Abstract
Background
Concerns about hyperkalemia limit the use of angiotensin‐converting enzyme inhibitors (ACE‐I) and angiotensin receptor blockers (ARBs), but guidelines conflict regarding potassium‐monitoring protocols. We quantified hyperkalemia monitoring and risks after ACE‐I/ARB initiation and developed and validated a hyperkalemia susceptibility score.
Methods and Results
We evaluated 69 426 new users of ACE‐I/ARB therapy in the Stockholm Creatinine Measurements (SCREAM) project with medication initiation from January 1, 2007 to December 31, 2010, and follow‐up for 1 year thereafter. Three fourths (76%) of SCREAM patients had potassium checked within the first year. Potassium >5 and >5.5 mmol/L occurred in 5.6% and 1.7%, respectively. As a comparison, we propensity‐matched new ACE‐I/ARB users to 20 186 new β‐blocker users in SCREAM: 64% had potassium checked. The occurrence of elevated potassium levels was similar between new β‐blocker and ACE‐I/ARB users without kidney disease; only at estimated glomerular filtration rate <60 mL/min per 1.73 m2 were risks higher among ACE‐I/ARB users. We developed a hyperkalemia susceptibility score that incorporated estimated glomerular filtration rate, baseline potassium level, sex, diabetes mellitus, heart failure, and the concomitant use of potassium‐sparing diuretics in new ACE‐I/ARB users; this score accurately predicted 1‐year hyperkalemia risk in the SCREAM cohort (area under the curve, 0.845, 95% CI: 0.840–0.869) and in a validation cohort from the US‐based Geisinger Health System (N=19 524; area under the curve, 0.818, 95% CI: 0.794–0.841), with good calibration.
Conclusions
Hyperkalemia within the first year of ACE‐I/ARB therapy was relatively uncommon among people with estimated glomerular filtration rate >60 mL/min per 1.73 m2, but rates were much higher with lower estimated glomerular filtration rate. Use of the hyperkalemia susceptibility score may help guide laboratory monitoring and prescribing strategies.
Keywords: angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, angiotensin‐converting enzyme inhibition, chronic kidney disease, hyperkalemia, potassium, risk score
Subject Categories: Hypertension, Epidemiology
Clinical Perspective
What Is New?
Largest study to date investigating electrolyte monitoring and rates of hyperkalemia during the first year of angiotensin‐converting enzyme inhibitors (ACE‐I) or angiotensin receptor blockers (ARB) therapy.
Reporting of adherence to guideline‐recommended electrolyte monitoring after ACE‐I and ARB initiation.
Comparison of rates of hyperkalemia among new ACE‐I or ARB users to new β‐blocker users.
Development of a tool to predict risk of hyperkalemia after ACE‐I or ARB initiation according to 6 readily available characteristics, with validation in a separate patient cohort.
What Are the Clinical Implications?
Many patients lack guideline‐recommended electrolyte monitoring after ACE‐I or ARB initiation.
Risks of hyperkalemia are fairly similar in new ACE‐I or ARB users compared with new β‐blocker users until estimated glomerular filtration rate <60 mL/min per 1.73 m2.
Use of the hyperkalemia risk tool may inform a personalized assessment of the risks and benefits of ACE‐I and ARB prescription as well as the development of efficient protocols for electrolyte monitoring.
Introduction
Angiotensin‐converting enzyme (ACE‐I) inhibitors and angiotensin receptor blockers (ARBs) have demonstrated efficacy in reducing blood pressure and proteinuria, slowing progression of kidney disease, and improving outcomes in patients with heart failure, diabetes mellitus, and post–myocardial infarction.1, 2, 3, 4, 5 However, these medications have also been associated with adverse events, including hyperkalemia.6, 7, 8, 9 Although adverse events can occur at any level of kidney function, the risk is thought to be greatest in those with chronic kidney disease and congestive heart failure.6 Thus, concern for adverse effects may lead to underutilization in the subgroups of patients who are expected to derive the greatest benefit.10
Clinical detection of hyperkalemia requires laboratory evaluation, and close laboratory monitoring is recommended during ACE‐inhibitor or ARB use. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline on chronic kidney disease evaluation and management recommends assessing glomerular filtration rate (GFR) and potassium within 1 week after initiating or increasing the dose of an ACE‐I or ARB, regardless of baseline potassium level.11 The 2004 Kidney Disease Outcomes Quality Initiative (KDOQI) suggests a more detailed algorithm: monitor laboratory values within the first 4 weeks after initiation or dose change for high‐risk patients (defined as having systolic blood pressure <120 mm Hg, potassium >4.5 mmol/L, or estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2) and within 4 to 12 weeks for all others.12 The American Heart Association recommends checking serum electrolytes and creatinine before and 1 week after starting an ACE‐I.13 Clinical trials suggest that the rate of hyperkalemia after initiating renin–angiotensin system blockade is low14, 15; however, the frequency of monitoring and rates of hyperkalemia in clinical practice are not well defined.
Using The Stockholm Creatinine Measurements (SCREAM) cohort (a healthcare utilization cohort from the region of Stockholm, Sweden), we investigated the frequency of laboratory monitoring as well as the occurrence of hyperkalemia among new users of ACE‐Is and ARBs. To evaluate rates of hyperkalemia in a similar population not on ACE‐I or ARB therapy, we also quantified these parameters in a propensity‐matched cohort of new users of β‐blocker therapy. Finally, we created a susceptibility score to help predict the development of hyperkalemia among new ACE‐I or ARB‐users, and validated the score's performance in new users of ACE‐I and ARB therapy in a separate US‐based healthcare utilization cohort.
Methods
Study Design
The SCREAM project is a repository of laboratory data of healthcare users in the region of Stockholm, Sweden who underwent creatinine testing.16 These data were linked to administrative databases with complete information on demographic data, healthcare utilization, diagnoses, vital status, and pharmacy‐dispensed prescription medicines, and cover the time period from January 1, 2006 to December 31, 2011. For the present study, we included all new users of an ACE‐I or an ARB with a serum creatinine and a potassium measurement on or within a year before the dispensation date. New use was defined as a first‐time ACE‐I or ARB dispensation (with no previous dispensation of an ACE‐I or an ARB recorded) between January 1, 2007 and December 31, 2010. This time period was chosen to ensure that the dispensation was not merely a continuation of an existing prescription and that a participant had a full year of follow‐up after medication dispensation. For the purposes of evaluating monitoring patterns, we excluded the 926 new users who died within a year of drug initiation, for a final study population of 69 426. The study consisted solely of de‐identified data and thus was deemed not to require informed consent. It was approved by regional institutional review boards and the Swedish National Board of Welfare in accordance with journal guidelines.
Patient Characteristics
Age and sex were obtained from the Stockholm regional healthcare data warehouse. Race was not collected in accordance with Swedish law; however, the vast majority of Swedish citizens are of white origin. Serum creatinine collected in clinical practice is uniformly traceable to isotope dilution mass spectroscopy standards, and it was converted to eGFR using the CKD‐EPI equation.17 Albuminuria was assessed in clinical practice using urine albumin‐to‐creatinine ratio (mg/g) in the year before inclusion. Comorbid conditions included diabetes mellitus, heart failure, coronary artery disease, cerebrovascular disease, and peripheral vascular disease, assessed using International Classification of Disease—Tenth Edition (ICD‐10) diagnostic codes as previously described.16 Medications assessed included diuretics (both loop and thiazide), nonsteroidal anti‐inflammatory drugs, spironolactone, β‐blockers, and other antihypertension medications. Medications were assumed to be concomitant if there was a pharmacy dispensation within the year before ACE‐I or ARB dispensation.
Study Outcomes
Ambulatory as well as inpatient potassium measurements were evaluated during the year after ACE‐I or ARB initiation. For the purposes of assessing monitoring frequency and the occurrence of hyperkalemia, only the first inpatient potassium was taken into account, since hospital‐dispensed ACE‐I or ARB therapy is not recorded in the Swedish prescription drug registry. Among participants with available potassium measures, we identified episodes of potassium levels >5 and >5.5 mmol/L.
Comparison Group
To assess whether outcomes were different in individuals who were not on ACE‐I or ARB therapy, we constructed a parallel cohort of new users of β‐blocker therapy in the same time period (January 1, 2007 to December 31, 2010), with a serum creatinine and a potassium measurement on or within a year before the dispensation date, and who were not on or previously on ACE‐I or ARB therapy (N=24 333). This cohort was also drawn from the SCREAM data and selection was conducted using methods identical to that of the new users of ACE‐I or ARB cohort. We then used propensity matching to 1:1 match new users of β‐blockers to new users of ACE‐I/ARB, with the following covariates used in the construction of the propensity score: age; sex; diabetes mellitus status; history of congestive heart failure; history of coronary artery disease, cerebrovascular disease, or peripheral vascular disease; use of diuretics; use of potassium‐sparing diuretics; use of other hypertension medications; baseline potassium; baseline eGFR; and whether a baseline albumin‐to‐creatinine ratio was available. Of the matched participants, we evaluated for the occurrence of potassium monitoring and, among those with monitoring, potassium >5 and >5.5 mmol/L.
Statistical Analysis
Baseline characteristics were displayed as mean and SD or median and interquartile range in the setting of a skewed distribution. Patient characteristics were stratified by the availability of potassium measurements in the year following ACE‐I or ARB initiation. The proportion experiencing elevated potassium levels during the year was further stratified by eGFR (≥90, 60–89, 45–59, 30–44, and <30 mL/min per 1.73 m2). The distribution of change in potassium from baseline value to the first check in the year following medication initiation was described using kernel density plots. Logistic regression was used to estimate the risk relationship between baseline characteristics, concomitant medications, and the development of hyperkalemia, adjusted for number of potassium checks as a surrogate for contact with the medical system (1, 2–4, and >4 checks in the year following medication initiation). A susceptibility score for the development of potassium >5.5 mmol/L in the year following medication initiation was developed among the 68 880 new users of ACE‐I or ARB therapy with baseline potassium <5 mmol/L. Inputs for the susceptibility score were selected based on factors that had significant coefficients in logistic regression (sex, baseline potassium level, eGFR, diabetes mellitus, heart failure, and the presence of K‐sparing diuretics) in order to predict the risk of hyperkalemia in the year after ACE‐I or ARB initiation. Discrimination and calibration were tested by calculating the area under the curve (C‐index) and plotting deciles of predicted risk against observed risk. The score was validated in a cohort of new ACE‐I and ARB users in Geisinger Health System using the same selection criteria and statistical analysis. Additional details regarding the electronic health record have been previously published.18 In brief, Geisinger is a rural health system in central and northeastern Pennsylvania. The study population included all patients over 18 years of age who are followed as primary care patients in the Geisinger Health System. Use of ACE‐I and ARB therapy was determined based on outpatient orders and medication lists. Demographic variables were assessed from the electronic medical record and comorbidities from International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes on or before medication prescription date. Analyses were performed in Stata 14 MP (College Station, TX).
Results
Baseline Characteristics
There were 69 426 patients who initiated ACE‐I or ARB therapy from January 1, 2007 to December 31, 2010 (Table 1). The majority of dispensations were for ACE‐I therapy (82%; N=56 943). Approximately half of the population were women (N=35 270, 50.8%), and the average age was 55 years. The average predispensation potassium level was 4.1 mmol/L, and 9% (N=6341) of the population had an eGFR <60 mL/min per 1.73 m2. Of those participants with an available assessment of albuminuria (23%; N=15 898), 20% had values between 30 and 299 mg/g, and 5% had values ≥300 mg/g.
Table 1.
Baseline Characteristics of Patients in the Stockholm Creatinine Measurements (SCREAM) Cohort Initiating ACE‐I or ARB, Stratified by the Presence of Potassium Monitoring in the Year Following Initial Medication Prescription
| Overall | No Potassium Monitoring | Potassium Measured at Least Once in the First Year | |
|---|---|---|---|
| Total, N (%) | 69 426 | 16 430 (24%) | 52 996 (76%) |
| Age, mean (SD), y | 55 (13) | 51 (13) | 56 (13) |
| Female, n (%) | 35 270 (51%) | 7923 (48%) | 27 347 (52%) |
| Diabetes mellitus, n (%) | 7099 (10%) | 1045 (6%) | 6054 (11%) |
| History of CHF, n (%) | 5162 (7%) | 365 (2%) | 4797 (9%) |
| History of CAD, CVD, or PVD, n (%) | 9940 (14%) | 1235 (8%) | 8705 (16%) |
| Use of NSAID, n (%) | 18 330 (26%) | 4003 (24%) | 14 327 (27%) |
| Use of other diuretics, n (%) | 14 987 (22%) | 2255 (14%) | 12 732 (24%) |
| Use of K‐sparing diuretics, n (%) | 2728 (4%) | 261 (2%) | 2467 (5%) |
| Use of β‐blockers, n (%) | 27 580 (40%) | 5184 (32%) | 22 396 (42%) |
| Use of other HTN meds, n (%) | 14 104 (20%) | 2765 (17%) | 11 339 (21%) |
| Potassium, mean (SD), mmol/L | 4.1 (0.4) | 4.1 (0.3) | 4.1 (0.4) |
| Potassium >5 mmol/L, n (%) | 546 (1%) | 94 (1%) | 452 (1%) |
| Potassium >5.5 mmol/L, n (%) | 78 (0.1%) | 13 (0%) | 65 (0%) |
| eGFR, mean (SD), mL/min per 1.73 m2 | 89 (20) | 94 (18) | 87 (21) |
| eGFR <60 mL/min per 1.73 m2, n (%) | 6341 (9%) | 703 (4%) | 5638 (11%) |
| Available ACR or dipstick, n (%) | 15 898 (23%) | 2932 (18%) | 12 966 (24%) |
| Albuminuria 30 to 300 mg/g, n (%) | 3106 (20%) | 443 (15%) | 2663 (21%) |
| Albuminuria >300 mg/g, n (%) | 792 (5%) | 70 (2%) | 722 (6%) |
Indication for ACE‐I/ARB defined as ACR ≥300 mg/g or a protein level of ++ on urine dipstick, a diagnosis of congestive heart failure, or a diagnosis of diabetes mellitus with ACR >30 mg/g or a protein level of + on urine dipstick. ACE‐I indicates angiotensin‐converting enzyme inhibitor; ACR, albumin‐to‐creatinine ratio; ARB, angiotensin receptor blocker therapy; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; HTN, hypertension; K, potassium; NSAID, nonsteroidal anti‐inflammatory drug; PVD, peripheral vascular disease.
Frequency of Potassium Monitoring
Only one third (34%; N=23 927) of new ACE‐I or ARB users had potassium checked within 1 month of initiation, and 76% (N=52 996) had potassium checked within the year after ACE‐I or ARB initiation. Overall, those with potassium measurements in the year following ACE‐I or ARB initiation were older, with more heart failure, diabetes mellitus, and coronary artery disease. They also had lower eGFR (87 mL/min per 1.73 m2 versus 94 mL/min per 1.73 m2, P<0.001) and higher prevalence of eGFR <60 mL/min per 1.73 m2 (10.6% versus 4.3%, P<0.001). Of note, the average baseline potassium level was the same among patients with and without potassium monitoring (4.1 mmol/L in both groups).
Incidence of Hyperkalemia in the Year After ACE‐I/ARB Initiation
Among SCREAM patients with potassium monitoring in the year following ACE‐I or ARB initiation (N=52 996), the occurrence of hyperkalemia was relatively low. Potassium >5 mmol/L occurred in 5.6% (N=2977), potassium >5.5 mmol/L occurred in 1.7% (N=924), and potassium >6 mmol/L occurred in 0.63% (N=334). Of those who developed potassium levels >5 mmol/L, 1000 participants had another level >5 mmol/L within the year; 278 of the 924 participants with potassium >5.5 mmol/L and 77 of the 334 participants with potassium >6.0 mmol/L had another episode, respectively. Hyperkalemia occurred much more frequently among persons with lower eGFR. For example, among persons with eGFR <30 mL/min per 1.73 m2, new users of ACE‐I or ARB therapy had a 55% and 29% 1‐year occurrence of potassium >5 and >5.5 mmol/L, respectively.
Comparison of Hyperkalemia Incidence in the Year After β‐Blocker Initiation
In a propensity‐matched analysis, 20 186 new users of β‐blockers had less potassium monitoring in the year after initiation compared with 20 186 new ACE‐I/ARB users (64% versus 74%) (Table S1). The occurrence of hyperkalemia was similar in new ACE‐I/ARB and β‐blocker users except in patients with low eGFR (Figure 1A and 1B). For example, new users of β‐blocker therapy had a 4.4% and 1.4% occurrence of potassium >5 and >5.5 mmol/L overall, similar to that observed among new ACE‐I/ARB users; however, among patients with an eGFR <30 mL/min per 1.73 m2, occurrence was 38% and 17%, respectively, compared with 51% and 29% in the matched ACE‐I/ARB users. In general, the change in potassium after medication initiation was shifted slightly to the right (increase in potassium) in new users of ACE‐I or ARBs compared to new users of β‐blockers, and this was more pronounced in lower eGFR strata (Figure S1).
Figure 1.
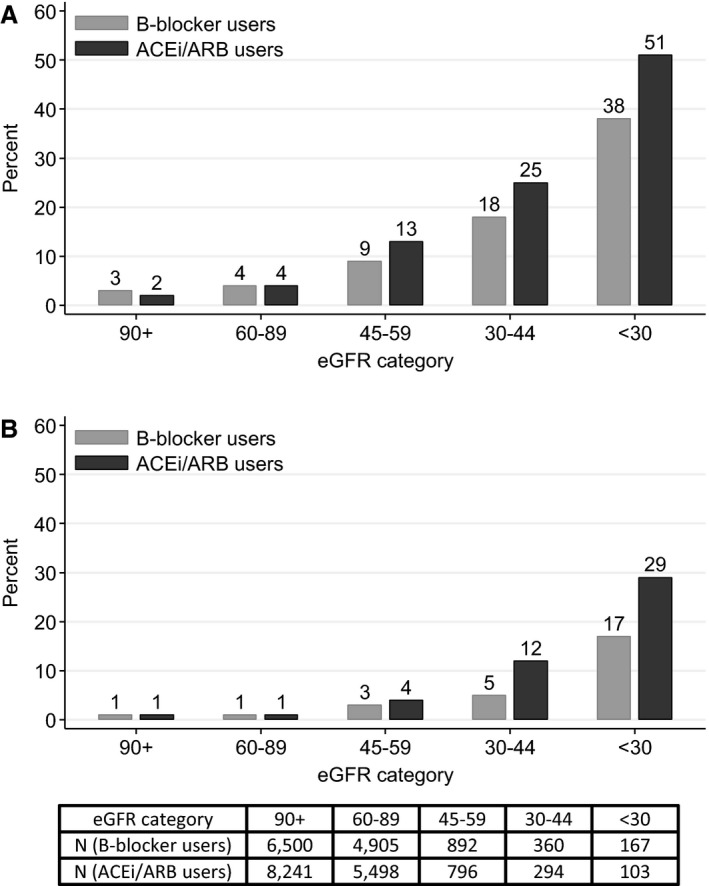
Proportion of propensity‐matched cohort of patients in the Stockholm Creatinine Measurements (SCREAM) cohort initiating or β‐blocker therapy (light gray; N=20 186) or angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy (dark gray; N=20 186) with (A) potassium >5 mmol/L and (B) potassium >5.5 mmol/L during the first year on therapy. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate.
Risk Factors for Hyperkalemia After ACE‐I/ARB Initiation
Risk factors for hyperkalemia included the presence of diabetes mellitus (odds ratio [OR]: 1.64 for potassium >5 mmol/L and 1.73 for potassium >5.5 mmol/L), congestive heart failure (OR: 1.57 and 1.76, respectively), and use of K‐sparing diuretics (OR: 2.06 and 2.16, respectively) (Table 2). Lower eGFR, whether in patients with eGFR >60 or <60 mL/min per 1.73 m2, was a strong risk factor for the occurrence of potassium >5 or >5.5 mmol/L. Women had significantly lower risk of hyperkalemia. Baseline potassium level was among the strongest risk factors, with each 0.1 mmol/L higher baseline potassium conferring a 19% (OR, 1.19, 95% CI: 1.17–1.20) and a 15% (OR 1.15, 95% CI: 1.13–1.17) higher risk for potassium >5 or >5.5 mmol/L, respectively. The use of ACE‐I compared with ARB therapy also appeared to confer a slightly higher risk for having potassium >5 mmol/L or potassium >5.5 mmol/L.
Table 2.
Adjusted Associations of Baseline Characteristics of Patients in the Stockholm Creatinine Measurements (SCREAM) Cohort With the Development of Mild (Potassium >5 mmol/L) and Moderate Hyperkalemia (Potassium >5.5 mmol/L) in the Year Following Initiation of ACE‐I or ARB (N=52 996)
| Mild Hyperkalemia | Moderate Hyperkalemia | |||
|---|---|---|---|---|
| Adjusted OR | P Value | Adjusted OR | P Value | |
| Age, per 10 y | 1.03 (0.99, 1.07) | 0.132 | 0.98 (0.92, 1.05) | 0.583 |
| Female | 0.83 (0.76, 0.90) | <0.001 | 0.83 (0.72, 0.96) | 0.014 |
| Potassium, per 0.1 mmol/L | 1.19 (1.17, 1.20) | <0.001 | 1.15 (1.13, 1.17) | <0.001 |
| eGFR <60, per −15 mL/min/1.73 m2 | 1.93 (1.80, 2.07) | <0.001 | 1.93 (1.76, 2.11) | <0.001 |
| eGFR 60+, per −15 mL/min/1.73 m2 | 1.24 (1.18, 1.31) | <0.001 | 1.24 (1.14, 1.35) | <0.001 |
| Diabetes mellitus | 1.64 (1.47, 1.82) | <0.001 | 1.73 (1.46, 2.05) | <0.001 |
| History of CHF | 1.57 (1.40, 1.76) | <0.001 | 1.76 (1.47, 2.12) | <0.001 |
| History of CAD, CVD, PVD | 1.12 (1.01, 1.24) | 0.031 | 1.12 (0.95, 1.32) | 0.192 |
| ACE‐I (vs ARB) | 1.17 (1.03, 1.32) | 0.012 | 1.26 (1.02, 1.56) | 0.036 |
| K‐sparing diuretics | 2.06 (1.80, 2.35) | <0.001 | 2.16 (1.77, 2.63) | <0.001 |
| Other diuretics | 1.12 (1.01, 1.24) | 0.032 | 1.24 (1.04, 1.48) | 0.014 |
| β‐Blockers | 1.03 (0.94, 1.13) | 0.531 | 0.94 (0.80, 1.09) | 0.406 |
| Other HTN meds | 0.95 (0.86, 1.05) | 0.314 | 1.12 (0.95, 1.31) | 0.179 |
Associations were additionally adjusted for the frequency of K check (<2, 2–4, >4) as a proxy for contact with the medical system. ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker therapy; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; HTN, hypertension; K, potassium; OR, odds ratio; PVD, peripheral vascular disease.
Discrimination and Calibration of a Hyperkalemia Susceptibility Score After ACE‐I/ARB Initiation
A susceptibility score for the development of potassium >5.5 mmol/L using sex, baseline potassium, eGFR, the presence of diabetes mellitus, heart failure, and concomitant use of K‐sparing diuretics showed excellent discrimination (C‐index, 0.854; 95% CI: 0.840–0.869) in the SCREAM cohort with good calibration (Figure 2A). Validation was performed in the US‐based Geisinger Health System, a population that was older than the SCREAM cohort, with a greater prevalence of diabetes mellitus, coronary artery disease, and diuretic use, and higher baseline potassium levels (Table S2). Discrimination and calibration were again excellent, with a c‐index of 0.818 (95% CI: 0.794–0.841) (Figure 2B). Of note, a slightly higher proportion of Geisinger patients had potassium checked in the year following ACE‐I or ARB initiation (79%), and the incidence of potassium >5 and >5.5 mmol/L was also slightly higher, at 11.6% and 2.8%.
Figure 2.
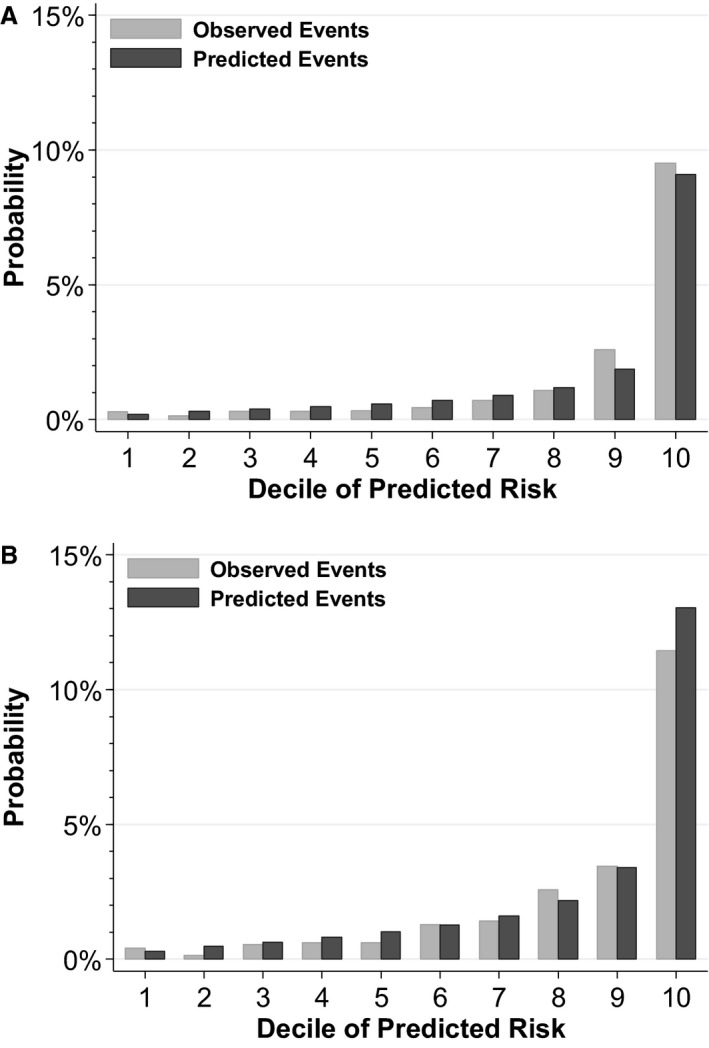
Calibration plot of observed vs predicted risk of potassium >5.5 mmol/L in the year following angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy by decile of predicted risk among patients in the (A) Stockholm Creatinine Measurements (SCREAM) development cohort (N=52 544), and (B) Geisinger Health System validation cohort (N=14 772). Reflects patients with baseline potassium levels <5 mmol/L.
Prediction of Hyperkalemia After ACE‐I or ARB Initiation
The susceptibility score–predicted probability of developing potassium >5.5 mmol/L in the year following ACE‐I or ARB initiation varied by patient profile (Table 3). Patients with relatively preserved eGFR or baseline potassium <4 mmol/L generally had low risk of developing potassium levels >5.5 mmol/L in the year following ACE initiation. Among all new users of ACE‐I or ARB therapy in the SCREAM cohort, 91.8% had a predicted risk <5%, 6.8% had a predicted risk between 5% and 20%, and 1.4% had a predicted risk >20%. The risks among new users without potassium checks in the year following medication initiation were lower: 95.6% had a predicted risk <5%, 3.7% had a predicted risk between 5% and 20%, and 0.6% had a predicted risk >20%.
Table 3.
Hyperkalemia Susceptibility Score: Predicted Probability of Developing Potassium Levels >5.5 mmol/L in the Year Following Initiation of ACE‐I or ARB Therapy for Various Patient Scenariosa
| Sex | Potassium | eGFR | DM | HF | K‐Sparing Diuretics | 1‐Year Risk of K >5.5 | |
|---|---|---|---|---|---|---|---|
| Scenario 1 | F | 4 | 55 | 1 | 0 | 0 | 2.1% |
| Scenario 2 | F | 3.5 | 45 | 1 | 0 | 0 | 2.0% |
| Scenario 3 | F | 4.5 | 20 | 1 | 0 | 0 | 26% |
| Scenario 4 | M | 4 | 55 | 1 | 0 | 0 | 3.0% |
| Scenario 5 | M | 4 | 55 | 1 | 1 | 0 | 8.2% |
| Scenario 6 | M | 4 | 55 | 1 | 1 | 1 | 18% |
ACE‐I indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HF, heart failure; K, potassium.
Risk can be calculated by transforming variables in the following manner:
Female=1 if female, 0 if male
K=(potassium level in mmol/L−4)×10
eGFR1=[60−(60 if eGFR >60, eGFR if eGFR <60)]/15
eGFR2=[60−eGFR if eGFR >60, 0 if eGFR ≤60]/15
DM, HF, and KSparing=1 if yes, 0 if no
Odds=e^(−4.450592−0.3347532×female+0.1314612×K+0.9125292×eGFR1+0.3386612×eGFR2+0.6625622×DM+1.067721×HF+0.9290511×Ksparing)
Probability=odds/(1+odds).
Discussion
In this large, population‐based study of new users of ACE‐I or ARB therapy, we demonstrate that adherence to guideline‐recommended electrolyte monitoring was poor. Less than one third of patients had a potassium measurement within 30 days of starting an ACE‐I or ARB, and only 76% had one in the year following medication initiation. On the other hand, the 1‐year occurrence of hyperkalemia was generally quite low in those with potassium measurements, the majority of whom had preserved eGFR, with only 1.7% developing potassium levels >5.5 mmol/L. People without potassium measurements tended to be of low predicted risk. These results suggest that more targeted protocols for monitoring potassium levels after ACE‐I/ARB initiation may be cost‐effective and safe.
There was a large increase in hyperkalemia risk among persons with low eGFR, a risk that was also present to a lesser extent in matched new users of β‐blocker therapy. This may suggest a need for heightened potassium monitoring in patients with reduced eGFR, even those taking medications thought to have only small effects on potassium, such as β‐blockers. Other strong risk factors for the development of hyperkalemia on ACE‐I or ARB therapy included higher baseline potassium levels, the concomitant use of K‐sparing diuretics, and the presence of diabetes mellitus or heart failure. The combination of these factors into a susceptibility score may allay provider concerns regarding the risk of hyperkalemia in low‐risk patients, encouraging greater prescription in patients with indications for ACE‐I or ARB therapy. Future work should focus on the risks and benefits of ACE‐I and ARB therapy in patients with higher risk scores, including whether use may be optimized with monitoring practices tailored to an individual patient's risk level.
Our findings of low rates of potassium monitoring among new ACE‐I and ARB users is at odds with various scientific societies’ recommendations, but nonetheless consistent with observations in American cohorts.18, 19 A recent study by Chang and colleagues evaluating the prevalence of hyperkalemia and monitoring patterns in patients on antihypertensive medications reported that 20% of the population did not have serum potassium checked within a 3‐year period, including 6% of patients on an ACE‐I and 5% of patients on an ARB.18 Raebel and colleagues evaluated a prevalent population of patients using ACE‐I or ARB therapy and found similar rates of monitoring over 1 year.19 Our study adds to the existing literature by reporting testing in new ACE‐I or ARB users, a population in which KDOQI, KDIGO, and American Heart Association guidelines provide specific recommendations for testing.11, 12, 13
Hyperkalemia is a noteworthy adverse event, with even modest elevations in potassium relating to mortality.20, 21, 22 Moreover, concern regarding the risk of hyperkalemia may preclude optimal prescribing practices. A Turkish study investigating barriers to use of ACE‐I and ARB therapy in chronic kidney disease patients reported that hyperkalemia was the most common reason for discontinuation of renin–angiotensin–aldosterone system blockers10; other studies have attributed underutilization to similar reasons.23 Although clinical trials of ACE‐I or ARB therapy have reported low rates of hyperkalemia in both treatment and control arms, populations tend to be highly selected and monitored and hyperkalemia defined by potassium levels as high as >6 mmol/L.24, 25 Our study reflects the “real world” use of ACE‐I and ARB, yet the overall rate of even mild forms of hyperkalemia (eg, >5 mmol/L) was relatively low, suggesting that the vast majority of persons prescribed these medications can be considered low‐risk. Our observed rates of hyperkalemia generally coincide with those reported in the general population without risk factors (≈2%).26 However, the increased risk with lower eGFR is also consistent with previous reports, where rates may be as high as 40%.27, 28 This variation in hyperkalemia risk supports recommendations that tailor monitoring strategies to the individual patient, such as that put forth by KDOQI in 2004.12 Use of a susceptibility score may further refine personalized monitoring strategies.
Our hyperkalemia susceptibility score provides a 1‐year estimate of the likelihood of developing potassium levels >5.5 mmol/L following ACE‐I or ARB initiation according to 6 commonly assessed clinical characteristics, and is easy to implement in an electronic medical record or trial protocol. We anticipate that risk prediction could be used both for medication safety as well as for optimizing ACE‐I and ARB use in target populations. Although many of the risk characteristics have been previously identified, an existing risk score did not incorporate sex or potassium, one of the strongest observed risk factors.24, 25, 29 In our study, male sex was also a risk factor for the development of hyperkalemia, potentially because of lower intake of potassium among women.30 Interestingly, we noted no difference in monitoring by baseline potassium level. Explicit consideration of baseline potassium (such as in a risk model) might improve monitoring practices after ACE‐I and ARB initiation.
Our study represents the largest study to date detailing risks among new users of ACE‐I and ARB therapy. We developed a simple susceptibility score using 6 commonly available clinical variables, and this risk prediction was accurate and well‐calibrated in both the development cohort and in a validation cohort in a different country with different laboratories and a different risk profile, demonstrating the robustness and generalizability of the model. However, there are certain limitations as well. We were only able to ascertain hyperkalemia risk in patients who had potassium monitoring. However, those without potassium monitoring were predicted to be low risk, providing additional confidence that the model aligns with provider expectations. We selected people who survived a year postinitiation, and thus may have slightly underestimated rates of hyperkalemia, which are related to death. We lacked uniform availability of certain variables that have been linked to the development of hyperkalemia previously, such as blood pressure and albuminuria.31 The population was primarily white, and there have been reports of lower risks among blacks.32 We did not separate different types of ACE‐I or ARB therapy and had no information on medication dose—previous studies have suggested that risk increases with higher dose29—nor did we have information regarding the reasons for medication initiation. Finally, potassium values may be calibrated differently at different laboratories in the development and validation cohorts. However, the prediction model performed very well in all categories of risk as well as in both the development and validation cohorts.
In conclusion, we report on a large, population‐based cohort of individuals living in Sweden, detailing the rates of potassium monitoring and hyperkalemia among new users of ACE‐I and ARB therapy. Potassium monitoring in new users of ACE‐I and ARB therapy did not meet current recommended guidelines, but rates of hyperkalemia within the first year of therapy appeared to be low in most patients, the majority of whom had preserved eGFR. A notable exception was patients with eGFR <30 mL/min per 1.73 m2, where rates were high for both patients on and off ACE‐I or ARB therapy. We propose an easy‐to‐implement hyperkalemia susceptibility score that may help tailor prescribing and monitoring practices in the clinical setting as well as aid in clinical trial design.
Sources of Funding
This study was supported by an institutional grant from AstraZeneca to Karolinska Institutet. We also acknowledge the support of Vifor Fresenius Medical Care Renal Pharma, Swedish Heart and Lung Foundation, the Stockholm County Council, Martin Rind's and Westman's Foundations, the National Institute of Diabetes and Digestive and Kidney Diseases (K08DK092287) and the United States National Kidney Foundation, which receives support from Relypsa.
Disclosures
Dr Lund received consulting honoraria from Relypsa, Vifor Fresenius Medical Care Renal Pharma, and AstraZeneca. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics of Propensity‐Matched Patients in the Serum Creatinine Measurements (SCREAM) Cohort Who Initiated ACE‐I or ARB Therapy and Those Who Initiated β‐Blocker Therapy Who Were Not on ACE‐I or ARB Therapy
Table S2. Baseline Characteristics of Patients Initiating Angiotensin‐Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Therapy in the Geisinger Health System, Stratified by the Presence of Serum Potassium Monitoring in the Year Following Initial Medication Prescription
Figure S1. Distribution of change in potassium from baseline to first value after medication initiation among propensity‐matched patients in the Serum Creatinine Measurements (SCREAM) cohort initiating β‐blocker therapy (dashed line; N=20 186) or ACE‐I or ARB therapy (solid line; N=20 186).
Data Access and Responsibility
Grams, Carrero, and Sang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2017;6:e005428 DOI: 10.1161/JAHA.116.005428.)28724651
References
- 1. Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P. Effect of the angiotensin‐converting‐enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin‐Converting‐Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. [DOI] [PubMed] [Google Scholar]
- 2. Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin‐dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA. 1994;271:275–279. [PubMed] [Google Scholar]
- 3. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 4. Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–86. [DOI] [PubMed] [Google Scholar]
- 5. The Heart Outcomes Prevention Evaluation Study Investigators . Effects of an angiotensin‐converting–enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 6. Maddirala S, Khan A, Vincent A, Lau K. Effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on serum potassium levels and renal function in ambulatory outpatients: risk factors analysis. Am J Med Sci. 2008;336:330–335. [DOI] [PubMed] [Google Scholar]
- 7. Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, Szabo Z, Kalantar‐Zadeh K, Kovesdy CP. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis. 2016;67:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wynckel A, Ebikili B, Melin JP, Randoux C, Lavaud S, Chanard J. Long‐term follow‐up of acute renal failure caused by angiotensin converting enzyme inhibitors. Am J Hypertens. 1998;11:1080–1086. [DOI] [PubMed] [Google Scholar]
- 9. Toto RD, Mitchell HC, Lee HC, Milam C, Pettinger WA. Reversible renal insufficiency due to angiotensin converting enzyme inhibitors in hypertensive nephrosclerosis. Ann Intern Med. 1991;115:513–519. [DOI] [PubMed] [Google Scholar]
- 10. Yildirim T, Arici M, Piskinpasa S, Aybal‐Kutlugun A, Yilmaz R, Altun B, Erdem Y, Turgan C. Major barriers against renin‐angiotensin‐aldosterone system blocker use in chronic kidney disease stages 3‐5 in clinical practice: a safety concern? Ren Fail. 2012;34:1095–1099. [DOI] [PubMed] [Google Scholar]
- 11. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;suppl:1–138. [Google Scholar]
- 12. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 13. Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS. Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation. 2001;104:1985–1991. [DOI] [PubMed] [Google Scholar]
- 14. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. [DOI] [PubMed] [Google Scholar]
- 15. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 16. Runesson B, Gasparini A, Qureshi AR, Norin O, Evans M, Barany P, Wettermark B, Elinder CG, Carrero JJ. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J. 2016;9:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD EPI Collaboration . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang AR, Sang Y, Leddy J, Yahya T, Kirchner HL, Inker LA, Matsushita K, Ballew SH, Coresh J, Grams ME. Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension. 2016;67:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raebel MA, McClure DL, Simon SR, Chan KA, Feldstein A, Andrade SE, Lafata JE, Roblin D, Davis RL, Gunter MJ, Platt R. Laboratory monitoring of potassium and creatinine in ambulatory patients receiving angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Pharmacoepidemiol Drug Saf. 2007;16:55–64. [DOI] [PubMed] [Google Scholar]
- 20. Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834–1842. [DOI] [PubMed] [Google Scholar]
- 23. Shirazian S, Grant CD, Mujeeb S, Sharif S, Kumari P, Bhagat M, Mattana J. Underprescription of renin‐angiotensin system blockers in moderate to severe chronic kidney disease. Am J Med Sci. 2015;349:510–515. [DOI] [PubMed] [Google Scholar]
- 24. Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–1903. [DOI] [PubMed] [Google Scholar]
- 25. Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. [DOI] [PubMed] [Google Scholar]
- 26. Weir MR, Rolfe M. Potassium homeostasis and renin‐angiotensin‐aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. [DOI] [PubMed] [Google Scholar]
- 27. Reardon LC, Macpherson DS. Hyperkalemia in outpatients using angiotensin‐converting enzyme inhibitors. How much should we worry? Arch Intern Med. 1998;158:26–32. [DOI] [PubMed] [Google Scholar]
- 28. Espinel E, Joven J, Gil I, Sune P, Renedo B, Fort J, Seron D. Risk of hyperkalemia in patients with moderate chronic kidney disease initiating angiotensin converting enzyme inhibitors or angiotensin receptor blockers: a randomized study. BMC Res Notes. 2013;6:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson ES, Weinstein JR, Thorp ML, Platt RW, Petrik AF, Yang X, Anderson S, Smith DH. Predicting the risk of hyperkalemia in patients with chronic kidney disease starting lisinopril. Pharmacoepidemiol Drug Saf. 2010;19:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, Saydah SH, Yang Q, Moshfegh AJ. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am J Clin Nutr. 2012;96:647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weinberg JM, Appel LJ, Bakris G, Gassman JJ, Greene T, Kendrick CA, Wang X, Lash J, Lewis JA, Pogue V, Thornley‐Brown D, Phillips RA; African American Study of H, Kidney Disease Collaborative Research G . Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med. 2009;169:1587–1594. [DOI] [PubMed] [Google Scholar]
- 32. Hayes J, Kalantar‐Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo‐ and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120:c8–c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Propensity‐Matched Patients in the Serum Creatinine Measurements (SCREAM) Cohort Who Initiated ACE‐I or ARB Therapy and Those Who Initiated β‐Blocker Therapy Who Were Not on ACE‐I or ARB Therapy
Table S2. Baseline Characteristics of Patients Initiating Angiotensin‐Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Therapy in the Geisinger Health System, Stratified by the Presence of Serum Potassium Monitoring in the Year Following Initial Medication Prescription
Figure S1. Distribution of change in potassium from baseline to first value after medication initiation among propensity‐matched patients in the Serum Creatinine Measurements (SCREAM) cohort initiating β‐blocker therapy (dashed line; N=20 186) or ACE‐I or ARB therapy (solid line; N=20 186).


