Abstract
Background
Cardiac troponin T and brain natriuretic peptide (BNP) are elevated in >50% of dialysis patients and are associated with poor outcomes. Few data investigated these associations in earlier chronic kidney disease (CKD).
Methods and Results
We studied whether CKD modified associations of elevated BNP, N‐terminal‐pro‐BNP, high‐sensitivity cardiac troponin T, coronary artery calcification, and left ventricular hypertrophy with all‐cause death and cardiovascular death/events in 3218 multiethnic individuals followed for 12.5 years, and whether biomarkers added prognostic information to traditional cardiovascular risk factors in CKD. Of the cohort, 279 (9%) had CKD. There were 296 deaths and 218 cardiovascular deaths/events. Of non‐CKD individuals, 7% died and 6% had cardiovascular death/event versus 32% and 30% of CKD participants, P<0.001 for both. The interaction between BNP and CKD on death was significant (P=0.01): the adjusted hazard ratio in CKD was 2.05, 95% CI (1.34, 3.14), but not significant in non‐CKD, 1.04 (0.76, 1.41). CKD modified the association of high‐sensitivity cardiac troponin T with cardiovascular death/event, adjusted hazard ratio 3.34 (1.56, 7.18) in CKD versus 1.65 (1.16, 2.35) in non‐CKD, interaction P=0.09. There was an interaction between N‐terminal‐pro‐BNP and CKD for death in those without prior cardiovascular disease. Addition of each biomarker to traditional risk factors improved risk prediction, except coronary artery calcification was not discriminatory for cardiovascular death/event in CKD.
Conclusions
Cardiac biomarkers, with the exception of coronary artery calcification, prognosticated outcomes in early‐stage CKD as well as, if not better than, in non‐CKD individuals, even after controlling for estimated glomerular filtration rate, and added to information obtained from traditional cardiovascular risk factors alone.
Keywords: cardiac biomarkers, cardiovascular outcomes, chronic kidney disease, coronary artery calcium, mortality, N‐terminal‐pro‐brain natriuretic peptide, troponin T
Subject Categories: Biomarkers, Clinical Studies, Nephrology and Kidney, Cardiovascular Disease, Mortality/Survival
Clinical Perspective
What Is New?
Cardiac biomarkers such as high‐sensitivity cardiac troponin T, brain natriuretic peptide, N‐terminal‐pro‐brain natriuretic peptide, coronary artery calcification, and left ventricular hypertrophy are more commonly elevated in individuals with chronic kidney disease, even at early stages identified by albuminuria in the setting of preserved glomerular filtration rate.
Each of these biomarkers, except for coronary artery calcification, prognosticate cardiovascular outcomes in chronic kidney disease patients at least as well, if not more powerfully, as in those without chronic kidney disease.
What Are the Clinical Implications?
A multimodality approach combining these circulating and imaging‐based cardiac biomarkers can be used to add to the prognostic information obtained from traditional risk factors alone to predict the likelihood of cardiovascular events in individuals with chronic kidney disease.
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality among patients with chronic kidney disease (CKD), and is almost twice as prevalent among those with CKD as those without it.1 Baseline levels of commonly tested plasma cardiac biomarkers including brain natriuretic peptide (BNP), cardiac troponin T (TnT), and N‐terminal‐pro‐BNP (NT‐pro‐BNP) can be elevated in asymptomatic patients with advanced CKD and end‐stage renal disease,2, 3, 4 and can thus be difficult to interpret clinically in these populations.5 Several studies suggest prognostic value of these biomarkers in hemodialysis patients for predicting all‐cause mortality and cardiovascular events.6, 7, 8 Few studies have assessed elevated levels of these biomarkers in earlier CKD stages and explored associations with outcomes in nondialysis CKD samples. These were limited by small sample sizes and event rates, inadequate control for confounding, and ethnic homogeneity.2, 5, 9, 10, 11, 12, 13, 14 Moreover, few data are available evaluating the prognostic value of TnT measured with new high‐sensitivity assays (hs‐TnT) in CKD.13, 14, 15 Importantly, most prior studies do not include stages 1 to 2 CKD as defined by albuminuria with preserved glomerular filtration rate (GFR), when early interventions might make the largest impact on cardiovascular outcomes.
It is not known whether cardiac imaging–based biomarkers such as coronary artery calcification (CAC) and left ventricular hypertrophy (LVH), also prevalent in CKD,16, 17, 18 should be used for cardiovascular risk prediction. CAC testing is recommended for assessing future cardiovascular risk in non‐CKD patients with intermediate risk, but its prognostic utility in CKD patients as an add‐on to the Framingham Risk score is not clear. LVH is prevalent in 75% of patients with advanced CKD,19 but studies reporting an association with cardiovascular events may be largely confounded by the presence of hypertension.19, 20
The specific aims of this study are to determine the following: (1) whether CKD modifies the association of detectable hs‐TnT, elevated BNP and NT‐pro‐BNP, CAC ≥100 Agatston units, and LVH with death and cardiovascular events; and (2) whether cardiac biomarkers differentially add to the prognostic ability of traditional Framingham cardiovascular risk factors in CKD versus non‐CKD individuals. We addressed these aims using a pre‐existing cohort that includes early stages of CKD defined by albuminuria with preserved estimated GFR (eGFR) in order to address existing knowledge gaps.
Materials and Methods
Participants
The DHS (Dallas Heart Study) is a longitudinal, multiethnic, population‐based study involving a probability sample of community‐dwelling residents of Dallas County, TX,21 approved by the University of Texas Southwestern Institutional Review Board. The study adheres to the Declaration of Helsinki. After providing informed consent, 6101 participants completed an in‐home visit to collect health‐related data. A probability‐based subset of 3398 persons aged 30 to 65 years underwent a second in‐home visit, providing fasting blood and first‐void urine. Of those, 2971 participants completed a third visit for advanced imaging. Our primary analysis included 3218 participants with samples for circulating cardiac biomarkers of interest, microalbuminuria, and sufficient data to estimate GFR. Of those, 2324 with available imaging studies were included in a secondary analysis involving CAC and LV mass.
Biomarker Measurements
Fasting venous blood was drawn via venipuncture into EDTA tubes, refrigerated for up to 4 hours at 4°C before centrifugation at 1430g for 15 minutes. Plasma was removed and frozen at −70°C until assays were performed. TnT levels were measured using a high‐sensitivity assay (hs‐TnT; Elecsys‐2010® Troponin T hs STAT; Roche Diagnostics, Indianapolis, IN). BNP (Biosite Inc, San Diego, CA) and NT‐pro‐BNP (Elecsys®; Roche Diagnostics) were measured using commercially available assays, previously described.22 Hs‐TnT was considered elevated if present at a concentration ≥3 ng/L, the limit of blank of the assay. BNP and NT‐pro‐BNP were defined as elevated if ≥75th sex‐based percentiles. For BNP, the 75th percentile cutoff was ≥15.4 pg/mL for women and ≥9.5 pg/mL for men; and for NT pro‐BNP, ≥76.1 pg/mL for women and ≥40.6 pg/mL for men. The 75th percentile threshold was selected because it is unbiased and would yield a prevalence of elevation roughly equivalent to hs‐TnT, which was above the limit of blank in 24% of DHS participants.
CAC was measured with electron‐beam computerized tomography on a single scanner (Imatron 150 XP; Imatron, Inc, San Francisco, CA) at 80% of the R‐R interval with 30‐cm field of view, 512 matrix with sharp kernel reconstruction.23 The mean of 2 consecutive measurements was used as the final score. If only 1 scan was performed, that measurement was designated as the final score. CAC was scored following the Multi‐Ethnic Study of Atherosclerosis protocol, and expressed in Agatston units.24 Clinically relevant CAC was defined as a score of ≥100 Agatston units, corresponding to moderate‐to‐high 10‐year cardiovascular event risk.25 LV mass was measured with cardiac magnetic resonance imaging using a Phillips Medical Systems (Best, the Netherlands) 1.5‐T Intera magnet and was indexed to body surface area.26 LVH was defined as normalized LV mass >89 g/m2 in men and >112 g/m2 in women, representing sex‐specific 97.5th percentiles from a healthy, phenotypically normal subpopulation of the DHS.26
Urinary and Kidney Function Measurements
A first‐void urine sample was used to measure spot urinary albumin and creatinine and calculate the urinary albumin‐to‐creatinine ratio (ACR), expressed as mg/g. Serum and urine creatinine concentrations were both determined by the alkaline picrate method, and, therefore, the 4‐variable Modification of Diet in Renal Disease study formula was used to derive eGFR.27, 28 CKD was defined as an eGFR <60 mL/min per 1.73 m2 or an ACR ≥17 mg/g in men or ≥25 mg/g in women.29, 30 CKD stage was defined by National Kidney Foundation guidelines: stage 1, ACR ≥17 mg/g in men or ≥25 mg/g in women and eGFR ≥90; stage 2, ACR ≥17 mg/g in men or ≥25 mg/g in women and eGFR 60 to 89; stage 3, eGFR 30 to 59; stage 4, eGFR 15 to 29; and stage 5, eGFR <15.29 Sensitivity analyses were also performed using the CKD‐EPI equation to derive eGFRs.31
Outcome Measures
The a priori primary outcome was all‐cause death. The secondary outcome, designed to reflect predictive impact of biomarkers for cardiovascular events, was a composite of cardiovascular death or cardiovascular event, defined as nonfatal myocardial infarction, stroke, cardiovascular revascularization (coronary artery bypass grafting or percutaneous coronary intervention), or hospitalization for congestive heart failure or atrial fibrillation. Death was ascertained using the National Death Index through December 31, 2013. Participants were labeled as having died of cardiovascular causes using International Statistical Classification of Diseases 10 codes I00 to I99.32 Cardiovascular events were adjudicated by DHS investigators through 2011. Two‐hundred fifty‐nine participants without adjudicated data for cardiovascular events were excluded from the survival analyses for the secondary outcome.
Statistical Analysis
Biomarker levels were compared among participants with versus without CKD using χ2 test for categorical and 2‐sample t test or Wilcoxon rank sum for continuous variables. For comparison across CKD stages, Cochran–Armitage trend test was used for categorical and Jonckheere–Terpstra test for continuous variables.
For the primary prespecified analysis including 3218 participants with available plasma biomarkers, all‐cause death and cardiovascular deaths/events were estimated using Kaplan–Meier curves, and compared between CKD and non‐CKD groups using the log‐rank test. Univariable and multivariable Cox proportional hazards models were used to determine the association between biomarkers, at the prespecified cut points, and outcomes. Effect modification of CKD on these associations was determined using interaction terms (CKD×biomarker), with an interaction P value of <0.1 considered significant. Multivariable models controlled for race and traditional cardiovascular risk factors (age, sex, diabetes mellitus, hypertension, current smoking, total cholesterol, and high‐density lipoprotein cholesterol).33 Finally, adjustment for log‐transformed eGFR was made to control for any potential effect of reduced renal clearance on biomarker levels, and separate models were also constructed to evaluate controlling for body mass index and ACR. Sensitivity analyses were performed excluding 236 participants (49 with CKD and 187 without CKD) with prior CVD (self‐reported history of myocardial infarction, revascularization, congestive heart failure, or stroke). Sensitivity analyses were also performed using the CKD‐EPI equation (instead of Modification of Diet in Renal Disease) to derive eGFRs. A prespecified secondary analysis was performed that included 2324 participants with imaging studies available for CAC and LVH assessment.
To determine whether biomarkers differentially add to the prognostic ability of traditional cardiovascular risk factors (base model), Harrell's c‐statistics were calculated and compared with and without the addition of biomarkers. Standard errors and 95% CI for the c‐statistics were computed with jackknife estimation.34 Nested models were compared with likelihood ratio tests after adding each biomarker at the prespecified cut points to the base model, then introducing new biomarkers to assess for improvement in model discrimination for risk prediction in CKD and non‐CKD groups.35, 36 Statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of Study Participants
Of the 3218 participants, 56% were female, 52% were black, 29% were white, 17% were Hispanic, and 2% were other races. There were 279 (8.7%) with CKD. Seventy‐six percent of those with CKD had stages 1 to 2, defined by albuminuria with eGFR ≥60 mL/min per 1.73 m2. Specifically, proportions with stage 1, 2, 3, and 4 to 5 were 50%, 26%, 20%, and 3%, respectively. The mean (±SD) age was 44.6±9.8 years (Table 1). Participants with CKD were older, had a higher proportion of men and blacks, and were more likely to be hypertensive, diabetic, and current smokers than those without CKD. Median blood pressure and body mass index were also higher in the CKD group (Table 1). There were no differences in high‐density lipoprotein cholesterol levels and the proportion with hyperlipidemia between groups.
Table 1.
Demographic and Clinical Variables by CKD Status
| Variables | No CKD (N=2939) | CKD (N=279) | P Value |
|---|---|---|---|
| Age, y, median (IQR) | 44.0 (37.0, 52.0) | 49.5 (40.0, 56.0) | <0.001 |
| Women, % | 1676 (57.0) | 137 (49.1) | 0.01 |
| Race/ethnicity, % | <0.001 | ||
| Black | 1485 (50.5) | 192 (68.8) | <0.001 |
| White | 876 (29.8) | 45 (16.1) | |
| Hispanic | 515 (17.5) | 38 (13.6) | |
| Other | 63 (2.1) | 4 (1.4) | |
| Smoker, % | 1309 (44.6) | 149 (53.4) | 0.006 |
| Hypertension, % | 976 (33.2) | 186 (66.7) | <0.001 |
| Diabetes mellitus, % | 286 (9.7) | 105 (37.6) | <0.001 |
| Hyperlipidemia, % | 382 (12.7) | 45 (16.2) | 0.11 |
| Prior cardiovascular disease, %a | 187 (6.4) | 49 (17.6) | <0.001 |
| Blood pressure, mm Hg | |||
| Systolic, median (IQR) | 123.5 (114.4, 134.2) | 135.1 (123.3, 153.1) | <0.001 |
| Diastolic, medium (IQR) | 77.7 (72.4, 83.6) | 82.0 (76.0, 90.4) | <0.001 |
| BMI, kg/m2, median (IQR) | 29.4 (25.4, 34.6) | 31.8 (27.4, 36.5) | <0.001 |
| ACR, mg/g, median (IQR) | 2.7 (1.8, 4.5) | 48.2 (26.6, 117.5) | <0.001 |
| GFR, mL/min per 1.73 m2, median (IQR) | 98.4 (85.8, 112.9) | 89.6 (61.7, 114.4) | <0.001 |
| Total cholesterol, mg/dL, median (IQR) | 177.0 (154.0, 203.0) | 176.0 (150.0, 200.0) | 0.85 |
| HDL cholesterol, mg/dL, median (IQR) | 48.0 (40.0, 57.0) | 47.0 (39.0, 55.0) | 0.33 |
| Cardiac biomarkers | |||
| Hs‐TnT ≥3 ng/L, % | 712 (24.2) | 166 (59.5) | <0.001 |
| BNP, pg/mL, mean (SD) | 10.9 (32.6) | 56.0 (316.4) | <0.001 |
| BNP, pg/mL, median (IQR) | 3.0 (0.1, 12.5) | 5.6 (0.1, 25.7) | <0.001 |
| NT‐pro‐BNP, pg/mL, mean (SD) | 53.6 (118.4) | 327.0 (1242.4) | <0.001 |
| NT‐pro‐BNP, pg/mL, median (IQR) | 27.9 (13.0, 56.5) | 55.0 (19.4, 157.1) | <0.001 |
| BNP ≥75%ile, %b | 707 (24.1) | 100 (35.8) | <0.001 |
| NT‐pro‐BNP ≥75%ile, %b | 668 (22.7) | 138 (49.5) | <0.001 |
| CAC ≥100 Agatston units, %c | 197 (8.5) | 45 (22.4) | <0.001 |
| LV mass, g, median (IQR)d | 155.9 (129.9, 186.2) | 183.4 (147.9, 232.0) | <0.001 |
| LV mass/BSA, g/m2, median (IQR)d | 79.5 (69.8, 92.1) | 92.4 (78.5, 111.2) | <0.001 |
| LVH, %d | 219 (9.4) | 69 (34.0) | <0.001 |
ACR indicates spot urine albumin‐to‐creatinine ratio; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; CAC, coronary artery calcification; CKD, chronic kidney disease; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; hs‐TnT, high‐sensitivity cardiac troponin T; IQR, interquartile range; LV, left ventricular; LVH, left ventricular hypertrophy; NT‐pro‐BNP, N‐terminal pro‐brain natriuretic peptide.
Prior cardiovascular disease was defined as self‐reported history of prior myocardial infarction, revascularization, heart failure, or stroke.
Derived from sex‐based cutoffs: BNP 75th percentile cutoff for women=15.4 pg/mL, for men=9.5 pg/mL; NT‐pro‐BNP 75th percentile cutoff for women=76.1 pg/mL, for men=40.6 pg/mL.
Included 2522 participants (2321 without CKD and 201 with CKD) with available CAC scores.
Included 2543 participants (2340 without CKD and 203 with CKD) with available cardiac magnetic resonance imaging measurements.
Univariable Associations of Biomarkers With CKD
Elevated circulating biomarkers, CAC, LV mass normalized to body surface area, and presence of LVH were all significantly associated with the presence of CKD, P<0.001 for all (Table 1). Across advancing stages of CKD, there were statistically significant graded increases in the plasma levels of both BNP and NT‐pro‐BNP (P for trend <0.001 for both, data not shown). Proportion with elevated hs‐TnT, BNP, NT‐pro‐BNP, CAC, and LVH also increased across advancing CKD stages, P for trend <0.001 for each (data not shown). Sensitivity analyses using the CKD‐EPI equation to derive eGFRs for the analysis of biomarker levels revealed similar differences between CKD and non‐CKD groups (Table S1).
All‐Cause Death and Cardiovascular Death or Event
There were 296 deaths during a median (interquartile range) follow‐up of 149.6 (145.8, 154.6) months. In the CKD group, 31.9% died compared with 7.0% in the non‐CKD group, P<0.001 (Table 2). Cardiovascular death/event was reached in 218 cases (29.7% of participants with versus 6.1% without CKD, P<0.001). Each component of the secondary outcome (cardiovascular death, nonfatal myocardial infarction, stroke, congestive heart failure hospitalization, cardiovascular revascularization, and atrial fibrillation) also occurred in a higher proportion of CKD individuals (Table 2). Sensitivity analysis excluding 236 participants with prior cardiovascular disease revealed similar findings (Table S2).
Table 2.
Outcome Measures by CKD Status
| Outcome Measure | Entire Cohort (N=3218) N (%) | No CKD (N=2939) N (%) | CKD (N=279) N (%) | P Value |
|---|---|---|---|---|
| All‐cause death | 296 (9.2) | 207 (7.0) | 89 (31.9) | <0.001 |
| Cardiovascular death or cardiovascular event | 218 (7.9) | 155 (6.1) | 63 (29.7) | <0.001 |
| Cardiovascular death | 48 (1.7) | 29 (1.1) | 19 (9.0) | <0.001 |
| Cardiovascular death or heart failure | 107 (3.3) | 66 (2.3) | 41 (14.7) | <0.001 |
| Nonfatal MI | 67 (2.4) | 53 (2.1) | 14 (6.6) | <0.001 |
| Stroke | 21 (0.8) | 12 (0.5) | 9 (4.3) | <0.001 |
| CHF hospitalization | 72 (2.6) | 45 (1.8) | 27 (12.7) | <0.001 |
| Cardiovascular revascularization | 69 (2.5) | 52 (2.0) | 17 (8.0) | <0.001 |
| Atrial fibrillation | 29 (1.1) | 22 (0.9) | 7 (3.3) | 0.005 |
CHF indicates congestive heart failure; CKD, chronic kidney disease; MI, myocardial infarction.
Effect Modification of CKD on Association of Biomarkers With Outcomes
Event rates for both primary and secondary outcomes were higher in participants with elevated plasma biomarkers than without in both CKD and non‐CKD groups (Figures 1 and 2). A statistically significant interaction was seen between CKD and the effect of BNP ≥75th percentile on all‐cause death such that the adjusted hazard ratio was intensified and remained significant in the CKD group but was not significant in the non‐CKD group (interaction P=0.01) (Table 3). There was also a significant interaction between the effect of CKD and both elevated BNP and detectable hs‐TnT on cardiovascular death/event, so that the magnitude of the associations was accentuated in CKD individuals (Table 3). The adjusted hazard ratios of elevated hs‐TnT and BNP for cardiovascular death/event in the CKD group were twice that in the non‐CKD group. Controlling for eGFR yielded similar associations, with no change in the interactions between CKD and BNP on either outcome. However, after adjusting for eGFR, the interaction of CKD×hs‐TnT for cardiovascular death/event became nonsignificant. Despite this, the adjusted hazard ratio of hs‐TnT for cardiovascular death/event remained almost twice as high in the CKD group as in the non‐CKD group. Additional models adjusting for ACR and body mass index separately produced similar results, with the exception of BNP (data not shown). When controlling for albuminuria, BNP remained significantly associated with cardiovascular death or event in CKD and non‐CKD individuals, but the CKD×BNP interaction became nonsignificant: adjusted hazard ratio (95% CI) was 1.60 (1.15, 2.23) in the non‐CKD and 2.59 (1.54, 4.37) in the CKD group, interaction P=0.12.
Figure 1.
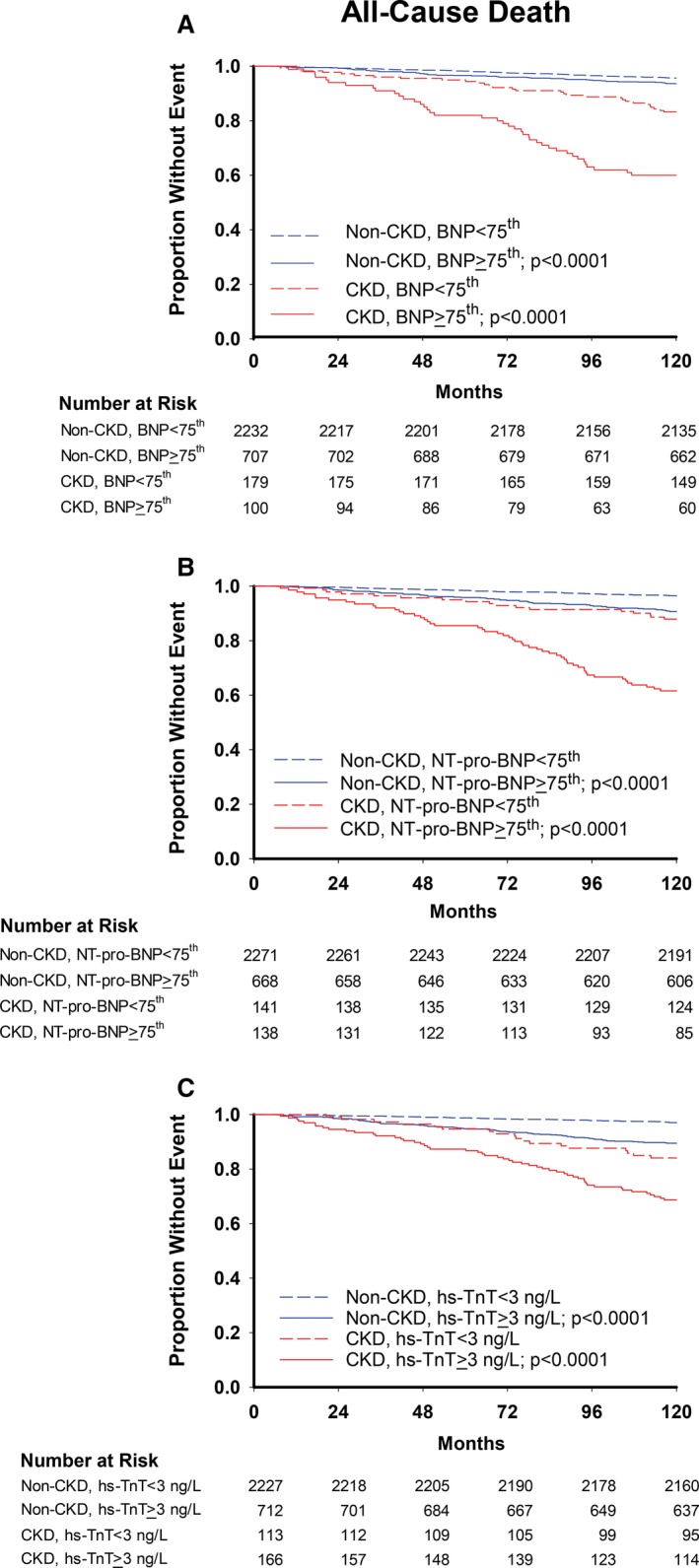
Kaplan–Meier curves for all‐cause death with (A) BNP, (B) NT‐pro‐BNP, and (C) hs‐TnT cutoffs. P values are for log‐rank tests comparing curves within CKD and non‐CKD groups. BNP indicates brain natriuretic peptide; CKD, chronic kidney disease; hs‐TnT, high‐sensitivity troponin T; NT‐pro‐BNP, N‐terminal‐pro‐brain natriuretic peptide.
Figure 2.
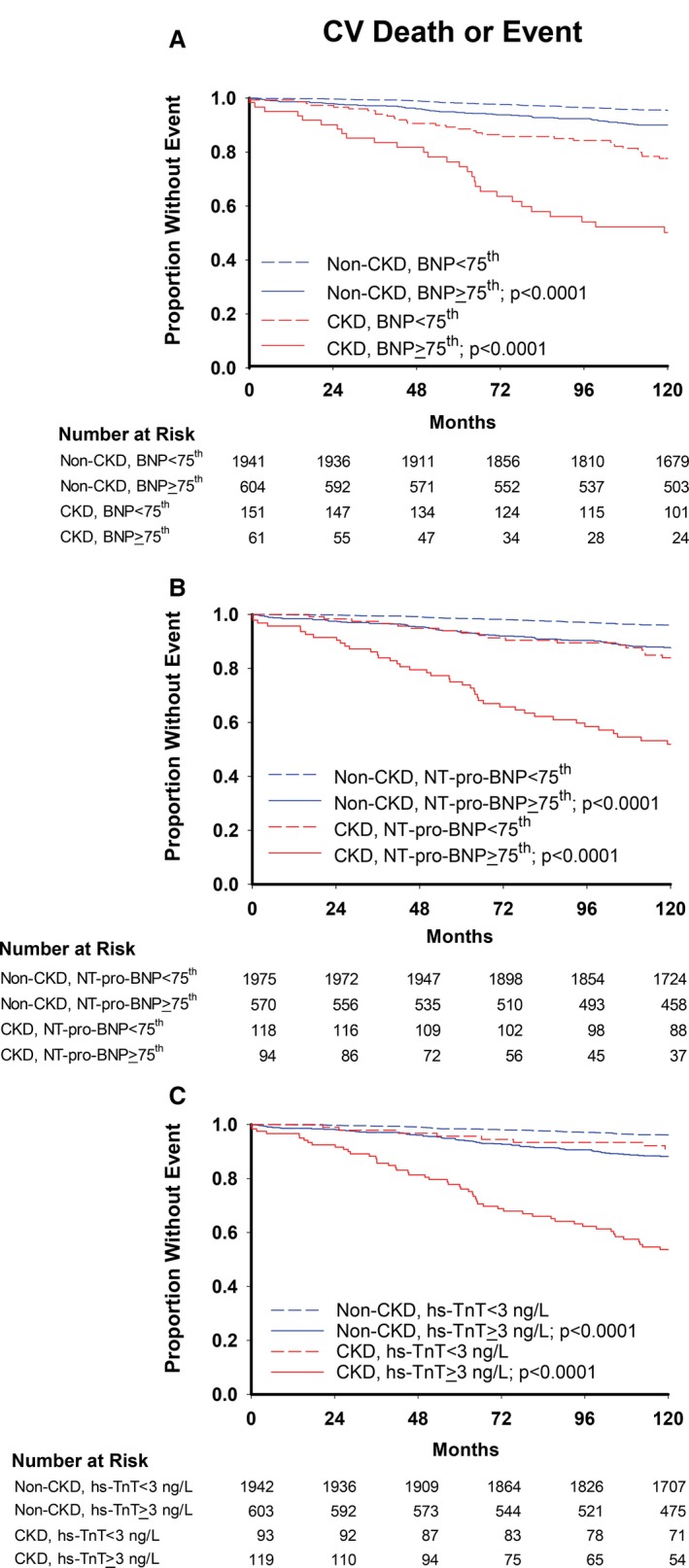
Kaplan–Meier curves for cardiovascular deaths or events with (A) BNP, (B) NT‐pro‐BNP, and (C) hs‐TnT cutoffs. P values are for log‐rank tests comparing curves within CKD and non‐CKD groups. BNP indicates brain natriuretic peptide; CKD, chronic kidney disease; hs‐TnT, high‐sensitivity troponin T; NT‐pro‐BNP, N‐terminal‐pro‐brain natriuretic peptide.
Table 3.
Associations of Biomarkers With Outcomes by CKD Status
| Exposure Variable | No CKD (N=2939) HR (95% CI) | CKD (N=279) HR (95% CI) | P Value for Interaction |
|---|---|---|---|
| All‐cause death | |||
| Hs‐TnT (≥3 ng/L) | |||
| Unadjusted | 3.16 (2.40, 4.15) | 2.73 (1.66, 4.50) | 0.62 |
| Adjusteda | 1.75 (1.29, 2.38) | 1.41 (0.84, 2.39) | 0.47 |
| Adjusted+eGFRb | 1.72 (1.27, 2.34) | 1.31 (0.77, 2.24) | 0.36 |
| Without prior CVDc | |||
| Unadjusted | 2.73 (2.00, 3.73) | 2.58 (1.48, 4.48) | 0.85 |
| Adjusteda | 1.55 (1.09, 2.20) | 1.37 (0.76, 2.47) | 0.71 |
| Adjusted+eGFRb | 1.52 (1.07, 2.16) | 1.26 (0.69, 2.29) | 0.57 |
| BNP ≥75th percentile | |||
| Unadjusted | 1.36 (1.01, 1.83) | 2.47 (1.63, 3.75) | 0.02 |
| Adjusteda | 1.04 (0.76, 1.41) | 2.05 (1.34, 3.14) | 0.01 |
| Adjusted+eGFRb | 1.04 (0.77, 1.42) | 1.97 (1.29, 3.02) | 0.02 |
| Without prior CVDc | |||
| Unadjusted | 1.02 (0.71, 1.47) | 2.13 (1.31, 3.47) | 0.02 |
| Adjusteda | 0.78 (0.53, 1.14) | 1.63 (0.99, 2.69) | 0.02 |
| Adjusted+eGFRb | 0.79 (0.54, 1.15) | 1.55 (0.93, 2.57) | 0.04 |
| NT‐pro‐BNP ≥75th percentile | |||
| Unadjusted | 2.57 (1.95, 3.38) | 3.66 (2.28, 5.88) | 0.21 |
| Adjusteda | 1.92 (1.44, 2.56) | 2.92 (1.80, 4.76) | 0.14 |
| Adjusted+eGFRb | 1.93 (1.44, 2.57) | 2.82 (1.73, 4.60) | 0.18 |
| Without prior CVDc | |||
| Unadjusted | 2.19 (1.59, 3.03) | 4.11 (2.39, 7.08) | 0.05 |
| Adjusteda | 1.72 (1.22, 2.41) | 3.20 (1.83, 5.60) | 0.06 |
| Adjusted+eGFRb | 1.73 (1.23, 2.43) | 3.10 (1.77, 5.44) | 0.08 |
| CAC ≥100d | |||
| Unadjusted | 5.36 (3.76, 7.64) | 3.39 (1.98, 5.80) | 0.16 |
| Adjusteda | 2.31 (1.55, 3.43) | 2.12 (1.20, 3.73) | 0.80 |
| Adjusted+eGFRb | 2.30 (1.54, 3.42) | 1.88 (1.05, 3.35) | 0.56 |
| LVHd | |||
| Unadjusted | 3.12 (2.12, 4.61) | 2.34 (1.38, 3.95) | 0.39 |
| Adjusteda | 1.61 (1.06, 2.44) | 1.57 (0.92, 2.68) | 0.94 |
| Adjusted+eGFRb | 1.61 (1.06, 2.45) | 1.52 (0.88, 2.60) | 0.86 |
| Cardiovascular death or event | |||
| Hs‐TnT (≥3 ng/L) | |||
| Unadjusted | 3.04 (2.21, 4.16) | 7.07 (3.37, 14.86) | 0.04 |
| Adjusteda | 1.65 (1.16, 2.35) | 3.34 (1.56, 7.18) | 0.09 |
| Adjusted+eGFRb | 1.63 (1.14, 2.32) | 3.13 (1.45, 6.76) | 0.12 |
| Without prior CVDc | |||
| Unadjusted | 2.54 (1.73, 3.73) | 7.45 (3.16, 17.61) | 0.03 |
| Adjusteda | 1.39 (0.91, 2.14) | 3.60 (1.48, 8.78) | 0.05 |
| Adjusted+eGFRb | 1.38 (0.90, 2.12) | 3.40 (1.39, 8.37) | 0.06 |
| BNP ≥75th percentile | |||
| Unadjusted | 2.27 (1.65, 3.13) | 2.80 (1.71, 4.60) | 0.49 |
| Adjusteda | 1.65 (1.19, 2.28) | 3.05 (1.83, 5.07) | 0.05 |
| Adjusted+eGFRb | 1.65 (1.19, 2.29) | 2.88 (1.70, 4.85) | 0.08 |
| Without prior CVDc | |||
| Unadjusted | 1.80 (1.20, 2.68) | 2.85 (1.58, 5.15) | 0.21 |
| Adjusteda | 1.33 (0.88, 2.01) | 3.10 (1.68, 5.71) | 0.02 |
| Adjusted+eGFRb | 1.33 (0.88, 2.01) | 2.95 (1.57, 5.54) | 0.04 |
| NT‐pro‐BNP ≥75th percentile | |||
| Unadjusted | 3.45 (2.52, 4.73) | 3.70 (2.17, 6.29) | 0.83 |
| Adjusteda | 2.60 (1.88, 3.60) | 2.75 (1.61, 4.70) | 0.86 |
| Adjusted+eGFRb | 2.60 (1.88, 3.60) | 2.64 (1.53, 4.53) | 0.97 |
| Without prior CVDc | |||
| Unadjusted | 2.71 (1.84, 3.99) | 4.00 (2.15, 7.45) | 0.29 |
| Adjusteda | 2.20 (1.47, 3.28) | 2.82 (1.50, 5.29) | 0.51 |
| Adjusted+eGFRb | 2.21 (1.48, 3.30) | 2.73 (1.45, 5.14) | 0.58 |
| CAC ≥100d | |||
| Unadjusted | 6.74 (4.51, 10.08) | 2.93 (1.51, 5.66) | 0.04 |
| Adjusteda | 2.76 (1.77, 4.29) | 1.22 (0.61, 2.47) | 0.04 |
| Adjusted+eGFRb | 2.76 (1.77, 4.30) | 1.15 (0.56, 2.35) | 0.03 |
| LVHd | |||
| Unadjusted | 4.38 (2.86, 6.72) | 4.13 (2.23, 7.67) | 0.88 |
| Adjusteda | 2.95 (1.86, 4.66) | 3.33 (1.77, 6.27) | 0.77 |
| Adjusted+eGFRb | 2.95 (1.86, 4.66) | 3.25 (1.72, 6.16) | 0.80 |
BNP indicates brain natriuretic peptide; CAC, coronary artery calcification; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; hs‐TnT, high‐sensitivity cardiac troponin T; LVH, left ventricular hypertrophy; NT‐pro‐BNP, N‐terminal pro‐brain natriuretic peptide.
Models adjusted for age, sex, race, diabetes mellitus, hypertension, smoking, total and high‐density lipoprotein (HDL) cholesterol.
Models adjusted for age, sex, race, diabetes mellitus, hypertension, smoking, total and HDL cholesterol, and eGFR.
Analysis excluding 236 participants with prior cardiovascular disease, defined as self‐reported history of prior myocardial infarction, revascularization, heart failure, or stroke (total N=2982).
Analysis including 2324 participants with available imaging studies for evaluation of CAC and LVH. Sensitivity analyses excluding participants with prior cardiovascular disease were not performed for CAC or LVH, given fewer numbers of events.
Sensitivity analyses excluding participants with prior CVD illustrated similar results for CKD effect modification, and also revealed a significant interaction between CKD and NT‐pro‐BNP for death, such that the hazard ratio for those with elevated NT‐pro‐BNP was 3.20 (1.83, 5.60) in the CKD group versus 1.72 (1.22, 2.41) in the non‐CKD group, interaction P=0.06 (Table 3). Results were similar when using CKD‐EPI equation–derived eGFRs (Table S3).
There was no significant CKD×CAC interaction for death, although CAC appeared less predictive of cardiovascular death/event in the CKD compared with the non‐CKD group, such that the association was no longer significant after adjustment for Framingham risk factors in the CKD group (Table 3). CKD did not modify the associations between LVH and the primary or secondary outcomes.
Prognostic Ability of Biomarkers
Figures 3 and 4 illustrate the c‐statistics of nested model comparisons to determine whether biomarkers differentially added to the prognostic ability of traditional cardiovascular risk factors (base model). (See Tables S4 and S5 for individual model c‐statistics, 95% CI). NT‐pro‐BNP added prognostic information for all‐cause death to the base model in both CKD and non‐CKD individuals. Hs‐TnT improved the prognostic discrimination for death in the non‐CKD but not in the CKD group. This was true for both 1 biomarker and 2 biomarker models (Figure 3A and 3B). As compared to the base model and the model containing hs‐TnT, addition of BNP or NT‐pro‐BNP improved the model fits in CKD, but adding hs‐TnT to BNP or NT‐pro‐BNP did not improve prognostication. In the non‐CKD group, all 2‐biomarker models were more discriminatory for all‐cause death than 1‐biomarker models (Figure 3A and 3B).
Figure 3.
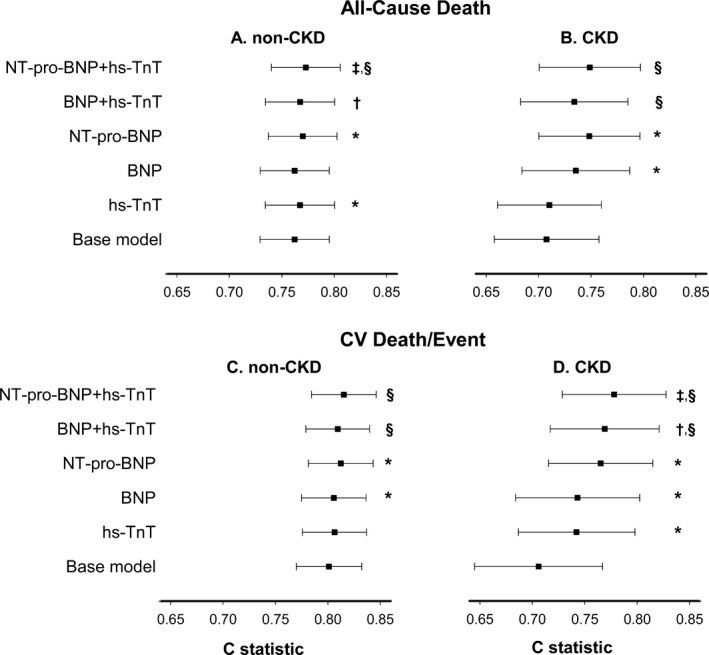
Differential prognostication of circulating biomarkers for all‐cause death in (A) non‐CKD and (B) CKD individuals; and for cardiovascular death or event in (C) non‐CKD and (D) CKD individuals. X‐axis represents Harrell's c‐statistics, and P values are for likelihood ratio tests comparing the nested models. BNP indicates brain natriuretic peptide; CKD, chronic kidney disease; hs‐TnT, high‐sensitivity troponin T; NT‐pro‐BNP, N‐terminal‐pro‐brain natriuretic peptide. *P<0.05 1 biomarker model compared with base model, including age, sex, race, diabetes mellitus, hypertension, smoking, and total and HDL cholesterol. † P<0.05 2 biomarker model compared to base model+BNP. ‡ P<0.05 2 biomarker model compared to base model+NT‐pro‐BNP. § P<0.05 2 biomarker model compared with base model+hs‐TnT.
Figure 4.
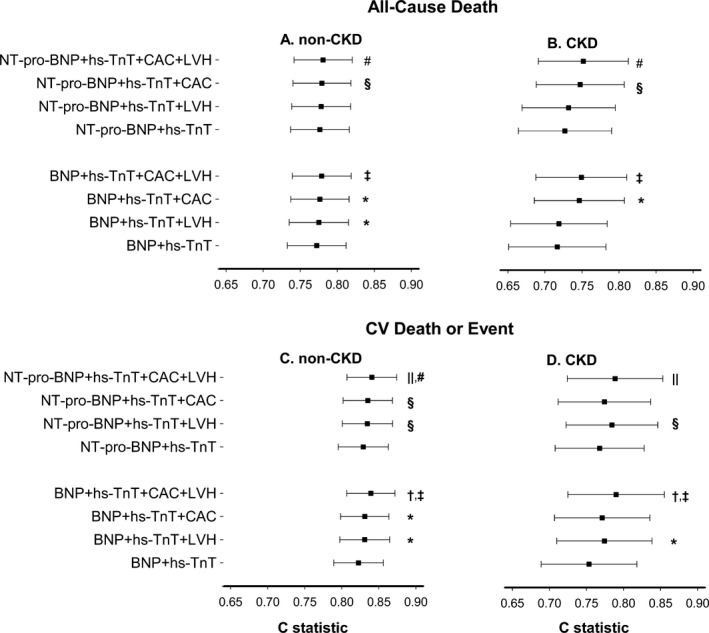
Differential prognostication of circulating and imaging biomarkers for all‐cause death in (A) non‐CKD and (B) CKD individuals; and for cardiovascular death or event in (C) non‐CKD and (D) CKD individuals. X‐axis represents Harrell's c‐statistics, and P values are for likelihood ratio tests comparing the nested models. BNP indicates brain natriuretic peptide; CAC, coronary artery calcification; CKD, chronic kidney disease; hs‐TnT, high‐sensitivity troponin T; LVH, left ventricular hypertrophy; NT‐pro‐BNP, N‐terminal‐pro‐brain natriuretic peptide. *P<0.05 3‐biomarker model compared with base model+BNP+hs‐TnT. † P<0.05 4‐biomarker model compared with base model+BNP+hs‐TnT+CAC. ‡ P<0.05 4‐biomarker model compared with base model+BNP+hs‐TnT+LVH. § P<0.05 3‐biomarker model compared with base model+NT‐pro‐BNP+hs‐TnT. || P<0.05 4‐biomarker model compared with base model+NT‐pro‐BNP+hs‐TnT+CAC. # P<0.05 4‐biomarker model compared with base model+NT‐pro‐BNP+hs‐TnT+LVH.
In models for cardiovascular death/event, both BNP and NT‐pro‐BNP added prognostic value to the base model for CKD and non‐CKD individuals (Figure 3C and 3D). Adding hs‐TnT to the base model improved the fit only for those with CKD. Two‐biomarker models were more discriminatory than 1‐biomarker models for cardiovascular death/event in the CKD group. In those without CKD, hs‐TnT did not add prognostic information for cardiovascular death/event to either the base model or the models containing only BNP or NT‐pro‐BNP (Figure 3C and 3D).
In the CKD group, adding LVH did not improve the prognostic discrimination of models containing 2 circulating biomarkers for all‐cause death (Figure 4A and 4B). However, in both CKD and non‐CKD participants, LVH did improve model fit for cardiovascular death/event when added to any of the 2‐biomarker models or models including CAC (Figure 4C and 4D). Adding CAC provided prognostic value for all‐cause death in both CKD and non‐CKD, but not for cardiovascular death/event in CKD participants.
Discussion
In this report from a large multiethnic population‐based cohort with a median follow‐up of 12.5 years, we found that (1) despite that levels of plasma and imaging cardiac biomarkers were more commonly elevated in CKD, these biomarkers, except for CAC, still prognosticated all‐cause death and cardiovascular death/event at least as well, if not better in CKD as in non‐CKD individuals; and (2) each biomarker added to the prognostic ability of traditional cardiovascular risk factors alone in those with CKD, except that CAC was not discriminatory for cardiovascular death/event. Our CKD sample is unique in that the majority were defined by albuminuria, with a minority defined by eGFR <60 mL/min per 1.73 m2, showing that these associations exist not only in those with reduced GFR but also in those with earlier stages of CKD when GFR is preserved.
Baseline chronic elevation of circulating cardiac biomarker levels has historically clouded clinical interpretation of these important tests in advanced CKD patients.2, 3, 4, 11, 12, 13, 37, 38, 39 The fractional plasma clearance of both BNP and NT‐pro‐BNP are reduced with declining eGFR, particularly for NT‐pro‐BNP.38, 40 The impact of renal clearance on circulating TnT concentrations is less certain.5 In this study, the majority of the CKD group was defined by albuminuria with preserved eGFR, where knowledge gaps in the literature exist. We extend the finding that these cardiac biomarkers are elevated in those with decreased GFR to a multiethnic CKD group primarily composed of those with preserved GFR, a sample not included in the majority of previous studies. In addition to decreased renal clearance, potential mechanisms for biomarker elevations in CKD patients could include chronic myocardial injury from altered hemodynamics, inflammation, endothelial dysfunction, and subendocardial ischemia in those with albuminuria.5
We show that despite the increased prevalence of elevated hs‐TnT, BNP, and NT‐pro‐BNP levels, each biomarker independently prognosticates hard clinical outcomes in CKD, and in some instances has even stronger associations with outcomes than in non‐CKD individuals. Studies of TnT in nondialysis CKD patients were limited by small sample sizes and low event rates, precluding adjustment for traditional risk factors and limiting results to unadjusted hazard ratios2, 12, 13, 14, 41, 42, 43, 44; only 3 investigated the prognostic value of the hs‐TnT assay in CKD patients, showing an association with incident heart failure44 and cardiovascular events.13, 14 Our study is the first to report from a multiethnic population‐based cohort that even after controlling for traditional cardiovascular risk factors, the magnitude of the association between hs‐TnT and cardiovascular outcomes was 2 times greater in those with CKD versus those without CKD, in a sample weighted towards those with albuminuria but preserved eGFR. Controlling for eGFR to account for potentially decreased renal clearance of biomarkers slightly attenuated the interaction between CKD and hs‐TnT on cardiovascular death/event, but the association remained twice as strong in the CKD group as in the non‐CKD group. This suggests that elevation in hs‐TnT and association with cardiovascular outcomes in CKD is not merely because of decreased clearance, and may reflect other mechanisms such as underlying myocardial strain, endothelial dysfunction, or subendocardial ischemia in albuminuric CKD.5 NT‐pro‐BNP has been associated with death9, 11, 15, 39, 45 and cardiovascular events in CKD samples.10, 11, 14, 38, 43, 44, 45 Elevated BNP, which has a shorter half‐life than NT‐pro‐BNP, was associated with cardiovascular events in 1 Japanese cohort with known CVD.11 In our adjusted models, elevated BNP was associated with all‐cause death in the CKD group, with no significant association in non‐CKD individuals, even after controlling for eGFR. This may reflect the fact that very low BNP levels in the non‐CKD group, which fall in a range of imprecision of the assay, do not allow discrimination of risk. We also show that in individuals without prior CVD, NT‐pro‐BNP was more strongly associated with death if CKD was present versus if absent. This association persisted after controlling for eGFR, suggesting mechanisms other than decreased renal clearance of NT‐pro‐BNP. Volume overload is a poor prognosticator in CKD, and despite decreased renal clearance of BNP or NT‐pro‐BNP in later stage CKD, elevated levels in earlier stages may reflect subclinical chronic volume overload in the setting of albuminuria.46 While hs‐TnT more specifically prognosticated cardiovascular outcomes, BNP was associated with both cardiovascular outcomes and all‐cause death, supporting this underlying pathophysiologic mechanism of chronic volume overload secondary to albuminuria even before decline in GFR.
Although traditional risk factors were more prevalent among those with CKD than without, addition of cardiac circulating biomarkers generally improved the prognostic ability of the base model that included traditional risk factors in CKD participants. The poorer performance of traditional cardiovascular risk factors alone before the addition of biomarkers for predicting outcomes highlights an opportunity to identify nontraditional risk factors specific to CKD populations to improve risk prediction.
Regarding cardiac imaging biomarkers, we confirm increased prevalence of LVH in CKD versus non‐CKD participants, even in those with albuminuria but without diminished eGFR.47, 48, 49 Previous studies reporting association of LVH with cardiovascular events may be largely confounded by the presence of hypertension.20 We show that although adding LVH does not improve mortality prediction in CKD, it does improve prediction for cardiovascular death/event, even after controlling for hypertension.
There are less data reporting unfavorable clinical outcomes of CAC in nondialysis CKD versus in end‐stage renal disease samples and were limited by low event rates, limited follow‐up, or ethnic homogeneity.16, 17 We show that although CAC ≥100 was more commonly present in those with CKD, it did not add prognostic value above traditional markers for cardiovascular outcomes in CKD individuals. In fact, CAC was less predictive of cardiovascular death/event in the CKD compared with the non‐CKD group. It is possible that underlying pathophysiologic differences in the development of CVD in CKD, such as medial versus intimal vessel calcification,50 may lead to other predisposing factors for cardiovascular events that would not be reflected in CAC scores. Alternatively, increased CAC may be a surrogate for other traditional cardiovascular risk factors in CKD patients, such that controlling for these factors resulted in a nonsignificant hazard ratio.5 Finally, the decreased number of participants with available CAC may have underpowered this assessment.
Despite the significance of our findings in a large multiethnic population‐based cohort, a few limitations deserve mentioning. The CKD group comprises a relatively low proportion of our cohort, but it is the largest cohort in the literature that contains all of the cardiac biomarkers of interest, a non‐CKD comparison group, and long‐term cardiovascular outcome measures for analysis. These findings should be validated in samples with larger numbers of individuals with CKD. Our sample included a lesser number of participants with stage 4 to 5 CKD, although the larger number with earlier stages of CKD addresses the knowledge gaps in the existing literature. In addition, time‐varying repeated measures of kidney function and cardiac biomarkers in relation to outcomes were not assessed. Future studies with serial biomarker evaluations are needed to investigate whether changing biomarker levels over time will affect cardiovascular outcomes. Finally, given that serum creatinine concentrations were determined by the alkaline picrate method, the Modification of Diet in Renal Disease equation was used to calculate eGFR. This could lead to potential misclassification of some CKD patients based on eGFR alone. However, only 3 participants were classified as having CKD by the Modification of Diet in Renal Disease but not by the CKD‐EPI equation, and sensitivity analyses using CKD‐EPI‐derived eGFRs yielded similar results.
Conclusion
We confirm that hs‐TnT, BNP, NT‐pro‐BNP, CAC, and LVH are more commonly elevated in CKD, even at early stages identified by albuminuria. Despite this, we demonstrate that in early stages of CKD with preserved GFR, each of these biomarkers, except for CAC, prognosticates outcomes at least as well, if not more powerfully, as in non‐CKD individuals, and adds to the prognostic information obtained from traditional risk factors alone. The lower performance of traditional risk models alone in those with CKD leaves room for further elucidation of the role of nontraditional risk factors to improve risk prediction in CKD patients.
Sources of Funding
The Dallas Heart Study was supported by a grant from the Donald W. Reynolds Foundation and by USPHS GCRC grant #M01‐RR00633 from NIH/NCRR‐CR. This work was supported in part by UT Southwestern O'Brien Kidney Research Core Center (NIDDK, P30DK079328). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105 to the University of Texas Southwestern Medical Center, as well as grant T32DK007257 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Disclosures
J. de Lemos has received grant support and consulting income from Roche Diagnostics and Abbott Diagnostics, and has served on end point committees for Siemen's Health Care Diagnostics and Radiometer. The remaining authors have no disclosures to report.
Supporting information
Table S1. Cardiac Biomarkers Across CKD Stages Based on eGFR Derived by CKD‐EPI Equation
Table S2. Outcome Measures by CKD Status Excluding Participants With Prior Cardiovascular Disease*
Table S3. Associations of Biomarkers With Outcomes by CKD Status Based on eGFR Derived by CKD‐EPI Equation
Table S4. C‐Statistics of Nested Models Comparing Prognostic Utility of Adding Circulating Biomarkers for Outcomes by CKD Status
Table S5. C‐Statistics of Nested Models Comparing Prognostic Utility of Adding Circulating and Imaging Biomarkers for Outcomes by CKD Status
(J Am Heart Assoc. 2017;6:e005235 DOI: 10.1161/JAHA.116.005235.)28679558
Parts of these data were presented in abstract form at the 50th Annual Meeting of the American Society of Nephrology on November 19, 2016 in Chicago, IL; at the Southern Society for Clinical Investigation Nephrology Young Investigators' Forum on February 10, 2017 in New Orleans, LA; and at the Young Investigators' Forum at the National Kidney Foundation Spring Clinical Meeting on April 18, 2017 in Orlando, FL.
References
- 1. United States renal data system: USRDS 2014 annual report volume 1: chronic kidney disease in the United States. 2014. Available at: https://www.usrds.org/2014/view/v1_04.aspx. Accessed 7/1/2016.
- 2. Abbas NA, John RI, Webb MC, Kempson ME, Potter AN, Price CP, Vickery S, Lamb EJ. Cardiac troponins and renal function in nondialysis patients with chronic kidney disease. Clin Chem. 2005;51:2059–2066. [DOI] [PubMed] [Google Scholar]
- 3. Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, Lamb EJ. B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46:610–620. [DOI] [PubMed] [Google Scholar]
- 4. Dubin RF, Li Y, He J, Jaar BG, Kallem R, Lash JP, Makos G, Rosas SE, Soliman EZ, Townsend RR, Yang W, Go AS, Keane M, Defilippi C, Mishra R, Wolf M, Shlipak MG; Investigators CS . Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross‐sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. 2013;14:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colbert G, Jain N, de Lemos JA, Hedayati SS. Utility of traditional circulating and imaging‐based cardiac biomarkers in patients with predialysis CKD. Clin J Am Soc Nephrol. 2015;10:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end‐stage renal disease. Circulation. 2002;106:2941–2945. [DOI] [PubMed] [Google Scholar]
- 7. Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LS, Creed I. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508–1515. [DOI] [PubMed] [Google Scholar]
- 8. Iliou MC, Fumeron C, Benoit MO, Tuppin P, Calonge VM, Moatti N, Buisson C, Jacquot C. Prognostic value of cardiac markers in ESRD: Chronic Hemodialysis and New Cardiac Markers Evaluation (CHANCE) study. Am J Kidney Dis. 2003;42:513–523. [DOI] [PubMed] [Google Scholar]
- 9. Vickery S, Webb MC, Price CP, John RI, Abbas NA, Lamb EJ. Prognostic value of cardiac biomarkers for death in a non‐dialysis chronic kidney disease population. Nephrol Dial Transplant. 2008;23:3546–3553. [DOI] [PubMed] [Google Scholar]
- 10. Astor BC, Yi S, Hiremath L, Corbin T, Pogue V, Wilkening B, Peterson G, Lewis J, Lash JP, Van Lente F, Gassman J, Wang X, Bakris G, Appel LJ, Contreras G. N‐terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: the African American Study of Kidney Disease and Hypertension (AASK). Circulation. 2008;117:1685–1692. [DOI] [PubMed] [Google Scholar]
- 11. Horii M, Matsumoto T, Uemura S, Sugawara Y, Takitsume A, Ueda T, Nakagawa H, Nishida T, Soeda T, Okayama S, Somekawa S, Ishigami K, Takeda Y, Kawata H, Kawakami R, Saito Y. Prognostic value of B‐type natriuretic peptide and its amino‐terminal proBNP fragment for cardiovascular events with stratification by renal function. J Cardiol. 2013;61:410–416. [DOI] [PubMed] [Google Scholar]
- 12. Goicoechea M, Garca de Vinuesa S, Gomez‐Campdera F, Gutierrez MJ, Blanco P, Amann R, Luno J. Clinical significance of cardiac troponin T levels in chronic kidney disease patients: predictive value for cardiovascular risk. Am J Kidney Dis. 2004;43:846–853. [DOI] [PubMed] [Google Scholar]
- 13. Hasegawa M, Ishii J, Kitagawa F, Kanayama K, Takahashi H, Ozaki Y, Yuzawa Y. Prognostic value of highly sensitive troponin T on cardiac events in patients with chronic kidney disease not on dialysis. Heart Vessels. 2013;28:473–479. [DOI] [PubMed] [Google Scholar]
- 14. Scheven L, de Jong PE, Hillege HL, Lambers Heerspink HJ, van Pelt LJ, Kootstra JE, Bakker SJ, Gansevoort RT; Group Ps . High‐sensitive troponin T and N‐terminal pro‐B type natriuretic peptide are associated with cardiovascular events despite the cross‐sectional association with albuminuria and glomerular filtration rate. Eur Heart J. 2012;33:2272–2281. [DOI] [PubMed] [Google Scholar]
- 15. Roberts MA, Hare DL, Sikaris K, Ierino FL. Temporal trajectory of B‐type natriuretic peptide in patients with CKD stages 3 and 4, dialysis, and kidney transplant. Clin J Am Soc Nephrol. 2014;9:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiu YW, Adler SG, Budoff MJ, Takasu J, Ashai J, Mehrotra R. Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int. 2010;77:1107–1114. [DOI] [PubMed] [Google Scholar]
- 17. Russo D, Corrao S, Battaglia Y, Andreucci M, Caiazza A, Carlomagno A, Lamberti M, Pezone N, Pota A, Russo L, Sacco M, Scognamiglio B. Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int. 2011;80:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russo D, Morrone LF, Imbriaco M, Pota A, Russo L, Scognamiglio B, Sorrentino R. Coronary artery calcification and outcomes in diabetic patients with and without chronic kidney disease. Blood Purif. 2013;36:17–20. [DOI] [PubMed] [Google Scholar]
- 19. Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end‐stage renal disease. Kidney Int. 1989;36:286–290. [DOI] [PubMed] [Google Scholar]
- 20. Chen SC, Chang JM, Liu WC, Huang JC, Tsai JC, Lin MY, Su HM, Hwang SJ, Chen HC. Echocardiographic parameters are independently associated with increased cardiovascular events in patients with chronic kidney disease. Nephrol Dial Transplant. 2012;27:1064–1070. [DOI] [PubMed] [Google Scholar]
- 21. Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH; Dallas Heart Study I . The Dallas Heart Study: a population‐based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. [DOI] [PubMed] [Google Scholar]
- 22. de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–753.e742. [DOI] [PubMed] [Google Scholar]
- 23. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 24. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 25. Baber U, de Lemos JA, Khera A, McGuire DK, Omland T, Toto RD, Hedayati SS. Non‐traditional risk factors predict coronary calcification in chronic kidney disease in a population‐based cohort. Kidney Int. 2008;73:615–621. [DOI] [PubMed] [Google Scholar]
- 26. Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. [DOI] [PubMed] [Google Scholar]
- 27. Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–513. [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 29. National Kidney F . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S46–S103. [PubMed] [Google Scholar]
- 30. Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–937. [DOI] [PubMed] [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; Ckd EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J; American Heart Association Statistics C, Stroke Statistics S . Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. [DOI] [PubMed] [Google Scholar]
- 33. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 34. Kremers W. SAS macro that calculates the c‐statistic (concordance, discrimination index) for survived data with time dependent covariates and corresponding SE and 100(1−α)% CI. Available at: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros. Accessed February 25, 2016.
- 35. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 36. Pepe MS, Kerr KF, Longton G, Wang Z. Testing for improvement in prediction model performance. Stat Med. 2013;32:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tagore R, Ling LH, Yang H, Daw HY, Chan YH, Sethi SK. Natriuretic peptides in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeFilippi CR, Fink JC, Nass CM, Chen H, Christenson R. N‐terminal pro‐B‐type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am J Kidney Dis. 2005;46:35–44. [DOI] [PubMed] [Google Scholar]
- 39. Levin A, Rigatto C, Barrett B, Madore F, Muirhead N, Holmes D, Clase CM, Tang M, Djurdjev O; Can PI . Biomarkers of inflammation, fibrosis, cardiac stretch and injury predict death but not renal replacement therapy at 1 year in a Canadian chronic kidney disease cohort. Nephrol Dial Transplant. 2014;29:1037–1047. [DOI] [PubMed] [Google Scholar]
- 40. Panteghini M, Clerico A. Understanding the clinical biochemistry of N‐terminal pro‐B‐type natriuretic peptide: the prerequisite for its optimal clinical use. Clin Lab. 2004;50:325–331. [PubMed] [Google Scholar]
- 41. Hayashi T, Kimura T, Yasuda K, Sasaki K, Obi Y, Rakugi H, Isaka Y. Cardiac troponin T elevation at dialysis initiation is associated with all‐cause and cardiovascular mortality on dialysis in patients without diabetic nephropathy. Clin Exp Nephrol. 2017;21:333–341. [DOI] [PubMed] [Google Scholar]
- 42. Michos ED, Wilson LM, Yeh HC, Berger Z, Suarez‐Cuervo C, Stacy SR, Bass EB. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta‐analysis. Ann Intern Med. 2014;161:491–501. [DOI] [PubMed] [Google Scholar]
- 43. Matsushita K, Sang Y, Ballew SH, Astor BC, Hoogeveen RC, Solomon SD, Ballantyne CM, Woodward M, Coresh J. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol. 2014;34:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bansal N, Hyre Anderson A, Yang W, Christenson RH, deFilippi CR, Deo R, Dries DL, Go AS, He J, Kusek JW, Lash JP, Raj D, Rosas S, Wolf M, Zhang X, Shlipak MG, Feldman HI. High‐sensitivity troponin T and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fu S, Luo L, Ye P, Yi S, Liu Y, Zhu B, Wang L, Xiao T, Bai Y. The ability of NT‐proBNP to detect chronic heart failure and predict all‐cause mortality is higher in elderly Chinese coronary artery disease patients with chronic kidney disease. Clin Interv Aging. 2013;8:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yilmaz Z, Yildirim Y, Oto F, Aydin FY, Aydin E, Kadiroglu AK, Yilmaz ME. Evaluation of volume overload by bioelectrical impedance analysis, NT‐proBNP and inferior vena cava diameter in patients with stage 3&4 and 5 chronic kidney disease. Ren Fail. 2014;36:495–501. [DOI] [PubMed] [Google Scholar]
- 47. Tucker B, Fabbian F, Giles M, Thuraisingham RC, Raine AE, Baker LR. Left ventricular hypertrophy and ambulatory blood pressure monitoring in chronic renal failure. Nephrol Dial Transplant. 1997;12:724–728. [DOI] [PubMed] [Google Scholar]
- 48. Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005;46:320–327. [DOI] [PubMed] [Google Scholar]
- 49. Nitta K, Iimuro S, Imai E, Matsuo S, Makino H, Akizawa T, Watanabe T, Ohashi Y, Hishida A. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:730–742. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cardiac Biomarkers Across CKD Stages Based on eGFR Derived by CKD‐EPI Equation
Table S2. Outcome Measures by CKD Status Excluding Participants With Prior Cardiovascular Disease*
Table S3. Associations of Biomarkers With Outcomes by CKD Status Based on eGFR Derived by CKD‐EPI Equation
Table S4. C‐Statistics of Nested Models Comparing Prognostic Utility of Adding Circulating Biomarkers for Outcomes by CKD Status
Table S5. C‐Statistics of Nested Models Comparing Prognostic Utility of Adding Circulating and Imaging Biomarkers for Outcomes by CKD Status


