Abstract
Background
Increasing age predisposes to both thromboembolic and bleeding events in patients with atrial fibrillation; therefore, balancing risks and benefits of antithrombotic strategies in older populations is crucial. We investigated 1‐year outcome with different antithrombotic approaches in very elderly atrial fibrillation patients (age ≥85 years) compared with younger patients.
Methods and Results
We accessed individual patients’ data from the prospective PREFER in AF (PREvention oF thromboembolic events‐European Registry in Atrial Fibrillation), compared outcomes with and without oral anticoagulation (OAC), and estimated weighed net clinical benefit in different age groups. A total of 6412 patients, 505 of whom were aged ≥85 years, were analyzed. In patients aged <85 years, the incidence of thromboembolic events was 2.8%/year without OAC versus 2.3%/year with OAC (0.5% absolute reduction); in patients aged ≥85 years, it was 6.3%/year versus 4.3%/year (2% absolute reduction). In very elderly patients, the risk of major bleeding was higher than in younger patients, but similar in patients on OAC and in those on antiplatelet therapy or without antithrombotic treatment (4.0%/year versus 4.2%/year; P=0.77). OAC was overall associated with weighted net clinical benefit, assigning weights to nonfatal events according to their prognostic implication for subsequent death (−2.19%; CI, −4.23%, −0.15%; P=0.036). We found a significant gradient of this benefit as a function of age, with the oldest patients deriving the highest benefit.
Conclusions
Because the risk of stroke increases with age more than the risk of bleeding, the absolute benefit of OAC is highest in very elderly patients, where it, by far, outweighs the risk of bleeding, with the greatest net clinical benefit in such patients.
Keywords: anticoagulation, atrial fibrillation, major bleeding, thromboembolic events, very elderly
Subject Categories: Ischemic Stroke, Arrhythmias, Aging, Anticoagulants, Intracranial Hemorrhage
Clinical Perspective
What is New?
We report real‐world data on very elderly patients (aged ≥85 years) with atrial fibrillation, and we show that, because the risk of stroke increases with age more than the risk of bleeding, in very elderly patients the absolute benefit of oral anticoagulation is highest, where it, by far, outweighs the risk of bleeding, and the net clinical benefit is highest.
What are the Clinical Implications?
Although the concern of bleeding often leads, in clinical practice, to the underutilization of oral anticoagulation in very elderly patients with atrial fibrillation, our study strongly supports the use of anticoagulant therapy also in these setting of patients to prevent thromboembolic events.
Introduction
Approaches to prevent cardioembolic complications of atrial fibrillation (AF) in older subjects may have a prominent impact on cardiovascular morbidity and mortality of these patients and on health economy. Indeed, there is a progressive aging of Western populations, with the proportion of individuals aged ≥85 years being expected to have a 3‐fold increase by the year 2035 worldwide.1 Advancing age predisposes to AF and is associated with increases of both thromboembolic risk and antithrombotic drug‐related bleeding once AF occurs.2, 3, 4
A previous randomized study (BAFTA [Birmingham Atrial Fibrillation Treatment of the Aged])5 has indicated that, among elderly patients (aged ≥75 years) with AF, the use of warfarin is associated with significant reduction of thromboembolic complications compared with antiplatelet therapy. Such results were replicated in a subgroup analysis on patients with AF and age ≥75 years from the AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trial,6 in which the non–vitamin K antagonist oral anticoagulant (NOAC), apixaban, was tested against aspirin in patients who had contraindications to anticoagulation with warfarin, were unwilling to take it or were considered unsuitable to it. Of note, a patient‐level meta‐analysis of 6 randomized trials in the setting of AF showed no interaction between effects of anticoagulant versus antiplatelet treatment on bleeding or ischemic end points and age (<75 versus ≥75 years).7 Despite this information, advanced age remains one of the major reasons for physicians to withhold oral anticoagulation from AF patients.
There is a broad interest in the best treatment of very elderly patients with cardiovascular diseases, usually defined as ≥85 years.8, 9, 10 Unfortunately, there has, so far, been no controlled trial with sufficient power in this age group to inform treatment of very elderly patients with AF. Therefore, we have explored this issue in a large, multicenter registry on AF patients. Moreover, because the absolute increase in mortality related to thromboembolic and hemorrhagic events is prominent in older populations, we have also here carried out a weighted net clinical benefit analysis, by adjusting each major nonfatal ischemic and hemorrhagic events for the specific risk of death of such adverse events, occurring with either oral anticoagulation or no anticoagulation.
Methods
For this study, we used individual patients’ data from PREFER in AF (the PREvention oF thromboembolic events‐European Registry in Atrial Fibrillation).11 PREFER in AF was a prospective, real‐world registry on 7228 AF patients from 461 hospitals and 7 European countries (Austria, France, Germany, Italy, Spain, Switzerland, and United Kingdom). Inclusion criteria were: age ≥18 years; at least 1 episode of AF in the previous year, as demonstrated by an ECG or by an implanted pacemaker/defibrillator; signed informed consent to adhere to the study. Patients were enrolled regardless of the type of AF and of current antithrombotic therapy (ie, also including patients on chronic oral anticoagulation). The first patient was enrolled in January 2012, with the last follow‐up visit being performed in January 2014. In order to avoid selection bias, at each center all patients were consecutively enrolled, without explicit exclusion criteria. The study design comprised a baseline visit at the time of patient recruitment and a clinical evaluation at 1‐year follow‐up. In this investigation, we only included patients with data from both the baseline and the 1‐year follow‐up visits available. Only documented stroke, systemic embolism, myocardial infarction (MI), or major bleeding events were considered as relevant outcome measures in this analysis, counting all events occurring after the baseline assessment.
Individual data were reported into an electronic case report form including various plausibility checks for the considered variables. On‐site verification of source data was done in ≈5% of the sites. Study management was overseen by a scientific steering committee; the registry was sponsored by Daiichi Sankyo Europe GmbH (Munich, Germany) through a contract research organization (SSS International Clinical Research GmbH, Munich, Germany) coordinating various local national contract research organizations. The study protocol was approved by each local site ethics committee, and the study design has been published.11
Definitions and End Points
For the purpose of this study, we specifically focused on very elderly patients (ie, patients aged ≥85 years). Very elderly patients were compared with younger patients, and exploratory analyses on extremely elderly patients (aged ≥90 years) were also performed.
Thromboembolic events (stroke, transient ischemic attack [TIA], and systemic embolism), myocardial infarction (MI), and major bleeding occurring within 1‐year follow‐up were considered as study end points. These outcome measures of thromboembolic and bleeding events essentially reflect the definitions used in the ENGAGE AF‐TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial,12 namely.
Stroke
Abrupt onset of a focal neurological deficit, generally distributed in the territory of a single brain artery (including the retinal artery), and that is not attributable to an identifiable nonvascular cause (ie, brain tumor or trauma). The deficit must either be characterized by symptoms lasting >24 hours or cause death within 24 hours of symptom onset. This stroke definition reflects the Statement for Healthcare Professionals From the American Heart Association/American Stroke Association,13 that incorporates the World Health Organization definition of stroke.14
Transient Ischemic Attack
Focal neurological deficit associated with symptoms lasting <24 hours.
Systemic embolic event
Abrupt episode of arterial insufficiency with clinical or radiologic documentation of arterial occlusion in the absence of other likely mechanisms (eg, atherosclerosis, instrumentation); venous thromboembolism and pulmonary embolism were also included in this outcome measure.
Major bleeding
Fatal bleeding and/or bleeding into a critical organ (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome) and/or clinically relevant bleeding with hemoglobin drop ≥2 g/dL; this is consistent with the definition of major bleeding from the International Society on Thrombosis and Haemostasis.15
Statistical Analysis
Descriptive statistics were performed first. Discrete characteristics are expressed as frequency counts and percentages (n, %), whereas continuous characteristics are expressed as means and SDs or medians lower and upper quartiles, where appropriate. We performed a complete case analysis, and assumed that missing data were missing at random.
Odds ratios (ORs) between events (yes/no) during the 1‐year follow up, as well as oral anticoagulant therapy (yes/no) were calculated by logistic regression, where outcome events were the dependent variables and anticoagulant therapy (yes/no) the independent variable. The following adjusting factors were included into the model: use of concomitant drugs (such as antiplatelet or nonsteroidal anti‐inflammatory drugs) at the baseline visit; vascular disease (eg, peripheral artery disease, MI, or aortic plaque) at any time preceding baseline; stroke/TIA/systemic embolic event at any time preceding baseline; major bleeding at any time preceding baseline; and stent implantation at any time preceding baseline. Analyses were adjusted for demographic/clinical variables, as indicated in Table 1. ORs, 95% CIs, and the corresponding P value are reported. All analyses are not confirmatory, but purely descriptive/exploratory, and therefore no adjustment for multiple testing was done.
Table 1.
Distribution of Demographic and Clinical Characteristics According to Age Groups
| Variable | Age <85 Y (N=5907) | Age ≥85 Y (N=505) | P Value |
|---|---|---|---|
| Female sex | 2260 (38.3) | 286 (56.6) | <0.0001 |
| BMI >30 kg/m2 | 1690 (29.6) | 59 (12.2) | <0.0001 |
| Systemic hypertension | 4267 (72.6) | 391 (78.5) | 0.0044 |
| Congestive heart failure | 1543 (28.2) | 210 (44.7) | <0.0001 |
| Previous TIA/stroke/thromboembolism | 857 (14.7) | 104 (20.9) | 0.0002 |
| Vascular disease | 1204 (22.0) | 138 (29.5) | 0.0002 |
| Chronic renal failure | 722 (12.5) | 120 (24.3) | <0.0001 |
| Left atrial dilatation (diameter >40 mm) | 3443 (70.3) | 316 (77.3) | 0.0030 |
| Chronic obstructive pulmonary disease | 653 (11.2) | 74 (14.8) | 0.014 |
| Antithrombotic therapies | |||
| No therapy | 349 (5.9) | 35 (6.9) | 0.410 |
| Oral anticoagulant | 4917 (83.2) | 393 (77.8) | 0.019 |
| VKA | 4556 (77.3) | 362 (71.7) | 0.0055 |
| NOAC | 361 (6.1) | 31 (6.1) | 0.9804 |
| Antiplatelet only | 641 (10.9) | 77 (15.3) | 0.0026 |
| Oral anticoagulant plus antiplatelet | 662 (11.2) | 50 (9.9) | 0.3699 |
Values are given as n (%). BMI indicates body mass index; NOAC, non–vitamin K antagonist oral anticoagulant; TIA, transient ischemic attack; VKA, vitamin K antagonist.
The weighted net clinical benefit with oral anticoagulant therapy versus no anticoagulation (ie, use of antiplatelet treatment or no antithrombotic drug) according to different age strata was evaluated as previously described.16, 17 In brief, the following adverse events were counted in the net clinical benefit: ischemic stroke; systemic embolism; MI; hemorrhagic stroke; and major bleeding (without hemorrhagic stroke). Incidence at 1‐year follow‐up was considered for each event. We included MI in the net clinical benefit because it is well established that in patients at high‐cardiovascular‐risk (such as AF patients) oral anticoagulation may prevent atherothrombotic events (also including atherothrombotic stroke and MI), and because previous analyses on the net clinical benefit of oral anticoagulation in patients with AF have already included MI as an outcome measure.16, 17 Both ST‐segment elevation and non‐ST‐segment elevation MIs were included as outcome measures in the net clinical benefit analysis, and MI was defined according to the classification at the time of the conduction of PREFER in AF (ie, Third Universal Definition of Myocardial Infarction18). The net clinical benefit was calculated as the weighted sum of crude incidence rates (IRs) in patients on oral anticoagulant therapy (OAC) minus the weighted sum of events in those without anticoagulant treatment: net clinical benefit=[IRischemic stroke_OAC+w1IRsystemic embolism_OAC+w2IRmyocardial infarction_OAC+w3IRhemorrhagic stroke_OAC+w4IRmajor extra‐cranial bleeding_OAC]−[IRischemic stroke_no OAC+w1IRsystemic embolism_no OAC+w2IRmyocardial infarction_no OAC+w3IRhemorrhagic stroke_no OAC+w4IRmajor extra‐cranial bleeding_no OAC], where OAC is oral anticoagulant therapy and w1, w2, w3, and w4 the death‐related weights associated with each type of event. Weights were calculated as the impact of each event on mortality, as derived from a recent analysis combining data from the ACTIVE and RE‐LY databases,17 and related to ischemic stroke (weight=1). Weights were thus 0.61 for systemic embolism, 0.89 for MI, 3.23 for hemorrhagic stroke, and 0.63 for major bleeding (without hemorrhagic stroke). The lower the value of the result in this calculation, the higher the net clinical benefit of anticoagulant therapy compared to no anticoagulation was assumed to be.16
All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC) with a 2‐tailed significance value of 0.05.
Results
Of 7228 patients enrolled in PREFER in AF, 6412 had both baseline and 1‐year follow‐up visits and were then included in this subanalysis. Of these, 505 patients were aged ≥85 years. Distribution of demographic and clinical features according to age is shown in Table 1. Compared with younger patients, those aged ≥85 years had a lower prevalence of body mass index >30 kg/m2 and a higher prevalence of female sex, systemic hypertension, congestive heart failure, previous stroke/TIA/systemic embolism, vascular disease, chronic renal failure, chronic obstructive pulmonary disease, and left atrial dilatation. In very elderly patients, the use of oral anticoagulation was lower than in those aged <85 years (78% versus 83%), whereas treatment with antiplatelet drugs only was more frequent (15% versus 11%). Of note, because of the time period in which PREFER in AF was performed, penetration of NOACs was scarce (6%) and similar in very elderly and younger patients. Treatment durations for oral anticoagulation (any type) were 3.8±4.5 years in patients aged <85 years and 4.7±4.8 years in those aged ≥85, and this difference was not statistically significant (P=0.054). Risk profiles of patients included and those excluded from the study because of the lack of data from both baseline and the 1‐year follow‐up visits were comparable (Table S1). In particular, very elderly patients included in our investigation had similar prevalence of CHA2DS2 –VASc descriptors, renal failure, and chronic obstructive pulmonary disease, as well as similar therapeutic patterns, than very elderly patients excluded; only prevalence of left atrial dilatation was lower in the latter.
Thromboembolic and Bleeding Outcomes in Very Elderly Patients
Incidence of thromboembolic and major bleeding events was first evaluated in the entire cohort of patients stratified by age strata, regardless of antithrombotic therapy. Mean follow‐up duration was 12±2 months. Among patients aged ≥85 years, incidence of stroke/TIA/systemic embolism was 4.8 per 100 patients/year (absolute numbers, 24 of 505 patients), significantly higher than in those aged <85 years (2.3 per 100 patients/year; 135 of 5907 patients; P=0.0006; Figure 1).When considering specific age strata, the rate of thromboembolic events in very elderly patients was higher compared with both patients aged <75 years (1.8 per 100 patients/year; 63 of 3454 patients; P=0.0001) and those between 75 and 84 years (3 per 100 patients/year; 72 of 2318 patients; P=0.047; Figure 1).
Figure 1.
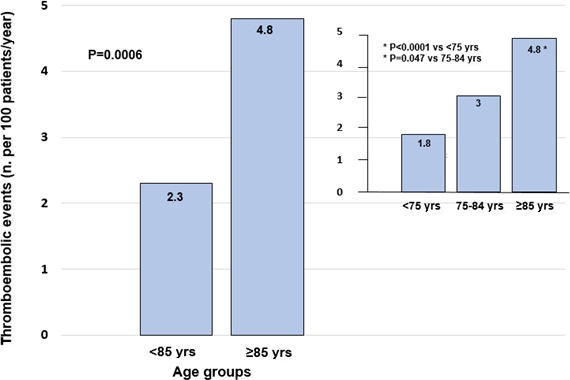
Incidence of thromboembolic events (stroke/TIA/systemic embolism) at 1 year in patients aged <85 and ≥85 years. Rates of thromboembolic events according to 3 age strata (<75, 75–84, and ≥85 years) are also depicted. TIA indicates transient ischemic attack.
Occurrence of major bleeding was 2.7 per 100 patients/year (absolute numbers, 161 of 5907 patients) in patients aged <85 years versus 4 per 100 patients/year (20 of 505 patients) in the very elderly population (P=0.11; Figure 2). Of note, this rate of major bleeding in very elderly patients was higher than in the subgroup aged <75 years (1.9 per 100 patients/year; 67 of 3450 patients; P=0.003), but similar to those between 75 and 84 years (3.9 per 100 patients/year; 94 of 2296 patients; P=0.98; Figure 2). Three sites of major bleeding were distinguished in PREFER in AF: gastrointestinal, intracerebral, and “other site” major bleeding. A total of 181 patients had at least 1 major bleeding event and 7 had major bleeding at different sites: 51% of major bleeding was gastrointestinal, 9% intracerebral, and 43% at other sites.
Figure 2.
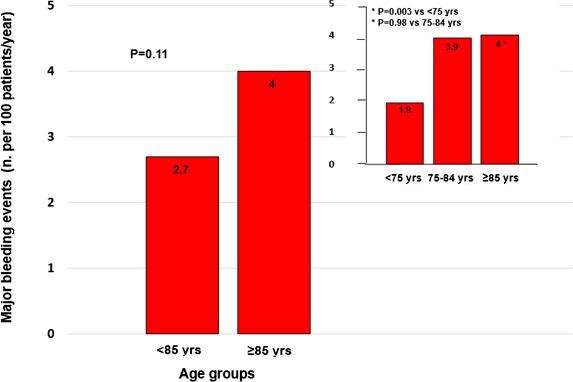
Incidence of major bleeding at 1 year in patients aged <85 and ≥85 years. Rates of major bleeding according to 3 age strata (<75, 75–84, and ≥85 years) are also depicted.
Incidence of Events According to Age and Anticoagulation Status
Because of their age, very elderly subjects have, by definition, a CHA2DS2‐VASc score ≥2. Incidence of thromboembolic and major bleeding events in patients aged ≥85 years versus younger those according to different antithrombotic strategies was evaluated in the cohort of 5058 patients with CHA2DS2‐VASc score ≥2: 4346 of these subjects were on OAC (vitamin K antagonists or NOACs) and 712 did not receive any anticoagulation (ie, were treated with antiplatelet only or no antithrombotic drug). Among very elderly patients (N=474), those 362 who were receiving anticoagulant treatment were younger (87.2±2.4 versus 87.9±2.5; P=0.008) and had a lower HAS‐BLED score (2.4±1.0 versus 2.7±0.9; P=0.021) than those without anticoagulation.
Adjusted ORs for stroke/TIA/systemic embolism, favoring OAC versus no anticoagulant therapy, were 0.74 (CI, 0.43, 1.25; P=0.26) in patients aged <85 years and 0.64 in those aged ≥85 years (CI, 0.24, 1.69; P=0.37). Of note, incidence of thromboembolic events in patients aged <85 years decreased from 2.8%/year without oral anticoagulation to 2.3%/year with anticoagulation (0.5% absolute reduction) and in those aged ≥85 years from 6.3%/year without anticoagulation to 4.3%/year with anticoagulation (2% absolute reduction; Figure 3A and Table 2). Such absolute difference was even more pronounced in the exploratory analysis performed on extremely elderly patients (aged ≥90 years; N=84), in whom the event rate was 11.5 per 100 patients/year without anticoagulation and 6.9 per 100 patients/year with anticoagulation (absolute percent difference 4.6%; OR, 0.57; CI 0.12, 2.74; P=0.48; Table 2).
Figure 3.
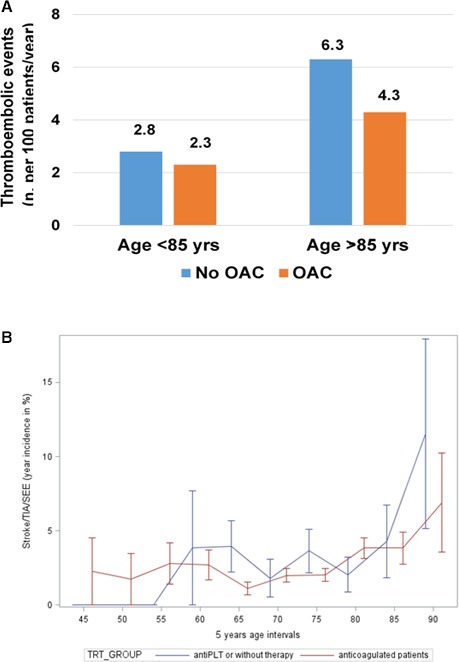
A, Incidence of thromboembolic events (stroke/TIA/SEE) in patients aged <85 and ≥85 years receiving OAC or no OAC (antiplatelet therapy only or no antithrombotic drug). B, Risk of thromboembolic events by 5‐year intervals of age increase in patients receiving OAC vs no OAC. antiPLT indicates antiplatelet; OAC, oral anticoagulant therapy; SEE, systemic embolic event; TIA, transient ischemic attack; TRT, .
Table 2.
Incidence of Events According to Age and Anticoagulation Status
| Event | Age <85 Y | Age ≥85 Y | Age ≥90 Y | |||
|---|---|---|---|---|---|---|
| No OAC (N=616) | OAC (N=3975) | No OAC (N=96) | OAC (N=371) | No OAC (N=26) | OAC (N=58) | |
| Thromboembolic events | 17 (2.8) | 91 (2.3) | 6 (6.3) | 16 (4.3) | 3 (11.5) | 4 (6.9) |
| Major bleeding | 21 (3.4) | 114 (2.9) | 4 (4.2) | 15 (4.0) | 2 (7.7) | 5 (8.6) |
Values are given as n (%). OAC indicates oral anticoagulant therapy.
Figure 3B indicates the risk of thromboembolic events by 5‐year intervals of age increase in patients receiving or not receiving OAC. The incremental risk of stroke/TIA/systemic embolism per 1 year of age increase among patients aged ≥85 years was ≈3‐fold lower in those on anticoagulant treatment versus those without anticoagulation: 0.43% (CI, −0.60%, −1.08%) versus 1.23% (CI, −0.58%, −3.03%; P=0.23).
Occurrence of major bleeding in patients not receiving anticoagulation (ie, being on antiplatelet treatment or without antithrombotic drugs) versus those receiving anticoagulant therapy was not significantly different either in the subgroup aged <85 years (3.4 versus 2.9 per 100 patients/year; P=0.74) and in the subgroup aged ≥85 years (4.2 versus 4.0 per 100 patients/year; P=0.77; adjusted OR, 0.89, 0.57, 1.34; P=0.59; Figure 4A and Table 2). This was confirmed in the exploratory analysis on the subset aged ≥90 years, in which major bleeding rates were 7.7 versus 8.6 per 100 patients/year (P=0.89; Table 2). In very elderly patients on oral anticoagulation, incidence of major bleeding was similar to the incidence in those receiving antiplatelet agents (4.1 per 100 patients/year [16 of 377 patients] versus 3.9 per 100 patients/year [3 of 74 patients]; adjusted OR, 1.05; 95% CI, 0.30, 3.68; P=0.75) and higher than in patients without antithrombotic therapy (4.1 per 100 patients/year [16 of 377 patients] versus 2.8 per 100 patients/year [1 of 34 patients]).
Figure 4.
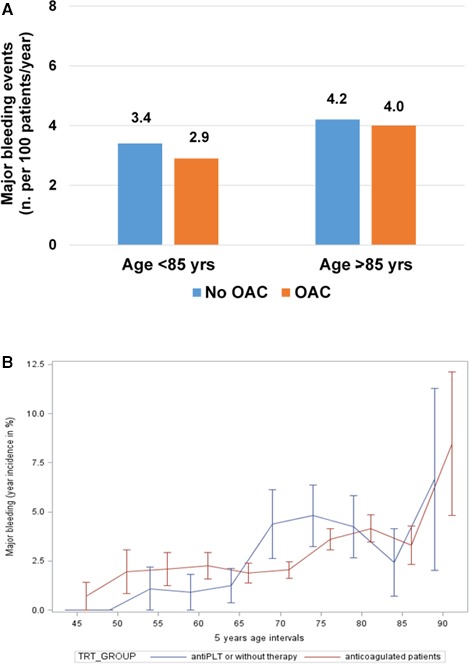
A, Incidence of major bleeding in patients aged <85 and ≥85 years receiving OAC or no OAC (antiplatelet therapy only or no antithrombotic drug). B, Risk of thromboembolic events by 5‐year intervals of age increase in patients receiving OAC vs no OAC. antiPLT indicates antiplatelet therapy; OAC, oral anticoagulant therapy; TRT, .
We also performed a separate analysis on the bleeding risk in patients receiving the combination of anticoagulant and antiplatelet therapy. Among patients on anticoagulant plus antiplatelet treatment and a CHA2DS2‐VASc score ≥2, those aged ≥85 years had the highest incidence of major bleeding: 6.4 per 100 patients/year (3 of 44 patients) versus 4.5 per 100 patients/year (27 of 571 patients) in those aged <85 years. However, the relative increase of major bleeding in patients on anticoagulant plus antiplatelet therapy versus those receiving an anticoagulant alone or an antiplatelet agent alone was similar in very elderly and younger patients (OR, 1.73; 95% CI, 0.49, 6.19; P=0.38 and OR, 1.83; 95% CI, 1.20, 2.80; P=0.025, respectively).
Figure 4B shows the risk of major bleeding by 5‐year intervals of age in patients receiving or not receiving oral anticoagulant treatment. The incremental risk of major bleeding per 1 year of age increase among patients aged ≥85 years was 0.74% in those with anticoagulation (CI, −0.08%, 1.56%; P=0.08) versus 1.95% in those without (CI, 0.34%, 3.55%; P=0.018).
Net Clinical Benefit of Anticoagulant Therapy
We then investigated the weighted net clinical benefit of anticoagulant therapy compared to no anticoagulant treatment. In this combined analysis of weighted ischemic and hemorrhagic events, the lower the relative number of oral anticoagulants versus no oral anticoagulants, the greater the benefit of the former versus the latter. In the overall population, this benefit was significant in favor of oral anticoagulants: the net clinical benefit, analyzed with the methodology described, of oral anticoagulant therapy versus no anticoagulant therapy was −2.19% (CI, −4.23%, −0.15%; P=0.036), favoring oral anticoagulant therapy. These results were consistent across age strata (Figure 5), with the point estimate of the net clinical benefit being more favorable in patients aged ≥85 years (−2.78%; CI, −9.13, 3.58) and ≥90 years (−8.02%; CI, −25.62, 9.59) compared with those aged <85 years (−1.92%; CI, −4.09, 0.24), although with wider CIs. We also performed an analysis on the net clinical benefit without MI as outcome measure, essentially showing similar results across different age strata (Figure S1).
Figure 5.
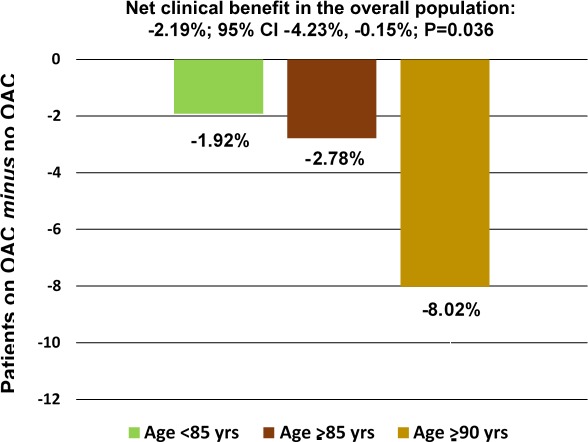
Net clinical benefit, adjusted for the mortality risk, of OAC vs no OAC (antiplatelet therapy only or no antithrombotic drug) according to different age strata, including ischemic stroke, systemic embolism, myocardial infarction, hemorrhagic stroke, and major bleeding as outcome measures. OAC indicates oral anticoagulant therapy.
Discussion
In this analysis of individual patients’ data from the real‐world, prospective PREFER in AF, we have found that in very elderly patients (aged ≥85 years): (1) The benefit of anticoagulant treatment over no anticoagulation for prevention of thromboembolic events is largely maintained, with anticoagulation being associated with the greatest absolute reduction of those complications; and (2) in the subgroup on anticoagulant therapy, the bleeding risk was not higher than that of patients receiving antiplatelet treatment. We have also found that the older the patients with AF, the higher was the weighted net clinical benefit in favor of anticoagulant therapy.
Previous data from the ENGAGE AF trial on warfarin‐treated patients indicated a 2‐ and 3‐fold elevation of thromboembolic events and major hemorrhagic complications, respectively, when comparing patients aged ≥75 years to those <65 years.19 This was also confirmed in the subanalyses on elderly patients from other phase III trials comparing NOACs versus warfarin in patients with nonvalvular AF20, 21, 22 and in a recent cohort study on patients at a service center receiving vitamin K antagonists.23 In the present investigation, we report on an evaluation of thromboembolic and bleeding outcomes of even older populations in the setting of AF. Our real‐world findings expand previous observations, showing that regardless of antithrombotic therapies in patients aged ≥85 years, overall risk of stroke/TIA/systemic embolism is significantly higher compared with any strata of younger age, that is, a 2.6‐fold elevation versus patients aged <75 years and a 1.6‐fold elevation versus those aged 75 to 84 years; conversely, the overall bleeding risk was higher than for patients with age <75 years, but similar to those between 75 and 84 years. Of note, in the very elderly population, there was an 0.8% excess absolute risk of thromboembolic events at 1 year compared with major bleeding events. Accordingly, the major concern in this setting of patients appears to be the thromboembolic risk rather than the propensity of bleeding related to anticoagulant treatment.
Advancing age also entails various challenges in antithrombotic therapy, that is, the presence of comorbidities further elevating the risk of thromboembolic and bleeding complications, a propensity to fall, cognitive impairment, impaired compliance, low body weight, and reduced renal function, with decreased drugs clearance, all making antithrombotic management more complex. Increasing age predisposes to both thromboembolic and bleeding events; therefore, balancing the risk and the benefit of different antithrombotic strategies in older populations is even more relevant. In particular, the concern of bleeding often leads, in clinical practice, to the underutilization of chronic anticoagulant therapy in older patients with AF, attributed to the perception that the hazards outweigh the benefits24; to this regard, each increasing decade has been previously associated with a 14% decrease in warfarin utilization.25 Results of our contemporary registry show that use of oral anticoagulant treatment in very elderly patients was still high (78%). This is consistent with other recent observational studies showing, in older AF populations, an improved penetration of chronic anticoagulant therapy.26, 27 A different subanalysis from PREFER in AF was not focused on outcome results, but on the prevalence of different antithrombotic strategies in octagenarians28; it showed that factors associated with oral anticoagulant utilization were previous ischemic stroke and heart failure, whereas higher age, previous bleeding, paroxysmal AF, chronic hepatic disease, lower autonomy, and problems for self‐care were associated with the nonuse of anticoagulant therapies. Previous studies have compared outcome of anticoagulant versus antiplatelet treatment in AF patients aged ≥75 years; in particular, the randomized BAFTA trial,5 performed in the setting of primary care, demonstrated that, compared with aspirin 75 mg daily, use of warfarin with a target international normalized ratio (INR) of 2 to 3 led to a significant 52% relative reduction of the composite primary end point, including stroke, systemic embolism, and intracranial hemorrhage. Similar findings were obtained in a prospective, observational investigation on patients aged ≥70 years,29 in the subgroup of patients aged ≥75 years from the SPAF (Stroke Prevention in Atrial Fibrillation) II study.30
Another cohort study24 described the prevalence of various treatment strategies (no antithrombotic therapy, antiplatelet treatment, or oral anticoagulant therapy) in AF patients aged ≥85 years, but no specific, adjusted correlation between different types of treatment and clinical outcome was done. Thus, we performed such evaluation in the very elderly population of PREFER in AF. We have observed that in patients aged ≥85 years, the use of anticoagulant therapy was associated with 36% risk reduction of thromboembolic events compared with no antithrombotic treatment or antiplatelet therapy. Of note, the reduction was similar (43%) in the subset of extremely elderly patients aged ≥90 years. Owing to the higher baseline risk profile of very elderly patients, those changes here translated into more‐pronounced absolute event reductions than in younger patients (number needed to treat for 1 year=50). Furthermore, in very elderly patients, oral anticoagulation significantly attenuated the incidence of thromboembolic complications per 1 year of age increase.
The bleeding risk related to anticoagulant therapy in older populations with AF is controversial and debated. In BAFTA,5 occurrence of major bleeding complications in the warfarin and aspirin arms was similar, but the study may have been underpowered for the evaluation of safety outcome measures and the high percentage of crossovers in patients randomized to warfarin (one third of patients) might have led here to an underestimation of the bleeding risk. Conversely, a subgroup analysis on patients aged ≥75 years from 6 randomized, clinical trials7 indicated a doubling of rates of major hemorrhages during the follow‐up in patients on warfarin compared with those on aspirin. However, 3 of those 6 trials had a higher upper limit of target INR (4.0–4.5 instead of 3), and this may have increased the propensity to bleeding in the warfarin arm. In our study, in patients aged ≥85 years, the incidence of major bleeding complications in patients on anticoagulant therapy was not different from that in patients treated with antiplatelet agents. As expected, very elderly patients not receiving anticoagulation were older and had a higher bleeding risk profile than those on anticoagulation; nevertheless, the lack of increase of major hemorrhages in the latter was maintained after adjustment for those potential confounders. Therefore, our results may mitigate concerns on the bleeding risk related to use of anticoagulant instead of antiplatelet therapy in very elderly patients with AF. Of note, previous randomized data indicated that antiplatelet treatment was associated with higher rates of adverse events than anticoagulant therapy in octogenarians with AF.31
The analysis on the net clinical benefit, in which both ischemic and bleeding events are included, appears crucial to carefully evaluate the benefit/risk ratio of any antithrombotic treatment, especially when such analysis can be obtained from prospective, real‐world investigations. A previous meta‐analysis on retrospective and prospective cohort studies had investigated the net clinical benefit of warfarin versus no warfarin use in patients with AF.32 However, only intracranial hemorrhage (and not extracranial bleeding) was counted in the net benefit, and thromboembolic events were not weighted for the mortality risk. Conversely, we have here evaluated the net clinical benefit, including both intra‐ and extracranial bleeding, and adjusting the incidence of both ischemic and bleeding complications by a mortality‐weighting factor, specific for each type of event.16 In the overall population, we have found a significant weighted net clinical benefit of OAC compared with antiplatelet or no antithrombotic treatment. Importantly, a gradient in this benefit according to classes of age increase was present, with the oldest patients getting the highest advantage.
We acknowledge limitations in our analyses. Bias in patients’ selection and residual confounding cannot be excluded, and we had no data on INR control during follow‐up in warfarin‐treated patients and on compliance to antithrombotic therapy. However, INR control in the PREFER in AF was assessed by collecting the last 3 INRs preceding enrollment, and these determinations are a reliable proxy for INR by the Rosendaal method; of note, an adequate INR control (ie, at least 2 of 3 INR values in the therapeutic range, between 2 and 3) was obtained in 72% of the overall population. Furthermore, the risk profile of our population reflects that only cardiology institutions participated in the registry, and very frail patients (ie, residents in nursing homes with multiple comorbidities and major functional disabilities) were excluded. Finally, given the very low prevalence of NOAC use, we could not compare the clinical outcome in patients receiving NOACs versus warfarin among the very elderly population. Indeed, in phase III randomized trials, there was no interaction between age and clinical benefit of NOACs compared to warfarin, but given their higher risk profile, the older populations generally achieved the greater absolute benefits with the use of NOACs.33
In conclusion, our study supports the use of anticoagulant therapy also in very elderly patients with AF. Because stroke risk dramatically increases with age, the absolute benefit of oral anticoagulation is highest in very elderly populations and this largely outweighs the bleeding risk, achieving the most favorable net clinical benefit as patients get older. Logical considerations and evidence‐base data make NOACs the anticoagulant drugs of choice in such patients.
Sources of Funding
This analysis of PREFER in AF was initiated by the Thrombosis Exchange Meeting in AF, TEAM in AF, funded and sponsored by Daiichi‐Sankyo Europe.
Disclosures
PREFER in AF is supported and coordinated by Daiichi Sankyo Europe. Patti is a speaker/consultant/on the advisory board for Bayer, Boehringer Ingelheim, BMS/Pfizer, Daiichi Sankyo, AstraZeneca, Sigma‐Tau, Malesci, and MSD. Lucerna is currently an employee of Daiichi Sankyo Europe. Siller‐Matula is a speaker/consultant/on the advisory board for Bayer, Daiichi Sankyo, Eli Lilly, and AstraZeneca and has received research funding from Roche. Kirchhof receives research support from the British Heart Foundation, the European Union, Leducq Foundation, and the German Centre for Heart Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies. F. Syeda, L. Fabritz, and P. Kirchhof are listed as inventors on a patent (WO2015/140571) held by the University of Birmingham on genotype‐specific antiarrhythmic drug therapy of AF. De Caterina reports fees, honoraria, and research funding from Sanofi‐Aventis, Boehringer Ingelheim, Bayer, BMS/Pfizer, Daiichi‐Sankyo, Novartis, and MSD. The remaining authors have no disclosures to report.
Supporting information
Table S1. Distribution of Demographic and Clinical Characteristics According to Age Groups in Patients Included Versus Those Excluded From the Study
Figure S1. Net clinical benefit, adjusted for the mortality risk, of OAC vs no OAC (antiplatelet therapy only or no antithrombotic drug) according to different age strata, including ischemic stroke, systemic embolism, hemorrhagic stroke, and major bleeding (without myocardial infarction) as outcome measures. OAC indicates oral anticoagulant therapy.
(J Am Heart Assoc. 2017;6:e005657 DOI:10.1161/JAHA.117.005657.)28736385
Contributor Information
Giuseppe Patti, Email: g.patti@unicampus.it.
Raffaele De Caterina, Email: rdecater@unich.it.
References
- 1. National Population Projections 2010‐Based Statistical Bulletin. London: Office for National Statistics; 2011. Available at: http://www.ons.gov.uk/ons/dcp171778_235886.pdf. Accessed November 1, 2016. [Google Scholar]
- 2. Andreotti F, Rocca B, Husted S, Ajjan RA, ten Berg J, Cattaneo M, Collet JP, De Caterina R, Fox KAA, Halvorsen S, Huber K, Hylek EM, Lip GYH, Montalescot G, Morais J, Patrono C, Verheugt FWA, Wallentin L, Weiss TW, Storey RF; on behalf of the ESC Thrombosis Working Group . Antithrombotic therapy in the elderly: expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J. 2015;36:3238–3249. [DOI] [PubMed] [Google Scholar]
- 3. Marinigh R, Lip GYH, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients. J Am Coll Cardiol. 2010;56:827–837. [DOI] [PubMed] [Google Scholar]
- 4. Edholm K, Ragle N, Rondina MT. Anti‐thrombotic management of atrial fibrillation in the elderly. Med Clin North Am. 2015;99:417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mant J, Hobbs R, Fletcher K, Roalfe A, Fitzmaurice D, Lip GYH, Murray E. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged study, BAFTA): a randomized controlled trial. Lancet. 2007;370:493–503. [DOI] [PubMed] [Google Scholar]
- 6. Ng KH, Shestakovska O, Connolly SJ, Eikelboom JW, Avezum A, Diaz R, Lanas F, Yusuf S, Hart RG. Efficacy and safety of apixaban compared with aspirin in the elderly: a subgroup analysis from the AVERROES trial. Age Ageing. 2016;45:77–83. [DOI] [PubMed] [Google Scholar]
- 7. van Walraven C, Hart RG, Connolly SJ, Austin PC, Mant J, Hobbs R, Koudstaal PJ, Petersen P, Perez‐Gomes F, Knottnerus JA, Boode B, Ezekowitz MD, Singer DE. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410–1416. [DOI] [PubMed] [Google Scholar]
- 8. Higuchi S, Kabeya Y, Matsushita K, Taguchi H, Ishiguro H, Kohshoh H, Yoshino H. Barthel index as a predictor of 1‐year mortality in very elderly patients who underwent percutaneous coronary intervention for acute coronary syndrome: better activities of daily living, longer life. Clin Cardiol. 2016;39:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dynina O, Vakili BA, Slater JN, Dynina O, Vakili BA, Slater JN, Sherman W, Ravi KL, Green SJ, Sanborn TA, Brown DL. In‐hospital outcomes of contemporary percutaneous coronary interventions in the very elderly. Catheter Cardiovasc Interv. 2003;58:351–357. [DOI] [PubMed] [Google Scholar]
- 10. Mizuno M, Kajimoto K, Sato N, Yumino D, Minami Y, Murai K, Munakata R, Asai K, Keida T, Sakata Y, Hagiwara N, Takano T; ATTEND Investigators . Clinical profile, management, and mortality in very‐elderly patients hospitalized with acute decompensated heart failure: an analysis from the ATTEND registry. Eur J Intern Med. 2016;27:80–85. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events–European Registry in Atrial Fibrillation (PREFER in AF). Europace. 2014;16:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 13. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 15. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 16. Renda G, di Nicola M, De Caterina R. Net clinical benefit of non‐vitamin K antagonist oral anticoagulant versus warfarin in phase III atrial fibrillation trials. Am J Med. 2015;128:1007–1014. [DOI] [PubMed] [Google Scholar]
- 17. Eikelboom JW, Connolly SJ, Hart RG, Wallentin L, Reilly P, Oldgren J, Yang S, Yusuf S. Balancing the benefits and risks of 2 doses of dabigatran compared with warfarin in atrial fibrillation. J Am Coll Cardiol. 2013;62:900–908. [DOI] [PubMed] [Google Scholar]
- 18. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 19. Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG, Nordio F, Murphy SA, Kimura T, Jin J, Lanz H, Mercuri M, Braunwald E, Antman EM. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE‐TIMI 48 trial. J Am Heart Assoc. 2016;5:e003432 DOI: 10.1161/JAHA.116.003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener H‐C, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123:2363–2372. [DOI] [PubMed] [Google Scholar]
- 21. Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR, Breithardt G, Singer DE, Becker RC, Hacke W, Paolini JF, Nessel CC, Mahaffey KW, Califf RM, Fox KAA. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;130:138–146. [DOI] [PubMed] [Google Scholar]
- 22. Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D, Granger CB, Hanna M, Held C, Husted S, Hylek EM, Jansky P, Lopes RD, Ruzyllo W, Thomas L, Wallentin L. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2004;35:1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kooistra HA, Calf AH, Piersma‐Wichers M, Kluin‐Nelemans HC, Izaks GJ, Veeger NJ, Meijer K. Risk of bleeding and thrombosis in patients 70 years or older using vitamin K antagonists. JAMA Intern Med. 2016;176:1176–1183. [DOI] [PubMed] [Google Scholar]
- 24. Wolff A, Shantsila E, Lip GYH, Lane DA. Impact of advanced age on management and prognosis in atrial fibrillation: insights from a population‐based study in general practice. Age Ageing. 2015;44:874–878. [DOI] [PubMed] [Google Scholar]
- 25. Brophy MT, Snyder KE, Gaehde S, Ives C, Gagnon D, Fiore LD. Anticoagulant use for atrial fibrillation in the elderly. J Am Geriatr Soc. 2004;52:1151–1156. [DOI] [PubMed] [Google Scholar]
- 26. Lip GYH, Laroche C, Dan G, Santini M, Kalarus Z, Rasmussen LH, Ioachim PM, Tica O, Boriani G, Cimaglia P, Diemberger I, Hellum CF, Mortensen B, Maggioni AP. ‘Real‐world’ antithrombotic treatment in atrial fibrillation: the EORP‐AF pilot survey. Am J Med. 2014;127:519–529. [DOI] [PubMed] [Google Scholar]
- 27. Hanon O, Vidal JS, Pisica‐Donose G, Benattar‐Zibi L, Bertin P, Berrut G, Corruble E, Derumeaux G, Falissard B, Forette F, Pasquier F, Pinget M, Ourabah R, Becquemont L, Danchin N. Therapeutic management in ambulatory elderly patients with atrial fibrillation: the S.AGES cohort. J Nutr Health Aging. 2015;19:219–227. [DOI] [PubMed] [Google Scholar]
- 28. Hanon O, Vidal J, Le Heuzey J, Kirchhof P, de Caterina R, Schmitt J, Laeis P, Mannucci PM, Marcucci M. Oral anticoagulant use in octogenarian European patients with atrial fibrillation: a subanalysis of PREFER in AF. Int J Cardiol. 2017;232:98–104. [DOI] [PubMed] [Google Scholar]
- 29. Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38:156–162. [DOI] [PubMed] [Google Scholar]
- 30. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet. 1994;343:687–691. [PubMed] [Google Scholar]
- 31. Rash A, Downes T, Portner R, Yeo WW, Morgan N, Channer KS. A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO). Age Ageing. 2007;36:151–156. [DOI] [PubMed] [Google Scholar]
- 32. Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta‐analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distribution of Demographic and Clinical Characteristics According to Age Groups in Patients Included Versus Those Excluded From the Study
Figure S1. Net clinical benefit, adjusted for the mortality risk, of OAC vs no OAC (antiplatelet therapy only or no antithrombotic drug) according to different age strata, including ischemic stroke, systemic embolism, hemorrhagic stroke, and major bleeding (without myocardial infarction) as outcome measures. OAC indicates oral anticoagulant therapy.


