Abstract
Background
The optimal initial noninvasive diagnostic testing strategy for stable coronary artery disease (CAD) is unknown. Although American guidelines recommend an exercise stress test as the first‐line test, European guidelines suggest that stress imaging (myocardial perfusion imaging or stress echocardiography) or coronary computed tomography angiography may be preferable. Understanding the relationship between the initial strategy and downstream yield of obstructive CAD and major adverse cardiac events may provide insight as to the optimal strategy.
Methods and Results
We conducted a population‐based retrospective cohort study of adults in Ontario, Canada, using health administrative and clinical data. The relationship between the initial testing strategy and obstructive CAD on invasive angiography was examined. Patients were then followed from their angiogram onward to determine whether they developed a composite end point of major adverse cardiac events. After adjusting for covariates, patients with initial myocardial perfusion imaging (odds ratio: 0.92; 95% confidence interval, 0.85, 1.00), coronary computed tomography angiography (odds ratio: 1.51; 95% confidence interval, 0.91, 2.49), or stress echo (odds ratio: 0.95; 95% confidence interval, 0.84, 1.08) did not a have significantly different yield of obstructive CAD compared with those with an initial exercise stress test. Furthermore, there was no significant difference in downstream major adverse cardiac events after invasive angiography among the 4 initial testing strategies after adjusting for clinically relevant covariates.
Conclusions
Our study found no evidence to suggest significant differences in either yield of obstructive CAD or downstream major adverse cardiac events in patients undergoing an initial noninvasive testing strategy with stress or anatomical imaging compared with those undergoing an initial exercise stress test.
Keywords: coronary computed tomography, exercise stress testing, myocardial perfusion imaging, noninvasive diagnostic testing, stable coronary artery disease
Subject Categories: Health Services, Quality and Outcomes, Computerized Tomography (CT), Echocardiography, Exercise Testing
Clinical Perspective
What Is New?
The optimal initial noninvasive diagnostic testing strategy for stable coronary artery disease is unknown.
Population‐based real‐world data comparing outcomes related to different initial strategies are scarce.
There is disagreement between American and European guidelines with regard to the optimal initial strategy.
Our population‐based retrospective cohort study of adults in Ontario, Canada (approximate population: 10.1 million), found no significant differences in either yield of obstructive coronary artery disease or downstream major adverse cardiovascular events in patients undergoing an initial noninvasive testing strategy with stress or anatomical imaging compared with those undergoing an initial simple exercise stress test.
What Are the Clinical Implications?
Our results do not support the routine initial use of stress imaging or coronary computed tomography angiography in the workup of stable coronary artery disease.
These findings highlight the need for future research to explore the reasons for the discrepancy between our real‐world findings and those of clinical efficacy studies.
Introduction
There are currently 4 cardiac noninvasive diagnostic tests for identifying coronary artery disease (CAD) that are clinically available and reimbursed in Ontario, Canada: graded exercise stress test (GXT), myocardial perfusion imaging (MPI), coronary computed tomography angiography (CCTA), and stress echocardiography (stress echo). There is disagreement between American and European guidelines with respect to which test should be initially used for the evaluation of patients with stable CAD (SCAD). Although the American guidelines recommend GXT as the first‐line test when it is possible to perform, the European guidelines suggest that stress imaging tests or CCTA may be a preferable initial approach in certain patient groups.1, 2, 3 Furthermore, although many studies have assessed the clinical efficacy of these tests in selected populations, none have assessed the comparative clinical effectiveness of these modalities in a head‐to‐head fashion in a population‐based study. With healthcare budgets rising sharply in many high‐income countries and with cardiac diagnostic testing and medical imaging contributing substantively to these costs, the assessment of comparative clinical effectiveness is important to assess the real‐world impact of these modalities.4, 5, 6, 7
The ideal outcome measure to assess the clinical effectiveness of cardiac noninvasive diagnostic tests is not currently established. The yield of obstructive CAD based on downstream invasive angiography has recently been used as an outcome measure in studies designed to assess the clinical effectiveness of noninvasive cardiac diagnostic tests.8, 9, 10 Recent registry data suggest that only ≈38% of those referred for invasive angiography in the United States for the diagnosis of SCAD had obstructive CAD on invasive angiography.11, 12 Consequently, understanding the relationship between the initial noninvasive cardiac diagnostic tests and subsequent downstream yield of obstructive CAD may provide insight as to which noninvasive test to use, thereby reducing unnecessary invasive angiograms and the resultant complications. The objective of this study was to determine whether a diagnostic strategy with an initial MPI, stress echo, or CCTA was independently predictive of a higher yield of obstructive CAD compared with GXT, after adjusting for clinically relevant covariates. To provide another metric of effectiveness, we also assessed a composite end point of major adverse cardiac events (MACE). Based on studies showing greater clinical efficacy, we hypothesized that a strategy with initial MPI, stress echo, or CCTA would lead to a higher yield of obstructive CAD compared with a strategy using initial GXT.
Methods
Design
We conducted a retrospective cohort study of patients undergoing an initial test in the calendar year 2012.
Derivation of the Cohort
Patients began entering the cohort on January 1, 2012 (see Figure 1). Inclusion criteria were age ≥20 years; receipt of 1 GXT, stress echo, CCTA, or MPI; and receipt of invasive angiography for the evaluation of SCAD. The first cardiac noninvasive diagnostic test after January 1, 2012, for each patient was deemed the index event or test. Index tests were identified between January 1, 2012, and December 31, 2012. We used a look‐back window of 20 years (back to January 1, 1992) to exclude patients with prior cardiovascular disease. Previous cardiovascular disease was defined by prior hospitalization for acute myocardial infarction, stroke, congestive heart failure, percutaneous coronary intervention, and coronary artery bypass grafting, using previously validated algorithms.13, 14, 15, 16, 17, 18, 19 We also used a 1‐year washout period such that patients who had 1 of the 4 noninvasive diagnostic tests during the 2011 calendar year were excluded. After the index event, patients were followed for a maximum of 6 months to identify whether they progressed to invasive angiography. Consequently, the observation window for invasive angiography was from January 1, 2012, to June 30, 2013, to provide a full 6 months of follow‐up for the last potential person entering the cohort on December 31, 2012. Furthermore, patients were followed for a maximum of 2 years after their invasive angiogram (until June 30, 2015) to ascertain MACE.
Figure 1.
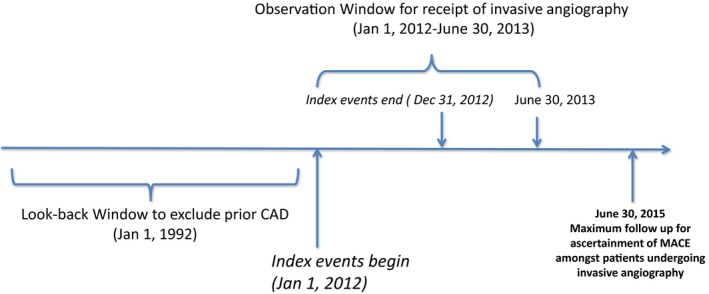
Schematic representation of the design of the cohort study. This figure demonstrates the design of our study and outlines the observation window, follow‐up period, and look‐back window for our study. CAD indicates coronary artery disease; MACE, major adverse cardiovascular events.
Data Sources
Information to identify patient receipt of cardiac noninvasive diagnostic CAD tests was obtained through medical claims data from the Ontario Health Insurance Plan (OHIP) physician claims database during the calendar year 2012. The OHIP physician claims database contains all physician reimbursement claims for GXTs, CCTAs, MPIs, and stress echos performed in Ontario. Physician specialty was determined by linking the OHIP database with the Institute for Clinical Evaluative Sciences (ICES) physician database. The Registered Persons Database (RPDB), a registry of Ontario residents who are registered for Ontario health insurance coverage, was used to obtain demographic information and all‐cause mortality. Median neighborhood income was obtained by linking the Census Area Profile with patients’ postal codes of residence from RPDB using the Postal Code Conversion File. Hospitalizations, including those for unstable angina and acute myocardial infarction, were determined using the Canadian Institutes for Health Information Discharge Abstract Database. The Cardiac Care Network (CCN) of Ontario Cardiac Registry was used to determine receipt of angiography, obstructive CAD status on angiography, and patient clinical covariate status. The CCN Cardiac Registry is an ongoing prospective registry storing clinical information on all invasive cardiac procedures in Ontario and has been used extensively in clinical research.20, 21, 22, 23 The registry contains detailed demographic, comorbidity, and procedural details, including coronary anatomy, that have been validated against chart abstraction and core laboratory verification.24, 25 All data were accessed and analyzed at ICES in Toronto, Canada. ICES has been deemed a prescribed entity by the government of Ontario. This status allows it to collect personal information without the need for informed consent; therefore, approved projects using ICES data, such as this one, are exempt from the requirement of informed consent.
Exposure
The exposure was the receipt of 1 of the 4 index noninvasive tests.
Outcomes
In those patients who underwent angiography for the assessment of SCAD, we determined whether their angiogram showed obstructive CAD, our primary outcome of interest. Obstructive CAD on angiography was defined as stenosis of ≥50% of the left main coronary artery or ≥70% of a major epicardial or branch vessel, according to CCN data in a manner previously described and validated.25, 26 Furthermore, as a secondary outcome, patients were followed after their invasive angiogram for the development of MACE, defined as a composite end point of all‐cause mortality and hospitalization for acute myocardial infarction or unstable angina.
Covariates
Covariates for inclusion in multivariable models were selected a priori based on clinical importance. Increasing age, male sex, diabetes mellitus, dyslipidemia, hypertension, lower income status, increased serum creatinine, and smoking are all cardiovascular risk factors associated with a higher risk of developing obstructive CAD.27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Presence of chronic obstructive pulmonary disease, peripheral vascular disease, and a Charlson comorbidity index score are all measures of comorbidity that may affect a clinician's decision‐making process with respect to choosing a suitable initial diagnostic test.38 Resting ECG abnormalities and Canadian Cardiovascular Society (CCS) angina symptoms are other important factors that may influence the decision regarding the optimal initial diagnostic test.2, 38, 39 Furthermore, each patient's risk was categorized as low (<10%), intermediate (10–20%), or high (>20%) based on a modified Framingham risk score, as described previously.11, 40 We used the demographic information in Table 1 to calculate this score. Each patient was assigned a total score based on the sum of scores for sex‐based age, dyslipidemia, hypertension, smoking, and diabetes mellitus.
Table 1.
Characteristics of Patients Who Underwent Angiography for Evaluation of SCAD by Index Cardiac Noninvasive Test
| CCTA (n=113) | GXT (n=6742) | MPI (n=6877) | Stress Echo (n=1735) | Total (N=15 467) | P Value | |
|---|---|---|---|---|---|---|
| Age, y, mean±SD | 62.8±9.8 | 61.4±10.5 | 64.6±10.9 | 63.1±10.7 | 63.0±10.8 | <0.001 |
| Serum creatinine, μmol/L, mean±SD | 78.1±18.6 | 83.2±48.7 | 94.1±102.9 | 83.7±59.6 | 88.2±79.2 | <0.001 |
| Diabetes mellitus (%) | 39 (34.5) | 1981 (29.4) | 2672 (38.9) | 513 (29.6) | 5205 (33.7) | <0.001 |
| Dyslipidemia (%) | 77 (68.8) | 4309 (65.1) | 4898 (71.7) | 1136 (67.9) | 10 420 (68.4) | <0.001 |
| Hypertension (%) | 76 (67.3) | 4097 (60.8) | 4838 (70.4) | 1082 (62.4) | 10 093 (65.3) | <0.001 |
| Female sex (%) | 49 (43.4) | 2415 (35.8) | 2822 (41.0) | 696 (40.1) | 5982 (38.7) | <0.001 |
| Charlson score, mean±SD | 0.19±0.53 | 0.15±0.60 | 0.27±0.83 | 0.17±0.70 | 0.21±0.73 | <0.001 |
| Smoking (%) | 0.62 | |||||
| Current | 21 (18.9) | 1312 (19.6) | 1272 (18.6) | 349 (20.4) | 2954 (19.2) | |
| Former | 26 (23.4) | 1763 (26.3) | 1853 (27.1) | 451 (26.3) | 4093 (26.6) | |
| Never | 64 (57.7) | 3536 (52.8) | 3646 (53.2) | 890 (52.0) | 8136 (52.9) | |
| ST‐segment changes at rest on ECG (%) | 7 (6.2) | 697 (10.3) | 822 (12.0) | 179 (10.3) | 1705 (11.0) | <0.001 |
| CCS symptom scale, stable angina (%) | <0.001 | |||||
| 0 | 23 (20.4) | 1100 (16.3) | 1588 (23.1) | 441 (25.4) | 3152 (20.4) | |
| 1 | 18 (15.9) | 1286 (19.1) | 1325 (19.3) | 292 (16.8) | 2921 (18.9) | |
| 2 | 42 (37.2) | 2866 (42.5) | 2587 (37.6) | 695 (40.1) | 6190 (40.0) | |
| 3 or 4 | 32 (28.3) | 1490 (22.1) | 1377 (20.0) | 307 (17.7) | 3204 (20.5) | |
| Income quintile (%) | <0.001 | |||||
| 1 | 15 (13.4) | 1130 (16.8) | 1255 (18.3) | 354 (20.4) | 2754 (17.9) | |
| 2 | 18 (16.1) | 1359 (20.2) | 1479 (21.6) | 324 (18.7) | 3180 (20.6) | |
| 3 | 24 (21.4) | 1497 (22.3) | 1435 (20.9) | 337 (19.5) | 3293 (21.4) | |
| 4 | 26 (23.2) | 1370 (20.4) | 1386 (20.2) | 354 (20.4) | 3136 (20.3) | |
| 5 | 19 (25.9) | 1363 (20.3) | 1302 (19.0) | 363 (21.0) | 3057 (19.8) | |
| Framingham risk category (%) | 0.03 | |||||
| High | 30 (26.6) | 2076 (30.8) | 2248 (32.7) | 506 (29.2) | 4860 (31.4) | |
| Intermediate | 59 (52.2) | 3198 (47.4) | 3140 (45.7) | 816 (47.0) | 7213 (46.7) | |
| Low | 24 (21.2) | 1468 (21.7) | 1489 (21.7) | 413 (23.8) | 3394 (21.9) |
CCS indicates Canadian Cardiovascular Society; CCTA, coronary computed tomography angiography; GXT, graded exercise stress test; MPI, myocardial perfusion imaging study; SCAD, stable coronary artery disease; stress echo, stress echocardiogram.
Statistical Analysis
Descriptive statistics
Characteristics of patients who underwent angiography for the evaluation of SCAD were compared with the different initial testing strategies using the χ2 test for categorical variables and analysis of variance for continuous variables.
Logistic regression analyses
Unadjusted analyses
Unadjusted analyses were performed using logistic regression models to assess the relationship of the index cardiac noninvasive tests with downstream obstructive CAD in those patients who underwent invasive angiography for the evaluation of SCAD. GXT was considered the reference test.
Adjusted analyses
We performed multivariable logistic regression analyses to examine the association between the index noninvasive cardiac diagnostic tests and downstream obstructive CAD in those patients who underwent angiography for the evaluation of SCAD, controlling for the clinical covariates listed earlier. Before performing the logistic regression analyses, all predictor variables were assessed for the presence of multicollinearity. We concluded that there was no significant multicollinearity among the predictor variables included in the models based on the fact that none of the variables had a variance inflation factor >4 or tolerance <0.25. Subsequently, subgroup analyses were performed for those at high, intermediate, and low modified Framingham risk scores.
Time‐to‐event analyses for MACE
We compared time to event (development of MACE) using Kaplan–Meier survival curves estimated for each of the 4 noninvasive test groups. These were compared across the 4 test groups using the log‐rank test. Subsequently, we utilized a Cox proportional hazards model to adjust for clinical covariates. We obtained direct adjusted or marginal survival curves by noninvasive test group using previously described methods.41, 42 We subsequently performed subgroup analyses for those at high, intermediate, and low modified Framingham risk scores.
A 2‐tailed value of P<0.05 was considered statistically significant. Analyses were performed with SAS version 9.3. This study was approved by the research ethics board at Sunnybrook Health Sciences Center.
Results
Cohort Creation
In 2012 in Ontario, 464 647 patients aged ≥20 years had GXT, MPI, stress echo, or CCTA. Of these, 45 711 were excluded for having 1 of the 4 cardiac noninvasive diagnostic tests in the preceding 12 months. An additional 69 443 patients were excluded for having a previous diagnosis of cardiovascular disease in the preceding 20 years. Of the remaining 349 493 patients, a total of 18 819 underwent subsequent invasive angiography. The final cohort consisted of 15 467 patients who underwent invasive angiography for the indication of SCAD (see Figure 2).
Figure 2.
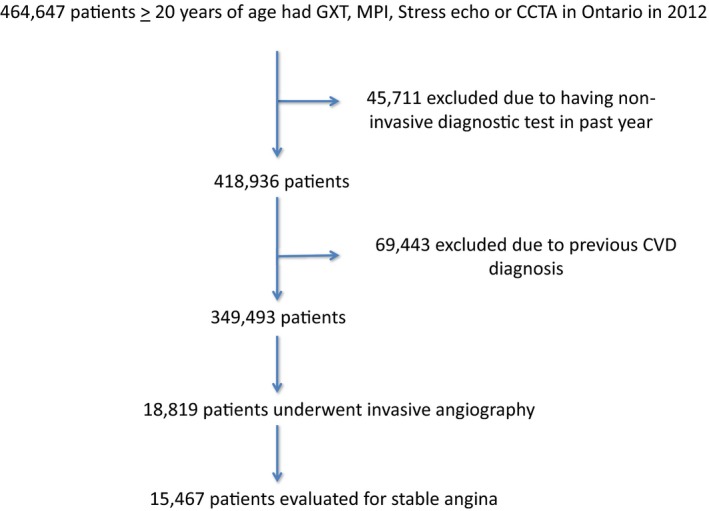
Derivation of the study cohort. The number of excluded patients is shown, as is the reason for their exclusion. Furthermore, the number of patients who ultimately underwent invasive angiography for the diagnosis of stable coronary artery disease is displayed. CCTA indicates coronary computed tomography angiography; CVD, cardiovascular disease; GXT, graded exercise stress test; MPI, myocardial perfusion imaging; stress echo, stress echocardiogram.
Progression to Angiography Among Patients With Various Initial Noninvasive Tests
Of the 349 493 patients who had a noninvasive cardiac diagnostic test in 2012 in Ontario, 1748 patients underwent initial CCTA, 175 900 underwent initial GXT, 128 622 underwent initial MPI, and 43 223 underwent initial stress echo. Within our observation period, 113 (6.5%) of those with initial CCTA versus 6877 (5.3%) with initial MPI, 1735 (4.0%) with initial stress echo, and 6742 (3.8%) with initial GXT received invasive angiography for the diagnosis of SCAD (P<0.001).
Characteristics of Patients Undergoing Angiography for the Evaluation of SCAD
Table 1 displays the characteristics of patients who underwent an initial noninvasive test and subsequent angiography in our cohort. Those patients who underwent an index MPI were, on average, the oldest (mean age: 64.6±10.9 versus 63.1±10.7 years for those with an index stress echo, 62.8±9.8 years for those with an index CCTA, and 61.4±10.5 for those with an index GXT; P<0.001). They also had the highest mean serum creatinine levels and were more likely to have diabetes mellitus, dyslipidemia, and hypertension (P<0.001). Patients with an index MPI also had a higher mean Charlson comorbidity index score. Finally, those with an index MPI had a higher proportion of patients classified as high risk according to the modified Framingham risk score (32.7% versus 30.8% for GXT, 29.2% for stress echo, and 26.6% for CCTA; P=0.03). Patients with an index CCTA were more likely both to be female and to have CCS class 3 to 4 angina symptoms. Those patients with an index CCTA were significantly more likely to reside in the top 20% of the Ontario's wealthiest neighborhoods.
Coronary anatomy
Table 2 summarizes the coronary anatomy of our patients at the time of angiography. Patients with an initial CCTA were significantly more likely to have left main disease on angiography (defined as luminal stenosis >50%). In contrast, there was no significant difference among the 4 initial testing strategies in 2‐ or 3‐vessel disease or in the rates of left anterior descending, right coronary artery, or circumflex stenosis.
Table 2.
Coronary Anatomy of the Study Patients at the Time of Invasive Angiography
| Anatomical Distribution of Significant Coronary Stenoses, n (%) | CCTA | GXT | MPI | Stress Echo | Total | P Value |
|---|---|---|---|---|---|---|
| n=113 | n=6742 | n=6877 | n=1735 | N=15 467 | ||
| Left anterior descending | 40 (36.4) | 2167 (32.8) | 2219 (32.8) | 546 (32.1) | 4972 (32.7) | 0.793 |
| Circumflex | 21 (19.1) | 1537 (23.2) | 1635 (24.2) | 384 (22.5) | 3577 (23.6) | 0.261 |
| Right coronary artery | 28 (25.5) | 1737 (26.3) | 1821 (26.9) | 423 (24.8) | 4009 (26.4) | 0.357 |
| Left main artery | 13 (11.8) | 380 (5.7) | 354 (5.2) | 77 (4.5) | 824 (5.4) | 0.004 |
| 2‐vessel disease | 10 (9.1) | 937 (14.2) | 965 (14.3) | 227 (13.3) | 2139 (14.1) | 0.346 |
| 3‐vessel disease | 11 (10.0) | 707 (10.7) | 774 (11.4) | 185 (10.9) | 1677 (11.0) | 0.545 |
CCTA indicates coronary computed tomography angiography; GXT, graded exercise stress test (GXT); MPI, myocardial perfusion imaging; stress echo, stress echocardiography.
Missing Data
Of the 15 467 patients in our cohort, 13 062 were ultimately utilized in our statistical models; therefore, 2405 patients were deleted because of incomplete variable records. When stratified according to risk, 3013 of 3529 high‐risk patients (516 deleted because of missing data), 5970 of 7105 intermediate‐risk patients (1135 deleted because of missing data), and 4079 of 4833 low‐risk patients (754 deleted because of incomplete data) were used in our models.
Yield of Obstructive CAD
Of those patients with an index CCTA, 54% had obstructive CAD on subsequent angiography compared with 47% with an index GXT and MPI and 45% with an index stress echo (P=0.18). On unadjusted analyses, patients undergoing an initial diagnostic strategy with MPI (odds ratio [OR]: 0.97; 95% confidence interval [CI], 0.91–1.04), CCTA (OR: 1.31; 95% CI, 0.89–1.92), or stress echo (OR: 0.92; 95% CI, 0.82–1.02) did not have statistically significantly different odds of having obstructive CAD compared with those whose initial diagnostic test was a GXT. This relative relationship persisted in the fully adjusted model. Patients undergoing initial MPI (OR: 0.92; 95% CI, 0.85–1.00), CCTA (OR: 1.51; 95% CI, 0.91–2.49), or stress echo (OR: 0.95; 95% CI, 0.84–1.08) did not have statistically significantly different odds of having obstructive CAD compared with those whose initial test was a GXT, after adjusting for clinically relevant covariates (see Table 3 and Figure 3).
Table 3.
Unadjusted and Adjusted Logistic Regression Models for the Outcome of Obstructive CAD in Patients Who Underwent Angiography for the Assessment of Stable CAD
| Odds Ratio (95% CI)* | P Value | |
|---|---|---|
| Coronary computed tomography angiography | ||
| Unadjusted | 1.31 (0.89–1.92) | 0.17 |
| Fully adjusted | 1.51 (0.91–2.49) | 0.11 |
| Myocardial perfusion imaging | ||
| Unadjusted | 0.97 (0.91–1.04) | 0.42 |
| Fully adjusted | 0.92 (0.85–1.00) | 0.05 |
| Stress echocardiogram | ||
| Unadjusted | 0.92 (0.82–1.02) | 0.11 |
| Fully adjusted | 0.95 (0.84–1.08) | 0.44 |
CAD indicates coronary artery disease.
* Graded exercise stress test as comparator.
Figure 3.
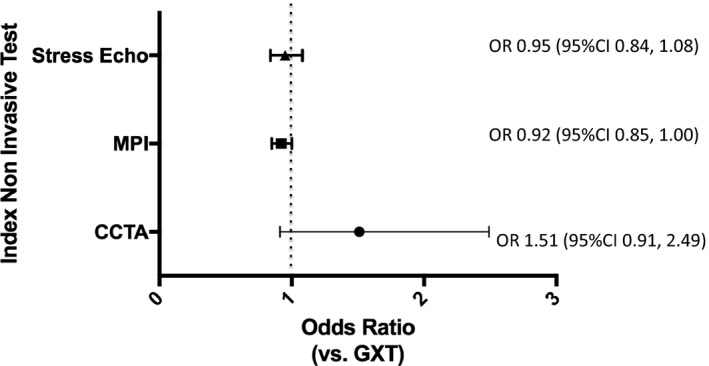
Yield of obstructive coronary artery disease (CAD) among the different index cardiac noninvasive diagnostic testing strategies. Multivariable model displaying the odds ratios and 95% confidence intervals of the yield of obstructive CAD among the different index cardiac noninvasive diagnostic tests in patients who underwent invasive angiography for the assessment of stable CAD (GXT is the reference test). Adjusted for age, sex, presence of diabetes mellitus, dyslipidemia, hypertension, income quintile, resting ECG abnormalities, Charlson comorbidity index score, serum creatinine, smoking history, Canadian Cardiovascular Society class angina symptom scale, chronic obstructive pulmonary disease, peripheral vascular disease, and modified Framingham risk score. CCTA indicates coronary computed tomography angiography; CI, confidence interval; GXT, graded exercise stress test; MPI, myocardial perfusion imaging; OR, odds ratio; stress echo, stress echocardiogram.
Subgroup analyses
Figure 4 displays the yield of obstructive CAD, stratified according to low‐, intermediate‐, and high‐risk patients. In the low‐risk subgroup, those with an initial CCTA were more likely to have obstructive CAD on subsequent angiography (OR: 2.22; 95% CI, 1.03–4.82). There was no statistically significant difference between those with initial MPI (OR: 0.98; 95% CI, 0.85–1.14), or stress echo (OR: 0.99; 95% CI, 0.79–1.24) compared with those who had an initial GXT. In the intermediate‐risk subgroup, there was no statistically significant difference between those with initial MPI (OR: 0.88; 95% CI, 0.78–1.00), CCTA (OR: 1.09; 95% CI, 0.50–2.38), and stress echo (OR: 0.97; 95% CI, 0.80–1.18) compared with those with an initial GXT. Finally, in the high‐risk subgroup, there was no statistically significant difference between any of the tests in terms of yield of obstructive CAD on subsequent angiography (initial CCTA [OR: 1.21; 95% CI, 0.37–3.99], MPI [OR: 0.89; 95% CI, 0.75–1.06], stress echo [OR: 0.84; 95% CI, 0.64–1.09]).
Figure 4.
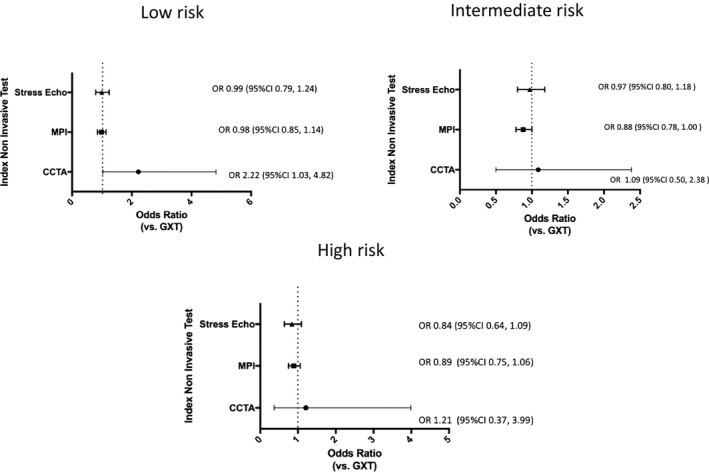
Yield of obstructive coronary artery disease (CAD) among the different index cardiac noninvasive diagnostic testing strategies stratified according to low, intermediate, and high modified Framingham risk. Multivariable model displaying the odds ratios and 95% confidence intervals of the yield of obstructive CAD among the different index cardiac noninvasive diagnostic tests in patients who underwent invasive angiography for the assessment of stable CAD (GXT is the reference test). Adjusted for age, sex, presence of diabetes mellitus, dyslipidemia, hypertension, income quintile, resting ECG abnormalities, Charlson comorbidity index score, serum creatinine, smoking history, Canadian Cardiovascular Society class angina symptom scale, chronic obstructive pulmonary disease, peripheral vascular disease, and modified Framingham risk score. CCTA indicates coronary computed tomography angiography; CI, confidence interval; GXT, graded exercise stress test; MPI, myocardial perfusion imaging; OR, odds ratio; stress echo, stress echocardiogram.
Major Adverse Cardiac Events
Patients were followed for a mean time of 1.89 years (SD: 0.42 year) after coronary angiography. Overall, 833 (5.4%) developed the composite end point during the follow‐up period. This included 9 patients with initial CCTA (7.9%), 358 with initial GXT (5.3%), 395 with initial MPI (5.7%), and 71 with initial stress echo (4.1%).
In the unadjusted model, those undergoing angiography with an index CCTA or MPI had a higher risk of developing the composite outcome than those with an initial GXT or stress echo (log‐rank P=0.03); however, after adjustment for clinical covariates, there was no significant difference in MACE among the 4 initial testing strategies (see Figure 5). In the adjusted stratified analysis, those in the low‐risk subgroup with an initial CCTA had a borderline significant increased risk of developing the composite outcome, whereas those with an initial MPI or stress echo had no significant difference in terms of risk of developing the composite outcome compared with those who had an initial GXT. There were no significant differences in terms of risk of developing the composite outcome between the initial testing strategies in the intermediate‐ and low‐risk subgroups after adjustment for relevant clinical covariates (see Figure 6).
Figure 5.
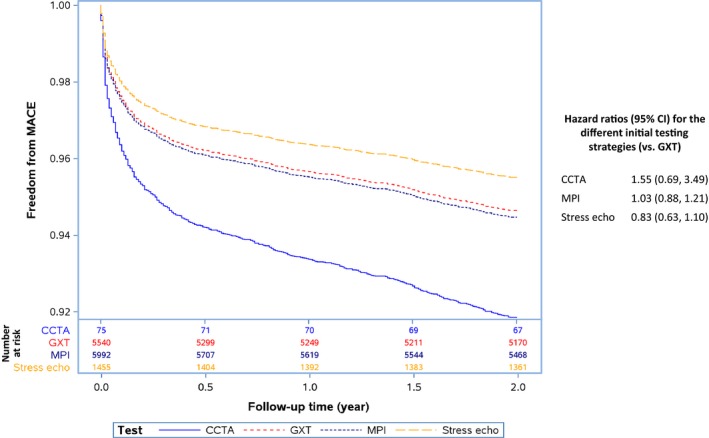
Adjusted Kaplan–Meier estimates of the composite end point of all‐cause mortality, acute myocardial infarction, and unstable angina. Adjusted for age, sex, presence of diabetes mellitus, dyslipidemia, hypertension, income quintile, resting ECG abnormalities, Charlson comorbidity index score, serum creatinine, smoking history, Canadian Cardiovascular Society class angina symptom scale, chronic obstructive pulmonary disease, peripheral vascular disease, and modified Framingham risk score. Number of patients at risk at 6‐month intervals is provided for each test below the main figure. CCTA indicates coronary computed tomography angiography; CI, confidence interval; GXT, graded exercise stress test; MPI, myocardial perfusion imaging; stress echo, stress echocardiogram.
Figure 6.
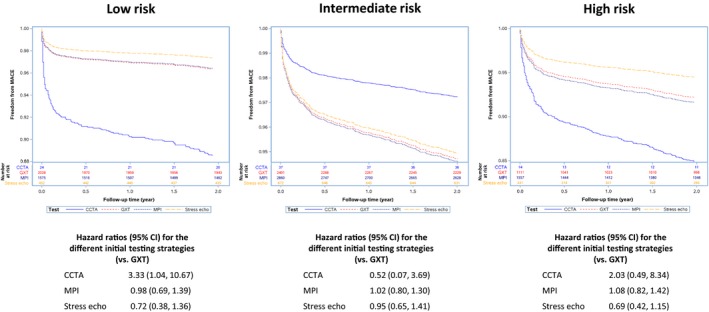
Adjusted Kaplan–Meier estimates of the composite end point of all‐cause mortality, acute myocardial infarction, and unstable angina stratified according to low, intermediate, and high Framingham risk. Clinical covariates included in the model were adjusted for age, sex, presence of diabetes mellitus, dyslipidemia, hypertension, income quintile, resting ECG abnormalities, Charlson comorbidity index score, serum creatinine, smoking history, Canadian Cardiovascular Society class angina symptom scale, chronic obstructive pulmonary disease, and peripheral vascular disease. Number of patients at risk at 6‐month intervals is provided for each test below the main figure. CCTA indicates coronary computed tomography angiography; CI, confidence interval; GXT, graded exercise stress test; MPI, myocardial perfusion imaging; stress echo, stress echocardiogram.
Discussion
The most common initial noninvasive test in our cohort was MPI. The prevalence of most major cardiovascular risk factors was higher in those with initial MPI compared with patients whose initial test was CCTA, stress echo, or GXT. Patients whose initial test was MPI tended to have a higher mean Charlson comorbidity index score. They also were more likely to have a higher modified Framingham risk score. Those with an index CCTA were most likely to be female and to have CCS class 3 to 4 symptoms. After adjusting for relevant covariates, patients with an initial testing strategy with MPI, CCTA or stress echo did not have a statistically significant different yield of obstructive CAD compared with patients with an initial testing strategy using a GXT. Furthermore, there was no significant difference in downstream rates of MACE among the 4 initial testing strategies.
Noninvasive Diagnostic Tests to Diagnose CAD
The optimal strategy to evaluate patients with suspected SCAD is currently uncertain. The recent proliferation of noninvasive cardiac diagnostic testing technology has led to various potential options for the noninvasive diagnosis of CAD. The 2012 Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease from the American College of Cardiology Foundation and American Heart Association task force on practice guidelines and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons recommended GXT as the first‐line test in patients who are able to exercise in the absence of significant ST‐T wave abnormalities.3 In contrast, the 2013 and 2014 European Society for Cardiology (ESC) guidelines on SCAD and revascularization are more liberal in their recommendations of stress and anatomic imaging as the initial testing strategy. Class 1 recommendations in the ESC documents are conferred to stress imaging when there are baseline ECG abnormalities, when patients are unable to exercise, and when stress imaging is available and there is local expertise. Furthermore, there is a class 1 recommendation for stress imaging for high‐risk patients and a class IIa indication for CCTA in the diagnosis of SCAD.1, 2
Many studies have examined the efficacy of CCTA, GXT, MPI, and stress echo in selected populations43, 44, 45; however, despite widespread clinical adoption of newer technologies, there have been limited data comparing their relative clinical effectiveness, specifically by comparing their real‐world downstream outcomes.4, 5 The lack of a clear demonstration of an effect on outcomes has led to calls for regulation aimed at controlling spending and improving quality.46, 47 Little comparative effectiveness research has been published on differing initial noninvasive diagnostic strategies that are currently available to clinicians.
Yield of Obstructive CAD
The optimal end point to assess clinical effectiveness is currently unknown. In recent years, the concept of yield of obstructive CAD has emerged as a surrogate end point designed to assess the clinical effectiveness of cardiac diagnostic tests. Multiple studies have linked obstructive CAD to an increase in mortality.3, 48, 49 Recent registry data reported an obstructive CAD rate of only 37.6% on invasive angiograms of patients being evaluated for SCAD.11 Given the potential risks associated with invasive angiography, it is important to assess the yield of obstructive CAD related to cardiac noninvasive diagnostic tests. The PROMISE trial reported that although more patients underwent catheterization, there were significantly fewer diagnostic angiograms showing nonobstructive CAD in the anatomic versus the functional groups.47 The yield of obstructive CAD was 72.1% in the anatomic group versus 47.5% in the functional group (P=0.02).47 Another recent study retrospectively collected data on 209 patients evaluated at chest pain clinics at 2 British hospitals. The first hospital evaluated patients with a strategy of GXT as the first and only cardiac noninvasive diagnostic test. The second used a “cardiac imaging” pathway that involved a combination of coronary artery calcium score, CCTA, MPI, and stress echo. They concluded that the cardiac imaging strategy resulted in fewer invasive angiograms and a higher yield of obstructive CAD.10 They did not analyze the yield of obstructive CAD among the different testing modalities in the imaging arm.
Major Adverse Cardiac Events
The relationship between a diagnostic imaging test and downstream “hard” cardiovascular outcomes such as mortality and nonfatal cardiovascular events is complex. Clinical decisions made after the imaging test have a significant impact on the development of such outcomes. Nonetheless, when comparing the clinical effectiveness of different diagnostic testing modalities, a recent position paper recommended examining such outcomes with the recognition that they are a reflection of the strategy and the entire diagnostic/therapeutic pathway initiated by a test rather than a reflection of the efficacy of the test itself with isolation of other downstream factors.4
This study, to our knowledge, is the first analysis to directly compare the yield of obstructive CAD and MACE based on strategies of initiation of the diagnostic pathway for SCAD with either a GXT, MPI, stress echo, or CCTA at the level of the entire adult population; therefore, we were able to assess the potential real‐world impact of the initial noninvasive testing strategies in the largest province in Canada (approximate adult population of 10.1 million). Our results indicate that an initial diagnostic strategy with MPI, CCTA, or stress echo did not result in a higher yield of obstructive CAD or differential downstream MACE compared with a GXT. The exception may be in the low‐risk CCTA group, for which our subgroup analysis indicated that those who had an initial CCTA were more likely to have obstructive CAD on downstream angiography compared with those with an initial GXT. This result needs to be interpreted with caution because it was the result of a post hoc subgroup analysis, and the level of statistical significance was borderline (P=0.047).
Another important finding in our study was that CCTAs were more likely to be performed in higher income individuals. This phenomenon is similar to trends observed in utilization of other diagnostic tests including magnetic resonance imaging in Ontario.50, 51, 52, 53 Higher income individuals may be more aware of emerging and new technologies and may be more likely to ask their physicians to order a newer modality such as CCTA.37, 51, 54
Significance
We expected that an initial diagnostic strategy with MPI, stress echo, or CCTA would have a significantly higher yield of obstructive CAD compared with a GXT. Our hypothesis was based on clinical efficacy studies showing superior accuracy of the former 3 modalities compared with GXT. Our results failed to support our hypothesis. Instead, our results showed that in a large contemporary real‐world population‐based cohort in Ontario, Canada, an initial noninvasive diagnostic strategy with a CCTA, stress echo, or MPI failed to result in a higher yield of obstructive CAD compared with an initial strategy with a GXT. Furthermore, there was no significant difference in downstream MACE between the different initial testing strategies. Consequently, within the context of the limitations of our study, our results do not support the routine initial use of stress imaging or CCTA in the workup of SCAD. These findings highlight the need for future research to explore the reasons for the discrepancy between our real‐world findings and those of clinical efficacy studies.
Limitations
Our study must be interpreted in the context of a number of limitations. First, we had limited access to granular clinical data; for example, we were able to detect whether or not a patient had a noninvasive test, but we did not have access to the result of that test. We assumed that those who were not referred for angiography had negative noninvasive tests. Second, our analysis focused on the yield of obstructive CAD. By its nature, it included only patients who underwent invasive angiography. We did not assess the patients whose diagnostic cascade was appropriately terminated with a negative noninvasive test. Third, our results reflect data from Ontario and may not be generalizable to other jurisdictions. Fourth, our analysis was limited to tests that are currently widely utilized in Ontario. We did not assess other emerging technologies such as stress cardiac magnetic resonance imaging and positron emission tomography nuclear imaging because of the absence of an associated physician billing code and/or limited clinical availability. Fifth, the number of patients who underwent angiography after having an index CCTA was small. Consequently, we were likely underpowered to show a statistically significant difference in yield of obstructive CAD compared with GXT. Finally, although we attempted to adjust for all known relevant confounders, the observational nature of this study raises the possibility of the presence of unknown confounders that may have inadvertently affected our results.
Conclusions
Our study found no evidence to suggest a higher yield of obstructive CAD with an initial testing strategy with stress imaging or CCTA compared with GXT. Furthermore, we found no evidence of improvements in downstream MACE with either a stress imaging or CCTA initial testing strategy compared with an initial GXT. These real‐world results, therefore, do not provide evidence to support the routine initial use of stress imaging or CCTA in the workup of SCAD.
Sources of Funding
This study was supported by operating grants from the Institute for Circulatory and Respiratory Health‐Canadian Institutes of Health Research (ICRH‐CIHR) Team Grant‐Chronic Disease Risk and Intervention Strategies (TCA 118349), a CIHR Foundation Grant (FDN‐143313) and a CIHR Operating Grant (MOP‐111035) in support of the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) initiative (www.canheart.ca). This study was also supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The funding organizations did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Dr Tu is supported by a Canada Research Chair in Health Services Research and an Eaton Scholar award. Dr Wijeysundera is supported by a Distinguished Clinician Scientist Award from the Heart and Stroke Foundation of Canada. Dr Austin is supported by a Career Scientist Award from the Heart and Stroke Foundation of Canada.
Disclosures
None.
Acknowledgments
The authors acknowledge that the clinical registry data used in this publication is from participating hospitals through the Cardiac Care Network of Ontario, which serves as an advisory body to the Minister of Health and Long‐Term Care (MOHLTC), is funded by the MOHLTC, and is dedicated to improving the quality, efficiency, access and equity in the delivery of the continuum of adult cardiac services in Ontario, Canada.
(J Am Heart Assoc. 2017;6:e005462 DOI: 10.1161/JAHA.116.005462.)28729409
References
- 1. Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Sousa Uva M, Achenbach S, Pepper J, Anyanwu A, Badimon L, Bauersachs J, Baumbach A, Beygui F, Bonaros N, De Carlo M, Deaton C, Dobrev D, Dunning J, Eeckhout E, Gielen S, Hasdai D, Kirchhof P, Luckraz H, Mahrholdt H, Montalescot G, Paparella D, Rastan AJ, Sanmartin M, Sergeant P, Silber S, Tamargo J, ten Berg J, Thiele H, van Geuns RJ, Wagner HO, Wassmann S, Wendler O, Zamorano JL. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517–592. [DOI] [PubMed] [Google Scholar]
- 2. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, Anderson JL. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 4. Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. Circ Cardiovasc Imaging. 2009;2:339–348. [DOI] [PubMed] [Google Scholar]
- 5. Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. JACC Cardiovasc Imaging. 2009;2:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. J Cardiovasc Comput Tomogr. 2009;3:212–223. [DOI] [PubMed] [Google Scholar]
- 7. Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. J Am Soc Echocardiogr. 2009;22:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dedic A, Rossi A, Ten Kate GJ, Neefjes LA, Galema TW, Moelker A, van Domburg RT, Schultz CJ, Mollet NR, de Feyter PJ, Nieman K. First‐line evaluation of coronary artery disease with coronary calcium scanning or exercise electrocardiography. Int J Cardiol. 2013;163:190–195. [DOI] [PubMed] [Google Scholar]
- 9. Chow BJ, Abraham A, Wells GA, Chen L, Ruddy TD, Yam Y, Govas N, Galbraith PD, Dennie C, Beanlands RS. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging. 2009;2:16–23. [DOI] [PubMed] [Google Scholar]
- 10. Demir OM, Bashir A, Marshall K, Douglas M, Wasan B, Plein S, Alfakih K. Comparison of clinical efficacy and cost of a cardiac imaging strategy versus a traditional exercise test strategy for the investigation of patients with suspected stable coronary artery disease. Am J Cardiol. 2015;115:1631–1635. [DOI] [PubMed] [Google Scholar]
- 11. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel MR, Dai D, Hernandez AF, Douglas PS, Messenger J, Garratt KN, Maddox TM, Peterson ED, Roe MT. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167:846–852.e2. [DOI] [PubMed] [Google Scholar]
- 13. Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, Maclagan LC, Guo H, Austin PC, Hogg W, Kapral MK, Wijeysundera HC, Atzema CL, Gershon AS, Alter DA, Lee DS, Jackevicius CA, Bhatia RS, Udell JA, Rezai MR, Stukel TA. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. [DOI] [PubMed] [Google Scholar]
- 14. Maclagan LC, Park J, Sanmartin C, Mathur KR, Roth D, Manuel DG, Gershon A, Booth GL, Bhatia S, Atzema CL, Tu JV. The CANHEART health index: a tool for monitoring the cardiovascular health of the Canadian population. CMAJ. 2014;186:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–296. [DOI] [PubMed] [Google Scholar]
- 16. Lee DS, Stitt A, Wang X, Yu JS, Gurevich Y, Kingsbury KJ, Austin PC, Tu JV. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51:e22–e26. [DOI] [PubMed] [Google Scholar]
- 17. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 19. Tu JV, Donovan LR, Lee DS, Wang JT, Austin PC, Alter DA, Ko DT. Effectiveness of public report cards for improving the quality of cardiac care: the EFFECT study: a randomized trial. JAMA. 2009;302:2330–2337. [DOI] [PubMed] [Google Scholar]
- 20. Tu JV, Bowen J, Chiu M, Ko DT, Austin PC, He Y, Hopkins R, Tarride JE, Blackhouse G, Lazzam C, Cohen EA, Goeree R. Effectiveness and safety of drug‐eluting stents in Ontario. N Engl J Med. 2007;357:1393–1402. [DOI] [PubMed] [Google Scholar]
- 21. Wijeysundera HC, Stukel TA, Chong A, Natarajan MK, Alter DA. Impact of clinical urgency, physician supply and procedural capacity on regional variations in wait times for coronary angiography. BMC Health Serv Res. 2010;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ko DT, Guo H, Wijeysundera HC, Natarajan MK, Nagpal AD, Feindel CM, Kingsbury K, Cohen EA, Tu JV. Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J Am Coll Cardiol. 2012;60:1876–1884. [DOI] [PubMed] [Google Scholar]
- 23. Wijeysundera HC, Trubiani G, Abrahamyan L, Mitsakakis N, Witteman W, Paulden M, van der Velde G, Kingsbury K, Krahn M. Specialized multi‐disciplinary heart failure clinics in Ontario, Canada: an environmental scan. BMC Health Serv Res. 2012;12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tu JV, Ko DT, Guo H, Richards JA, Walton N, Natarajan MK, Wijeysundera HC, So D, Latter DA, Feindel CM, Kingsbury K, Cohen EA. Determinants of variations in coronary revascularization practices. CMAJ. 2012;184:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwalm JD, Wijeysundera HC, Tu JV, Guo H, Kingsbury KJ, Natarajan MK. Influence of coronary anatomy and SYNTAX Score on the variations in revascularization strategies for patients with multivessel disease. Can J Cardiol. 2014;30:1155–1161. [DOI] [PubMed] [Google Scholar]
- 26. Ko DT, Tu JV, Austin PC, Wijeysundera HC, Samadashvili Z, Guo H, Cantor WJ, Hannan EL. Prevalence and extent of obstructive coronary artery disease among patients undergoing elective coronary catheterization in New York State and Ontario. JAMA. 2013;310:163–169. [DOI] [PubMed] [Google Scholar]
- 27. Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
- 28. Steyn K, Sliwa K, Hawken S, Commerford P, Onen C, Damasceno A, Ounpuu S, Yusuf S. Risk factors associated with myocardial infarction in Africa: the INTERHEART Africa study. Circulation. 2005;112:3554–3561. [DOI] [PubMed] [Google Scholar]
- 29. Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, Zhang X, Yusuf S. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case‐control study. Lancet. 2006;368:647–658. [DOI] [PubMed] [Google Scholar]
- 30. Lanas F, Avezum A, Bautista LE, Diaz R, Luna M, Islam S, Yusuf S. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation. 2007;115:1067–1074. [DOI] [PubMed] [Google Scholar]
- 31. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. [DOI] [PubMed] [Google Scholar]
- 32. Teo KK, Liu L, Chow CK, Wang X, Islam S, Jiang L, Sanderson JE, Rangarajan S, Yusuf S. Potentially modifiable risk factors associated with myocardial infarction in China: the INTERHEART China study. Heart. 2009;95:1857–1864. [DOI] [PubMed] [Google Scholar]
- 33. Guo J, Li W, Wang Y, Chen T, Teo K, Liu LS, Yusuf S. Influence of socioeconomic status on acute myocardial infarction in the Chinese population: the INTERHEART China study. Chin Med J (Engl). 2012;125:4214–4220. [PubMed] [Google Scholar]
- 34. Hemann BA, Bimson WF, Taylor AJ. The Framingham Risk Score: an appraisal of its benefits and limitations. Am Heart Hosp J. 2007;5:91–96. [DOI] [PubMed] [Google Scholar]
- 35. Kraus JF, Borhani NO, Franti CE. Socioeconomic status, ethnicity, and risk of coronary heart disease. Am J Epidemiol. 1980;111:407–414. [DOI] [PubMed] [Google Scholar]
- 36. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 37. Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–1367. [DOI] [PubMed] [Google Scholar]
- 38. Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Winters WL Jr, Yanowitz FG, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Lewis RP, O'Rourke RA, Ryan TJ. ACC/AHA guidelines for exercise testing: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation. 1997;96:345–354. [DOI] [PubMed] [Google Scholar]
- 39. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:3097–3137. [DOI] [PubMed] [Google Scholar]
- 40. Mohareb MM, Qiu F, Cantor WJ, Kingsbury KJ, Ko DT, Wijeysundera HC. Validation of the appropriate use criteria for coronary angiography: a cohort study. Ann Intern Med. 2015;162:549–556. [DOI] [PubMed] [Google Scholar]
- 41. Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35:437–443. [DOI] [PubMed] [Google Scholar]
- 42. Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. [DOI] [PubMed] [Google Scholar]
- 43. Shaw LJ, Berman DS. Functional versus anatomic imaging in patients with suspected coronary artery disease. Cardiol Clin. 2009;27:597–604. [DOI] [PubMed] [Google Scholar]
- 44. Shaw LJ, Tandon S, Rosen S, Mieres JH. Evaluation of suspected ischemic heart disease in symptomatic women. Can J Cardiol. 2014;30:729–737. [DOI] [PubMed] [Google Scholar]
- 45. Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iglehart JK. Health insurers and medical‐imaging policy—a work in progress. N Engl J Med. 2009;360:1030–1037. [DOI] [PubMed] [Google Scholar]
- 47. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al‐Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Bono D. Investigation and management of stable angina: revised guidelines 1998. Joint Working Party of the British Cardiac Society and Royal College of Physicians of London. Heart. 1999;81:546–555. [PMC free article] [PubMed] [Google Scholar]
- 50. Roifman I, Wijeysundera HC, Austin PC, Maclagan LC, Rezai MR, Wright GA, Tu JV. Temporal trends in the utilization of noninvasive diagnostic tests for coronary artery disease in Ontario between 2008 and 2014: a population‐based study. Can J Cardiol. 2017;33:279–282. [DOI] [PubMed] [Google Scholar]
- 51. You JJ, Venkatesh V, Laupacis A. Better access to outpatient magnetic resonance imaging in Ontario—but for whom? Open Med. 2009;3:e22–e25. [PMC free article] [PubMed] [Google Scholar]
- 52. Demeter S, Reed M, Lix L, MacWilliam L, Leslie WD. Socioeconomic status and the utilization of diagnostic imaging in an urban setting. CMAJ. 2005;173:1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roifman I, Rezai MR, Wijeysundera HC, Chow BJ, Wright GA, Tu JV. Utilization of cardiac computed tomography angiography and outpatient invasive coronary angiography in Ontario, Canada. J Cardiovasc Comput Tomogr. 2015;9:567–571. [DOI] [PubMed] [Google Scholar]
- 54. Alter DA, Iron K, Austin PC, Naylor CD. Socioeconomic status, service patterns, and perceptions of care among survivors of acute myocardial infarction in Canada. JAMA. 2004;291:1100–1107. [DOI] [PubMed] [Google Scholar]


