Abstract
Background
Several studies have suggested that cytomegalovirus infection is likely associated with an increased relative risk of cardiovascular disease (CVD); however, the results are inconsistent. We aimed to provide a systematic review and meta‐analysis of community‐based prospective studies assessing the association between cytomegalovirus infection and relative risk of CVD.
Methods and Results
We searched Medline and EMBASE to retrieve prospective studies that reported risk estimates of the association between cytomegalovirus infection and relative risk of CVD. The search yielded 10 articles including a total of 34 564 participants and 4789 CVD patients. Overall, exposure to cytomegalovirus infection was associated with a 22% (relative risk: 1.22, 95% CI: 1.07–1.38, P=0.002) increased relative risk of future CVD. We estimated that 13.4% of CVD incidence could be attributable to cytomegalovirus infection.
Conclusions
In conclusion, cytomegalovirus infection is associated with a significantly increased relative risk of CVD.
Keywords: cardiovascular disease risk factors, cytomegalovirus, infectious disease, meta‐analysis, prospective cohort study, virus
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
Our study suggests that exposure to cytomegalovirus infection is associated with a 22% (relative risk: 1.22, 95% CI: 1.07–1.38, P=0.002) increased risk for future development of cardiovascular disease. We estimate that 13.4% of the cardiovascular disease incidence can be attributed to a cytomegalovirus infection.
What Are the Clinical Implications?
Given the high prevalence and incidence of cytomegalovirus infection and the burden of cardiovascular disease in the population, our research provides an incentive to develop a vaccine for cytomegalovirus as a potential preventive measure for cardiovascular disease.
Introduction
The current study aims to examine the relative risk of cardiovascular disease (CVD) in cytomegalovirus‐infected persons by summarizing the currently available prospective evidence.
CVD is the leading cause of morbidity and mortality in the population worldwide. The occurrence of CVD in populations is incompletely explained by traditional cardiovascular risk factors, and the identification of additional risk factors of CVD would have profound implications for the development of new preventative strategies that could improve public health.
Cytomegalovirus is a DNA virus that belongs to the herpes family of virus.1 Cytomegalovirus infection is widely distributed in the population.2 Moreover, previous studies have provided evidence that infection with cytomegalovirus may play a role in the development of atherosclerosis. For example, researchers have detected cytomegalovirus DNA in atherosclerotic plaques,3, 4, 5 and the presence of cytomegalovirus has been correlated with restenosis in patients who have undergone coronary atherectomy or angioplasty.6, 7 Other studies found the level of serum cytomegalovirus DNA was higher in patients with stable coronary artery disease and acute coronary syndrome than in healthy controls8, 9; cytomegalovirus IgG seropositivity was associated with future risk of stroke after adjusting for other risk factors.10 Furthermore, there is growing evidence implying an important role of this virus in vascular pathology by introducing slow but persistent inflammation in the vessel wall.11
Despite these studies, whether cytomegalovirus infection increases the relative risk of CVD remains uncertain. In the Framingham Heart Study, researchers found that cytomegalovirus IgG seropositivity is not associated with incidence of CVD during 10 years of follow‐up.12 On the contrary, cytomegalovirus IgG seropositivity was associated with a slight excess risk of subsequent myocardial infarction, stroke, or cardiovascular death in HOPE (Heart Outcomes Prevention Evaluation) study patients.13 While several studies found that cytomegalovirus infection is linked to a higher relative risk of CVD,14, 15 negative results were also reported in other studies.12, 16 Therefore, we conducted a systemic review and a meta‐analysis to determine the relationship between cytomegalovirus infection and relative risk of future CVD events. We also performed subgroup analysis by different population features and study characteristics.
Methods
Search Strategy and Study Selection
Two authors (H.W. and J.B.) independently searched Medline and EMBASE for studies reporting the association between cytomegalovirus infection and risk of CVD morbidity and mortality up to October 2016. Our overall search strategy included key words for cytomegalovirus infection (eg, cytomegalovirus, cytomegalovirus infection, cytomegalovirus IgG, cytomegalovirus antibody, and cytomegalovirus seropositive) and CVD (eg, CVD, cardiovascular disease mortality, ischemic heart disease [IHD], coronary heart disease, coronary artery disease, myocardial infarction, stroke, and heart failure). In addition, we searched the reference lists of all retrieved articles and relevant reviews. The eligibility of studies was assessed through a 3‐step process. First, 2 independent reviewers performed an initial screening of all titles and abstracts according to the following criteria: (1) original research articles published with English language were included, and other types of articles, including reviews, editors, commentaries and meta‐analyses, or published with other languages were excluded; (2) population‐based prospective studies reporting the relationship between cytomegalovirus infection and CVD risk were included without age limitation, and articles focused on other exposure or outcomes, or designed as a retrospective (case–control, cross‐sectional, or nested case–control) study were excluded. The full texts of all potentially relevant articles were then reviewed, and studies were included if they met the following criteria: (1) predefined diagnosis criteria for both cytomegalovirus infection and CVD; and (2) reported a risk estimate (by univariate or multivariate statistic) and 95% CI (eg, hazard ratio or relative risk [RR] relating cytomegalovirus infection to subsequent CVD events). Finally, discrepancies were resolved by consensus or consultation with a third reviewer. Also, we assessed agreement between reviewers with the kappa statistics.17
Data Extraction and Quality Assessment
Two authors (H.W. and J.B.) independently abstracted the characteristics and risk estimates of the included studies by using a predesigned data abstraction form. The form included questions on the primary authors' name, years of publication, numbers of total participants and CVD patients, years of follow‐up, and the characteristics of cytomegalovirus infection and outcome. The methods for the ascertainment of outcomes were carefully reviewed for each included study, and were classified as referencing to secure records (hospital records, autopsy records, death certificates, or telephone interviews) or by International Classification of Diseases codes. To assess risk of bias of individual studies, we performed study quality assessment according to the Newcastle‐Ottawa scale, which was recommended by the Cochrane guidelines. The detailed criteria for assessing study quality and the quality score of each included study are presented in Table 1.
Table 1.
Quality Score Assessment Criteria for the Included Studies
| Score | Gkrania‐Klotsas et al, 201218 | Haider et al, 200212 | Fagerberg et al, 199916 | Simanek et al, 201119 | |
|---|---|---|---|---|---|
| Selection | |||||
| (1) Representativeness of the exposed cohort | |||||
| (a) Truly representative of the individuals exposed to CMV infection in the community | 2 | 2 | 2 | 2 | 2 |
| (b) Somewhat representative of the individuals exposed to CMV infection in the community | 1 | … | … | … | … |
| (c) Selected group of users (eg, nurses, volunteers) | 0 | … | … | … | … |
| (d) No description of the derivation of the cohort | 0 | … | … | … | … |
| (2) Selection of the nonexposed cohort | |||||
| (a) Drawn from the same community as the exposed cohort | 2 | … | … | 2 | 2 |
| (b) Drawn from a different source | 1 | … | … | … | … |
| (c) No description of the derivation of the nonexposed cohort | 0 | … | … | … | … |
| (3) Ascertainment of exposure | |||||
| (a) Laboratory test | 2 | 2 | 2 | 2 | 2 |
| (b) Medical record | 1 | … | … | … | … |
| (c) Written self report | 0 | … | … | … | … |
| (4) Demonstration that outcome of interest was not present at start of study | |||||
| (a) Yes | 1 | 1 | 1 | 1 | |
| (b) No | 0 | … | … | 0 | … |
| Comparability | |||||
| (1) Comparability of cohorts on the basis of the design or analysis | |||||
| (a) Study controls for age and any additional factor | 2 | 2 | 2 | 2 | 2 |
| (b) Study controls for any confounding factor | 1 | … | … | … | … |
| (c) No adjustment | 0 | … | … | … | … |
| Outcome | |||||
| (1) Assessment of outcome | |||||
| (a) Referencing to secure records | 3 | … | 3 | 3 | … |
| (b) Record linkage | 2 | 2 | … | … | 2 |
| (c) Self report | 1 | … | … | … | … |
| (d) No description | 0 | … | … | … | … |
| (2) Was follow‐up long enough for outcomes to occur? | |||||
| (a) Yes (≥4 y) | 1 | 1 | 1 | 1 | 1 |
| (b) No | 0 | … | … | … | … |
| Total score | 13 | 12 | 13 | 12 | 12 |
| Quality levela | High | High | Medium | High | |
| Score | Roberts et al, 201014 | Spyridopoulos et al, 201620 | Corrado et al, 200621 | Smieja et al, 200313 | Elkind et al, 201010 | |
|---|---|---|---|---|---|---|
| Selection | ||||||
| (1) Representativeness of the exposed cohort | ||||||
| (a) Truly representative of the individuals exposed to CMV infection in the community | 2 | 2 | 2 | 2 | 2 | 2 |
| (b) Somewhat representative of the individuals exposed to CMV infection in the community | 1 | … | … | … | … | … |
| (c) Selected group of users (eg, nurses, volunteers) | 0 | … | … | … | … | … |
| (d) No description of the derivation of the cohort | 0 | … | … | … | … | … |
| (2) Selection of the nonexposed cohort | ||||||
| (a) Drawn from the same community as the exposed cohort | 2 | 2 | 2 | 2 | 2 | 2 |
| (b) Drawn from a different source | 1 | … | … | … | … | … |
| (c) No description of the derivation of the nonexposed cohort | 0 | … | … | … | … | … |
| (3) Ascertainment of exposure | ||||||
| (a) Laboratory test | 2 | 2 | 2 | 2 | 2 | 2 |
| (b) Medical record | 1 | … | … | … | … | … |
| (c) Written self report | 0 | … | … | … | … | … |
| (4) Demonstration that outcome of interest was not present at start of study | ||||||
| (a) Yes | 1 | 1 | 1 | 1 | 1 | 1 |
| (b) No | 0 | … | … | … | … | … |
| Comparability | ||||||
| (1) Comparability of cohorts on the basis of the design or analysis | ||||||
| (a) Study controls for age and any additional factor | 2 | 2 | … | 2 | 2 | 2 |
| (b) Study controls for any confounding factor | 1 | … | 1 | … | … | … |
| (c) No adjustment | 0 | … | … | … | … | … |
| Outcome | ||||||
| (1) Assessment of outcome | ||||||
| (a) Referencing to secure records | 3 | … | 3 | 3 | … | 3 |
| (b) Record linkage | 2 | 2 | … | … | 2 | … |
| (c) Self report | 1 | … | … | … | … | … |
| (d) No description | 0 | … | … | … | … | … |
| (2) Was follow‐up long enough for outcomes to occur? | ||||||
| (a) Yes (≥4 y) | 1 | 1 | 1 | 1 | 1 | 1 |
| (b) No | 0 | … | … | … | … | … |
| Total score | 13 | 12 | 12 | 13 | 12 | 13 |
| Quality levela | High | High | High | Medium | High | |
CMV indicates cytomegalovirus.
Quality level was defined as low (≤7), medium (8–10), or high (≥11) according to quality score.
Data Synthesis and Analysis
The principal estimate was the RR. The hazard ratio, a type of RR, was directly considered as RR.22 For each study included, we retrieved the reported RR estimates and the corresponding 95% CIs for the assessed outcomes. If several estimates were reported in the same study, we chose the most adjusted estimate that may reduce the impact of confounding factors. We calculated a pooled RR estimate across all studies by a random‐effects model that assumes that individual studies are estimating different association effects. We adopted this model for it is probably the most conservative analysis to account for variance within and between studies and take into account the presence of heterogeneity into their calculations.23 Heterogeneity between studies was assessed using Cochran Q statistics and I 2 statistics.24 We considered the result for heterogeneity to be significant at P<0.10 (2‐sided) for the Q statistics. Heterogeneity was classified as low (I2<25%), moderate (I2<50%), or high (I2>50%). To detect publication bias that may affect the cumulative evidence, we visually examined the asymmetry of funnel plots in which the log estimates were plotted against their standard errors. Furthermore, we also employed an Egger regression test to calculate P values for quantifying publication bias. To assess the influence of each individual study on the pooled estimate, we performed sensitivity analysis by omitting 1 study at a time and recalculating the pooled RRs of the remaining studies. In addition, we carried out multiple subgroup analyses by different study characteristics. Finally, the overall quality of the evidence at each outcome level was assessed by the GRADE approach.25 Analyses were performed with Stata Version 12.0 (StataCorp LP, College Station, TX).
We calculated absolute risk differences associated with cytomegalovirus infection by multiplying the background incidence rate of CVDs in the general population with (estimated RR−1), in which the RR was derived from this meta‐analysis. Population‐attributable risk was calculated based on the following equation: Population‐attributable risk %=100×Pe (RR−1)/(Pe [RR−1]+1), for which Pe is the prevalence of the exposure (cytomegalovirus seropositivity) in the population and the RR was derived from this meta‐analysis. Ethical approval was not required.
Results
Literature Search
Our literature search yielded 9323 articles. After the initial screening of titles and abstracts, a total of 9270 articles were excluded, leaving 53 articles for retrieval. Full text assessment of these articles resulted in 10 eligible articles that met our inclusion criteria, including a total of 34 564 participants and 4789 CVD patients. The inter‐reviewer reliability for the study selection was almost perfect (κ=0.97). The procedure for identifying the studies is illustrated in Figure 1.
Figure 1.
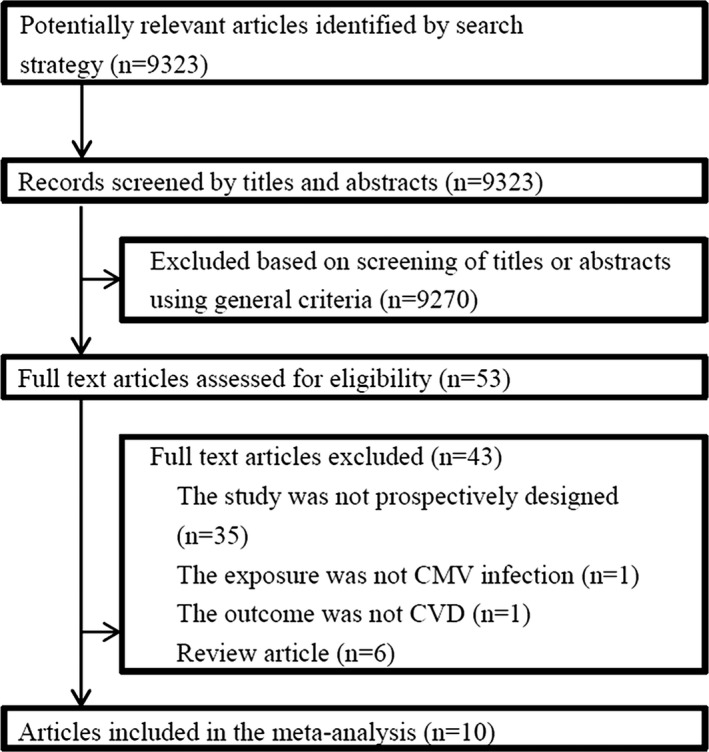
Flowchart on the selection of eligible studies. CMV indicates cytomegalovirus; CVD, cardiovascular disease.
Description of Studies
Study‐specific characteristics are shown in Table 2. Population characteristics are shown in Table 3. The studies were conducted in the United States,10, 12, 14, 19 United Kingdom,15, 18, 20 Canada,13 Sweden16, and Italy.21 Nine studies included men and women,10, 12, 13, 14, 15, 18, 19, 20, 21 and the other 1 study included only men.16
Table 2.
Study Characteristics of the Included Studies
| Author | Country | Cohort Name | Follow‐up | Total Participants | Total CVD Patients | CVD Type | Adjustment |
|---|---|---|---|---|---|---|---|
| Gkrania‐Klotsas et al, 201218 | UK | Cancer–Norfolk Cohort | 12 | 11 022 | 1356 | IHD, cardiovascular death | Age, sex, smoking, SBP, DBP, LDL, HDL, triglycerides, prevalent diabetes mellitus, family history, educational level, occupation, Townsend index, use of antihypertensives, statins, or glucose‐control medications, alcohol use, body mass index, C‐reactive protein |
| Haider et al, 200212 | US | Framingham Heart Study cohort | 10 | 1187 | 199 | IHD, stroke, cardiovascular death | Age, sex, body mass index, total cholesterol and HDL, diabetes mellitus, smoking, and hypertension |
| Fagerberg et al, 199916 | Sweden | NR | 6.5 | 152 | 58 | IHD, stroke, cardiovascular death | Smoking and the presence of previous CVD and for group allocation in the underlying multiple risk factor intervention study |
| Simanek et al, 201119 | US | National Health and Nutrition Examination Survey | 13.8 | 14 153 | 1542 | Cardiovascular death | Age, sex, race/ethnicity, country of origin, education level, body mass index (kg/m2), smoking status, diabetes mellitus status, and C‐reactive protein level |
| Smieja et al, 200313 | Canada | Heart Outcomes Prevention Evaluation | 4.5 | 3168 | 905 | IHD, stroke, cardiovascular death | Age, sex, smoking, ramipril, diabetes mellitus, hypertension, and hypercholesterolemia |
| Savva et al, 201315 | UK | ERSC Healthy Aging Study | 18 | 511 | 138 | Cardiovascular death | Date of birth and sex |
| Roberts et al, 201014 | US | Sacramento Area Latino Study on Aging | 9 | 1329 | 220 | Cardiovascular death | Age, sex, and education, myocardial infarction, congestive heart failure, stroke, dementia, liver/renal disease, diabetes mellitus, malignancy, and leukemia or lymphoma |
| Elkind et al, 201010 | US | The Northern Manhattan Study Mitchell | 7.6 | 1625 | 67 | Stroke | Age, sex, race/ethnicity, high school education, systolic blood pressure, HDL, LDL, blood glucose level, moderate alcohol use, cigarette smoking status, waist circumference, physical activity, and coronary artery disease |
| Spyridopoulos et al, 201620 | UK | The Newcastle 85+ study | 6 | 749 | 184 | Cardiovascular death | Sex |
| Corrado et al, 200621 | Italy | NR | 5 | 668 | 120 | IHD, stroke, cardiovascular death | Age, male sex, obesity, hypertension, diabetes mellitus, smoking habit, family history of CAD, and dyslipidemia |
CAD indicates coronary artery disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; ERSC Economic and Social Research Council; HDL, high‐density lipoprotein; IHD, ischemic heart disease; LDL, low‐density lipoprotein; NR, not reported; SBP, systolic blood pressure.
Table 3.
Population Characteristics of the Included Studies
| Author | Age | Male Sex (%) | BMI | CMV% | Obesity (%) | Smoke (%) | Diabetes Mellitus (%) | Hypertension (%) | Dyslipidemia (%) | Family History of CAD (%) | Previous CV Events (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gkrania‐Klotsas et al, 201218 | 58.5 | 43.9 | NR | 58.4 | NR | 52.7 | 2.8 | NR | NR | 36.0 | 0.0 |
| Haider et al, 200212 | 69.0 | 38.0 | 26.7 | 68.8 | NR | 22.0 | 8.0 | 61.0 | NR | NR | 0.0 |
| Fagerberg et al, 199916 | 65.7 | 100.0 | 26.7 | 84.6 | NR | 36.2 | 6.9 | NR | NR | NR | 29.2 |
| Simanek et al, 201119 | 47.8 | 47.8 | NR | 66.7 | 23.5 | 55.7 | 6.0 | NR | NR | NR | NR |
| Smieja et al, 200313 | 66.0 | 73.3 | 28.0 | 25.0 | NR | 14.2 | 38.5 | 46.8 | NR | NR | 87.8 |
| Savva et al, 201315 | 74.1 | 49.0 | NR | 70.0 | NR | 67.5 | NR | NR | NR | NR | NR |
| Roberts et al, 201014 | 70.6 | 40.0 | NR | 70.4 | NR | NR | 47.0 | 67.1 | NR | NR | NR |
| Elkind et al, 201010 | 68.4 | 35.1 | NR | 85.4 | NR | 16.8 | 21.0 | 73.5 | 60.0 | NR | 20.9 |
| Spyridopoulos et al, 201620 | 85+ | 38.5 | 24.3 | 85.6 | NR | NR | 13.6 | 57.1 | NR | NR | 53.6 |
| Corrado et al, 200621 | 59.5 | 48.8 | NR | 33.7 | 16.6 | NR | 24.0 | 55.2 | 75.2 | 57.2 | NR |
BMI indicates body mass index; CAD, coronary artery disease; CMV, cytomegalovirus; CV, cardiovascular; NR, not reported.
The ascertainment of cytomegalovirus infection varied across studies. All studies ascertained cytomegalovirus infection by serum cytomegalovirus IgG levels with various laboratory assays. Cytomegalovirus infection was defined as being positive for cytomegalovirus IgG antibody in 9 studies.10, 12, 13, 15, 16, 18, 19, 20, 21 The other one defined exposure as the highest quartile of cytomegalovirus IgG titer.14
The method of outcome ascertainment varied across studies. Five studies ascertained CVD by referencing to secure records,10, 12, 13, 16, 21 while the other 5 studies identified CVD events through International Classification of Diseases codes.14, 15, 18, 19, 20 The range of time to follow‐up for the cohort studies was between 4.5 and 18 years.
The quality of studies included in meta‐analyses was assessed by applying the Newcastle‐Ottawa scale for cohort studies. Table 1 lists details of how the criteria were applied to the included studies reporting cytomegalovirus infection and CVD risk as well as the scores assigned to each included study. Overall the level was adequate, with 3 of the 10 studies scoring 1310, 12, 21 and 7 scoring 12.13, 14, 15, 16, 18, 19, 20 Lower‐scoring studies had identified CVD events through International Classification of Diseases codes,13, 14, 15, 18, 19 had not demonstrated that outcome of interest was not present at start of study,16 or had not fully controlled for confounding factors.20 All studies had drawn noncases from the same population as cases, had adequate follow‐up time (≥4 years) between exposure assessment and outcomes, and also reported that outcomes were ascertained by medical records or record linkage.
Systematic Review of Evidence
Of the 10 estimates, 4 reported that cytomegalovirus infection was associated with a significantly increased risk of CVD,13, 14, 15, 20 4 that cytomegalovirus infection was associated with a nonsignificantly increased CVD risk,10, 18, 19, 21 and 2 that cytomegalovirus infection was associated with a nonsignificantly decreased CVD risk.12, 16 No study included in this systematic review reported that cytomegalovirus infection was associated with a significantly decreased risk of CVD.
Meta‐Analysis
Figure 2 displays the results of the meta‐analysis of the 10 studies. On pooling the retrieved measures of association, we found that prior cytomegalovirus infection was associated with a 22% increase in the relative risk of CVD (RR, 1.22; 95% CI 1.07–1.38; P=0.002), with evidence of moderate heterogeneity between studies (I2=44.8%, Q=16.3, P=0.061). By pooling together the reported prevalence of cytomegalovirus infection of the included studies, we estimated that 70.1% of all individuals in the population are infected by cytomegalovirus. Using the risk estimate from our meta‐analysis, we estimated that 13.4% (95% CI, 12.0–14.5%) of CVD events could be attributable to cytomegalovirus infection.
Figure 2.
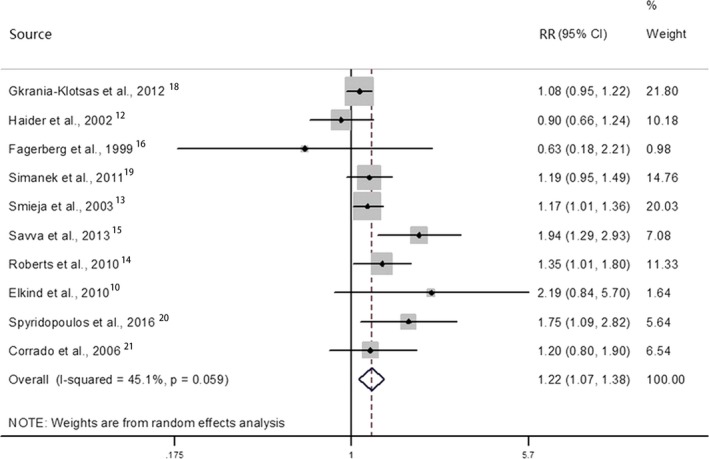
Association between CMV infection and risk of CVDs. Relative risks (RRs) in the individual studies are indicated by the data markers (shaded boxes around the data markers reflect the statistical weight of the study); 95% CIs are indicated by the error bars. The pooled‐effect estimate with its 95% CI is depicted as a diamond. CMV indicates cytomegalovirus; CVDs, cardiovascular diseases.
Begger funnel plots (Figure 3) did not show obvious asymmetry, and Egger test did not support the existence of publication bias (t=1.34, P=0.217). A sensitivity analysis of omitting 1 study in each turn showed that none of the individual studies influenced the pooled RR qualitatively (Figure 4). According to the GRADE guideline, the quality of evidence was leveled as low.
Figure 3.
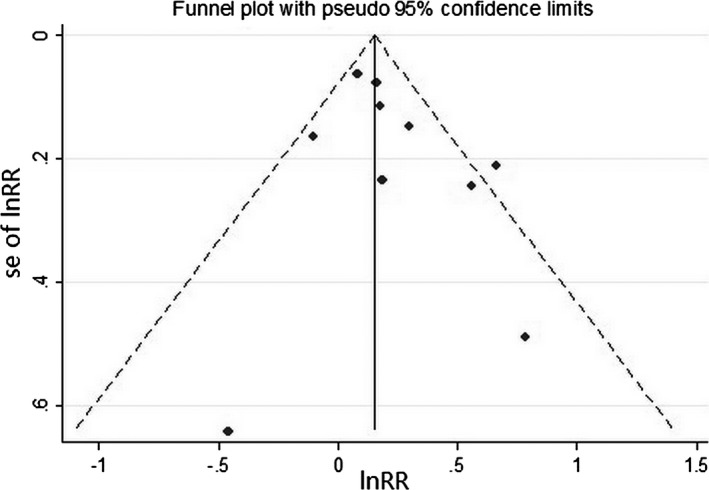
Funnel plots for bias assessment. Each point indicates an individual study. Funnel plots did not show obvious asymmetry. lnRR indicates natural logarithm of relative risk; se of lnRR, standard error of natural logarithm of relative risk.
Figure 4.
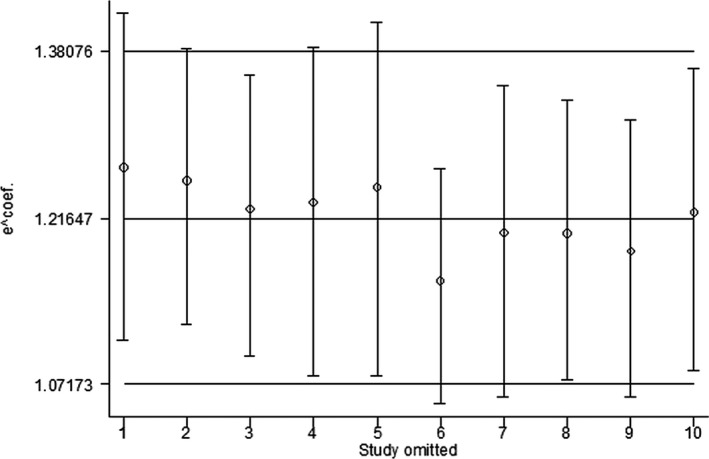
Sensitivity analysis by omitting 1 study at a time. The figure indicates that none of the individual studies influenced the pooled RR qualitatively. RR indicates relative risk.
Subgroup Analyses
Figure 5 displays the results for subgroup analyses by different outcomes. Cardiovascular mortality results were available from 6 studies14, 15, 16, 19, 20, 26 with a pooled RR of 1.30 (95% CI, 1.03–1.66; P=0.029) from a random‐effect model (Figure 3). A high‐level heterogeneity was found with an I2=68.7% (Cochrane Q statistic=15.95, P=0.007). Most of the studies found an RR above 1.00 except 1 study.16 According to the GRADE guideline, the quality of evidence was leveled as very low because of serious imprecision and inconsistency.
Figure 5.
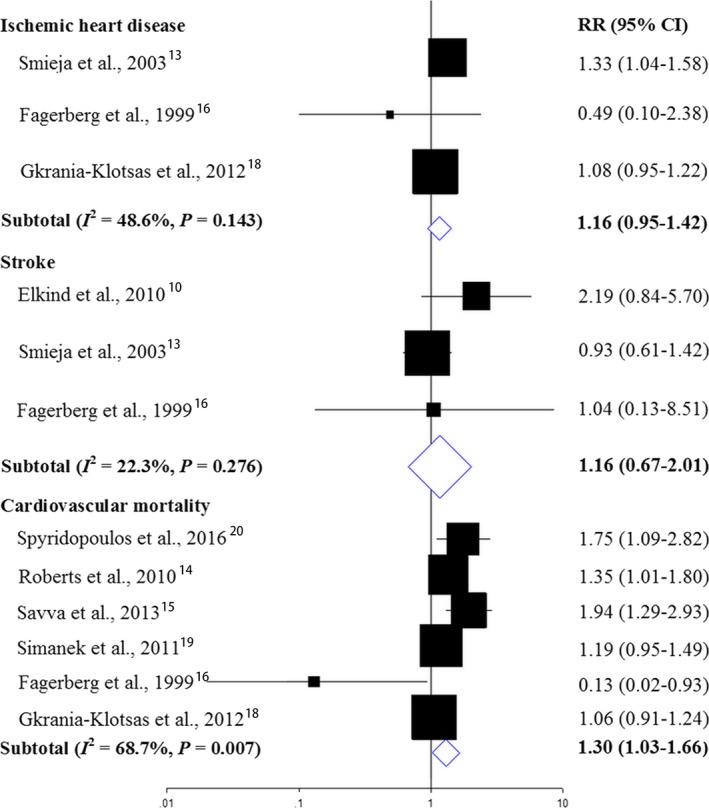
Associations between CMV infection and relative risk of IHD, stroke, and cardiovascular mortality. Relative risks (RRs) in the individual studies are indicated by the data markers. The size of the data markers indicates the weight of the study. The diamond data markers indicate the pooled RRs. CMV indicates cytomegalovirus; IHD, ischemic heart disease.
IHD (including coronary artery disease, coronary heart disease, and myocardial infarction) risk results were available from 3 studies.13, 16, 18 Of the 3 estimates, 2 reported that cytomegalovirus infection was associated with slightly increased relative risk of IHD,13, 18 and 1 that cytomegalovirus infection was associated with moderately decreased relative risk of IHD.16 Evidence synthesis for IHD reported a slightly increased RR in those with cytomegalovirus infection (RR, 1.16; 95% CI, 0.95–1.42; P=0.145). According to the GRADE guideline, the quality of evidence was leveled as very low because of serious imprecision.
Three estimates were available for stroke risk.10, 13, 16 Two reported that cytomegalovirus infection was associated with slightly or moderately increased RR of stroke,10, 16 and 1 that cytomegalovirus infection was associated with slightly decreased risk of stroke.13 Pooled estimate indicated a slightly increased stroke risk in those with cytomegalovirus infection (RR, 1.16; 95% CI, 0.67–2.01; P=0.145). According to the GRADE guideline, the quality of evidence was leveled as very low because of serious imprecision.
In addition, we also performed subgroup analyses according to population characteristics (Table 4). All subgroups indicated that cytomegalovirus infection was associated with increased RR of CVD, which suggested consistency across population characteristics.
Table 4.
Results for Subgroup Analyses
| Subgroup | Included Study | Total Participants | RR (95% CI) | P Value |
|---|---|---|---|---|
| Mean age (y) | ||||
| ≥70 | Ref.14, 15, 20 | 2589 | 1.58 (1.26–1.99) | <0.001 |
| <70 | Ref.10, 12, 13, 16, 18, 19, 21 | 31 975 | 1.12 (1.03–1.21) | 0.010 |
| Male percentage | ||||
| ≥40% | Ref.13, 14, 15, 16, 18, 19, 21 | 3561 | 1.20 (1.07–1.35) | 0.002 |
| <40% | Ref.10, 12, 20 | 31 003 | 1.38 (0.78–2.43) | 0.268 |
| Follow‐up (y) | ||||
| ≥10 | Ref.12, 15, 18, 19 | 26 874 | 1.17 (0.95–1.46) | 0.147 |
| <10 | Ref.10, 13, 14, 16, 20, 21 | 7690 | 1.26 (1.09–1.45) | 0.001 |
| Total participants | ||||
| ≥1000 | Ref.10, 12, 13, 14, 18, 19 | 32 484 | 1.14 (1.03–1.26) | 0.010 |
| <1000 | Ref.15, 16, 20, 21 | 2080 | 1.51 (1.09–2.09) | 0.014 |
RR indicates relative risk.
Discussion
To our knowledge, this meta‐analysis is the largest review, including nearly 35 000 individuals and 5000 CVD patients, assessing the relationship between cytomegalovirus infection and risk of future CVD events. We found that cytomegalovirus seropositivity was associated with a 22% increase in the risk of CVDs (RR, 1.22; 95% CI 1.07–1.38; P=0.002). Furthermore, cytomegalovirus infection contributes to 13.4% of the epidemic of CVD events in the general population.
Although the current study has focused on cytomegalovirus, a large body of published work has suggested that various bacterial and viral infections might be associated with increased risk of CVD. Substantial evidence has been gathered to support the effect of Chlamydia pneumonia and influenza in CVD,27, 28 and the current study provides further evidence supporting the hypothesis that infectious pathogens including cytomegalovirus contribute to the epidemic of CVD in the general population.
Our study found significantly increased risk of mortality in cytomegalovirus‐infected patients, but the results were nonsignificant for IHD and stroke. It should be noted that the sample sizes did not reach optimal size in IHD and stroke subgroups. According to GRADE guidelines, the results were subjected to serious imprecision, and the quality of evidence was therefore classified as very low. Future studies may improve the quality of evidence. There are several potential explanations for the observed association between cytomegalovirus seropositivity and increased risk of CVD events. The most important was its role in thrombosis. Cytomegalovirus can directly infect cells of the vessel wall, where they could persist in a latent state or replicate at a low level.29 It was shown that cytomegalovirus infection of endothelial cells causes the appearance of procoagulant activity on these cells.30 In addition, there was data suggesting that cytomegalovirus can directly interact with prothrombinase proteins and substitute for synthetic procoagulant phospholipid vesicles to catalyze the generation of thrombin.31 Furthermore, as the infection persists, it is well suited to induce proinflammatory cytokines, including tumor necrosis factor‐α and IL‐6, which are independently associated with CVD. Also, a recent study suggests that a substantial proportion of the relation between cytomegalovirus and mortality was mediated by circulating tumor necrosis factor‐α and IL‐6 levels. The high cytomegalovirus antibody levels probably reflect more frequent cytomegalovirus reactivation and higher levels of replication, leading to an increase in the proinflammatory cytokines tumor necrosis factor‐α and IL‐6 and enhanced vascular damage and plaque instability.14
There are several strengths of the current study. Our meta‐analysis was based on several prospectively designed, population‐based cohort studies. The combined sample size was large, and the follow‐up period was long enough. We combined the estimates from the fully adjusted models of each included study in our analyses to reduce the impact of confounding. Despite these strengths, there were also several limitations that should be noted. First, it should be noted that seropositivity represents prior infection. This is insufficient to assess the viral infection activity or acute infection. Future studies should base infection on quantitative DNA to detect ongoing infection as active infection might increase one's risk for CVD. Second, the included studies were limited to those designed as a population‐based prospective study and published in English. Therefore, our results may potentially be exposed to publication and language bias. Nonetheless, the included studies had generally satisfactory designs, methods, and outcomes, and all the included studies were of high quality. Furthermore, both funnel plot and Egger test indicated there was no evidence of publication bias. Third, most of the studies that were included were carried out in Europe and the United States, and this limits the direct generalization of our findings. Future high‐quality prospective studies conducted on Asian and African populations are required to confirm our findings. Fourth, in spite of the large number of participants, the individual participant data were not available, and we were not able to explore the dose–response relationship between cytomegalovirus infection and CVD risk, or seek for interaction between cytomegalovirus infection and other risk factors. Also, event numbers were low for some outcomes (IHD and stroke); therefore, the power of subgroup analyses was limited. Nonetheless, the consistency of the evidence overall supports a real association between cytomegalovirus infection and CVD risk. Fifth, while our study demonstrated that cytomegalovirus infection may predispose to CVD events, chemoprophylaxis would not be feasible because of the common asymptomatic course of the infection, and there are no effective vaccines available currently. Finally, use of International Classification of Diseases codes to ascertain outcomes in several studies is another limitation of the data.
In conclusion, this meta‐analysis provides strong evidence that cytomegalovirus infection is a significant risk factor for CVD. Given the high prevalence and incidence of cytomegalovirus infection and CVD in the general population, our research provides an impetus to develop a childhood vaccine as part of the fight against CVD.
Author Contributions
All authors contributed to data collection and wrote the manuscript. Hu and Liu drafted the study protocol. Wang, Peng, Bai, and Huang extracted data. Wang and Peng performed the analyses. Wang, Peng, and Bai drafted the paper. All authors critically reviewed the paper. Wang and Liu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Wang and Liu are the guarantors of the paper. All authors approved the current manuscript to be published, attested that they contributed substantially to the current work, and disclosed that there was no writing assistance.
Disclosures
None.
Acknowledgments
We thank Haijiao Wang, Luohe Central Hospital, Luohe, China, for his advice and substantive comments on an earlier draft of this article.
(J Am Heart Assoc. 2017;6:e005025 DOI: 10.1161/JAHA.116.005025.)28684641
Contributor Information
Haoran Wang, Email: washingtonhr@163.com.
Dongliang Liu, Email: dongliang003@live.cn.
References
- 1. Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–470. [DOI] [PubMed] [Google Scholar]
- 2. Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. [DOI] [PubMed] [Google Scholar]
- 3. Xenaki E, Hassoulas J, Apostolakis S, Sourvinos G, Spandidos DA. Detection of cytomegalovirus in atherosclerotic plaques and nonatherosclerotic arteries. Angiology. 2009;60:504–508. [DOI] [PubMed] [Google Scholar]
- 4. Melnick JL, Hu C, Burek J, Adam E, DeBakey ME. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J Med Virol. 1994;42:170–174. [DOI] [PubMed] [Google Scholar]
- 5. Melnick JL, Petrie BL, Dreesman GR, Burek J, McCollum CH, DeBakey ME. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983;2:644–647. [DOI] [PubMed] [Google Scholar]
- 6. Blum A, Giladi M, Weinberg M, Kaplan G, Pasternack H, Laniado S, Miller H. High anti‐cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post‐coronary balloon angioplasty restenosis. Am J Cardiol. 1998;81:866–868. [DOI] [PubMed] [Google Scholar]
- 7. Zhou YF, Leon MB, Waclawiw MA, Popma JJ, Yu ZX, Finkel T, Epstein SE. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med. 1996;335:624–630. [DOI] [PubMed] [Google Scholar]
- 8. Nikitskaya E, Lebedeva A, Ivanova O, Maryukhnich E, Shpektor A, Grivel JC, Margolis L, Vasilieva E. Cytomegalovirus‐productive infection is associated with acute coronary syndrome. J Am Heart Assoc. 2016;5:e003759 DOI: 10.1161/JAHA.116.003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu R, Moroi M, Yamamoto M, Kubota T, Ono T, Funatsu A, Komatsu H, Tsuji T, Hara H, Hara H, Nakamura M, Hirai H, Yamaguchi T. Presence and severity of Chlamydia pneumoniae and cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J. 2006;47:511–519. [DOI] [PubMed] [Google Scholar]
- 10. Elkind MS, Ramakrishnan P, Moon YP, Boden‐Albala B, Liu KM, Spitalnik SL, Rundek T, Sacco RL, Paik MC. Infectious burden and risk of stroke: the northern Manhattan study. Arch Neurol. 2010;67:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Popovic M, Smiljanic K, Dobutovic B, Syrovets T, Simmet T, Isenovic ER. Human cytomegalovirus infection and atherothrombosis. J Thromb Thrombolysis. 2012;33:160–172. [DOI] [PubMed] [Google Scholar]
- 12. Haider AW, Wilson PW, Larson MG, Evans JC, Michelson EL, Wolf PA, O'Donnell CJ, Levy D. The association of seropositivity to Helicobacter pylori, Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: a prospective study. J Am Coll Cardiol. 2002;40:1408–1413. [DOI] [PubMed] [Google Scholar]
- 13. Smieja M, Gnarpe J, Lonn E, Gnarpe H, Olsson G, Yi Q, Dzavik V, McQueen M, Yusuf S; Heart Outcomes Prevention Evaluation Study I . Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:251–257. [DOI] [PubMed] [Google Scholar]
- 14. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow‐up. Am J Epidemiol. 2010;172:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savva GM, Pachnio A, Kaul B, Morgan K, Huppert FA, Brayne C, Moss PA; Medical Research Council Cognitive F, Ageing S . Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12:381–387. [DOI] [PubMed] [Google Scholar]
- 16. Fagerberg B, Gnarpe J, Gnarpe H, Agewall S, Wikstrand J. Chlamydia pneumoniae but not cytomegalovirus antibodies are associated with future risk of stroke and cardiovascular disease: a prospective study in middle‐aged to elderly men with treated hypertension. Stroke. 1999;30:299–305. [DOI] [PubMed] [Google Scholar]
- 17. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 18. Gkrania‐Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Higher immunoglobulin G antibody levels against cytomegalovirus are associated with incident ischemic heart disease in the population‐based EPIC‐Norfolk cohort. J Infect Dis. 2012;206:1897–1903. [DOI] [PubMed] [Google Scholar]
- 19. Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all‐cause and cardiovascular disease‐related mortality in the United States. PLoS One. 2011;6:e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spyridopoulos I, Martin‐Ruiz C, Hilkens C, Yadegarfar ME, Isaacs J, Jagger C, Kirkwood T, von Zglinicki T. CMV seropositivity and T‐cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell. 2016;15:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corrado E, Rizzo M, Tantillo R, Muratori I, Bonura F, Vitale G, Novo S. Markers of inflammation and infection influence the outcome of patients with baseline asymptomatic carotid lesions: a 5‐year follow‐up study. Stroke. 2006;37:482–486. [DOI] [PubMed] [Google Scholar]
- 22. Spruance SL, Reid JE, Grace M, Samore M. Hazard ratio in clinical trials. Antimicrob Agents Chemother. 2004;48:2787–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langer G, Meerpohl JJ, Perleth M, Gartlehner G, Kaminski‐Hartenthaler A, Schunemann H. [GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables]. Z Evid Fortbild Qual Gesundhwes. 2012;106:357–368. [DOI] [PubMed] [Google Scholar]
- 26. Gkrania‐Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population‐based European prospective investigation of Cancer‐Norfolk cohort. Clin Infect Dis. 2013;56:1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Filardo S, Di Pietro M, Farcomeni A, Schiavoni G, Sessa R. Chlamydia pneumoniae‐mediated inflammation in atherosclerosis: a meta‐analysis. Mediators Inflamm. 2015;2015:378658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warren‐Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–610. [DOI] [PubMed] [Google Scholar]
- 29. Epstein SE, Zhu J, Najafi AH, Burnett MS. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119:3133–3141. [DOI] [PubMed] [Google Scholar]
- 30. Pryzdial EL, Wright JF. Prothrombinase assembly on an enveloped virus: evidence that the cytomegalovirus surface contains procoagulant phospholipid. Blood. 1994;84:3749–3757. [PubMed] [Google Scholar]
- 31. Etingin OR, Silverstein RL, Friedman HM, Hajjar DP. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990;61:657–662. [DOI] [PubMed] [Google Scholar]


