels | Total stents | Type of CPGs | |
|---|---|---|---|---|
| Bare stent | Covered stent | |||
| L-RA | 5 | 7 | 5 | 2 |
| R-RA | 5 | 5 | 3 | 2 |
| L-SA | 7 | 11 | 8 | 3 |
| R-SA | 1 | 5 | 2 | 3 |
| L-CA | 4 | 6 | 3 | 3 |
| SMA | 3 | 5 | 2 | 3 |
| CT | 1 | 1 | 0 | 1 |
| Total | 26 | 40 | 23 | 17 |
L-RA: Left renal artery; R-RA: Right renal artery; L-SA: Left subclavian artery; R-SA: Right subclavian artery; L-CA: Left common artery; SMA: Superior mesenteric artery; CT: Celiac trunk; CPGs: Chimney and/or periscope grafts.
The complete angiographies revealed two Type I endoleaks, one Type III endoleak, and one Type IV endoleak. We managed these complications with the techniques described in Table 4. Among these patients, two patients were under observation for Type I endoleaks with low flow. For one patient of advanced age who had several severe accompanying diseases, the procedure time was kept short. In addition, the risk of paraplegia may have increased if the proximal ends were completely sealed. Based on these factors, observations were made. The other patient was initially diagnosed with a Type I endoleak after his first endovascular management. The chimney technique was applied to reconstruct his L-SA. Considering that the chimney technique itself may also create Type I endoleak, we did not engage in further measures to seal the low Type I endoleak in the procedure. However, in one case, we failed with a left renal stent occlusion. The instant technical success was 96% (25/26 branches).
Table 4.
Intraoperative immediate complications and managements
| Immediate complications | n | Managements | Intraoperative outcomes |
|---|---|---|---|
| Endoleak | |||
| Type I | 4 | Cuff were implanted in two patients Two under observation | Disappeared Stable |
| Type II | 1 | Covered stent was implanted | Disappeared |
| Type III | 3 | Two expanded with tri-lobe balloon One under observation | Disappeared Stable |
| Type IV | 1 | Under observation | Stable |
| Brachial thrombosis | 1 | Thrombectomy | Patent |
| External iliac artery rupture | 1 | Right iliac-femoral bypass | Patent |
| Left renal stent occlusion | 1 | Recanalization | Failed |
Other intraoperative complications included the following: brachial thrombosis and external iliac artery rupture. No patients died during the procedures, and the 30-day mortality was also 0.
The postoperative eGFRs ranged from 17.5 to 122.9 ml·min−1·1.73 m−2 (mean, 76.3 ± 27.8 ml·min−1·1.73 m−2). The eGFR decreased by more than 25% in three patients. One patient suffered contrast-induced nephropathy and was transferred to the intensive care unit for hemofiltration and recovered 3 weeks later.
Follow-up and later complications (>30 days)
All patients were followed up. The mean follow-up time was 26.1 ± 10.1 months with a range of 2–39 months [Table 5]. Stent migration was not observed in any patient during the follow-up. One patient died of an aneurysm rupture due to a Type I endoleak. He was one of the two patients who were under observation for Type I endoleaks during the procedure. During the follow-up, the size of the aneurysm was found to increase. We suggested that the patient underwent the second procedure. However, he and his family refused the procedure and decided on conservative management. Finally, he died after 2 years. Two patients suffered myocardial infarction and died at 12 and 15 months. One patient suffered an occlusion in the right renal stent in the 5th month after his first procedure. This renal stent was returned to patency after the secondary procedure. The primary and secondary patencies were 92% and 96%, respectively.
Table 5.
Follow-up and later complications (>30 days) (N=22)
| Follow-up | Outcomes |
|---|---|
| Mean follow-up, months (range) | 26.1 ± 10.1 (2–39 months) |
| Endoleak, n (%) | |
| Type I | 2 (9) |
| Type IV | 1 (5) |
| Primary target vessel patency (%) | 92 |
| Secondary target vessel patency (%) | 96 |
| Death caused by, n (%) | |
| Myocardial infarction | 2 (9) |
| Aneurysm rupture for Type I endoleak | 1 (5) |
The overall survival rates were 95%, 84%, and 84% at 12, 24, and 36 months, respectively [Figure 1].
Figure 1.
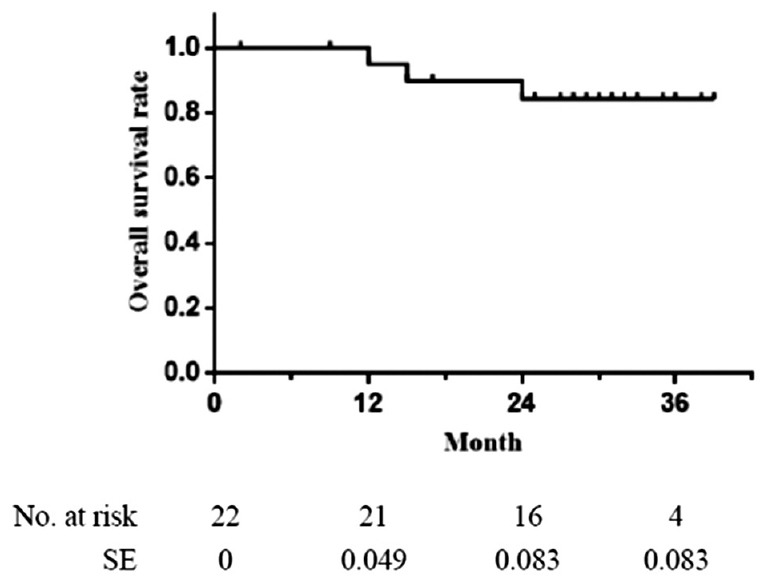
The overall survival rate. One-year: 95%; 2-year: 84%; 3-year: 84%. SE: Standard error.
DISCUSSION
The chimney/periscope technique was first introduced by Greenberg et al.[7] as an adjunctive procedure to address challenging anatomic situations for which open surgical interventions presented high physiologic risks. Because of the lack of need for customization compared with fenestrated grafts,[8] the CPG has exhibited better applicability in emergent cases.[9] In addition, this technique can also be used in selected complex procedures, such as reconstruction of the arch branches,[10] management of juxtarenal aortic aneurysms,[11] and treatment of Type I endoleaks after prior endovascular aortic aneurysm repair.[12] There were two emergent cases (18%, n = 4) and 18 selective (82%, n = 18) cases in our study, which confirmed that this technique could be applied in various clinical situations with satisfactory outcomes. Other authors have also adopted this procedure to address emergencies.[11]
Increasing numbers of reports in the literature have demonstrated promising short-/mid-term outcomes and high rates of technical success (≥95%).[10,13,14] A prospective study by Pecoraro et al.[11] reported that the primary patencies at 12, 24, and 36 months were 94%, 94%, and 93%, respectively, and the secondary patencies at 12, 24, and 36 were 97%, 96%, and 96%, respectively. In the present study, a 26.1-month follow-up yielded a primary target vessel patency of 92% and a secondary patency of 96%, and these values are similar to those reported in the previous literature. Our instant technical success was 96%, which was also similar to that reported in the previous literature.
Type I endoleak is associated with the gutters created by CPGs when they are in apposition between the aortic wall and the main body graft.[15] Both the stent size and the overlap, which is between the main body graft and the CPGs, play key roles in preventing Type I endoleak. Several experts[16,17] have proposed different formulas to choose the matched graft size, while not all Type I endoleaks can be prevented by an ideal graft size. For the management of Type I endoleaks in EVARs with the chimney technique, Coscas et al.[18] adopted ballooning, whereas we deployed a cuff for two patients and maintained observation with the other two. However, one patient died of an aneurysm rupture during the follow-up, and he was only one of the two patients who were under observation. Thus, Type I endoleaks are definitely worth maintaining close follow-ups and exercising managements over time.
Although chimney/periscope techniques have the potential to generate Type I endoleaks, they can also be applied to address Type I endoleaks after endovascular management as previous reports[12,19,20] have mentioned. There were three patients with Type I endoleaks due to prior endovascular aortic repairs in this study. Two of these patients underwent procedures with chimney/periscope techniques, and the other patient was treated with procedures involving the simultaneous application of the chimney/periscope and fenestrated technique. All of these patients achieved satisfactory outcomes without any stent stenosis or occlusion during the follow-up.
Lee et al.[21] reported that some patients (32.6%, 14/43) experienced acute kidney injury after chimney repairs of juxtarenal aneurysms. In this study, only 14% of these patients experienced renal dysfunction, and this percentage is lower than that reported by Lee and similar to the rate that was reported by Donas et al. (13.4%).[22] Various factors may affect renal function, such as age, baseline chronic kidney disease, the female gender, renal artery stenosis, and intraoperative manipulations.[11,21,23] To protect renal function, we cannot change age, baseline chronic disease, or gender, but we can eliminate stenosis with dilatation. We routinely perform hydration in the perioperative period. Both full knowledge of the lesion and a detailed preoperative plan can reduce the chances of unnecessary manipulations during the procedure and result in reduced use of contrast agents and reduced procedure time.
In addition, contrast-induced nephropathy (CIN) is also a cause of renal failure. Kawatani et al.[24] reported that 7.8% of 167 patients developed CIN after endovascular stent graft placement, and these authors concluded that impaired left ventricular function was the greatest risk factor for CIN (odds ratio 9.34, P = 0.018, and 95% confidence interval = 1.46–59.70). In our study, one patient suffered from CIN and was transferred into the intensive care unit for hemofiltration. He recovered 3 weeks later. However, he had no history of impaired left ventricular function. Although the pathological mechanisms of CIN remain unclear, attention should be given to CIN when offering interventions for patients with complex aortic pathologies.
In our study, one patient suffered brachial thrombosis and another experienced an external iliac artery rupture. The former indicated that adequate heparin was required. Whereas, the latter complication suggested that careful preformation was necessary.
There are several limitations to this study. The number of patients in this study was limited, and the analysis was retrospective. The stents used in this study and the types of diseases were numerous and diverse. Therefore, more experience and long-term follow-ups are necessary to confirm the final outcomes.
In conclusion, chimney/periscope techniques can be used to tackle complex aortic pathologies, but the indications must be strictly controlled and more experience is required.
Financial support and sponsorship
This study was supported by a grant of National Natural Science Foundation of China (No. 81270399).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
The content of this paper has been partly presented at the 43rd Annual Symposium on Vascular and Endovascular Issues, Techniques and Horizons (VEITH symposium™) as an oral presentation and then been published in Vascular (2016; 24 [1 s]) as an abstract style.
REFERENCES
- 1.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-term outcomes of abdominal aortic aneurysm in the medicare population. N Engl J Med. 2015;373:328–38. doi: 10.1056/NEJMoa1405778. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years'follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet. 2016;388:2366–74. doi: 10.1016/S0140-6736(16)31135-7. doi: 10.1016/S0140-6736(16)31135-7. [DOI] [PubMed] [Google Scholar]
- 3.Georgiadis GS, van Herwaarden JA, Antoniou GA, Giannoukas AD, Lazarides MK, Moll FL, et al. Fenestrated stent grafts for the treatment of complex aortic aneurysm disease: A mature treatment paradigm. Vasc Med. 2016;21:223–38. doi: 10.1177/1358863X16631841. doi: 10.1177/1358863X16631841. [DOI] [PubMed] [Google Scholar]
- 4.Banno H, Cochennec F, Marzelle J, Becquemin JP. Comparison of fenestrated endovascular aneurysm repair and chimney graft techniques for pararenal aortic aneurysm. J Vasc Surg. 2014;60:31–9. doi: 10.1016/j.jvs.2014.01.036. doi: 10.1016/j.jvs.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Donas KP, Torsello G, Bisdas T, Osada N, Schonefeld E, Pitoulias GA. Early outcomes for fenestrated and chimney endografts in the treatment of pararenal aortic pathologies are not significantly different: A systematic review with pooled data analysis. J Endovasc Ther. 2012;19:723–8. doi: 10.1583/JEVT-12-3952MR.1. doi: 10.1583/JEVT-12-3952MR.1. [DOI] [PubMed] [Google Scholar]
- 6.Yaoguo Y, Zhong C, Lei K, Yaowen X. Treatment of complex aortic aneurysms with fenestrated endografts and chimney stent repair: Systematic review and meta-analysis. Vascular. 2017;25:92–100. doi: 10.1177/1708538115627718. doi: 10.1177/1708538115627718. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003;38:990–6. doi: 10.1016/s0741-5214(03)00896-6. doi: 10.1016/S0741. [DOI] [PubMed] [Google Scholar]
- 8.Buck DB, van Herwaarden JA, Schermerhorn ML, Moll FL. Endovascular treatment of abdominal aortic aneurysms. Nat Rev Cardiol. 2014;11:112–23. doi: 10.1038/nrcardio.2013.196. doi: 10.1038/nrcardio.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trellopoulos G, Georgakarakos E, Pelekas D, Papachristodoulou A, Argyriou C, Georgiadis GS, et al. Chimney and periscope technique for emergent treatment of spontaneous aortic rupture. Ann Vasc Surg. 2014;28:1324–8. doi: 10.1016/j.avsg.2014.01.013. doi: 10.1016/j.avsg.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Guo W, Liu X, Jia X, Xiong J, Wang L, et al. The single-centre experience of the supra-arch chimney technique in endovascular repair of type B aortic dissections. Eur J Vasc Endovasc Surg. 2013;45:633–8. doi: 10.1016/j.ejvs.2013.02.016. doi: 10.1016/j.ejvs.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Igari K, Kudo T, Toyofuku T, Inoue Y. The outcomes of endovascular aneurysm repair with the chimney technique for juxtarenal aortic aneurysms. Ann Thorac Cardiovasc Surg. 2016;22:174–80. doi: 10.5761/atcs.oa.16-00026. doi: 10.5761/atcs.oa.16-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donas KP, Telve D, Torsello G, Pitoulias G, Schwindt A, Austermann M, et al. Use of parallel grafts to save failed prior endovascular aortic aneurysm repair and type ia endoleaks. J Vasc Surg. 2015;62:578–84. doi: 10.1016/j.jvs.2015.04.395. doi: 10.1016/j.jvs.2015.04.395. [DOI] [PubMed] [Google Scholar]
- 13.Pecoraro F, Veith FJ, Puippe G, Amman-Vesti B, Bettex D, Rancic Z, et al. Mid- and longer-term follow up of chimney and/or periscope grafts and risk factors for failure. Eur J Vasc Endovasc Surg. 2016;51:664–73. doi: 10.1016/j.ejvs.2016.01.010. doi: 10.1016/j.ejvs.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Xue Y, Sun L, Zheng J, Huang X, Guo X, Li T, et al. The chimney technique for preserving the left subclavian artery in thoracic endovascular aortic repair. Eur J Cardiothorac Surg. 2015;47:623–9. doi: 10.1093/ejcts/ezu266. doi: 10.1093/ejcts/ezu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniou GA, Schiro A, Antoniou SA, Farquharson F, Murray D, Smyth JV, et al. Chimney technique in the endovascular management of complex aortic disease. Vascular. 2012;20:251–61. doi: 10.1258/vasc.2011.ra0056. doi: 10.1258/vasc.2011.ra0056. [DOI] [PubMed] [Google Scholar]
- 16.Chou HW, Chan CY, Wang SS, Wu IH. How to size the main aortic endograft in a chimney procedure. J Thorac Cardiovasc Surg. 2014;147:1099–101. doi: 10.1016/j.jtcvs.2013.10.049. doi: 10.1016/j.jtcvs.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Matteo J, Cunningham J. Size matters! A reliable endovascular method to avoid infolding and endoleaks when reconstructing aortic bifurcations using stent grafts. Vascular. 2012;20:124–8. doi: 10.1258/vasc.2011.oa0340. doi: 10.1258/vasc.2011.oa0340. [DOI] [PubMed] [Google Scholar]
- 18.Coscas R, Becquemin JP, Majewski M, Mayer J, Marzelle J, Allaire E, et al. Management of perioperative endoleaks during endovascular treatment of juxta-renal aneurysms. Ann Vasc Surg. 2012;26:175–84. doi: 10.1016/j.avsg.2010.10.021. doi: 10.1016/j.avsg.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Montelione N, Pecoraro F, Puippe G, Chaykovska L, Rancic Z, Pfammatter T, et al. A12-year experience with chimney and periscope grafts for treatment of type I endoleaks. J Endovasc Ther. 2015;22:568–74. doi: 10.1177/1526602815586972. doi: 10.1177/1526602815586972. [DOI] [PubMed] [Google Scholar]
- 20.Kim NH, Kim WC, Jeon YS, Cho SG, Hong KC. Repair of type I endoleak by chimney technique after endovascular abdominal aortic aneurysm repair. Ann Surg Treat Res. 2014;86:274–7. doi: 10.4174/astr.2014.86.5.274. doi: 10.4174/astr.2014.86.5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JT, Varu VN, Tran K, Dalman RL. Renal function changes after snorkel/chimney repair of juxtarenal aneurysms. J Vasc Surg. 2014;60:563–70. doi: 10.1016/j.jvs.2014.03.239. doi: 10.1016/j.jvs.2014.03.239. [DOI] [PubMed] [Google Scholar]
- 22.Donas KP, Lee JT, Lachat M, Torsello G, Veith FJ. PERICLES Investigators. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies: The PERICLES registry. Ann Surg. 2015;262:546–53. doi: 10.1097/SLA.0000000000001405. doi: 10.1097/SLA.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kawatani Y, Nakamura Y, Mochida Y, Yamauchi N, Hayashi Y, Taneichi T, et al. Contrast medium induced nephropathy after endovascular stent graft placement: An examination of its prevalence and risk factors. Radiol Res Pract 2016. 2016:5950986. doi: 10.1155/2016/5950986. doi: 10.1155/2016/5950986. [DOI] [PMC free article] [PubMed] [Google Scholar]


