Abstract
Here I identify two gaps in cochlear implants that have been limiting their performance and acceptance. First, cochlear implant performance has remained largely unchanged, despite the number of publications tripling per decade in the last 30 years. Little has been done so far to address a fundamental limitation in the electrode-to-neuron interface, with the electrode size being a thousand times larger than the neuron diameter while the number of electrodes being a thousand times less. Both the small number and the large size of electrodes produce broad spatial activation and poor frequency resolution that limit current cochlear implant performance. Second, a similarly rapid growth in cochlear implant volume has not produced an expected decrease in unit price in the same period. The high cost contributes to low market penetration rate, which is about 20% in developed countries and less than 1% in developing countries. I will discuss changes needed in both research strategy and business practice to close the gap between prosthetic and normal hearing as well as that between haves and have-nots.
Index Terms: Biotechnology, Cochlear implant, Man-machine interface, Monopoly, Neural Engineering, Prosthetics, Public healthcare, Supply and demand
I. Performance vs. publication
Figure 1 shows that the annual number of cochlear implant related publications grew linearly from 1980 to 1995, then grew exponentially in each succeeding decade. Following the linear growth trend in publications, the average cochlear implant performance improved from essentially 0% recognition with the single-electrode 3M/House device in early 1980s to ~80% correct in the middle 1990s, largely thanks to advances in signal processing [1, 2]. In contrast to the exponential growth trend in publications, cochlear implant performance has remained largely unchanged in the last 30 years. Indeed, efforts in developing new stimulation strategies such as fine structure coding, current steering, and electric field focusing have not led to significant improvement in cochlear implant performance [3–6]. Other efforts focused on front-end sound processing from directional microphone to noise reduction, and cosmetic improvement from colorful looks to water resistance. Arguably, the two most successful applications during the last two decades were likely the adoption of bilateral cochlear implants to improve mostly sound localization [7, 8], and the combination with a hearing aid to complement and enhance cochlear implant speech performance in patients who have residual low-frequency acoustic hearing [9, 10].
Fig. 1.
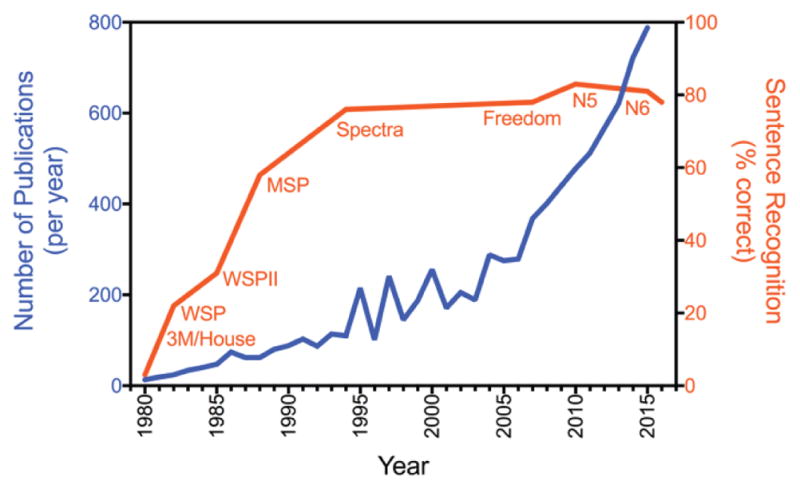
Contrast between cochlear implant research and performance measures. The annual number of cochlear implant related publications (blue line with units on the left y-axis) and the average implant user’s sentence recognition (orange line with percent correct scores on the right y-axis) is plotted as a function of year since 1980. The number of publications was retrieved from PubMed on November 28, 2016 (www.pubmed.gov using “cochlear” and “implant” as keywords). Cochlear implant performance used percent sentence recognition in quiet scores including the 3M/House and Cochlear’s devices from the early Wearable Speech Processor (WSP) and Mini Speech Processor (MSP) to more recent Spectra and Freedom processors [11] and the latest N5 [12] and N6 processors (FDA Premarket Approval P130016). Data from N5 and N6 used AzBio scores, which were converted into HINT scores using Figure 7B [12]. Devices from other manufacturers produced similar performance [13].
II. The electrode-to-neuron interface problem
I believe the root cause of the lack of improvement in speech understanding is the lack of progress in the design of cochlear electrodes. In the last 30 years, electrode contacts have changed shape from balls and rings to plates, while electrode arrays have evolved to have different length, thickness or straight vs. curved forms [14, 15], but none has changed the size and number mismatch between electrodes and neurons. Figure 2 shows that there is a 1,000 times mismatch in both the size (1 μm vs. 1 mm) and the number (12–24 vs. 35,000) between present electrodes and auditory neurons. This mismatch in electrode-to-neuron interface, compounded further by the degraded nature of auditory neurons with as much as 95% reduction in numbers in actual patients who obtain cochlear implants [16, 17], is responsible for current implant users’ poor frequency resolution [18] and the significant gaps between normal and prosthetic hearing [11, 19]. There is no evidence that current cochlear implant users have produced a pitch percept equivalent to a pure tone in normal hearing, let alone harmonic pitches that require separation of several pure tones with positive integer multiples of a fundamental frequency. The same interface problem underlies all other neural prostheses.
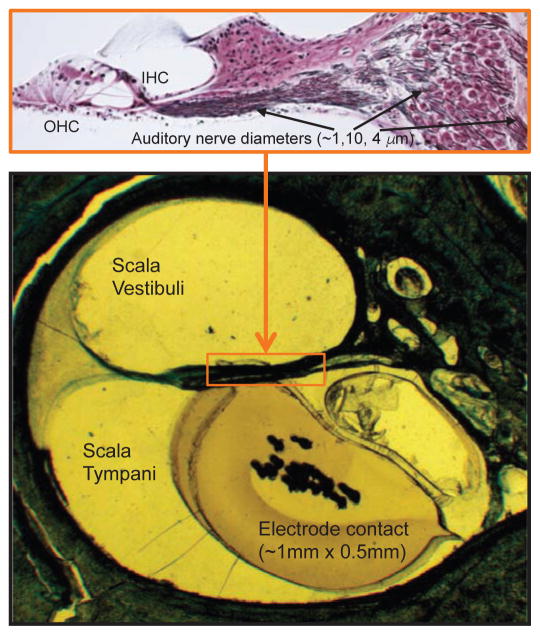
Fig. 2.
Mismatch between cochlear electrodes and auditory neurons. The bottom panel shows an electrode contact of the Nurotron device inserted in a cadaver human temporal bone [13]. The top panel shows the enlarged organ of Corti where outer hair cells (OHC) and inner hair cells (IHC) are displayed on the left, with 95% of the auditory neurons, displayed on the right, being connected to the inner hair cells (picture courtesy of MC Liberman). The diameter is about 1 μm for the terminal process of the auditory neurons, 10 μm for the cell body, and 4 μm for the central process projecting to the brain. Each inner hair cell is innervated by 10–20 auditory neurons, which have the same characteristic frequency but different spontaneous discharges, thresholds and dynamic ranges [20].
III. Volume vs. price
To demonstrate the second gap, I used publically available data from the Cochlear Corporation, which is currently the largest cochlear implant manufacturer and which accounts for 60–70% of market share worldwide. Figure 3 shows that the market size has been growing exponentially, tripling the volume per decade, since the early 1990s. However, the exponential growth in volume has not produced a significant decrease in unit price. I believe this disconnected relationship between volume and price is due to a lack of competition in the market. This high price has severely limited accessibility and affordability of cochlear implants. For example, there is still a 30-fold gap between annual income ($1000 or less) in developing countries and the unit price. Even in the United States, Medicare reimburses ~$20,000 for a cochlear implant, leaving hospitals losing money for operating on a Medicare patient, patients paying a significant balance from their pocket, or patients having to settle for lowered quality of life for not being able to afford the device. At present, although as many as 14 million people worldwide can potentially benefit from the cochlear implant, the current market penetration is still about 20% in developed countries and less than 1% in developing countries [21].
Fig. 3.
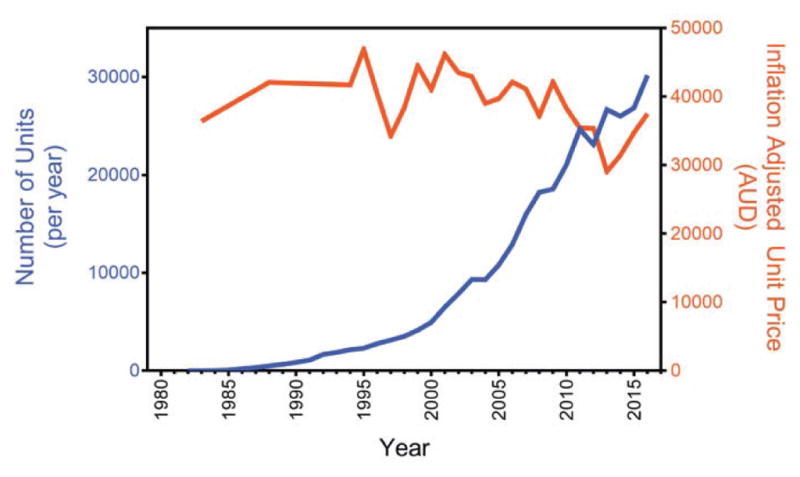
Contrast between cochlear implant volume and price. The number of annual cochlear implants sold by Cochlear Corporation (blue line with units on the left y-axis) and the unit price (orange line with units on the right y-axis) is plotted as a function of year. The unit price was estimated by dividing the annual revenue by the annual volume and had been adjusted for inflation according to 2016 Australian (AUD) dollars. Data from 1994 to 2016 were extracted from Cochlear Annual Reports (http://www.cochlear.com/wps/wcm/connect/intl/about/investor/annual-reports). Earlier data were extracted other sources [11].
IV. Solutions
Most recent research has focused on expanding the cochlear implant utility and indications, with little being done to fundamentally solve the electrode-to-neuron interface problem. Relatively minor improvements included pre-curved electrode arrays to get stimulation sites closer to the cochlear modiolus, thus to the auditory neurons [22] and atraumatic surgical techniques to preserve and promote the health of residual auditory neurons [23]. Several innovative lines of research are also emerging to change this direction, such as injecting neurotrophin or stem cells to attract neurons to grow toward electrodes [24, 25], using needle or thin-film electrodes to penetrate the auditory nerve bundle [26, 27], or optogenetically stimulating the auditory nerve [28]. Unfortunately, these new strategies are still in their exploratory stages and are years, if not decades, away from human application. A contract to develop a promising approach, i.e., penetrating electrodes, was terminated prematurely by the NIH (N263-2009-00024C) because no manufacturer was willing to take it to a human clinical trial.
While the price of cochlear implants has remained relatively unchanged worldwide, the price has come down at least in one market recently. With approval of a high-quality domestic device in China in 2011 [13], the unit cost for both domestic and foreign devices, reflected by the Chinese government tender program, dropped from US$25,000 in 2011 to US$6,030 in 2016. In 2017, the unit price was further dropped to US$5,490, with all four companies, i.e., Advanced Bionics, Cochlear, Med El and Nurotron, competing for the tender and three of them winning a tender at a similar price. This drop in unit price means that the same amount of money, which helped only one deaf child five years ago, can now help four or five of them now. The exponentially decreasing trend in the unit price and the active participation by multiple companies in the tender program further suggest that the current low price is unlikely to be a one-shot event to gain the market share, rather the low price is sustainable and will likely spread from only one market to the rest of the world. To ultimately address the performance and affordability gaps, biomedical engineers need to pursue innovative research, manufacturers need to undertake risky research and development projects, and regulatory agencies need to foster competition.
V. Conclusion
Cochlear implants were developed by visionary and bold physicians and engineers, and are now widely considered as the most successful neural prosthesis. Although the cochlear implant has initially served as the catalyst for many other applications from restoring vision to relieving pain, the cochlear implant field, as a whole, has become quite conservative and incremental recently. Lessons learned and solutions proposed here will help not only push forward the cochlear implant field but also apply to the other neural implants. It is even more important than ever to keep pushing the technology, science, regulatory and public aspects to improve these neural implants, given the emergent needs and new efforts in this realm across government, industry and academia, e.g., the BRAIN Initiative, DARPA Neuroprosthetics, and NIH Stimulating Peripheral Activity to Relieve Conditions (SPARC) programs.
Acknowledgments
The work was partially supported by the National Institutes of Health (P30-DC008369 and RO1-DC015587) and the Thomas and Misako Yuen Family Foundation.
The author thanks Phillip Tran, Bin He and two anonymous reviewers for comments on the manuscript. The conclusion section of the manuscript was based on comments from the reviewers. The author also thanks Charlie Liberman for providing a high-resolution picture of the organ of Corti used in Fig. 2 and discussing physical properties of the auditory neurons. The author has an equity interest in the Nurotron Biotechnology Inc., whose business is related to work discussed here. The terms of his interest have been reviewed and approved by the University of California, Irvine, in accordance with its conflict of interest policies.
Biography
 Fan-Gang Zeng (S’88-M’91-SM’07-F’11) is Director of the Center for Hearing Research and Professor of Anatomy and Neurobiology, Biomedical Engineering, Cognitive Sciences and Otolaryngology – Head and Neck Surgery at the University of California Irvine. He is a leading researcher in hearing science and technology, with 236 publications, 10523 citations and an h-index of 50 (Google Scholar, May 3, 2017). He led development of the Nurotron 26-electrode cochlear implant (SFDA approval in 2011 and CE Mark in 2012) and SoundCure tinnitus suppressor (FDA clearance and CE Mark in 2011). He has consulted for NIH, NSF, DOD, and numerous other public and private agencies. He is the Editorial Board’s Chairman of the Hearing Journal and has been on the editorial board for 6 academic magazines. He severed as the Chair of the 2005 International Conference for Auditory Prostheses and edited 3 volumes on cochlear implants and tinnitus for Springer Handbook of Auditory Research. He holds 12 patents and has been on the Advisory Board and helped raise $80M for 5 medical device companies in US and China..
Fan-Gang Zeng (S’88-M’91-SM’07-F’11) is Director of the Center for Hearing Research and Professor of Anatomy and Neurobiology, Biomedical Engineering, Cognitive Sciences and Otolaryngology – Head and Neck Surgery at the University of California Irvine. He is a leading researcher in hearing science and technology, with 236 publications, 10523 citations and an h-index of 50 (Google Scholar, May 3, 2017). He led development of the Nurotron 26-electrode cochlear implant (SFDA approval in 2011 and CE Mark in 2012) and SoundCure tinnitus suppressor (FDA clearance and CE Mark in 2011). He has consulted for NIH, NSF, DOD, and numerous other public and private agencies. He is the Editorial Board’s Chairman of the Hearing Journal and has been on the editorial board for 6 academic magazines. He severed as the Chair of the 2005 International Conference for Auditory Prostheses and edited 3 volumes on cochlear implants and tinnitus for Springer Handbook of Auditory Research. He holds 12 patents and has been on the Advisory Board and helped raise $80M for 5 medical device companies in US and China..
References
- 1.Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991 Jul 18;352:236–8. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- 2.Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995 Oct 13;270:303–4. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- 3.Magnusson L. Comparison of the fine structure processing (FSP) strategy and the CIS strategy used in the MED-EL cochlear implant system: speech intelligibility and music sound quality. Int J Audiol. 2011 Apr;50:279–87. doi: 10.3109/14992027.2010.537378. [DOI] [PubMed] [Google Scholar]
- 4.Firszt JB, Holden LK, Reeder RM, Skinner MW. Speech recognition in cochlear implant recipients: comparison of standard HiRes and HiRes 120 sound processing. Otol Neurotol. 2009 Feb;30:146–52. doi: 10.1097/MAO.0b013e3181924ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buechner A, Brendel M, Krueger B, Frohne-Buchner C, Nogueira W, Edler B, et al. Current steering and results from novel speech coding strategies. Otol Neurotol. 2008 Feb;29:203–7. doi: 10.1097/mao.0b013e318163746. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson GS, Dawson PK, Borden LZ. Within-subjects comparison of the HiRes and Fidelity120 speech processing strategies: speech perception and its relation to place-pitch sensitivity. Ear Hear. 2011 Mar-Apr;32:238–50. doi: 10.1097/AUD.0b013e3181fb8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litovsky RY, Parkinson A, Arcaroli J, Peters R, Lake J, Johnstone P, et al. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004 May;130:648–55. doi: 10.1001/archotol.130.5.648. [DOI] [PubMed] [Google Scholar]
- 8.van Hoesel RJ, Tyler RS. Speech perception, localization, and lateralization with bilateral cochlear implants. J Acoust Soc Am. 2003 Mar;113:1617–30. doi: 10.1121/1.1539520. [DOI] [PubMed] [Google Scholar]
- 9.von Ilberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Sturzebecher E, et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999 Nov-Dec;61:334–40. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- 10.Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hearing Research. 2008 Aug;242:164–71. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng FG, Rebscher S, Harrison W, Sun X, Feng HH. Cochlear Implants: System Design, Integration and Evaluation. IEEE Rev Biomed Eng. 2008;1:115–142. doi: 10.1109/RBME.2008.2008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runge CL, Henion K, Tarima S, Beiter A, Zwolan TA. Clinical Outcomes of the Cochlear Nucleus((R)) 5 Cochlear Implant System and SmartSound 2 Signal Processing. J Am Acad Audiol. 2016 Jun;27:425–40. doi: 10.3766/jaaa.15021. [DOI] [PubMed] [Google Scholar]
- 13.Zeng FG, Rebscher SJ, Fu QJ, Chen H, Sun X, Yin L, et al. Development and evaluation of the Nurotron 26-electrode cochlear implant system. Hear Res. 2015 Apr;322:188–199. doi: 10.1016/j.heares.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Spelman FA. Cochlear electrode arrays: past, present and future. Audiol Neurootol. 2006;11:77–85. doi: 10.1159/000090680. [DOI] [PubMed] [Google Scholar]
- 15.Rebscher SJ, Hetherington A, Bonham B, Wardrop P, Whinney D, Leake PA. Considerations for design of future cochlear implant electrode arrays: electrode array stiffness, size, and depth of insertion. J Rehabil Res Dev. 2008;45:731–47. doi: 10.1682/jrrd.2007.08.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006 Aug;116:1310–20. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 17.Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997 Sep;117:220–8. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- 18.Zeng FG, Tang Q, Lu T. Abnormal pitch perception produced by cochlear implant stimulation. PLoS One. 2014;9:e88662. doi: 10.1371/journal.pone.0088662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson BS. A Quest for Quality: Closing Contrasts Between Prosthetic and Normal Hearing. Hearing Journal. 2016;69:10–12. [Google Scholar]
- 20.Liberman MC. Single-neuron labeling in the cat auditory nerve. Science. 1982 Jun 11;216:1239–41. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- 21.Zeng FG. Cochlear implants: Why don’t more people use them? Hearing Journal. 2007 Mar;60:48–49. [Google Scholar]
- 22.Briggs RJ, Tykocinski M, Lazsig R, Aschendorff A, Lenarz T, Stover T, et al. Development and evaluation of the modiolar research array--multi-centre collaborative study in human temporal bones. Cochlear Implants Int. 2011 Aug;12:129–39. doi: 10.1179/1754762811Y0000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshraghi AA. Prevention of cochlear implant electrode damage. Curr Opin Otolaryngol Head Neck Surg. 2006 Oct;14:323–8. doi: 10.1097/01.moo.0000244189.74431.df. [DOI] [PubMed] [Google Scholar]
- 24.Pinyon JL, Tadros SF, Froud KE, ACYW, Tompson IT, Crawford EN, et al. Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Sci Transl Med. 2014 Apr 23;6:233ra54. doi: 10.1126/scitranslmed.3008177. [DOI] [PubMed] [Google Scholar]
- 25.Nayagam BA, Backhouse SS, Cimenkaya C, Shepherd RK. Hydrogel limits stem cell dispersal in the deaf cochlea: implications for cochlear implants. J Neural Eng. 2012 Dec;9:065001. doi: 10.1088/1741-2560/9/6/065001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middlebrooks JC, Snyder RL. Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci. 2010 Feb 3;30:1937–46. doi: 10.1523/JNEUROSCI.4949-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillman T, Badi AN, Normann RA, Kertesz T, Shelton C. Cochlear nerve stimulation with a 3-dimensional penetrating electrode array. Otol Neurotol. 2003 Sep;24:764–8. doi: 10.1097/00129492-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Moser T. Optogenetic stimulation of the auditory pathway for research and future prosthetics. Curr Opin Neurobiol. 2015 Oct;34:29–36. doi: 10.1016/j.conb.2015.01.004. [DOI] [PubMed] [Google Scholar]