Abstract
Background
Individuals with Major Depressive Disorder (MDD) are characterized by biases in attention to negative emotional material. While there is evidence that anomalous functioning in frontocingulate regions may underlie these biases, we know little about the neural correlates of negative emotional biases in depressed adolescents.
Methods
Eighteen adolescents diagnosed with MDD and 21 matched healthy control (CTL) adolescents underwent fMRI while performing an emotional distractor task. On each trial participants were presented with task-relevant house pairs and task-irrelevant face pairs. Participants indicated whether the house pairs were identical while ignoring the face pairs, which were either fearful, sad, or neutral.
Results
Despite equivalent behavioral performance (response time and accuracy) between groups, adolescents with MDD exhibited greater activation in frontocingulate regions, including dorsal anterior cingulate cortex (dACC) and inferior frontal gyrus/middle frontal gyrus (IFG/MFG), and occipitoparietal regions, including lateral occipital cortex and superior parietal lobule when ignoring fearful versus neutral faces. Response times to these trial conditions also correlated negatively with activation in IFG/MFG and lateral occipital cortex suggesting these regions are recruited in order to effectively ignore emotional distractors. Groups did not differ when ignoring sad versus neutral faces or fearful versus sad faces.
Conclusions
Adolescents with MDD recruit both cognitive control and visual attention regions to a greater degree than do CTL adolescents, reflecting greater cognitive demand when downregulating threat-related stimuli.
Keywords: adolescence, depression, executive function, selective attention, anterior cingulate cortex, occipital cortex
Introduction
Major depressive disorder (MDD) is the leading cause of disability among adolescents (1). Moreover, adolescent-onset MDD is highly recurrent: 40 percent of depressed adolescents have a subsequent episode of depression within 3 years, and 50 percent experience recurrent episodes throughout adulthood (2,3,4). These high relapse rates highlight the inadequacy of current treatments for adolescent depression, arguably due in part to our lack of knowledge about brain function in adolescent MDD (5,6). Thus, it is critical that we examine neural mechanisms that underlie depression in adolescence and develop treatments based on models of adolescent, rather than adult, MDD.
Investigators have posited that biases in attention to and memory for negative stimuli contribute to the onset of depression (7,8). Attentional biases may be due to the increased salience of negative stimuli for depressed individuals, and/or to difficulties in inhibiting the processing of, or disengaging from, negative information (8). Consistent with these formulations, depressed adolescents have been found to exhibit specific impairments in performing cognitive tasks in an emotional context. For example, depressed adolescents attend more to negative than to neutral or positive stimuli on a dot-probe task (9), and show greater interference when required to ignore negative information in an emotional N-back task (10). These behavioral studies indicate that depressed adolescents not only have difficulty disengaging from relevant negative information, but are also impaired in their ability to attend to non-emotional material in the presence of negatively valenced distractor, or task-irrelevant, stimuli.
The neural correlates underlying biased processing of emotional information, and in particular negative information, in the context of performing cognitive tasks in adolescent MDD are not well understood. Studies that have assessed depressed adults as they complete cognitive tasks with emotional stimuli indicate that anomalous recruitment of frontocingulate cognitive control regions, including dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), and dorsal anterior cingulate cortex (dACC), may underlie difficulties in the processing of emotionally salient stimuli (11). For example, Fales et al. (12) found that, compared with nondepressed controls, depressed adults showed greater amygdala activity while attending to task-relevant neutral stimuli and ignoring negative distractors (fearful faces) than when ignoring neutral distractors (neutral faces). Moreover, only the nondepressed adults showed the predicted pattern of increased DLPFC recruitment when ignoring fearful faces. Similarly, when ignoring emotional distractors during an emotional oddball task, depressed adults showed attenuated recruitment of bilateral IFG, superior frontal gyrus, and middle frontal gyrus (MFG) relative to nondepressed controls (13). Together, these findings support a neural model of adult depression in which greater activation of frontocingulate cognitive control regions (e.g., DLPFC, IFG, dACC) is needed to suppress hyperactive emotion generative regions (e.g., amygdala) in order to appropriately process cognitively demanding conflicting stimuli and to reallocate attention (14,15). This model, however, has not been tested systematically in adolescent depression.
Although several researchers have examined the neural correlates of either emotion processing or cognitive inhibition in adolescent MDD (16,17); see also (18,19), few investigators have assessed the interaction of these two processes in depressed adolescents. Given that the PFC and ACC regions continue to mature both structurally and functionally during adolescence (20,21), understanding their functioning in the context of the cognitive processing of emotional stimuli in adolescents is critical to our understanding of the development of early-onset MDD. To date, only one fMRI study has directly assessed cognitive inhibition during emotional processing in depressed adolescents (22). In that study, adolescents performed a Go-NoGo task with negatively and positively valenced primes (sad and happy faces, respectively) that were presented prior to the presentation of the go and no-go targets. Interestingly, compared with healthy controls, depressed adolescents, despite equivalent task performance, showed reduced DLPFC and occipital cortex activation only to no-go targets that followed sad faces. Thus, while these findings indicate that DLPFC hypoactivation may underlie failed suppression of mood-congruent stimuli, it is not clear whether this result generalizes to other forms of negative stimuli. In particular, fearful faces, as a proxy for threat, have been shown to elicit negative emotional processing biases (23) and have also been used to probe amygdala hyperactivation in both depressed adolescents (24,25,26) and depressed adults (12,27). It is unclear whether the documented difficulties of depressed adolescents in inhibiting negative stimuli are due to heightened attention to sad stimuli, to threat-related stimuli, or to both types of stimuli. In examining this question, therefore, it is important to use a task that includes both mood-congruent (sad) and threat-related (fearful) emotional stimuli separately in order to elucidate the stimulus-specific nature of negative emotional biases in adolescent MDD and to test neural cognitive-emotion models of adult MDD, most of which have been formulated on the basis of findings with fearful face stimuli, in adolescents.
The present study was designed both to extend to adolescent depression previous findings obtained with depressed adults suggesting that frontocingulate dysfunction underlies difficulties disengaging from negative emotional stimuli, and to elucidate the involvement of mood-congruent (sad) and threat-related (fearful) stimuli in this processing bias. Specifically, we examined the neural correlates of attention to task-relevant neutral stimuli in the presence of salient but irrelevant negative emotional (sad and fearful) and neutral distractors in depressed and nondepressed adolescents. We hypothesized that depressed adolescents would show greater interference when presented with sad and fearful face distractors than would healthy control adolescents, indexed by longer response latencies and/or decreased accuracy. We hypothesized further that adolescents with MDD would exhibit hypoactivation in frontocingulate regions known to be involved in cognitive control and conflict processing (e.g., DLPFC, IFG, dACC), and hyperactivation in brain regions implicated in negative emotion reactivity (e.g., amygdala) during successful inhibition of negative (sad and fearful face) distractors.
Methods and Materials
Participants
Forty-six adolescents (ages 12-17 years) were recruited to participate in this study. Seven participants (3 MDD, and 4 CTL) were excluded from the analysis due to: 1) motion artifacts(1 MDD); 2) failure to record behavioral responses(3 CTL); and 3) poor behavioral performance (overall accuracy<50%; 2 MDD, 1 CTL). Thus, we report results from 39 participants(18 diagnosed with MDD (16 female) and 21 CTLs (16 female). The 7 excluded participants did not differ from the 39 included participants with respect to age or symptom severity, as assessed by scores on the Children's Depression Inventory (CDI (28); both ps>.23). Nine of the CTL and 13 of the MDD participants also completed an additional fMRI task, as described in Colich et al. (22). The order of fMRI tasks was counter-balanced across all participants. MDD participants met DSM-IV (DSM-IV-TR; 29) criteria for MDD and had CDI scores above the clinical threshold of 12 (30). Participants were recruited from the local community via media advertisements; depressed adolescents were also recruited through the Pediatric Mood Disorders Clinic at Stanford University. Participants were compensated for their participation. Exclusion criteria for all participants included: 1) contraindications to scan (metal implants, braces, etc.); 2) history of major neurological disorder; 3) any primary diagnosis other than MDD, including diagnoses of bipolar disorder, schizophrenia, or attention deficit hyperactivity disorder. For CTL adolescents, exclusion criteria also included any past or current Axis I disorder. MDD participants who were taking psychotropic medications were instructed to continue their normal medication regimen throughout their participation in this study. This study was approved by Stanford University's IRB, and informed written consent and assent was obtained from parent and child.
Clinical Assessment
At the first laboratory session, adolescents and the accompanying parent were administered the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL; 31) to confirm diagnosis of MDD in the MDD group and to rule out any past or current Axis I disorder in the CTL group. This interview was also used to assess present or lifetime Axis I disorder in all participants. All interviews were conducted by research staff who had established excellent symptom and diagnostic reliability (κ>0.9). Final diagnostic decisions were made by a board-certified child and adolescent psychiatrist (M.K.S.). In addition, all adolescents completed the CDI (28), a self-report measure of depressive symptoms, the State Trait Anxiety Inventory (STAI-T; 32), a self-report measure of trait anxiety, and the Tanner Staging questionnaire (33,34), a self-report measure of pubertal status.
Neuroimaging Data Acquisition
Details concerning data acquisition are presented in the Supplement.
fMRI Task Design
We used a modified version of an emotional distractor task (35) that has been used in previous studies of depression and anxiety (12,36). In this modified task, participants saw four images presented around a fixation cross: one pair of images (houses or faces) presented on a vertical plane and the other pair (faces or houses) presented horizontally. Participants were instructed to make judgments only about whether or not the two houses presented on each trial were identical (they were identical on half of the trials, and were presented either the horizontal or vertical planes on half of the trials). The face pairs were task distractors and the faces in each pair were neutral, sad, or fearful. Further task design details are presented in the Supplement.
fMRI Data Analysis
A whole-brain voxel-wise GLM analysis was conducted for each participant for each run, including regressors for each emotion distractor valence (neutral, sad, fearful) for trials on which the participant responded correctly, and a regressor for the inaccurate trials in order to remove these trials from the implicit baseline. Within-group and between-group Z statistical images were thresholded at Z>2.3 with a cluster probability of p<0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory. In contrast to previous studies that have used this task (12,35,36), we assessed only correct trials and conducted whole-brain analyses in order to be able to identify activation in neural regions that are necessary for the successful inhibition of emotional face distractors. Details concerning data preprocessing and additional data analysis details are presented in the Supplement.
Results
Participant Characteristics
Demographic and clinical characteristics of the MDD and CTL groups are presented in Table 1. The two groups did not differ in age, gender, pubertal stage, or ethnicity (ps>.1). As expected, the MDD group had significantly higher CDI and STAI-T scores than did the CTL group (all ps<.001). Half of the MDD participants had a comorbid anxiety disorder, and half were taking psychotropic medications at the time of the scan (7 of the 9 individuals with comorbid anxiety were taking psychotropic medications; for details about specific medications and anxiety disorder diagnoses see Tables S1 and S2). Importantly, there were no significant differences between the comorbid and non-comorbid, or between the medicated and unmedicated, MDD participants with respect to either the behavioral or the neural data (all ts<1.768, ps>.096). In the Supplement we present means and SDs for participants with and without medications, and with and without comorbid anxiety diagnoses, and the statistics associated with the t-test comparisons between the medication and comorbidity subgroups (Tables S3 and S4).
Table 1. Demographic Information and Clinical Characteristics.
| CTL (n = 21) | MDD (n = 18) | p-value | |
|---|---|---|---|
| Age | 14.78 (1.36) | 15.44 (1.17) | 0.12 |
| Gender, Female (n) | 16 | 15 | 0.27 |
| Tanner Stage | 4.1 (.53) | 4.2 (.60) | 0.51 |
| Caucasian (%) | 48% | 40% | 0.56 |
| CDI Total | 4.00 (6.04) | 25.33 (8.57) | < .001 |
| STAI-T Total | 32.52 (8.30) | 63.11 (7.84) | < .001 |
| Any Comorbid Anxiety Dx (n) | 0 | 9 | |
| Psychotropic Medication use (n) | 0 | 9 |
Note: Standard deviations are presented in parentheses. CTL = control participants; MDD = depressed participants; CDI = Children's Depression Inventory.
Behavioral Data
Results for task accuracy and response time are presented in the Supplement.
fMRI Data
In the main text we present only between-group comparisons; we present all within-group comparisons in the Supplement.
Sad versus Neutral Distractors
In order to examine group differences in neural regions involved in ignoring task-irrelevant sad versus neutral faces, we conducted a whole-brain ANOVA (Group [MDD, CTL] repeated over Distractor Valence [Sad, Neutral]). This voxel-wise ANOVA yielded no significant clusters.
Fearful versus Neutral Distractors
In order to examine group differences in neural regions involved in ignoring task-irrelevant fearful versus neutral faces, we conducted a whole-brain ANOVA (Group [MDD, CTL] repeated over Distractor Valence [Fearful, Neutral]). This voxel-wise ANOVA yielded a significant interaction of group and distractor valence in four large clusters: dACC, right IFG/middle frontal gyrus (IFG/MFG), left lateral occipital cortex, and right lateral occipital cortex/superior parietal lobule (SPL; see Table 2 for peak coordinates and statistics). Follow-up t-tests were conducted to determine the nature of the interaction in each of the four clusters. Across all four clusters, the MDD group showed significantly greater recruitment of these regions than did the CTL group when ignoring fearful relative to neutral face distractors (ts(37)<2.493, ps<.017). In the dACC and right lateral occipital/SPL, the MDD group compared to the CTL group showed reduced activation when ignoring neutral faces (ts(37)>2.265 ps<.029; see Figure 1). Furthermore, among the MDD only there was significantly greater activation in all four clusters when ignoring fear versus neutral faces (ts(17)>2.142, ps<.047), whereas among the CTL group only there was no significant differences between ignoring fear versus neutral faces (ts(20)<1.589, ps>.128; see Figure 1).
Table 2.
Cluster peaks for significant group (CTL, MDD) by distractor valence (neutral, fearful) interaction.
| Brain Region | MNI Coordinates | Z-Value | # of voxels | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| R lateral occipital cortex/superior parietal lobule | 34 | -66 | 28 | 4.15 | 2273 |
| R MFG/IFG | 56 | 10 | 38 | 4.15 | 1786 |
| L lateral occipital cortex | -22 | -66 | 30 | 4.44 | 1723 |
| Anterior cingulate cortex | -10 | 38 | 20 | 3.70 | 1292 |
Note: Coordinates represent voxels in each region with the most significant magnitude, identified at Z>2.3, with a (corrected) cluster-significance threshold of p = .05. MNI = Montreal Neurological Institute (RPI)). Voxel size is 3.4 × 3.4 × 4 mm.
Figure 1.
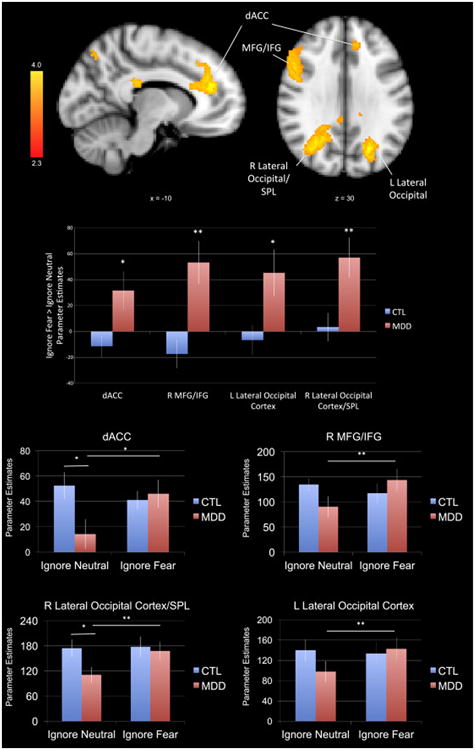
A group (MDD, CTL) by distractor valence (fearful, neutral) analysis of variance yielded greater activation in four clusters encompassing dACC, left MFG/IFG, left lateral occipital gyrus and right lateral occipital gyrus/superior parietal lobule during successful ignore fearful relative to ignore neutral trials in the MDD group relative to the CTL group. Activation maps (left) are thresholded at Z>2.3 and corrected for multiple comparisons using a cluster-based p<.05. MNI coordinates are indicated for slice distance (in mm). Parameter estimates (showing the amount of signal change measured in arbitrary units) of BOLD signal response were extracted from each significant cluster and plotted in the bar graph. We present the parameter estimates for the contrast of ignore fear > ignore neutral for each ROI in order to be consistent with previous studies that have used a similar task. We also present the parameter estimates for ignore fear and ignore neutral conditions separately for each ROI. * p<.05 and ** p<.01. MDD = participants with Major Depressive Disorder; CTL = control participants; dACC = dorsal anterior cingulate cortex; MFG = medial frontal gyrus; IFG = inferior frontal gyrus.
Fearful versus Sad Distractors
Using a whole-brain 2 (Group [MDD, CTL]) × 2 (Distractor Valence [Fear, Sad] and [Sad, Fear]) ANOVA, we examined group differences in neural regions involved in ignoring task-irrelevant fear versus sad faces. These voxel-wise ANOVAs yielded no significant clusters.
Correlation of Neural Activation with Task Performance
There was no significant relation across or within groups between task accuracy and parameter estimates in any ROI. In contrast, however, analyses of response time yielded similar findings for both fearful and neutral distractor faces: for all participants, greater recruitment of the IFG/MFG and bilateral lateral occipital clusters while ignoring these irrelevant stimuli was associated with a faster response to indicate whether the two houses on each trial were identical (see Table 3). There was no association between response times and parameter estimates for dACC activation for either valence of the task-irrelevant face stimuli.
Table 3. RT – Parameter Estimate Correlations.
| dACC | L lsateral occipital | R lateral occipital | R MFG/IFG | |
|---|---|---|---|---|
| Ignore neutral RT | ||||
| r | -0.048 | -0.316* | -0.41** | -0.374* |
| p-value | 0.770 | 0.050 | 0.010 | 0.019 |
| Ignore fearful RT | ||||
| r | -0.195 | -0.418** | -0.458** | -0.500** |
| p-value | 0.234 | 0.008 | 0.003 | 0.001 |
p ≤ .05
p ≤ .01
Correlation of Neural Activation with Depression/Anxiety Symptoms
None of the correlations between levels of neural activation in regions that showed within- or between-groups differences during successful ignoring of fearful versus neutral face distractors and symptoms of depression and anxiety were statistically significant. The results of these analyses are presented in the Supplement.
Discussion
This is the first study to use fMRI to directly examine cognitive inhibition in the context of processing both mood-congruent and threat-related stimuli in adolescents with MDD. Although both of these classes of stimuli have been implicated in adult models of MDD, our findings suggest that down-regulating threat-related stimuli (i.e., fearful faces) evokes greater neural challenge than did regulating mood-congruent stimuli in brain regions related to cognitive control and visual attention in adolescents with MDD. Despite equivalent behavioral performance (response times and accuracy) between groups, depressed adolescents showed greater activation in frontocingulate cognitive control regions, including the IFG/MFG and dACC, and occipitoparietal regions involved in visual attention, including bilateral lateral occipital cortices and right SPL, when ignoring fearful versus neutral distractors (Fig. 1). Surprisingly, however, when separating neural activation for fear neutral distractor trials from activation for neutral distractor trials, we found that interaction of group and valence was driven by fear relative to neutral distractors within the MDD group. Further, the only group differences were in the neutral condition, in which CTL adolescents showed greater activation in dACC and right SPL than did MDD adolescents. Our results build on earlier work concerning the role of frontocingulate dysfunction in cognitive-emotional models of MDD, suggest refinements of these models to include occipitoparietal attention regions, and highlight important directions for future work.
In the present study, depressed adolescents exhibited hyperactivation in the dACC, IFG, and MFG when ignoring fearful relative to neutral distractors compared to healthy controls (Fig. 1). Whereas dACC activity has been found to be associated with conflict monitoring, attention allocation, and the integration of task-relevant information (41,42), recruitment of IFG/MFG is associated more strongly with cognitive control (20). Our finding of greater recruitment of these regions for depressed adolescents in the presence of fearful relative to neutral distractors suggests that, compared with their nondepressed peers, depressed adolescents experience heightened conflict monitoring and attentional demands when attempting to ignore irrelevant yet salient face distractors, and a greater need for sustained attention to the task-relevant stimuli in order to achieve equivalent levels of behavioral performance. Moreover, our results are consistent with patterns of increased ACC activation in depressed adults relative to healthy controls when successfully inhibiting negative word distractors (43), suggesting heightened conflict monitoring in both depressed adults and adolescents. There are discrepancies between hyper- and hypo-recruitment of cognitive control regions in MDD, however, in studies of both adult and adolescent MDD (19,44). Interestingly, whereas we found hyperactivity in IFG/MFG in MDD participants when they ignored task-irrelevant fear faces, we previously found hypoactivity in DLPFC in an overlapping sample of MDD adolescents when they were primed with sad and happy faces before performing a less cognitively demanding response inhibition task (22). Together, these findings point to nuanced functional differences among prefrontal regions that may be sensitive to task demands, with hypoactive DLPFC reflecting response inhibition deficits (45) and hyperactive IFG/MFG reflecting the need for more cognitive resources in depressed adolescents.
Contrary to our predictions, we did not find group differences in amygdala or other regions involved in emotion generation in either the task-irrelevant sad or fearful conditions, compared with the task-irrelevant neutral condition. The processing of emotional faces activates brain regions at multiple levels of information processing, including not only limbic structures such as the amygdala, but also visual areas related to stimulus processing and attention, including occipital and parietal regions involved early in the processing stream (46,47,48). In our study, depressed adolescents exhibited heightened recruitment of the lateral occipital cortex and SPL, suggesting that depressed adolescents exhibit greater interference in the presence of threat-related stimuli and, consequently, require greater neural recruitment of these regions to disengage from task-irrelevant stimuli. Importantly, recent work with both adults and adolescents with MDD has highlighted functional abnormalities in regions related to visual processing and attention (49,50,51,52) during emotional face processing. The documentation of direct white matter projections between frontal and occipital regions in humans (53) further indicates that these regions and connections may be important to study in MDD. Thus, our results suggest that models of MDD-related cognitive biases for negative material should incorporate not only frontocingulate cognitive control regions, but also their interactions with occipitoparietal visual attention regions.
Our interpretations of greater recruitment in cognitive control and visual attention regions during successful inhibition of salient negative emotional distractors relative to neutral distractors in adolescent MDD are further supported by significant correlations between neural activation in IFG/MFG and bilateral lateral occipital cortices and response times during the neutral and fear conditions. Given the strong negative correlations between activation in these regions and response times, it is likely that these regions facilitate attention to task-relevant stimuli (houses), as opposed to task-irrelevant stimuli (distracting faces). Importantly, however, the lack of a significant association between recruitment of dACC and response times during these task conditions suggests a different function of the dACC. In this context, it is possible that dACC recruitment reflects conflict monitoring and the integration of task information, and thus plays a role in allocating resources according to task demands (54).
We did not find either behavioral or neural within- or between-group differences in the sad versus neutral face distractor contrast. It is important to note, however, that the stimuli were presented for only 250ms. Thus, our findings are consistent with previous work demonstrating that biases toward depressogenic stimuli occur only when stimuli are presented for longer durations (typically greater than 1000ms; 23,55); in contrast, biases towards threat-related stimuli typically occur when stimuli are presented for shorter durations (typically 500ms or less; 56). It is possible, therefore, that we would have found greater interference for sad distractors in the MDD group if we had used longer durations for stimulus presentation. Of course, altering the task design in this way would have changed the cognitive demands of the task and would likely have resulted in ceiling-level performance for both groups, eliminating significant group differences in neural regions underlying attentional and cognitive control processes.
Similarly, it is important to raise the issue of possible differences in levels of arousal between fear and sad face stimuli. Prior research using the same stimuli suggests that fearful and sad faces are equivalent in terms of valence, but that fearful faces are more arousing than sad faces (57,58). Further, there is evidence that arousal levels influence attention or perception (59). Thus, it is possible that fearful faces evoked higher levels of arousal in the depressed participants and that the differences in neural activation between ignoring fear and ignoring sad distractors relative to neutral distractors are due to differences in arousal level. As a related point, highly arousing positively valenced faces may elicit similar effects as fearful faces, which is consistent with our previous finding of hypoactivation in DLPFC to sad relative to happy face primes (22). Unfortunately, we did not obtain valence and arousal ratings from our participants, nor did we include positively valenced faces in this task. Future studies should obtain ratings of valence and arousal for all stimuli in order to examine whether neutral patterns are attributable to valence or arousal, and/or to depression-related effects on cognitive control and attention to negatively valenced stimuli.
It is noteworthy that the different patterns of neural activation exhibited by the MDD and CTL participants when ignoring fearful versus neutral distractors are consistent with previous reports of differences in selective attention to threat-related stimuli in adolescents and adults with anxiety and comorbid anxiety/depression, but not in adolescents/adults with pure depression (9,60). Although half of our depressed adolescent participants had a comorbid anxiety disorder, our findings did not differ as a function of this comorbidity (Table S3). Furthermore, the rate of comorbid anxiety disorders in our depressed sample is similar to the rate at which adolescent depression presents with comorbid anxiety in the general community (60%; 61,62). Our depressed adolescent sample thus reflects the distribution of anxiety disorders in the population of adolescents with depression typically seen in outpatient clinics, strengthening the generalizability of our results. Nevertheless, it will be important in future research to recruit and assess the neural functioning of separate samples of adolescents with pure and comorbid MDD.
Finally, it is important to note that when examining neural activation for fear and neutral distractor trials separately, group differences were present in the neutral condition where CTL adolescents showed greater activation in dACC and right SPL when ignoring neutral distractors than adolescents with MDD. Several studies have demonstrated that neutral facial expressions are not always processed as neutral (63), particularly in children (64,65,66), but also in clinical populations (67,68). These studies, however, involve attending to emotional faces rather than actively avoiding emotional distractors. Thus, it may be that CTLs require greater neural recruitment to inhibit processing of ambiguous neutral faces in order to accurately perform the task. It will be important in future studies that researchers consider whether neutral faces should be used to represent a neutral control condition.
Although this is the first study to carefully assess different negative stimuli to better understand the neural mechanisms underlying cognitive inhibition of emotional distractors, there are three primary limitations of this investigation. First, our sample size is relatively small, and we were underpowered to examine effects of comorbidity/medication on our findings. Second, half of our MDD participants were taking psychotropic medication. Although we found no significant differences in neural activation between the medicated and unmedicated adolescents (Supplemental Table S4), medication status may nevertheless have contributed to our findings (26,69). Third, given the high accuracy rates on our task, we did not have a sufficient number of error trials for each individual to analyze neural activations to incorrect responses. It is possible that hyper-recruitment of cognitive control regions underlies successful inhibition only of irrelevant stimuli, and that if our models included inaccurate trials, we would have found the opposite pattern. It will be important in future research to include more trials across all conditions to ensure that there will be a sufficient number of error trials to be included in the analyses.
Despite these limitations, this study documents aberrant function in frontocingulate and occipitoparietal regions underlying cognitive processing when depressed adolescents attempt to ignore distracting negative stimuli. These findings suggest heightened conflict monitoring and attentional demands when depressed adolescents successfully inhibit salient negative stimuli. These results are important in refining current neural models of adolescent MDD, including highlighting the role of visual attention regions along with cognitive control regions, and identifying neural processes that may contribute to negative cognitive biases in adolescent MDD.
Supplementary Material
Acknowledgments
We thank Maria Lemus for her assistance in scheduling and running the participants. We also thank the adolescents and their families who participated in our study. This work was supported by an NSF Graduate Fellowship to NLC, by a grant from the American Foundation for Suicide Prevention PDF-1-064-13 to TCH, by NIMH grants R01MH101545 to IHG, F32MH090617 to LCFR, K01MH106805 to SJO, and K23MH085919 and R01MH106581 to MKS, by the National Alliance for Research in Schizophrenia and Affective Disorders Distinguished Investigator Award to IHG and Young Investigator Awards to LCFR and SJO, by the Hope for Depression Research Foundation to IHG and LCFR, and by the Klingenstein Third Generation Foundation Fellowship Award to SJO.
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major Depression in the National Comorbidity Survey–Adolescent Supplement: Prevalence, Correlates, and Treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37–44.e2. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/S0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- 3.Lewinsohn PM, Rohde P, Seeley JR. Treatment of adolescent depression: Frequency of services and impact on functioning in young adulthood. Depress Anxiety. 1998;7(1):47–52. doi: 10.1002/(sici)1520-6394(1998)7:1<47::aid-da6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Rao U, Hammen C, Daley SE. Continuity of depression during the transition to adulthood: a 5-year longitudinal study of young women. J Am Acad Child Adolesc Psychiatry. 1999;38(7):908–915. doi: 10.1097/00004583-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Curry J, Silva S, Rohde P, et al. Recovery and recurrence following treatment for adolescent major depression. Arch Gen Psychiatry. 2011;68(3):263–269. doi: 10.1001/archgenpsychiatry.2010.150.Recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: A meta-analysis. Psychol Bull. 2006;132(1):132–149. doi: 10.1037/0033-2909.132.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. Philadelphia: University of Pennsylvania Press; 1967. [Google Scholar]
- 8.Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cogn Emot. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hankin BL, Gibb BE, Abela JRZ, Flory K. Selective attention to affective stimuli and clinical depression among youths: role of anxiety and specificity of emotion. J Abnorm Psychol. 2010;119(3):491–501. doi: 10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey BJ. Altered Emotional Processing in Pediatric Anxiety, Depression, and Comorbid Anxiety-Depression. J Abnorm Child Psychol. 2005;33(2):165–177. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- 11.Disner SG, Beevers CG, Haigh EaP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 12.Fales CL, Barch DM, Rundle MM, et al. Altered Emotional Interference Processing in Affective and Cognitive-Control Brain Circuitry in Major Depression. Biol Psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, LaBar KS, Smoski M, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 15.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diler RS, Pan LA, Segreti A, Ladouceur CD, Forbes E, Cela SR, et al. Differential Anterior Cingulate Activity during Response Inhibition in Depressed Adolescents with Bipolar and Unipolar Major Depressive Disorder. J Can Acad Child Adolesc Psychiatry. 2014;23(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- 17.Halari R, Simic M, Pariante CM, Papdopoulos A, Cleare A, Brammer M, et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. J Child Psychol Psychiatry. 2009;50(3):307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- 18.Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5(4):307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry. 2015;72(10):1045–1053. doi: 10.1001/jamapsychiatry.2015.1376. [DOI] [PubMed] [Google Scholar]
- 20.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 2013;33(46):18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colich NL, Foland-Ross LC, Eggleston C, Singh MK, Gotlib IH. Neural Aspects of Inhibition Following Emotional Primes in Depressed Adolescents. J Clin Child Adolesc Psychol. 2015;4416:1–10. doi: 10.1080/15374416.2014.982281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews A, MacLeod C. Cognitive Vulnerability to Emotional Disorders. Annu Rev Clin Psychol. 2005;1(1):167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 24.Beesdo K, Lau JYF, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, et al. Amygdala Function and 5-HTT Gene Variants in Adolescent Anxiety and Major Depressive Disorder. Biol Psychiatry. 2009;65(4):349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, et al. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169(4):381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/S0006-3223(01)01263-X. [DOI] [PubMed] [Google Scholar]
- 28.Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington DC: American Psychiatric Press; 2000. text rev. [Google Scholar]
- 30.Allgaier AK, Frühe B, Pietsch K, Saravo B, Baethmann M, Schulte-Körne G. Is the Children's Depression Inventory Short version a valid screening tool in pediatric care? A comparison to its full-length version. J Psychosom Res. 2012;73(5):369–374. doi: 10.1016/j.jpsychores.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman J, Birmaher B, Brent D, Rau U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 33.Marshall Wa, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall Wa, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuilleumier P, Vuilleumier P, Armony JL, Driver J, Dolan RJ, et al. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/S0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 36.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 37.Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 38.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 39.Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 40.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 42.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/CABN.3.4.255. [DOI] [PubMed] [Google Scholar]
- 43.Eugène F, Joormann J, Cooney RE, Atlas LY, Gotlib IH. Neural correlates of inhibitory deficits in depression. Psychiatry Res. 2010;181(1):30–35. doi: 10.1016/j.pscychresns.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61(3):677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Kadota H, Sekiguchi H, Takeuchi S, Miyazaki M, Kohno Y, Nakajima Y. The role of the dorsolateral prefrontal cortex in the inhibition of stereotyped responses. Exp Brain Res. 2010;203(3):593–600. doi: 10.1007/s00221-010-2269-4. [DOI] [PubMed] [Google Scholar]
- 46.Beevers CG, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: A functional magnetic resonance imaging study. Neuroscience. 2010;167(1):97–103. doi: 10.1016/j.neuroscience.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 48.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc Cogn Affect Neurosci. 2009;4(4):387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henje Blom E, Connolly CG, Ho TC, LeWinn KZ, Mobayed N, Han L, Paulus MP, et al. Altered insular activation and increased insular functional connectivity during sad and happy face processing in adolescent major depressive disorder. J Affect Disord. 2015;178:215–223. doi: 10.1016/j.jad.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, et al. Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biol Psychiatry. 2015;9(1):635–646. doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho TC, Zhang S, Sacchet MD, Weng H, Connolly CG, Henje Blom E, et al. Fusiform Gyrus Dysfunction is Associated with Perceptual Processing Efficiency to Emotional Faces in Adolescent Depression: A Model-Based Approach. Front Psychol. 2016;1(7):40. doi: 10.3389/fpsyg.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1(10):1–17. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forkel SJ, Thiebaut de Schotten M, Kawadler JM, Dell'Acqua F, Danek A, Catani M. The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex. 2014;56:73–84. doi: 10.1016/j.cortex.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Shenhav A, Botvinick M, Cohen J. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. J Abnorm Psychol. 2004;113(3):386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- 56.Frewen PA, Dozois DJA, Joanisse MF, Neufeld RWJ. Selective attention to threat versus reward: meta-analysis and neural-network modeling of the dot-probe task. Clin Psychol Rev. 2008;28(2):307–337. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Johnsen BH, Thayer JF, Hugdahl K. Affective judgment of the Ekman faces: A dimensional approach. J Psychophysiol. 1995;9(3):193–202. [Google Scholar]
- 58.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, FL: University of Florida; 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instructional Manual. [Google Scholar]
- 59.Mather M, Sutherland MR. Arousal-Biased Competition in Perception and Memory. Perspect Psychol Sci. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kircanski K, Joormann J, Gotlib IH. Attention to Emotional Information in Social Anxiety Disorder With and Without Co-Occurring Depression. Cognit Ther Res. 2015;39(2):153–161. doi: 10.1007/s10608-014-9643-7. [DOI] [Google Scholar]
- 61.Essau CA. Comorbidity of depressive disorders among adolescents in community and clinical settings. Psychiatry Res. 2008;158(1):35–42. doi: 10.1016/j.psychres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Kessler RC, Avenevoli S, Costello EJ, et al. Prevalence, Persistence, and Sociodemographic Correlates of DSM-IV Disorders in the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2012;69(4):372–380. doi: 10.1001/archgenpsychiatry.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biol Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Thomas KM, Drevets WC, Whalen PJ, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49(4):309–316. doi: 10.1016/S0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 65.Tottenham N, Phuong J, Flannery J, Gabard-durnam L, Goff B. A Negativity BIas for Ambiguous Facial Expression Valence during Childhood: Converging Evidence from Behavior Facial Corrugator Muscle Responses. Emotion. 2014;13(1):92–103. doi: 10.1037/a0029431.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lobaugh NJ, Gibson E, Taylor MJ. Children recruit distinct neural systems for implicit emotional face processing. Neuroreport. 2006;17(2):215–219. doi: 10.1097/01.wnr.0000198946.00445.2f. [DOI] [PubMed] [Google Scholar]
- 67.Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Res - Neuroimaging. 2006;148(1):55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Yoon KL, Zinbarg RE. Interpreting neutral faces as threatening is a default mode for socially anxious individuals. J Abnorm Psychol. 2008;117(3):680–685. doi: 10.1037/0021-843X.117.3.680. [DOI] [PubMed] [Google Scholar]
- 69.Singh MK, Chang KD. The Neural Effects of Psychotropic Medications in Children and Adolescents. Child Adolesc Psychiatr Clin N Am. 2012;21(4):753–771. doi: 10.1016/j.chc.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


