Abstract
Background
Physical activity reduces the risk of vascular disease. This benefit is not entirely explained through an effect on vascular risk factors. We examined the relationship of physical activity and characteristics of the carotid artery wall in patients with vascular disease or risk factors.
Methods and Results
Cross‐sectional analyses were performed in 9578 patients from the SMART (Second Manifestations of Arterial Disease) study, a prospective cohort study among patients with vascular disease or risk factors. Physical activity was assessed using questionnaires. Carotid intima‐media thickness and carotid artery stenosis of both common carotid arteries was measured. In a subset of 3165 participants carotid diastolic diameter and distension were assessed. Carotid stiffness was expressed as the distensibility coefficient and Young's elastic modulus. Regression analyses adjusted for vascular risk factors showed that physical activity was inversely associated with diastolic diameter (fifth versus first quintile B=−0.13 mm; 95% CI, −0.21 to −0.05) and decreased risk of carotid artery stenosis (relative risk, 0.58; 95% CI, 0.48–0.69). A light level of physical activity was associated with less carotid stiffness (second versus first quintile; Young's elastic modulus B=−0.11 kPa−1×10−3; 95% CI, −0.16 to −0.06; distensibility coefficient B=0.93 kPa×103; 95% CI, 0.34–1.51), but there was no additional benefit with increasing levels of physical activity. In patients with vascular disease, physical activity was inversely associated with common carotid intima‐media thickness, but not in patients with vascular risk factors.
Conclusions
In patients with vascular disease or risk factors, increased physical activity was associated with smaller carotid diastolic diameter, decreased risk of carotid artery stenosis, and less carotid stiffness, but it only showed benefits on carotid intima‐media thickness in patients with vascular disease.
Keywords: carotid intima‐media thickness, carotid stenosis, physical activity, stiffness
Subject Categories: Ultrasound, Exercise
Clinical Perspective
What is New?
In patients with vascular disease or vascular risk factors, a higher level of physical activity is associated with less vascular aging, and this association is independent of vascular risk factors.
Increased physical activity is associated with smaller carotid diastolic diameter; this association has not been previously described.
In patients with vascular disease, physical activity was inversely associated with carotid intima‐media thickness, but not in patients with vascular risk factors.
Our results suggest that there is a sex difference, as physical activity had no benefits on carotid stiffness and carotid intima‐media thickness in women, whereas these associations were significant in men.
What are the Implications?
Our results suggest that increased physical activity is important for vascular health, not only associated with vascular risk factors, but also directly, even in patients with vascular disease.
Studies investigating the effects of physical activity on vascular disease risk should also consider including a measurement of vascular aging, as vascular risk factors do not fully capture the benefits of physical activity.
Vascular aging can be assessed with several markers; more studies are needed to provide evidence for which marker can best be selected for a particular study population.
Sex differences should be examined in future studies.
Introduction
Regular physical activity is associated with a lower risk of vascular disease and mortality in healthy men and women,1, 2, 3 as well as in patients with vascular disease.4 However, the mechanisms by which physical activity influences this risk have not been completely elucidated. It has been attributed to beneficial effects on the traditional risk factors for vascular disease, such as blood pressure,5 body mass index (BMI), and lipid profile,6 and its effects on systemic inflammation7 and platelet aggregation.8 Physical activity may also have direct positive effects on the vasculature structure and function through an improvement in endothelial function.8, 9 In a recent study in patients with vascular disease or vascular risk factors, associations between physical activity and the risk of future vascular events did not change after additional adjustment for traditional risk factors.4 This finding suggests that other mediators than traditional risk factors such as the direct effects on the vasculature function and structure may be more important in these patients.
Characteristics of the carotid artery wall such as carotid intima‐media thickness (CIMT), carotid artery stenosis (CAS), end‐diastolic lumen diameter, and stiffness are measures of vasculature function and structure.10 Increased levels of these characteristics are known to increase the risk for stroke, other cardiovascular events, and mortality.11, 12, 13, 14 Several studies conducted in the general population investigated the association of physical activity with CIMT15, 16, 17, 18 and markers of stiffness.19, 20, 21, 22, 23 Most studies found an association between a higher level of physical activity and less carotid or aortic stiffness,20, 21, 22 lower CIMT,15, 16, 18 and less progression of CIMT,18 although some studies did not show these associations.17, 19, 23 Studies in patients with vascular disease are not available; however, there is circumstantial evidence that the benefits of physical activity on CIMT in patients with vascular risk factors24 and on endothelial function are more pronounced in patients with vascular disease.9 The aim of the current study was to investigate the associations between physical activity and characteristics of the carotid wall in patients with vascular disease or vascular risk factors, a population at high risk for (recurrent) vascular events and mortality.
Methods
Participants
Data were used from patients enrolled in the SMART (Second Manifestations of Arterial Disease) study, an ongoing single‐center prospective cohort trial in patients with vascular disease or risk factors for vascular disease. From 1996 onwards, patients were invited to participate if they were newly referred to the University Medical Center Utrecht in The Netherlands for treatment of vascular disease or vascular risk factors.25 During a 1‐day visit to our medical center, an extensive vascular screening was performed, including a physical examination, ultrasonography of the carotid arteries, overnight fasting venous blood and urine sampling, assessment of risk factors, medical history, functioning, physical activity, and medication use. Written informed consent was obtained from all participants. The study was approved by the medical ethics committee of the University Medical Center Utrecht.
For the current study, 10 128 consecutive patients included between September 1996 and February 2013 were studied; 550 patients (5%) had to be excluded because of missing data for physical activity, CIMT, or CAS, leaving 9578 patients for the present analysis.
Measurements of carotid diastolic diameter and distension were performed until 2003, when 3300 patients were included, of whom 135 patients (4%) had to be excluded because of missing data for physical activity. Therefore, 3165 patients were included in the analyses of the associations between physical activity and diastolic diameter and stiffness.26
Physical Activity
At baseline, patients completed a questionnaire on their usual pattern of leisure‐time physical activity during a regular week in the past year. A previously validated questionnaire27 was used and one question regarding the amount and intensity of activity in sport was added. Patients were asked how many hours per week they spent on leisure‐time physical activities, with focus on sport (eg, swimming and running) or other physical activities (eg, walking, cycling, gardening, and leisure‐time physical activity). Housekeeping and work‐related physical activity were not included.
Each activity was assigned a specific metabolic equivalent (MET) intensity derived from the Compendium of Physical Activity.28 The MET intensity is based on the rate of energy expenditure. One MET represents the energy expenditure for an individual at rest, whereas a 10 MET activity requires 10 times the resting energy expenditure (brisk walking is estimated to be about 3.5–4.0 METs).28 For each type of leisure‐time physical activity, the amount per week was calculated by multiplying the time spent on this activity in hours per week by the derived MET intensity of the activity, expressed in MET hours per week. The total amount of physical activity per week was the sum of these values.
Characteristics of the Carotid Wall
Structure of the carotid wall
Presence of atherosclerosis in the carotid arteries was assessed at baseline by measuring common CIMT and CAS. Ultrasonography was performed with a 10‐MHz linear array transducer (ATL Ultramark 9) by well‐trained and certified ultrasound technicians at the Department of Radiology, University Medical Center Utrecht. Mean common CIMT (referred to as CIMT throughout the text) was calculated for each patient based on 6 far wall measurements of the left and right common carotid arteries as previously described.29 The reference point for measurement of the CIMT was the beginning of the dilatation of the carotid bulb. The end‐diastolic lumen diameter of the carotid artery (referred to as diastolic diameter throughout the text) was also used as a marker of the structure of the carotid wall.
The degree of the CAS was assessed on both sides with color Doppler–assisted duplex scanning. The severity of CAS was evaluated on the basis of blood flow velocity patterns.30 The greatest stenosis observed on the right or the left side of the common or internal carotid artery was taken to determine the severity of carotid artery disease. CAS ≥50% was defined as peak systolic velocity >150 cm/s.
Function of the carotid artery wall
Stiffness was measured by distension of both common carotid arteries. The distension of an artery is the change in systolic diameter relative to the diastolic diameter during the cardiac cycle. The displacement of the walls of the left and right common carotid artery was measured with a wall track system (Scanner 200, Pie Medical Imaging) equipped with a 7.5‐MHz linear array transducer and vessel wall moving detector system. After the patients rested for at least 5 minutes in the supine position, the left and right carotid arteries were examined separately. Measurements were performed in the distal common carotid artery 2 cm proximal to the origin of the carotid bulb, as described elsewhere.29 Coefficients of variation for intraobserver and interobserver variability of distension and end‐diastolic lumen diameter measurements were all <10%.29 Distensibility coefficient (DC) and Young's elastic modulus (YEM) were used as the primary stiffness measures and were calculated as previously described.26 DC is the relative change in diameter with pressure, and increasing DC indicates decreasing stiffness. YEM is the pressure per square millimeter required for (theoretical) 100% extension.31
Covariables and Definitions
Covariables included demographic characteristics (age and sex), medical history, and risk factors for vascular disease (current smoking, current alcohol use, hypertension, diabetes mellitus, hyperlipidemia, and BMI). Hypertension was defined as the use of blood pressure–lowering drugs or blood pressure >140/90 mm Hg. Diabetes mellitus was defined as a referral diagnosis of diabetes mellitus, self‐reported diabetes mellitus, use of glucose‐lowering agents, or glucose ≥7.0 mmol/L and glucose‐lowering therapy within 1 year after inclusion. Hyperlipidemia was defined as fasting total cholesterol >5.0 mmol/L, fasting low‐density lipoprotein cholesterol >3.2 mmol/L, or lipid‐lowering drug use. Height and weight were measured and BMI was calculated (kg/m2).
Data Analysis
Data were analyzed with SPSS version 22.0 (IBM Corp). CIMT and YEM were skewed on the original scale and were normalized by log‐transformation. We first calculated baseline characteristics for the total population (n=9578) and for the population with carotid stiffness markers (n=3165). Next, linear regression was used to investigate the associations between physical activity and characteristics of the carotid artery wall. In model 1, we estimated this association adjusted for age and sex. In model 2, we additionally adjusted for smoking and current alcohol consumption. In model 3, we additionally adjusted for BMI, presence of diabetes mellitus, presence of hypertension, and presence of hyperlipidemia, as they could confound the relationship but could also be intermediates. For the association between physical activity at baseline and presence of CAS, we used Poisson regression models with log‐link function and robust standard errors to estimate relative risks and accompanying CIs rather than odds ratios, which overestimate the relative risk, particularly for outcomes that are common.32
First, we investigated the associations between physical activity and characteristics of the carotid artery wall using quintiles of physical activity in the total population and in the population with carotid stiffness markers (n=3165). To investigate whether there were linear trends, we also considered these quintiles as ordinal variables in the analysis (P trend). Second, we performed stratified analyses to evaluate whether the relationship between physical activity and characteristics of the carotid artery wall differed by: (1) the presence of vascular risk factors or vascular disease, (2) age, (3) sex,16 (4) smoking status, and (5) BMI.24 We also calculated interaction terms and considered interaction present if P<0.10. To investigate whether the observed associations between physical activity and the characteristics of the carotid artery wall were explained by previous carotid interventions, we performed an additional analysis excluding patients with a history of a carotid intervention (n=124; 1.3% of the total population). We used ANCOVA to calculate adjusted means of the characteristics of the carotid artery wall across the different quintiles of physical activity.
Results
At baseline, the mean age of the total population (n=9578) was 56.6 (SD, 12.4) years, and 67% of patients were men (Table 1). The median level of physical activity in the total population was 17.4 MET hours per week (10th–90th percentile, 0.0–55.5). In the population with carotid stiffness markers (n=3165), fewer patients had coronary artery disease and more patients had peripheral artery disease and the amount of physical activity was lower compared with the total group (Table 2). In the lowest quintile of physical activity, more patients had peripheral artery disease or diabetes mellitus and more patients were current smokers as compared with those in the highest quintile (Tables 1 and 2). Patients in the highest quintile of physical activity more often had coronary artery disease than patients in the lowest quintile (Tables 1 and 2).
Table 1.
Baseline Characteristics of Total Study Population
| Total | Quintiles of Physical Activity, METh/w | |||||
|---|---|---|---|---|---|---|
| 1 <3.8 | 2 3.8–12.3 | 3 12.3–23.0 | 4 23.0–39.3 | 5 >39.3 | ||
| No. | 9578 | 1911 | 1926 | 1908 | 1917 | 1916 |
| Age, y | 56.6 (12.4) | 56.9 (12.7) | 55.6 (12.3) | 55.0 (12.6) | 56.5 (12.5) | 59.1 (11.4) |
| Male sex, % | 67 | 63 | 65 | 67 | 68 | 72 |
| Physical activity, METh/wa | 17.4 (0.0–55.5) | 0.0 (0.0–3.4) | 8.0 (4.0–11.7) | 17.4 (13.6–21.8) | 29.6 (24.1–37.0) | 55.5 (41.6–95.8) |
| Manifestation at baseline, % | ||||||
| Vascular risk factors | 32 | 32 | 36 | 35 | 31 | 27 |
| Cerebrovascular disease | 14 | 14 | 15 | 14 | 15 | 14 |
| Coronary artery disease | 33 | 24 | 29 | 33 | 36 | 42 |
| Abdominal aortic aneurysm | 3 | 3 | 2 | 2 | 2 | 2 |
| Peripheral arterial disease | 8 | 12 | 8 | 8 | 7 | 6 |
| Multiple manifestations of vascular disease | 10 | 15 | 10 | 9 | 9 | 9 |
| Other variables, % | ||||||
| Hypertensionb | 66 | 69 | 65 | 65 | 64 | 68 |
| Diabetes mellitusc | 19 | 27 | 20 | 18 | 17 | 15 |
| Hyperlipidemiad | 77 | 82 | 79 | 78 | 75 | 74 |
| Current smoking | 30 | 44 | 32 | 28 | 24 | 22 |
| Current alcohol consumption | 71 | 57 | 69 | 75 | 76 | 76 |
| BMI, kg/m2 | 26.9 (4.3) | 27.5 (5.1) | 27.2 (4.4) | 26.6 (4.1) | 26.5 (4.1) | 26.6 (3.8) |
| Carotid markers | ||||||
| CIMT, mma | 0.83 (0.60–1.18) | 0.85 (0.62–1.22) | 0.82 (0.60–1.15) | 0.82 (0.60–1.17) | 0.83 (0.60–1.15) | 0.86 (0.62–1.18) |
| CAS >50%, % | 10 | 15 | 10 | 8 | 9 | 8 |
Characteristics are presented as mean±SD, unless otherwise specified. BMI indicates body mass index; CAS, carotid artery stenosis; CIMT, carotid intima‐media thickness; METh/w, metabolic equivalent hours per week.
Median (10th–90th percentile).
Hypertension was defined as blood pressure–lowering drug use or blood pressure >140/90 mm Hg.
Diabetes mellitus was defined as a referral diagnosis of diabetes mellitus, self‐reported diabetes mellitus, use of glucose‐lowering agents, or glucose ≥7.0 mmol/L and glucose‐lowering therapy within 1 year after inclusion.
Hyperlipidemia was defined as fasting total cholesterol >5.0 mmol/L, fasting low‐density lipoprotein cholesterol >3.2 mmol/L, or lipid‐lowering drug use.
Table 2.
Baseline Characteristics of the Population With Carotid Stiffness Markers
| Total | Quintiles of Physical Activity, METh/w | |||||
|---|---|---|---|---|---|---|
| 1 0.0 | 2 0.1–9.1 | 3 9.2–18.4 | 4 18.5–32.0 | 5 >32.0 | ||
| No. | 3165 | 713 | 553 | 631 | 634 | 634 |
| Age, y | 55.7 (12.8) | 56.8 (12.8) | 54.8 (12.3) | 54.3 (12.6) | 54.6 (13.2) | 57.7 (12.6) |
| Male sex, % | 69 | 65 | 68 | 69 | 68 | 75 |
| Physical activity, METh/wa | 13.4 (0.0–48.1) | 0.0 | 5.5 (1.9–8.0) | 13.4 (10.0–17.3) | 24.0 (18.5–29.6) | 48.1 (34.6–88.8) |
| Manifestation at baseline, % | ||||||
| Vascular risk factors | 33 | 29 | 38 | 35 | 37 | 26 |
| Cerebrovascular disease | 14 | 15 | 14 | 12 | 12 | 14 |
| Coronary artery disease | 26 | 19 | 24 | 30 | 28 | 33 |
| Abdominal aortic aneurysm | 3 | 4 | 3 | 2 | 4 | 4 |
| Peripheral arterial disease | 11 | 16 | 11 | 10 | 9 | 10 |
| Multiple manifestations of vascular disease | 13 | 18 | 11 | 11 | 10 | 13 |
| Other variables, % | ||||||
| Hypertensionb | 61 | 64 | 58 | 59 | 60 | 63 |
| Diabetes mellitusc | 22 | 27 | 24 | 19 | 20 | 19 |
| Hyperlipidemiad | 83 | 85 | 82 | 84 | 82 | 83 |
| Current smoking | 35 | 47 | 35 | 32 | 29 | 28 |
| Current alcohol consumption | 69 | 59 | 66 | 76 | 75 | 72 |
| BMI, kg/m2 | 26.5 (4.1) | 26.9 (4.7) | 26.6 (4.0) | 26.5 (4.2) | 26.1 (3.7) | 26.4 (3.8) |
| Carotid markers | ||||||
| CIMT, mma | 0.83 (0.60–1.23) | 0.85 (0.62–1.25) | 0.83 (0.60–1.23) | 0.80 (0.60–1.22) | 0.80 (0.58–1.18) | 0.87 (0.60–1.27) |
| Diastolic diameter, mm | 7.78 (1.10) | 7.91 (1.15) | 7.74 (1.13) | 7.69 (1.04) | 7.70 (1.11) | 7.86 (1.07) |
| Distension, mm | 0.44 (0.15) | 0.43 (0.15) | 0.44 (0.15) | 0.44 (0.15) | 0.44 (0.16) | 0.43 (0.15) |
| Distensibility coefficient, kPa−1×10−3 | 15.3 (7.1) | 14.3 (7.2) | 16.0 (7.3) | 16.0 (7.2) | 15.7 (7.2) | 14.6 (6.7) |
| Young's elastic modulus, kPa×103 a | 0.66 (0.37–1.22) | 0.70 (0.38–1.41) | 0.63 (0.35–1.13) | 0.64 (0.37–1.13) | 0.65 (0.36–1.19) | 0.67 (0.38–1.19) |
Characteristics presented as mean±SD, unless otherwise specified. BMI indicates body mass index; CIMT, carotid intima‐media thickness; METh/w, metabolic equivalent hours per week.
Median (10th–90th percentile).
Hypertension was defined as blood pressure–lowering drug use or blood pressure >140/90 mm Hg.
Diabetes mellitus was defined as a referral diagnosis of diabetes mellitus, self‐reported diabetes mellitus, use of glucose‐lowering agents, or glucose ≥7.0 mmol/L and glucose‐lowering therapy within 1 year after inclusion.
Hyperlipidemia was defined as fasting total cholesterol >5.0 mmol/L, fasting low‐density lipoprotein cholesterol >3.2 mmol/L, or lipid‐lowering drug use.
CIMT and CAS (n=9578)
Linear regression analysis adjusted for age, sex, smoking, and alcohol consumption showed that a higher level of physical activity was associated with a lower ln CIMT (fifth versus first quintile B=−0.018 mm; 95% CI −0.033 to −0.003) and a lower risk of CAS >50% (relative risk, 0.58; 95% CI, 0.48–0.69) (Table 3). The relationship between physical activity and CIMT was no longer significant in model 3.
Table 3.
Associations of Physical Activity With CIMT and CAS (n=9578)
| Quintiles of Physical Activity, METh/w | No. | Model 1 | Model 2 | Model 3 | P Trend |
|---|---|---|---|---|---|
|
Ln CIMT, mm B (95% CI) | |||||
| 1 (<3.8) | 1911 | Reference | Reference | Reference | |
| 2 (3.8–12.3) | 1926 | −0.016 (−0.031 to −0.002) | −0.009 (−0.023 to 0.006) | −0.004 (−0.018 to 0.011) | |
| 3 (12.3–23.0) | 1908 | −0.019 (−0.033 to −0.004) | −0.008 (−0.023 to 0.007) | −0.002 (−0.016 to 0.013) | |
| 4 (23.0–39.3) | 1917 | −0.026 (−0.040 to −0.011) | −0.013 (−0.028 to 0.001) | −0.003 (−0.018 to 0.011) | |
| 5 (>39.3) | 1916 | −0.030 (−0.045 to −0.016) | −0.018 (−0.033 to −0.003) | −0.008 (−0.023 to 0.006) | 0.32 |
|
CAS >50% (n=936) Relative risk (95% CI) | |||||
| 1 (<3.8) | 1911 | Reference | Reference | Reference | |
| 2 (3.8–12.3) | 1926 | 0.70 (0.59–0.84) | 0.79 (0.67–0.94) | 0.81 (0.69–0.96) | |
| 3 (12.3–23.0) | 1908 | 0.58 (0.49–0.70) | 0.67 (0.56–0.81) | 0.67 (0.56–0.81) | |
| 4 (23.0–39.3) | 1917 | 0.60 (0.50–0.72) | 0.71 (0.60–0.85) | 0.74 (0.62–0.88) | |
| 5 (>39.3) | 1916 | 0.48 (0.40–0.58) | 0.58 (0.48–0.69) | 0.59 (0.49–0.71) | <0.001 |
Linear (B [95% CI]) or Poisson (relative risk [95% CI]) regression model. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, smoking status, and current alcohol consumption. Model 3: adjusted for age, sex, smoking status, current alcohol consumption, body mass index, presence of diabetes mellitus, presence of hypertension, and presence of hyperlipidemia. P trend reflects the linear trend using quintiles in model 3. CAS indicates carotid artery stenosis; CIMT, carotid intima‐media thickness; METh/w, metabolic equivalent hours per week.
Diastolic Diameter and Stiffness Markers (n=3165)
Linear regression analysis adjusted for age, sex, smoking, and alcohol consumption showed that a higher level of physical activity was associated with a lower diastolic diameter (fifth versus first quintile B=−0.13 mm; 95% CI, −0.23 to −0.03) (Table 4). A light level of physical activity was associated with a lower YEM (second versus first quintile B=−0.11 kPa−1×10−3; 95% CI, −0.16 to −0.06) and higher DC (B=0.93 kPa×103; 95% CI, 0.34–1.51), but there was no additional benefit with a higher level of physical activity (Table 4). In model 3, the associations between physical activity and diastolic diameter, ln YEM, and DC were attenuated but remained statistically significant (Table 4).
Table 4.
Associations of Physical Activity With Other Characteristics of the Carotid Wall (n=3165)
| Quintiles of Physical Activity, METh/w | No. | Model 1 | Model 2 | Model 3 | P Trend |
|---|---|---|---|---|---|
| Diastolic diameter, mm | |||||
| 1 (0.0) | 713 | Reference | Reference | Reference | |
| 2 (0.1–9.1) | 553 | −0.11 (−0.21 to −0.01) | −0.09 (−0.19 to 0.01) | −0.06 (−0.16 to 0.04) | |
| 3 (9.2–18.4) | 631 | −0.15 (−0.25 to −0.05) | −0.12 (−0.21 to −0.02) | −0.09 (−0.19 to 0.00) | |
| 4 (18.5–32.0) | 634 | −0.14 (−0.24 to −0.04) | −0.10 (−0.20 to −0.01) | −0.07 (−0.16 to 0.03) | |
| 5 (>32.0) | 634 | −0.16 (−0.26 to −0.07) | −0.13 (−0.23 to −0.03) | −0.10 (−0.20 to −0.01) | 0.04 |
| Ln Young's elastic modulus, kPa×103 | |||||
| 1 (0.0) | 713 | Reference | Reference | Reference | |
| 2 (0.1–9.1) | 553 | −0.10 (−0.15 to −0.06) | −0.11 (−0.16 to −0.06) | −0.09 (−0.14 to −0.05) | |
| 3 (9.2–18.4) | 631 | −0.08 (−0.12 to −0.03) | −0.08 (−0.12 to −0.03) | −0.07 (−0.11 to −0.02) | |
| 4 (18.5–32.0) | 634 | −0.06 (−0.10 to −0.01) | −0.06 (−0.10 to −0.01) | −0.04 (−0.09 to 0.00) | |
| 5 (>32.0) | 634 | −0.09 (−0.13 to −0.04) | −0.09 (−0.14 to −0.04) | −0.08 (−0.12 to −0.03) | 0.02 |
| Distensibility coefficient, kPa−1×10−3 | |||||
| 1 (0.0) | 713 | Reference | Reference | Reference | |
| 2 (0.1–9.1) | 553 | 0.94 (0.36–1.52) | 0.93 (0.34–1.51) | 0.66 (0.13–1.19) | |
| 3 (9.2–18.4) | 631 | 0.71 (0.15–1.27) | 0.62 (0.05–1.19) | 0.42 (−0.09 to 0.94) | |
| 4 (18.5–32.0) | 634 | 0.53 (−0.04 to 1.08) | 0.45 (−0.11 to 1.02) | 0.18 (−0.34 to 0.69) | |
| 5 (>32.0) | 634 | 0.64 (0.08–1.20) | 0.60 (0.04–1.17) | 0.59 (−0.14 to 0.90) | 0.54 |
Linear (B [95% CI]) or Poisson (relative risk [95% CI]) regression model. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, smoking status, and current alcohol consumption. Model 3: adjusted for age, sex, smoking status, current alcohol consumption, body mass index, presence of diabetes mellitus, presence of hypertension, and presence of hyperlipidemia. P trend reflects the linear trend using quintiles in model 3. METh/w indicates metabolic equivalent hours per week.
Stratified Analysis
The relationship between physical activity and CIMT, CAS, and diastolic diameter were stronger in patients with vascular disease than in patients with vascular risk factors (Figure 1 and Table S1), although the P value for interaction was significant for CIMT only (P=0.0003).
Figure 1.
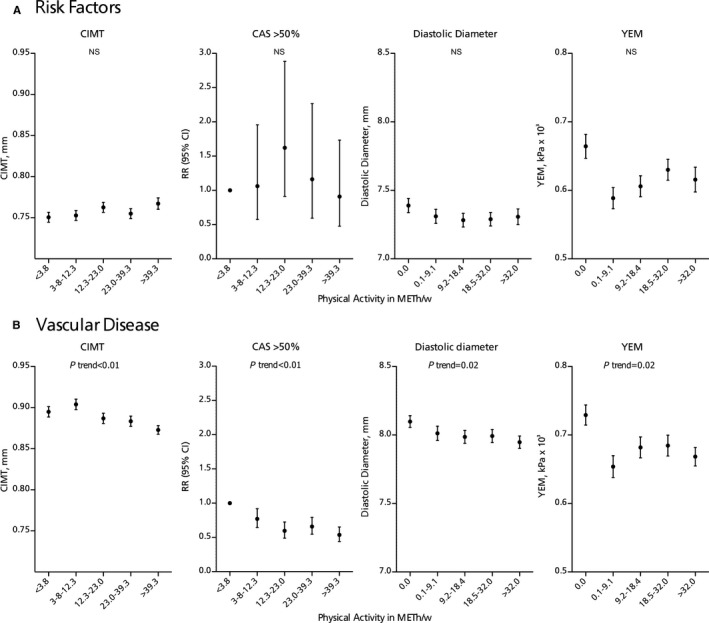
Adjusted means (SEM) of carotid intima‐media thickness (CIMT), diastolic diameter, and Young's elastic modulus (YEM) and relative risk (RR [95% CI]) of carotid stenosis per quintile of physical activity in patients with (A) vascular risk factors or (B) vascular disease (see Table S1). Analyses are adjusted for age and sex, smoking, and alcohol consumption. P trend reflects the linear trend using quintiles. CAS indicates carotid artery stenosis; METh/w, metabolic equivalent hours per week; NS, not significant.
Figure 2 shows the associations between physical activity and CIMT in men and women among different age categories. When we stratified for sex, there were no associations between physical activity and CIMT, YEM, and DC in women, whereas in men these associations were significant (Table S2). The P value for interaction was significant for CIMT (P=0.02). In older patients (>60 years), the relationship between physical activity and CIMT, diastolic diameter, and stiffness markers (Table S3) appeared to be stronger than in younger patients. The P values for interaction were significant for the stiffness markers (YEM P=0.08; DC P=0.01). Associations of physical activity with DC were stronger in nonsmokers and in patients with a normal BMI compared with current smokers and patients with a high BMI, while the associations with other characteristics were not different (Tables S4 and S5).
Figure 2.
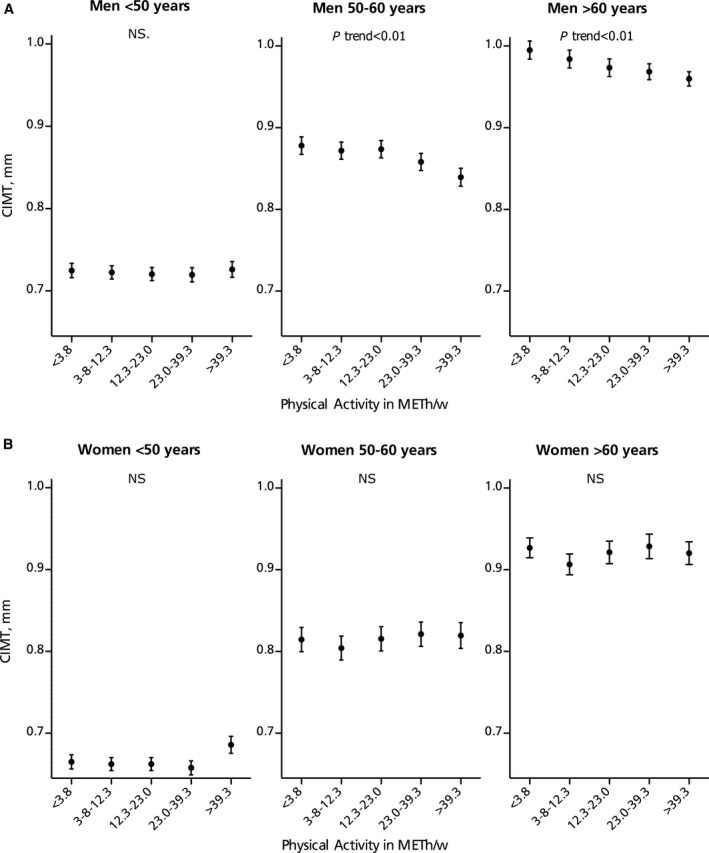
Adjusted means (SEM) of carotid intima‐media thickness (CIMT) per quintile of physical activity in (A) men and (B) women across age categories (see Tables S2 and S3). Analyses are adjusted for age, smoking, and alcohol consumption. P trend reflects the linear trend using quintiles. METh/w indicates metabolic equivalent hours per week; NS, not significant.
To explore potential cohort or period effects—because of the long inclusion period—we performed stratified analyses based on inclusion period. The total cohort was divided into 3 equal inclusion periods, each about 5.5 years, and the cohort with diastolic diameter and stiffness measurements was split into 2 periods of about 3.4 years. The associations with physical activity for the different measures in these periods were similar and the P values for interaction were not significant (data not shown), suggesting that the long inclusion period did not influence the observed associations. Exclusion of patients with a history of carotid interventions resulted in minor differences in the associations with physical activity (data not shown).
Discussion
In this large cohort of patients with vascular disease or vascular risk factors, we observed that a higher level of leisure‐time physical activity was associated with a lower risk of CAS and smaller end‐diastolic lumen diameter of the carotid artery. In addition, we found that patients with a light level of physical activity already had less carotid stiffness, whereas there was no additional benefit in patients with a higher level of physical activity. In patients with vascular disease, physical activity was inversely associated with common CIMT, but not in patients with vascular risk factors.
This study is among the first to examine the benefits of physical activity on characteristics of the carotid artery wall in patients with vascular disease or vascular risk factors, a patient population at high risk for (recurrent) vascular events and mortality. Our results suggest that there is a direct relationship between physical activity and the carotid artery wall and that increased physical activity is important in the prevention of vascular aging, even in patients with vascular risk factors or vascular disease. Therefore, patients with vascular disease and risk factors should be encouraged to perform physical activity. Moreover, in our study, even small amounts of physical activity had benefits on carotid artery stiffness. Our results expand the findings of previous studies in the general population, which have described that physical activity is associated with a lower risk of CAS33 and lower arterial stiffness.20, 21, 22
The benefits of physical activity on diastolic diameter of the carotid artery have not been previously described. Diastolic diameter is generally regarded as a measure of carotid artery structure, but vessel elasticity is the chief determinant of resting vessel size. Diastolic diameter can therefore also be viewed as a measure of arterial stiffness. Vascular risk factors such as blood pressure and smoking have been associated with a diastolic diameter enlargement.34 In a prospective study in young adults with metabolic syndrome, diameter enlargement and stiffening of the carotid artery preceded an increase in CIMT.35 This finding suggests that diastolic diameter enlargement and CIMT reflect different phases of carotid artery remodeling. This may also explain the difference in benefits on diastolic diameter versus CIMT in our study; the benefits on diastolic diameter were similar in patients with vascular disease and vascular risk factors, whereas the benefits on CIMT were present only in patients with vascular disease.
In our study, associations with the characteristics of the carotid artery wall were stronger in patients with vascular disease than in patients with vascular risk factors, especially for CIMT. These results suggest that the effects of physical activity on the carotid artery wall are stronger in patients with vascular disease than in patients with vascular risk factors. A similar result has been reported on the association of cardiorespiratory fitness, which is largely dependent on physical activity, with CIMT.24 In patients with vascular risk factors, the magnitude of the association was higher than in patients without vascular risk factors. In addition, there is evidence that patients with vascular disease may be more amenable to improvement in endothelial function as a result of physical exercise than healthy persons.9
Associations with CIMT were also stronger in men than in women and in patients with a higher age. BMI and smoking status did not influence this relationship. A sex difference has been previously reported for CIMT.16 The difference in relationship with higher age and male sex could be explained by the findings in an earlier study that suggested stronger associations in patients with vascular risk factors, as both age and male sex are important risk factors for vascular disease.24 However, contrary to this study, the associations in our study were not stronger for smoking and high BMI. The high prevalence of these risk factors in our study population could explain these results. Another explanation might be that these risk factors become less important in the presence of vascular disease.
Study Limitations
Limitations of the study are first the cross‐sectional design, which did not allow us to discern causality from consequence. We cannot determine which comes first. It is also possible that the observed associations are a consequence of the severity of vascular disease and vascular aging leading to decreased physical activity, and not a consequence of physical activity itself. Second, physical activity was measured by a self‐report questionnaire, which can be biased by patient recall and social desirability.36 This could have led to misclassification, especially in the most physically active group, and to an underestimation of the benefits of physical activity. Third, the influence of residual confounding cannot be excluded.
Conclusions
In this large cohort of patients with vascular disease and vascular risk factors, a higher level of physical activity was associated with a lower risk of carotid stenosis, a smaller diastolic diameter, and less carotid stiffness, even in patients with vascular disease. These results suggest that physical activity is important for vascular health and therefore support recommendations to increase physical activity, also, and perhaps especially, in patients with vascular disease.
Appendix
SMART study group
We gratefully acknowledge the contribution of the SMART research nurses; R. van Petersen (data manager); B. G. F. Dinther (vascular manager); and the participants of the SMART study group: A. Algra, MD, PhD; Y. van der Graaf, MD, PhD; D. E. Grobbee, MD, PhD; G. E. H. M. Rutten, MD, PhD, Julius Center for Health Sciences and Primary Care; F. L. J. Visseren, MD, PhD, Department of Internal Medicine; G. J. de Borst, MD, PhD, Department of Vascular Surgery; L. J. Kappelle, MD, PhD, Department of Neurology; T. Leiner, MD, PhD, Department of Radiology; P. A. Doevendans, MD, PhD, Department of Cardiology.
Sources of Funding
Funding for this paper was received as part of grants from the Netherlands Organisation for Scientific Research‐Medical Sciences (NWO‐MW; project No. 904‐65‐095 and project No. 904‐61‐154). The NWO‐MW had no role in the design, data collection, data analyses, or data interpretation of the study or writing of the report.
Disclosures
None.
Supporting information
Table S1. Associations Between Physical Activity and Characteristics of the Carotid Wall in Individuals With Vascular Risk Factors or Vascular Disease
Table S2. Associations Between Physical Activity and Characteristics of the Carotid Wall in Men and Women
Table S3. Associations Between Physical Activity and Characteristics of the Carotid Wall in Age Categories
Table S4. Associations Between Physical Activity and Characteristics of the Carotid Wall in Current Smokers and Nonsmokers
Table S5. Associations Between Physical Activity and Characteristics of the Carotid Wall in Participants With Normal BMI and High BMI
(J Am Heart Assoc. 2017;6:e005143 DOI: 10.1161/JAHA.116.005143.)28736388
References
- 1. Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta‐analysis. Stroke. 2003;34:2475–2481. [DOI] [PubMed] [Google Scholar]
- 2. Woodcock J, Franco OH, Orsini N, Roberts I. Non‐vigorous physical activity and all‐cause mortality: systematic review and meta‐analysis of cohort studies. Int J Epidemiol. 2011;40:121–138. [DOI] [PubMed] [Google Scholar]
- 3. Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta‐analysis. Circulation. 2011;124:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boss HM, Kappelle LJ, van der Graaf Y, Kooistra M, Visseren FL, Geerlings MI. Physical activity and vascular events and mortality in patients with vascular disease. Med Sci Sports Exerc. 2015;47:2359–2365. [DOI] [PubMed] [Google Scholar]
- 5. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 6. Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA. Exercise training and blood lipids in hyperlipidemic and normolipidemic adults: a meta‐analysis of randomized, controlled trials. Eur J Clin Nutr. 1999;53:514–522. [DOI] [PubMed] [Google Scholar]
- 7. Ford ES. Does exercise reduce inflammation? Physical activity and C‐reactive protein among U.S. adults. Epidemiology. 2002;13:561–568. [DOI] [PubMed] [Google Scholar]
- 8. Sherman DL. Exercise and endothelial function. Coron Artery Dis. 2000;11:117–122. [DOI] [PubMed] [Google Scholar]
- 9. Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108:845–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. [DOI] [PubMed] [Google Scholar]
- 11. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 12. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 13. van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all‐cause mortality: the Hoorn study. J Am Coll Cardiol. 2014;63:1739–1747. [DOI] [PubMed] [Google Scholar]
- 14. Polak JF, Sacco RL, Post WS, Vaidya D, Arnan MK, O'Leary DH. Incident stroke is associated with common carotid artery diameter and not common carotid artery intima‐media thickness. Stroke. 2014;45:1442–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. [DOI] [PubMed] [Google Scholar]
- 16. Stensland‐Bugge E, Bønaa KH, Joakimsen O, Njølstad I. Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: the Tromsø study. Stroke. 2000;31:574–581. [DOI] [PubMed] [Google Scholar]
- 17. Bertoni AG, Whitt‐Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;169:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kozakova M, Palombo C, Morizzo C, Nolan JJ, Konrad T, Balkau B; the RISC Investigators . Effect of sedentary behaviour and vigorous physical activity on segment‐specific carotid wall thickness and its progression in a healthy population. Eur Heart J. 2010;31:1511–1519. [DOI] [PubMed] [Google Scholar]
- 19. Schmitz KH, Arnett DK, Bank A, Liao D, Evans GW, Evenson KR, Stevens J, Sorlie P, Folsom AR. Arterial distensibility and physical activity in the ARIC study. Med Sci Sports Exerc. 2001;33:2065–2071. [DOI] [PubMed] [Google Scholar]
- 20. Palve KS, Pahkala K, Magnussen CG, Koivistoinen T, Juonala M, Kahonen M, Lehtimaki T, Ronnemaa T, Viikari JSA, Raitakari OT. Association of physical activity in childhood and early adulthood with carotid artery elasticity 21 years later: the cardiovascular risk in Young Finns Study. J Am Heart Assoc. 2014;3:e000594 DOI: 10.1161/JAHA.113.000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andersson C, Lyass A, Larson MG, Spartano NL, Vita JA, Benjamin EJ, Murabito JM, Esliger DW, Blease SJ, Hamburg NM, Mitchell GF, Vasan RS. Physical activity measured by accelerometry and its associations with cardiac structure and vascular function in young and middle‐aged adults. J Am Heart Assoc. 2015;4:e001528 DOI: 10.1161/JAHA.114.001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laursen AS, Hansen AL, Wiinberg N, Brage S, Sandbæk A, Lauritzen T, Witte DR, Jørgensen ME, Johansen NB. Higher physical activity is associated with lower aortic stiffness but not with central blood pressure: the ADDITION‐Pro Study. Medicine (Balitmore). 2015;94:e485–e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caviezel S, Dratva J, Schaffner E, Schindler C, Endes S, Autenrieth CS, Wanner M, Martin B, de Groot E, Gaspoz J‐M, Künzli N, Probst‐Hensch N, Schmidt‐Trucksäss A. Carotid stiffness and physical activity in elderly—a short report of the SAPALDIA 3 cohort study. PLoS One. 2015;10:e0128991–e0128999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scholl J, Bots ML, Peters SAE. Contribution of cardiorespiratory fitness, relative to traditional cardiovascular disease risk factors, to common carotid intima‐media thickness. J Intern Med. 2014;277:439–446. [DOI] [PubMed] [Google Scholar]
- 25. Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y. Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol. 1999;15:773–781. [DOI] [PubMed] [Google Scholar]
- 26. Dijk JM, Algra A, van der Graaf Y, Grobbee DE, Bots ML; SMART Study Group . Carotid stiffness and the risk of new vascular events in patients with manifest cardiovascular disease. The SMART study. Eur Heart J. 2005;26:1213–1220. [DOI] [PubMed] [Google Scholar]
- 27. Pols MA, Peeters PH, Ocké MC, Slimani N, Bueno‐de‐Mesquita HB, Collette HJ. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int J Epidemiol. 1997;26(suppl 1):S181–S189. [DOI] [PubMed] [Google Scholar]
- 28. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498. [DOI] [PubMed] [Google Scholar]
- 29. Simons PC, Algra A, Bots ML, Grobbee DE, van der Graaf Y. Common carotid intima‐media thickness and arterial stiffness: indicators of cardiovascular risk in high‐risk patients. The SMART Study (Second Manifestations of ARTerial disease). Circulation. 1999;100:951–957. [DOI] [PubMed] [Google Scholar]
- 30. Elgersma OEH, van Leersum M, Buijs PC, van Leeuwen MS, van de Schouw YT, Eikelboom BC, van der Graaf Y. Changes over time in optimal duplex threshold for the identification of patients eligible for carotid endarterectomy. Stroke. 1998;29:2352–2356. [DOI] [PubMed] [Google Scholar]
- 31. Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255. [DOI] [PubMed] [Google Scholar]
- 32. Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ. 2012;184:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stein RA, Rockman CB, Guo Y, Adelman MA, Riles T, Hiatt WR, Berger JS. Association between physical activity and peripheral artery disease and carotid artery stenosis in a self‐referred population of 3 million adults. Arterioscler Thromb Vasc Biol. 2014;35:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crouse JR, Goldbourt U, Evans G, Pinsky J, Sharrett AR, Sorlie P, Riley W, Heiss G. Risk factors and segment‐specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1996;27:69–75. [DOI] [PubMed] [Google Scholar]
- 35. Ferreira I, Beijers HJ, Schouten F, Smulders YM, Twisk JW, Stehouwer CD. Clustering of metabolic syndrome traits is associated with maladaptive carotid remodeling and stiffening: a 6‐year longitudinal study. Hypertension. 2012;60:542–549. [DOI] [PubMed] [Google Scholar]
- 36. Le Grande MR, Elliott PC, Worcester MU, Murphy BM, Goble AJ. An evaluation of self‐report physical activity instruments used in studies involving cardiac patients. J Cardiopulm Rehabil Prev. 2008;28:358–369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Associations Between Physical Activity and Characteristics of the Carotid Wall in Individuals With Vascular Risk Factors or Vascular Disease
Table S2. Associations Between Physical Activity and Characteristics of the Carotid Wall in Men and Women
Table S3. Associations Between Physical Activity and Characteristics of the Carotid Wall in Age Categories
Table S4. Associations Between Physical Activity and Characteristics of the Carotid Wall in Current Smokers and Nonsmokers
Table S5. Associations Between Physical Activity and Characteristics of the Carotid Wall in Participants With Normal BMI and High BMI


