Abstract
Background
The aim of our study was to evaluate the relationship of pulse pressure (PP), a raw index of arterial stiffness, with noninvasively determined coronary flow reserve (CFR) and its components, in patients with angiographically normal epicardial coronary arteries.
Methods and Results
The study population included 398 patients without angiographic evidence of coronary stenosis, who underwent high‐dose dipyridamole stress echocardiography with transthoracic‐derived CFR evaluation on the left anterior descending artery. CFR was calculated as the ratio between high‐dose dipyridamole and resting coronary diastolic peak velocities. Patients were divided into 2 groups: the first group included the first and second PP tertiles (n=298, PP ≤60 mm Hg) and the second group included the highest PP tertile (n=100, PP >60 mm Hg). Mean blood pressure, systolic blood pressure (both P<0.0001), age (P<0.002), and left ventricular mass index (P=0.013) were higher in the highest PP tertile, which also showed higher resting coronary flow velocity (31.6±9.6 cm/s versus 27.7±6.4 cm/s, P<0.0001) and marginally lower CFR (2.5±0.6 versus 2.6±0.6, P=0.044). Hyperemic coronary flow velocity did not differ between the 2 groups. By separate multiple linear regression analyses, after adjusting for sex, age, the highest systolic blood pressure tertile (≥140 mm Hg), left ventricular mass index, and cardiovascular risk factors, the highest PP tertile was associated with resting coronary flow velocity (P=0.003) and only marginally with hyperemic coronary flow velocity (P<0.02), whereas its association with CFR was not significant.
Conclusions
In patients without epicardial coronary artery stenosis, the highest PP tertile is associated with an increased coronary flow velocity at rest.
Keywords: coronary flow reserve, coronary flow resting velocity, pulse pressure, stress echocardiography
Subject Categories: High Blood Pressure, Echocardiography, Remodeling
Introduction
Pulse pressure (PP) is a function of systolic and diastolic blood pressure (BP), is dependent on stroke volume and arterial wall elastic properties, and reflects the difficulty in distending conduit arteries.1, 2, 3 Increased PP exhibits a wide range of values, depending on increased systolic BP, decreased diastolic BP, or both. PP is a raw index of arterial stiffness, since loss of arterial elasticity demands greater force to accommodate blood flow, leading to increased systolic BP, increased cardiac workload, and consequent cardiac4 and vascular remodeling.5 Arterial wall stiffening affects peripheral small arteries and microcirculation.6, 7, 8, 9 In addition, remodeling of small arteries provokes elevated peripheral vascular resistances, which can also contribute to arterial stiffness.10, 11 As a consequence, arterial stiffness is known to be a predictor of adverse cardiovascular outcomes,12, 13 whereas PP could be considered as an independent marker of preclinical cardiovascular disease.14, 15
Coronary flow reserve (CFR) represents the maximal increase in coronary flow above the normal resting volume when coronary arteries are maximally dilated.16, 17, 18 CFR is the difference between the basal, autoregulated coronary flow and the maximal flow, at any given perfusion pressure,19, 20 but it is calculated as the ratio between hyperemic and resting coronary flow. To date, CFR can be easily assessed by transthoracic Doppler echocardiography by recording Doppler‐derived flow velocities with great feasibility on the mid‐distal left anterior descending artery (LAD).21 CFR of LAD has shown excellent compliance with invasive Doppler flow wire and optimal reproducibility.22, 23 Several conditions can be associated with decreased CFR, such as a significant epicardial coronary artery stenosis, but also aging, aortic valve stenosis, left ventricular (LV) hypertrophy, hypertrophic and dilated cardiomyopathy, and even isolated coronary microvascular dysfunction.20, 21, 22, 23 Although previous studies have shown an inverse relationship between arterial stiffness and CFR,24, 25 limited information is available on the relationships between PP and CFR, in particular in patients who have cardiovascular risk factors but are free of obstructive coronary artery disease.26, 27, 28 Accordingly, the aim of our study was to evaluate the relationships of PP with noninvasively determined CFR and its components, ie, resting and hyperemic coronary flow velocities, in patients without angiographic evidence of epicardial coronary artery stenosis.
Methods
Study Population
The study population included 398 patients with absence of angiographic evidence of any degree of stenosis in epicardial coronary arteries, recruited from the EPIC (Echo Persantine International Cooperative Study) data set collected in 4 Italian centers between August 2003 and July 2015. All patients underwent dipyridamole stress echo, which was performed before (within 15 days) coronary angiography. The indications for coronary angiography were chest pain not responsive to cardiac treatment (n=197), abnormal exercise electrocardiography (n=125), or abnormal myocardial perfusion scintigraphy (n=76). Patients with angiographic evidence of epicardial coronary artery stenosis, previous coronary revascularizations, wall motion abnormalities detected at rest or during stress, or suboptimal echocardiographic imaging during stress were excluded.
Diagnosis of arterial hypertension was established according to 2013 guidelines of the European Society of Hypertension/European Society of Cardiology.29 Diagnosis of diabetes mellitus was defined according to the guidelines of the American Diabetes Association.30 Hypercholesterolemia was defined according to the European guidelines on cardiovascular disease prevention in clinical practice.31 Cigarette smoking habit was established according to self‐report. Data of antihypertensive and/or anti‐ischemic treatments, if any, at the testing time were collected for each patient. Moreover, BP was measured in patients in the supine position by a cuff sphygmomanometer (average of 3 measurements) before the beginning of the echocardiographic examination and this measurement was chosen for calculating PP and obtaining an almost simultaneous evaluation of echocardiographic parameters and LV afterload. All patients signed an informed consent. The study was approved by the institutional ethics committee.
Procedures
Echocardiographic examinations at rest and during stress were performed with commercially available ultrasound machines (Sequoia C256 Acuson Siemens, Mountain View, CA; Sonos 5500‐7500 Philips Ultrasound, Andover, MA; Vivid System 7, GE, Horten, Norway) equipped with a miltifrequency phased‐array sector scan probe and harmonic capability. Echocardiographic examination at rest was used for LV quantitative analysis in agreement with 2015 American Society of Echocardiography and European Association of Cardiovascular Imaging recommendations.32 LV ejection fraction was calculated from 2‐dimensional echocardiographic‐derived LV end‐diastolic and end‐systolic volumes (from apical 4‐ and 2‐chamber views) measured by the modified Simpson rule. LV mass was calculated by 2‐dimensional–guided M‐mode echocardiography or from direct 2‐dimensional longitudinal long‐axis view and normalized for body surface area (g/m2). LV hypertrophy was defined as a LV mass index >115 g/m2 in men and >95 g/m2 in women. Regional wall motion was assessed according to the standard 17‐segment model as recommended by the American Society of Echocardiography and European Association of Cardiovascular Imaging recommendations33 at rest and at a high dose of dipyridamole. Regional wall motion was visually assessed for each segment individually, considering both endocardial excursion and systolic thickening, and each segment was graded according to the semiquantitative scoring system. Wall motion score index (WMSI) was calculated.
Transthoracic echocardiography allows the recording of flow velocities of the LAD with a high feasibility.22 Transthoracic echocardiography–derived CFR of the LAD has shown excellent concordance with invasive Doppler flow wire22 and optimal reproducibility.34 Of note, by using this methodology, we sample pulsed Doppler‐derived velocities in the distal LAD, which represent good correlates of coronary microvascular function (Figure 1). During high‐dose dipyridamole (up to 0.84 mg over 6 minutes), 12‐lead electrocardiographic monitoring and 2‐dimensional echocardiography were executed according to standardized protocols,33, 35 and BP and electrocardiography were recorded at 1‐minute intervals. Coronary flow in the mid‐distal LAD was examined in the low parasternal long‐axis cross section under the guidance of color Doppler flow mapping, and attention was taken to maintain a constant incident angle (<30°) between coronary flow and the Doppler beam during the overall exam duration.36 All examinations were digitally stored to make the off‐line review and measurement processes easier. Coronary flow velocity measurements were analyzed according to standardized procedures. Coronary flow velocities were measured at least twice both at rest and at hyperemic stress (corresponding to high‐dose dipyridamole, before aminophylline injection). For each measurement, 3 optimal patterns of diastolic peak velocities were measured and averaged. CFR was calculated as the ratio between hyperemic and resting diastolic peak flow velocities. Quality check of performances in the participating centers was performed, as previously reported.37 Intraobserver and interobserver reproducibility of Doppler‐derived measurements of both resting and hyperemic coronary flow velocities was <10%.38
Figure 1.
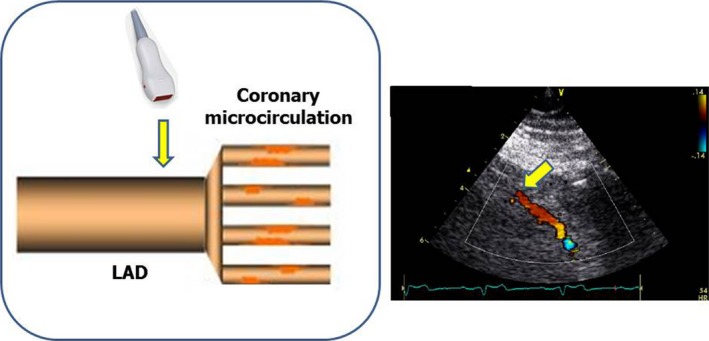
Illustration of the physiopathological meaning of coronary flow velocity in the distal left anterior descending artery (LAD) by transthoracic echocardiography: coronary flow reserve is the ratio between hyperemic and resting coronary flow velocities. In the absence of hemodynamically significant LAD stenosis, coronary flow reserve reflects the function of coronary microcirculation. In the presence of coronary microvascular dysfunction, resting coronary flow velocity of the distal LAD is increased. Left panel, schema illustrating that echo‐Doppler sampling of distal LAD is just upstream coronary microcirculation. Right panel, the real sampling of distal LAD by transthoracic Doppler echo.
Coronary angiography was obtained by multiple views according to the standard Judkins or Sones procedures. At least 2 orthogonal views for the right and at least 5 views (including 2 orthogonal views) for the left coronary arteries were analyzed. Additional appropriate views were acquired in case of side branches superimposition or foreshortening of a given segment.
Statistical Analysis
Statistical analysis was performed by SPSS version 12 (SPSS Inc, Chicago, IL). Data are presented as mean±SD. Descriptive statistical analyses were assessed by 1‐factor ANOVA and χ2 distribution with computation of exact P value by Monte Carlo method. The study population was divided into PP tertiles: the first PP tertile (PP ≤50 mm Hg, n=188), the second PP tertile (PP between 50 and ≤60 mm Hg, n=110), and the highest PP tertile (HPPT) (PP >60 mm Hg, n=100). Univariate correlates of a given variable were evaluated by least squares linear regression. Separate multiple linear regression analyses were performed to study correlates of CFR, resting and hyperemic coronary flow velocities after adjusting for confounders. Multicollinearity was also examined by computation of in‐model tolerance. The null hypothesis was rejected at 2‐tailed P<0.05.
Results
No significant difference was found in CFR, resting coronary flow velocity, and hyperemic flow velocity between the first and second tertiles. The HPPT showed a significantly higher resting coronary flow velocity in comparison with both the first and second tertiles (both P<0.0001, cumulative P<0.0001), while no significant difference was found for what concerns CFR and hyperemic coronary flow velocity (Figure 2). Accordingly, we decided to divide the study population into 2 groups: the merged lowest and middle tertiles were compared with the HPPT.
Figure 2.
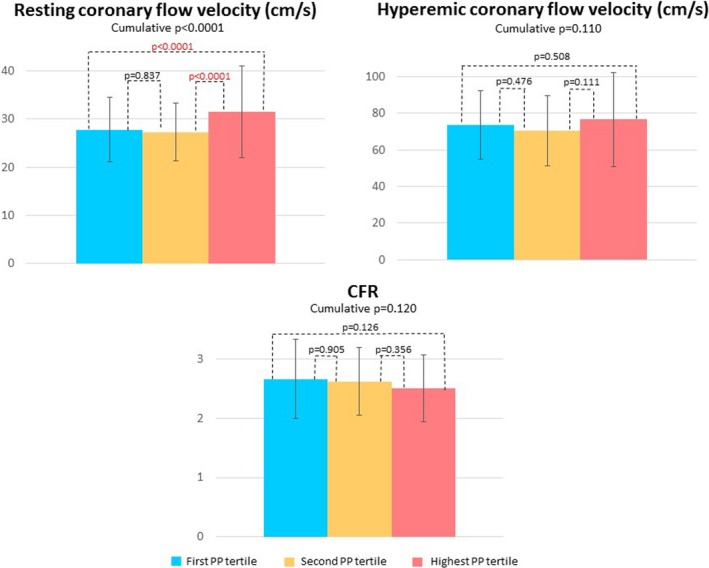
Graphs showing coronary flow reserve (CFR) and resting and hyperemic coronary flow velocity trends according to subdivision in pulse pressure (PP) tertiles: the first PP tertile (PP ≤50 mm Hg), the second PP tertile (50< PP ≤60 mm Hg) and the highest PP tertile (PP >60 mm Hg). Data are presented as mean value with SD bars and P value.
The clinical characteristics and echocardiographic data of the 2 study groups are presented in Table 1. The 2 groups were comparable for sex distribution, body mass index, diastolic BP, and heart rate. Mean BP and systolic BP (both P<0.0001) and age (P<0.002) were higher in the HPPT group. Among cardiovascular risk factors, only arterial hypertension was more prevalent in the HPPT group (P<0.0002). Antihypertensive drugs (angiotensin‐converting enzyme inhibitors, diuretics, β‐blockers, and calcium channel blockers) used were all long‐acting agents (data not in table).
Table 1.
Clinical and Echo‐Doppler Data of the Study Population
| First and Second PP Tertiles (n=298) | Highest PP Tertile (n=100) | P Value | |
|---|---|---|---|
| Male/female sex, No. | 148/150 | 38/62 | 0.06 |
| Age, y | 60.0±13.5 | 64.7±10.7 | <0.002 |
| Body mass index, kg/m2 | 26.3±3.5 | 26.8±4.1 | 0.235 |
| Systolic BP, mm Hg | 132.3±12.3 | 158.1±16.4 | <0.0001 |
| Diastolic BP, mm Hg | 81.9±10.2 | 81.8±12.4 | 0.892 |
| Mean BP, mm Hg | 98.7±10.2 | 107.2±13.2 | <0.0001 |
| Heart rate, beats per min | 69.1±8.2 | 68.6±10.1 | 0.622 |
| Arterial hypertension, No. (%) | 56.7 (169) | 78 (78) | <0.0002 |
| Diabetes mellitus, No. (%) | 20.5 (61) | 19 (19) | 0.863 |
| Hypercholesterolemia, No. (%) | 46.9 (140) | 53 (53) | 0.354 |
| Cigarette smoking, No. (%) | 26.8 (80) | 32 (32) | 0.388 |
| Cardiac therapy, No. (%) | 44.3 (132) | 54 (54) | 0.117 |
| LV ejection fraction, % | 60.4±5.9 | 61.7±6.0 | 0.060 |
| LV mass index, g/m2 | 109.7±23.5 | 118.5±24.5 | 0.013 |
| Rest WMSI | 1.01±0.06 | 1.01±0.03 | 0.393 |
| Peak WMSI | 1.01±0.06 | 1.00±0.01 | 0.238 |
| ΔWMSI | 0±0.01 | 0±0.03 | 0.403 |
| Resting coronary flow velocity, cm/s | 27.7±6.4 | 31.6±9.6 | <0.0001 |
| Hyperemic coronary flow velocity, cm/s | 72.5±19.0 | 76.7±25.8 | 0.087 |
| CFR | 2.6±0.6 | 2.5±0.6 | 0.044 |
Δ indicates difference between wall motion score index (WMSI) at rest and at a high dose of dipyridamole; BP, blood pressure; CFR, coronary flow reserve; LV, left ventricular; PP, pulse pressure.
HPPT patients had a higher LV mass index (P=0.013). LV hypertrophy was identified in 40% (40 of 100) of the patients in the HPTT group and in 39.6% of patients (118 of 298) in the other group (P=0.943). There were no significant differences between the 2 groups in LV ejection fraction, WMSI at rest, WMSI at the high dose of dipyridamole, and difference between WMSI at rest and at the high dose of dipyridamole. Coronary flow velocity at rest was higher (P<0.0001), while CFR was reduced (P=0.044) in the HPPT group, whereas coronary flow hyperemic velocity did not differ significantly between the 2 groups (Figure 3).
Figure 3.
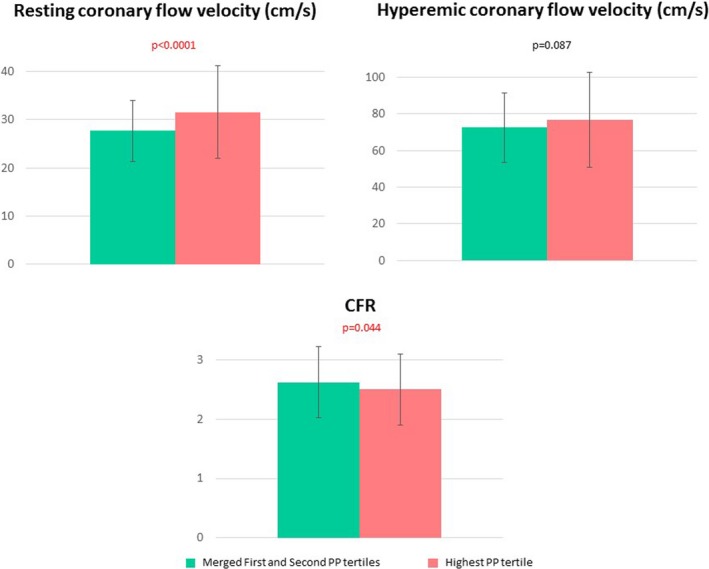
Graphs showing coronary flow reserve (CFR) and resting and hyperemic coronary flow velocities in the highest pulse pressure (PP) tertile compared with the merged first and second tertiles. Data are presented as mean value with SD bars and P value.
Univariate Relations and Multiple Linear Regression Analyses
In the pooled population, PP was significantly related to CFR (r=−0.14, P<0.01) and resting (r=0.20, P<0.0001) but not hyperemic coronary flow velocity (r=0.03, P=NS).
Separate multiple linear regression analyses were performed to identify the determinants of CFR, resting and hyperemic coronary flow velocities (Table 2). By these analyses, after adjusting for sex, age, the highest systolic BP tertile (≥140 mm Hg), HPPT, LV mass index, and presence of cardiovascular risk factors, CFR was associated with age (P<0.0001), LV mass index (P=0.013), and the highest systolic BP tertile (P<0.02). Considering the 2 CFR components, resting coronary flow velocity was associated with HPPT (P=0.003) and LV mass index (P<0.0001), whereas hyperemic coronary velocity was associated with diabetes mellitus status (P=0.022) and HPPT (P<0.02). Replacing the highest systolic BP tertile with the highest diastolic BP tertile (≥85 mm Hg), this parameter was shown to be associated with resting coronary flow velocity (standardized β coefficient=0.134, P=0.003) but not hyperemic coronary flow velocity (β=0.079, P=0.250) and CFR (β=−0.015, P=0.809) (data not in table).
Table 2.
Multiple Linear Regression Analyses
| Dependent Variable | Correlate | Standardized β Coefficient | P Value |
|---|---|---|---|
| Resting coronary flow velocity, cm/s | Female sex | 0.101 | 0.08 |
| Age, y | 0.086 | 0.213 | |
| Highest systolic BP tertile, mm Hg | 0.040 | 0.554 | |
| Highest PP tertile, mm Hg | 0.190 | 0.003 | |
| LV mass index, g/m2 | 0.259 | <0.0001 | |
| Arterial hypertension | −0.042 | 0.533 | |
| Diabetes mellitus | −0.090 | 0.130 | |
| Hypercholesterolemia | 0.038 | 0.543 | |
| Smoking | −0.026 | 0.666 | |
| Cumulative R 2=0.186, SEE=6.24 cm/s, P<0.0001 | |||
| Hyperemic coronary flow velocity, cm/s | Female sex | 0.016 | 0.103 |
| Age, y | −0.121 | 0.102 | |
| Highest systolic BP tertile, mm Hg | −0.107 | 0.134 | |
| Highest PP tertile, mm Hg | 0.159 | <0.02 | |
| LV mass index, g/m2 | −0.126 | 0.060 | |
| Arterial hypertension | −0.061 | 0.393 | |
| Diabetes mellitus | −0.145 | 0.022 | |
| Hypercholesterolemia | −0.035 | 0.600 | |
| Smoking | −0.048 | 0.441 | |
| Cumulative R 2=0.087, SEE=19.1 cm/s, P=0.007 | |||
| CFR | Female sex | −0.024 | 0.692 |
| Age, y | −0.284 | <0.0001 | |
| Highest systolic BP tertile, mm Hg | −0.153 | <0.02 | |
| Highest PP tertile, mm Hg | 0.010 | 0.867 | |
| LV mass index, g/m2 | −0.150 | 0.013 | |
| Arterial hypertension | −0.001 | 0.988 | |
| Diabetes mellitus | −0.073 | 0.209 | |
| Hypercholesterolemia | −0.078 | 0.202 | |
| Smoking | −0.040 | 0.490 | |
| Cumulative R 2=0.226, SEE=0.53, P<0.0001 | |||
BP indicates blood pressure; CFR, coronary flow reserve; LV, left ventricular; PP, pulse pressure.
Discussion
Our multicenter study demonstrates that in a large population sample of patients free of epicardial coronary artery stenosis but with a high prevalence of cardiovascular risk factors, hyperemic coronary flow and CFR are only marginally reduced in HPPT, which conversely exerts its influence on resting coronary flow velocity. Moreover, HPPT and resting coronary flow velocity are associated after adjusting for confounders.
In the absence of obstructive stenosis of epicardial coronary arteries, the reduction of transthoracic Doppler‐derived CFR can be ascribed to an isolated dysfunction of coronary microcirculation.39, 40 Under these circumstances, the detrimental effect of cardiovascular risk factors including arterial hypertension is recognized.23, 41, 42 It is also of interest that CFR, being the ratio between hyperemic and resting coronary flow,39 can be pathologically reduced due to the increase of denominator (coronary blood flow at rest) or the decrease of nominator (hyperemic coronary blood flow). Coronary flow at rest represents coronary autoregulation, ie, the intrinsic tendency of the heart to maintain constant coronary blood flow despite changes in arterial perfusion pressure.43 Obviously, a truly reduced coronary flow reactivity corresponds to a reduction of hyperemic response but not to an increase of coronary flow at rest, the latter often observed under pressure‐overload conditions.39 In the present study, we observed the influence of PP on the 2 different CFR components, resting and hyperemic coronary flow velocities, in relation to the concurrence of other physiopathological determinants.
Increased PP is a raw but recognized marker of arterial stiffness in the clinical setting.10, 11, 14 Arterial stiffness, which is a natural consequence of aging, is accelerated in arterial hypertension and is primarily caused by reduced elasticity and excessive fibrosis, which involves both large and small arteries. In conduit arteries, vascular stiffening is associated with hemodynamic damage of peripheral tissues, while, in the resistance circulation, fibrosis and stiffening can alter endothelial function, leading to elevated vasomotor tone and vascular rarefaction and thus impairing tissues perfusion.5 This explains why our patients with PP >60 mm Hg, identified by HPPT, presented higher coronary flow velocity at rest and reduced CFR. It is worth noting that we investigated coronary flow velocities on distal LAD and upstream coronary microcirculation, therefore before the beginning of resistance coronary microvessels (Figure 1). Higher coronary flow velocity at rest could then be interpreted as a compensatory mechanism to overcome higher arterial stiffness, expressed by an elevated PP.
Previous studies have underlined the relationship between arterial stiffness or related indices with CFR and its components. In particular, increased fractional PP was found to be associated with impaired CFR and diastolic dysfunction in hypertensive patients with normal coronary arteries.26, 27 Ikonomidis et al28 observed an independent association between pulse wave velocity, another good surrogate of arterial stiffness,44 and CFR in never‐treated hypertensive patients. Our results extend these findings since the impact of PP was primarily exerted on increased coronary flow velocity at rest, whereas the hyperemic coronary flow response did not differ significantly in comparison with patients having PP ≤60 mm Hg.
Multivariate analyses provided further information by testing the associations of CFR and its 2 different components with several possible correlates such as sex, age, the highest systolic BP tertile (≥140 mm Hg), HPTT, LV mass, and cardiovascular risk factors.
CFR was not associated with HPPT but with age and LV mass index, confirming previous results.23, 45, 46, 47 CFR was also marginally associated with the highest systolic BP tertile, confirming the role of systolic hypertension on CFR impairment.46 CFR was not associated with sex, in agreement with a positron emission tomography–derived observation in a large sample of patients of both sexes with suspected coronary artery disease.48
Coronary flow velocity at rest was positively associated with HPPT, while the highest systolic BP tertile did not enter the model. The association between resting coronary flow velocity and HPPT, then, is not merely due to the elevated values of systolic BP, which is crucial for the characterization of PP but not the only determinant. The association with HPPT is largely justified by the influence of increased arterial stiffness on coronary flow at rest: more rigid the arterial wall, higher the workload required to keep an adequate coronary flow.5 Coronary flow velocity at rest was also associated with LV mass, which can increase the extravascular resistance of coronary microvessels by the compressive force of myocardial fibrosis.39, 45, 47, 49 Choudhury et al47 observed that in patients with hypertensive LV hypertrophy, CFR impairment, measured with positron emission tomography, was mainly due to an increased baseline myocardial blood flow, whereas the blunted response to dipyridamole did not achieve statistical significance. The association between coronary flow velocity at rest and female sex showed a trend, but did not achieve statistical significance. Of note, Kobayashi et al50 found a reduction of CFR in women, mainly due to elevated resting coronary flow.
Hyperemic coronary flow velocity was marginally associated with HPPT but not with the highest systolic BP tertile. Also, the negative impact of diabetes mellitus on hyperemic coronary flow was evident, as previously observed.41 Dipyridamole‐induced coronary hyperemia is endothelium independent but coronary blood flow increase may beget further flow‐induced vasodilation, which is endothelium dependent.39 In addition, the association of CFR and LV mass index in our study was mainly driven by the influence exerted by resting but not hyperemic velocity. This is a controversial aspect, as a detrimental effect of LV hypertrophy on hyperemic coronary flow has been shown in some39, 46 but not in other studies.47 The lack of effect of LV mass on hyperemic coronary flow in our study could have been at least partially influenced by the high prevalence of LV hypertrophy in both the HPPT and the control group (about 40%).
In subsequent models, by replacing the highest systolic BP tertile with the highest diastolic BP tertile (≥85 mm Hg), the latter was associated only with coronary flow velocity at rest. Coronary blood flow mostly occurs during diastole51 and, at rest, diastolic BP is a good surrogate of diastolic perfusion pressure.52, 53
Study Limitations
The main limitation of this study is the use of transthoracic echo–derived CFR, which has a recognized lower reproducibility than intracoronary methods but also has the advantage of being more well tolerated in relation to its noninvasive nature. Another limitation of the study can be considered the high prevalence of LV hypertrophy as well as cardiovascular risk factors. Our patients, despite having normal epicardial coronary arteries, all underwent coronary angiography because of angina symptoms or equivalents (positive inducible ischemia tests), thus representing a high‐risk group for development of cardiovascular events. A possible “center” effect could be considered as another limitation, as there was no central reading in this multicenter study. Standard echocardiography and CFR measurement were interpreted in the peripheral centers and entered directly into the data bank. This system allowed substantial sparing of human and technologic resources, but it was also the logical prerequisite for a large‐scale study, designed to represent the realistic performance of the test rather than the results of a single laboratory—or even a single person—working in a highly dedicated echo laboratory. Because the assessment of the echocardiograms was qualitative and subjective, variability in reading the echocardiograms might have modulated the results of individual centers,54 especially for some parameters (eg, LV mass, which can be assessed with accuracy only with quantitative, relatively elaborated assessment). However, all of our readers in individual centers had a long‐lasting experience in echocardiography, passed the quality control in stress echo reading as previously described,55 and had extensive experience on execution and interpretation of CFR through joint reading sessions.
Perspectives
Increased PP, as an expression of arterial stiffness, shows a main association with increased coronary flow velocity at rest. When interpreting CFR in selected populations, we should consider separately the behavior of its 2 different components: coronary flow at rest and after maximally induced vasodilation. Factors that interfere with resting coronary flow should be differentiated from those directly affecting the hyperemic flow (Figure 4). In fact, when there is a CFR impairment, it is important to evaluate whether this reduction is an expression of elevated resting flow velocity, a display of high pressure overload and elevated arterial stiffness, or an indication of reduced hyperemic flow, the latter remaining the only expression of impaired coronary reactivity. Moreover, these findings may have important clinical implications, as they can lead to the identification of patients at risk for development of cardiovascular remodeling and subsequent ischemic heart disease.
Figure 4.
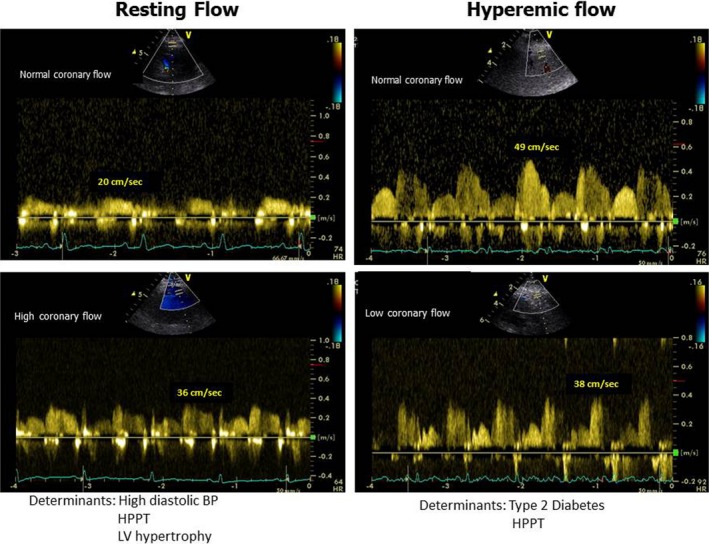
Schema depicting the main determinants of coronary flow at rest and after dipyridamole‐induced hyperemia in 4 different patients without obstructive coronary artery disease. Left panel, normal (upper) and abnormally high coronary flow velocity at rest. Right panel, normal (upper) and abnormally low hyperemic coronary flow velocity. BP indicates blood pressure; HPPT, highest pulse pressure tertile; LV, left ventricular.
Conclusions
Our study tried to identify a phenotype of patients at high risk for the development of coronary events. These patients could benefit from more aggressive management of cardiovascular risk factors, in particular, BP control and administration of drugs for cardiac prevention (eg, statins). Since the fibrogenic process is progressive and potentially reversible,5 the identification of patients at risk, also at a preclinical stage, and timely treatment might avoid the progression of cardiovascular impairment.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005710 DOI: 10.1161/JAHA.117.005710.)28663250
References
- 1. Girerd X, Laurent S, Pannier B, Asmar R, Safar M. Arterial distensibility and left ventricular hypertrophy in patients with sustained essential hypertension. Am Heart J. 1991;122:1210–1214. [DOI] [PubMed] [Google Scholar]
- 2. Safar ME. Pulse pressure in essential hypertension: clinical and therapeutic implications. J Hypertens. 1989;7:769–776. [DOI] [PubMed] [Google Scholar]
- 3. Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieere P, Guize L. Pulse pressure: a predictor of long‐term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. [DOI] [PubMed] [Google Scholar]
- 4. Roman M, Ganau A, Saba P, Pini R, Pickering T, Devereux R. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. [DOI] [PubMed] [Google Scholar]
- 5. Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. 2016;32:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. AlGhatrif M, Lakatta EG. The conundrum of arterial stiffness, elevated blood pressure, and aging. Curr Hypertens Rep. 2015;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snijder MB, Stronks K, Agyemang C, Busschers WB, Peters RJ, van den Born BJ. Ethnic differences in arterial stiffness the Helius study. Int J Cardiol. 2015;191:28–33. [DOI] [PubMed] [Google Scholar]
- 9. Safar M. Peripheral pulse pressure, large arteries, and microvessels. Hypertension. 2004;44:121–122. [DOI] [PubMed] [Google Scholar]
- 10. Nilsson PM, Boutouyrie P, Cunha P, Kotsis V, Narkiewicz K, Parati G, Rietzschel E, Scuteri A, Laurent S. Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens. 2013;31:1517–1526. [DOI] [PubMed] [Google Scholar]
- 11. Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–1021. [DOI] [PubMed] [Google Scholar]
- 12. Hansen TW, Staessen JA, Torp‐Pedersen C, Rasmussen S, Li Y, Dolan E, Thijs L, Wang JG, O'Brien E, Ibsen H, Jeppesen J. Ambulatory arterial stiffness index predicts stroke in a general population. J Hypertens. 2006;24:2247–2253. [DOI] [PubMed] [Google Scholar]
- 13. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 14. de Simone G, Roman MJ, Alderman MH, Galderisi M, de Divitiis O, Devereux RB. Is high pulse pressure a marker of preclinical cardiovascular disease? Hypertension. 2005;45:575–579. [DOI] [PubMed] [Google Scholar]
- 15. Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, Glynn RJ. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801–807. [DOI] [PubMed] [Google Scholar]
- 16. Hoffman JIE. Maximal coronary flow and the concept of coronary flow reserve. Circulation. 1984;70:153–159. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann JIE. Transmural myocardial perfusion. Prog Cardiovasc Dis. 1987;24:429–464. [DOI] [PubMed] [Google Scholar]
- 18. Blomster JI, Svedlund S, U Westergren H, Gan LM. Coronary flow reserve as a link between exercise capacity, cardiac systolic and diastolic function. Int J Cardiol. 2016;217:161–166. [DOI] [PubMed] [Google Scholar]
- 19. Gould LK, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87–94. [DOI] [PubMed] [Google Scholar]
- 20. Haraldsson I, Gan LM, Svedlund S, Wittfeldt A, Råmunddal T, Angerås O, Albertsson P, Matejka G, Omerovic E. Non‐invasive evaluation of coronary flow reserve with transthoracic Doppler echocardiography predicts the presence of significant stenosis in coronary arteries. Int J Cardiol. 2014;176:294–297. [DOI] [PubMed] [Google Scholar]
- 21. Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, Morioka S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation. 1998;97:1557–1562. [DOI] [PubMed] [Google Scholar]
- 22. Caiati C, Zedda N, Montaldo C, Bina A, Iliceto S. Contrast‐enhanced transthoracic second harmonic echo Doppler with adenosine: a noninvasive, rapid and effective method for coronary flow reserve assessment. J Am Coll Cardiol. 1999;34:122–130. [DOI] [PubMed] [Google Scholar]
- 23. Galderisi M, Rigo F, Gherardi S, Cortigiani L, Santoro C, Sicari R, Picano E. The impact of aging and atherosclerotic risk factors on transthoracic coronary flow reserve in subjects with normal coronary angiography. Cardiovasc Ultrasound. 2012;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saito M, Okayama H, Nishimura K, Ogimoto A, Ohtsuka T, Inoue K, Hiasa G, Sumimoto T, Higaki J. Possible link between large artery stiffness and coronary flow velocity reserve. Heart. 2008;94:e20. [DOI] [PubMed] [Google Scholar]
- 25. Gullu H, Erdogan D, Caliskan M, Tok D, Yildirim E, Ulus T, TuranSezgin A, Muderrisoglu H. Interrelationship between noninvasive predictors of atherosclerosis: transthoracic coronary flow reserve, flow‐mediated dilation, carotid intima‐media thickness, aortic stiffness, aortic distensibility, elastic modulus, and brachial artery diameter. Echocardiography. 2006;23:835–842. [DOI] [PubMed] [Google Scholar]
- 26. Mahfouz RA, Elawady W, Abdu M, Salem A. Associations of fractional pulse pressure to aortic stiffness and their impact on diastolic function and coronary flow reserve in asymptomatic diabetic patients with normal coronary angiography. Cardiol J. 2013;20:605–611. [DOI] [PubMed] [Google Scholar]
- 27. Mahfouz RA. Relation of coronary flow reserve and diastolic function to fractional pulse pressure in hypertensive patients. Echocardiography. 2013;30:1084–1090. [DOI] [PubMed] [Google Scholar]
- 28. Ikonomidis I, Lekakis J, Papadopoulos C, Triantafyllidi H, Paraskevaidis I, Georgoula G, Tzortzis S, Revela I, Kremastinos DT. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never‐treated patients with essential hypertension. Am J Hypertens. 2008;21:806–813. [DOI] [PubMed] [Google Scholar]
- 29. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck‐Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Rydén L, Sirenko Y, Stanton A, Struijker‐Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2221. [DOI] [PubMed] [Google Scholar]
- 30. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:s5–s10. [DOI] [PubMed] [Google Scholar]
- 31. Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28:2375–2414. [DOI] [PubMed] [Google Scholar]
- 32. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;15:233–270. [DOI] [PubMed] [Google Scholar]
- 33. Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. [DOI] [PubMed] [Google Scholar]
- 34. Cortigiani L, Rigo F, Galderisi M, Gherardi S, Bovenzi F, Picano E, Sicari R. Diagnostic and prognostic value of Doppler echocardiographic coronary flow reserve in the left anterior descending artery in hypertensive and normotensive patients. Heart. 2011;97:1758–1765. [DOI] [PubMed] [Google Scholar]
- 35. Dal Porto R, Faletra F, Picano E, Pirelli S, Moreo A, Varga A. Safety, feasibility, and diagnostic accuracy of accelerated high‐dose dipyridamole stress echocardiography. Am J Cardiol. 2001;87:520–524. [DOI] [PubMed] [Google Scholar]
- 36. Rigo F. Coronary flow reserve in stress‐echo lab. From pathophysiologic toy to diagnostic tool. Cardiovasc Ultrasound. 2005;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rigo F, Gherardi S, Galderisi M, Pratali L, Cortigiani L, Sicari R, Picano E. The prognostic impact of coronary flow‐reserve assessed by Doppler echocardiography in non‐ischaemic dilated cardiomyopathy. Eur Heart J. 2006;27:1319–1323. [DOI] [PubMed] [Google Scholar]
- 38. Rigo F, Sicari R, Gherardi S, Djordjevic‐Dikic A, Cortigiani L, Picano E. The additive prognostic value of wall motion abnormalities and coronary flow reserve during dipyridamole stress echo. Eur Heart J. 2008;29:79–88. [DOI] [PubMed] [Google Scholar]
- 39. Galderisi M, D'Errico A. Beta‐blockers and coronary flow reserve: the importance of a vasodilatory action. Drugs. 2008;68:579–590. [DOI] [PubMed] [Google Scholar]
- 40. Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- 41. Galderisi M, Capaldo B, Sidiropulos M, D'Errico A, Ferrara L, Turco A, Guarini P, Riccardi G, de Divitiis O. Determinants of reduction of coronary flow reserve in patients with type 2 diabetes mellitus or arterial hypertension without angiographically determined epicardial coronary stenosis. Am J Hypertens. 2007;20:1283–1290. [DOI] [PubMed] [Google Scholar]
- 42. Galderisi M, de Simone G, Cicala S, Parisi M, D'Errico A, Innelli P, de Divitiis M, Mondillo S, de Divitiis O. Coronary flow reserve in hypertensive patients with hypercholesterolemia and without coronary heart disease. Am J Hypertens. 2007;20:177–183. [DOI] [PubMed] [Google Scholar]
- 43. Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. [DOI] [PubMed] [Google Scholar]
- 44. Lekakis JP, Zakopoulos NA, Protogerou AD, Papaioannou TG, Kotsis VT, Pitiriga VCh, Tsitsirikos MD, Stamatelopoulos KS, Papamichael CM, Mavrikakis ME. Arterial stiffness assessed by pulse wave analysis in essential hypertension: relation to 24‐h blood pressure profile. Int J Cardiol. 2005;102:391–395. [DOI] [PubMed] [Google Scholar]
- 45. Schäfer S, Kelm M, Mingers S, Strauer BE. Left ventricular remodeling impairs coronary flow reserve in hypertensive patients. J Hypertens. 2002;20:1431–1437. [DOI] [PubMed] [Google Scholar]
- 46. Rimoldi O, Rosen SD, Camici PG. The blunting of coronary flow reserve in hypertension with left ventricular hypertrophy is transmural and correlates with systolic blood pressure. J Hypertens. 2014;32:2465–2471. [DOI] [PubMed] [Google Scholar]
- 47. Choudhury L, Rosen SD, Patel D, Nihoyannopoulos P, Camici P. Coronary vasodilator reserve in primary and secondary left ventricular hypertrophy. A study with positron emission tomography. Eur Heart J. 1997;18:108–116. [DOI] [PubMed] [Google Scholar]
- 48. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marcus ML, Mueller TM, Gascho JA, Kerber RE. Effects of cardiac hypertrophy secondary to hypertension on the coronary circulation. Am J Cardiol. 1979;44:1023–1028. [DOI] [PubMed] [Google Scholar]
- 50. Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, Lee DP, Stefanick M, Yeung AC, Tremmel JA. Effect of sex differences on invasive measures of coronary microvascular dysfunction in patients with angina in the absence of obstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Archie JP. Intramyocardial pressure: effect of preload on transmural distribution of systolic coronary blood flow. Am J Cardiol. 1975;35:904–911. [DOI] [PubMed] [Google Scholar]
- 52. Duncker DJ, Koller A, Merkus D, Canty JM Jr. Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis. 2015;57:409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schultz MG, Abhayaratna WP, Marwick TH, Sharman JE. Myocardial perfusion and the J curve association between diastolic blood pressure and mortality. Am J Hypertens. 2013;26:557–566. [DOI] [PubMed] [Google Scholar]
- 54. Hoffmann R, Lethen H, Marwick T, Arnese M, Fioretti P, Pingitore A, Picano E, Buck T, Erbel R, Flachskampf FA, Hanrath P. Analysis of inter‐institutional observer agreement in interpretation of dobutamine stress echocardiograms. J Am Coll Cardiol. 1996;27:330–336. [DOI] [PubMed] [Google Scholar]
- 55. Picano E, Landi P, Bolognese L, Chiarandà G, Chiarella F, Seveso G, Sclavo MG, Gandolfo N, Previtali M, Orlandini A; on behalf of the EPIC study group . Prognostic value of dipyridamole echocardiography early after uncomplicated myocardial infarction: a large scale multicenter trial. Am J Med. 1993;11:608–618. [DOI] [PubMed] [Google Scholar]


