Abstract
Background
Despite higher thromboembolism risk, women with atrial fibrillation have lower oral anticoagulation (OAC) use compared to men. The influence of the CHA 2 DS 2‐VASc score or the introduction of non–vitamin K OACs on this relationship is not known.
Methods and Results
Using the PINNACLE National Cardiovascular Data Registry from 2008 to 2014, we compared the association of sex with OAC use (warfarin or non–vitamin K OACs) overall and by CHA 2 DS 2‐VASc score and examined temporal trends in OAC use by sex. Multivariable regression models assessed the association between sex and OAC use in those with CHA 2 DS 2‐VASc scores ≥2. Temporal analyses assessed changes in OAC use by sex over time. Of the 691 906 atrial fibrillation patients, 48.5% were women. Women were significantly less likely than men to use any OAC overall (56.7% versus 61.3%; P<0.001) and at all levels of CHA 2 DS 2‐VASc score (adjusted risk ratio 9% to 33% lower, all P<0.001). Compared to other thromboembolic risk factors, female sex was associated with lower use of OAC (risk ratio 0.90, 95%CI 0.90‐0.91). Over time, non–vitamin K OAC use increased at a slightly higher rate in women (56.2% increase per year, 95%CI 54.6% to 57.9%) compared to men (53.6% increase per year, 95%CI 52.0% to 55.2%), yet women remained less likely to receive any OAC at all time points (P<0.001).
Conclusions
Among patients with atrial fibrillation, women were significantly less likely to receive OAC at all levels of the CHA 2 DS 2‐VASc score. Despite increasing non–vitamin K OAC use, women had persistently lower rates of OAC use compared to men over time.
Keywords: anticoagulants, atrial fibrillation, non‐vitamin K oral anticoagulants, sex differences, warfarin, women
Subject Categories: Women, Anticoagulants, Arrhythmias
Clinical Perspective
What Is New?
Women with nonvalvular atrial fibrillation are significantly less likely to receive oral anticoagulation (warfarin or non–vitamin K oral anticoagulation) compared to men at all levels of thromboembolic risk.
Non–vitamin K oral anticoagulation use has increased in women at a slightly faster pace than in men, yet women remained significantly less likely to receive any oral anticoagulation over time.
What Are the Clinical Implications?
A risk‐treatment paradox for women with atrial fibrillation exists, suggesting that those at increased thromboembolic risk are less likely than men to receive guideline‐concordant therapy.
Underrecognition of female sex as a thromboembolic risk factor does not fully explain these sex differences and suggest that clinical guidelines may be applied differently in women and men.
Interventions aimed at increasing appropriate oral anticoagulation use, particularly in women, are needed.
Introduction
Despite a higher risk of stroke, women with nonvalvular atrial fibrillation (AF) receive less oral anticoagulation (OAC) than men.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Possible explanations for decreased OAC use in women include underrecognition of their higher thromboembolic risk or concern for bleeding risk on warfarin in female patients.11, 12
Recent advancements in AF care may have addressed these concerns. First, the CHA2DS2‐VASc score for thromboembolic risk stratification incorporates female sex as an independent risk factor for thromboembolic events.13 Its incorporation into current AF guidelines may have increased provider awareness of higher thromboembolic risk in women and thus increased OAC use.14, 15 Second, the development of non–vitamin K oral anticoagulants (NOAC) has expanded treatment options for patients with AF. NOACs have a lower risk of major bleeding and equivalent stroke rates compared to warfarin.16, 17, 18, 19, 20 Therefore, it is possible that sex differences in OAC use have diminished with the introduction of NOACs.
To assess the impact of these advancements on OAC use in female AF patients, we examined the association of individual thromboembolic risk factors with OAC use in the National Cardiovascular Data Registry (NCDR®) PINNACLE Registry of outpatient cardiology practices. Rates of OAC use were evaluated by sex according to their CHA2DS2‐VASc scores, controlling for estimated bleeding risk. Finally, temporal trends in overall and individual OAC (warfarin and NOAC) use by sex were assessed. Understanding these relationships can determine the influence of the CHA2DS2‐VASc score and NOAC use on the sex gap in OAC provision and suggest future directions for improvement.
Methods
Data Source
The NCDR® PINNACLE Registry consists of consecutive patients from cardiology practices in the United States that voluntarily participate and submit data as part of a national office‐based cardiovascular quality improvement program.21, 22 Data are collected at the point of care using a validated electronic medical record‐mapping algorithm for patients with hypertension (HTN), coronary artery disease, congestive heart failure, and AF.23, 24 Registry data quality is maintained through data definitions, standard data collection and transmission, and periodic data quality checks.23, 24, 25
Study Population
Between May 1, 2008 and December 31, 2014, 848 931 patients with their first documented AF diagnosis within the registry were identified. Patients were excluded for missing data on sex (n=1439, 0.2%), reversible causes of AF (cardiac surgery, hyperthyroidism, pregnancy, pneumonia; n=1100, 0.1%), other indications for OAC (mechanical heart valve, valvular heart surgery, systemic embolization; n=6206, 0.7%), or documented contraindication to OAC (medical reasons or patient preference; n=24 893, 2.9%), as these would have impacted decisions to initiate OAC. Patients were excluded if they had a CHA2DS2‐VASc score ≤1 (n=123 387, 14.5%), leaving a final study cohort of 691 906 patients with high thromboembolic risk14, 15 (Figure 1).
Figure 1.
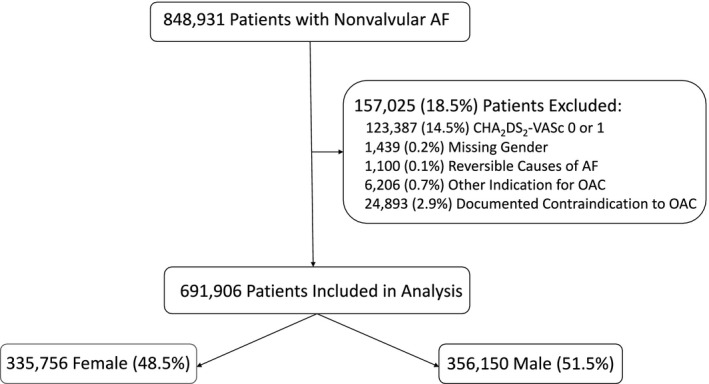
Study cohort. Reversible causes of AF include cardiac surgery, hyperthyroidism, pregnancy, and pneumonia. Other indications for OAC include mechanical heart valve or systemic embolization. Documented contraindications included both medical and patient preferences. AF indicates non‐valvular atrial fibrillation; OAC, oral anticoagulants.
Outcomes
The primary predictor variable for all analyses was patient sex. The primary outcome was prescription of any OAC defined as warfarin or NOAC (apixaban, dabigatran, and rivaroxaban) within 1 year of the patient's first encounter in the PINNACLE registry with a diagnosis of AF. Secondary outcomes of interest were use by OAC class: warfarin or NOACs. For patients with multiple OAC prescriptions within the first year of an AF diagnosis, the first prescribed OAC class was used.
Estimation of Thromboembolic Risk
According to practice guidelines, a CHA2DS2‐VASc score was calculated for each patient as the summation of his or her risk factor points.13 Risk factors receiving 1 point per factor included female sex, age 65 to 74 years, history of congestive heart failure, HTN, diabetes mellitus, or vascular disease. Risk factors receiving 2 points per factor included age ≥75 years or a history of prior transient ischemic attack or stroke.13 The variables included in the CHA2DS2‐VASc score were defined according to NCDR® PINNACLE data standards.13, 26 Congestive heart failure was defined as symptoms of heart failure or left ventricular ejection fraction <40%. Vascular disease was defined by the presence of any of the following: peripheral arterial disease, peripheral vascular disease, history of myocardial infarction, prior coronary artery bypass surgery, percutaneous coronary angioplasty, or percutaneous coronary intervention.13
Estimation of Bleeding Risk
Bleeding risk was estimated using the modified HAS‐BLED score (mHAS‐BLED).27 The mHAS‐BLED score is a total of the patient's bleeding risk factors including: HTN (diagnosis of hypertension, or at least 2 prior encounters with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg within 2 years), abnormal renal function (creatinine ≥2.3 mg/dL), previous stroke, major bleeding history (intracranial hemorrhage or nonintracranial major hemorrhage) or anemia, age ≥65 years, concomitant use of medications predisposing to bleeding (antiplatelets or nonsteroidal anti‐inflammatory drugs), and alcohol abuse history.
Statistical Analysis
Patient and practice level characteristics were compared between women and men using chi‐squared tests for categorical variables and Student t test for continuous variables. Continuous variables were summarized as median (interquartile range [IQR]) or mean±SD, whereas categorical variables were summarized as percentages and frequencies.
First, the associations of female sex and the other components of the CHA2DS2‐VASc score with OAC use were examined. Models were adjusted for individual components of the CHA2DS2‐VASc score (congestive heart failure, HTN, age, etc) and additional patient (race, insurance type [private versus nonprivate], mHAS‐BLED, and rhythm control therapy), provider (physician versus other provider), and clinic (total number of physicians at site and proportion of female patients at site) characteristics. Next, the fully adjusted models were stratified by CHA2DS2‐VASc strata (score=2, 3, 4, 5, and ≥6) with the individual components of the score removed as covariates to estimate adjusted risk ratios (RR) by sex within each CHA2DS2‐VASc stratum.
Because of the number of missing variables for estimating bleeding risk, we conducted a sensitivity analysis excluding the mHAS‐BLED estimate from the multivariable models. The CHA2DS2‐VASc was incorporated into the American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines toward the end of the study timeframe (2014), so we performed a sensitivity analysis stratifying by the CHADS2 score, which does not include female sex, vascular disease, or the age category 65 to 74 as risk factors.28, 29
To assess temporal trends in OAC use overall and by class in women and men, we used multivariable regression with calendar quarter as a categorical independent variable and the first quarter of 2010 as our referent group. The cohort was limited to patients who received an OAC prescription after 2010 when the Food and Drug Administration approved the first NOAC (dabigatran). In both women and men we multiplied the adjusted RR for each quarter by the observed OAC use for the reference quarter to obtain quarterly risk‐adjusted proportions of patients receiving OAC. A sex‐by‐quarter interaction term was included in the models to test whether the uptake in OAC use differed in women and men. To examine whether OAC use by sex changed significantly after guideline updates were published, we examined these relationships using the quarter prior to publication of the guidelines as the reference quarter (July to September 2010 for European Society of Cardiology and January to March 2014 for American Heart Association/American College of Cardiology) and compared rates of OAC use in the subsequent 4 quarters.
All models accounted for clustering of patients by provider and practice using Generalized Estimating Equations. To directly estimate RRs, we used the Zou method by specifying a Poisson distribution and including a robust variance estimate in our models.30, 31, 32, 33
All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). The Harvard Clinical Research Institute was the primary analytic center for this analysis.
Results
Baseline Characteristics in Women and Men
Our final study cohort included 691 906 patients with AF and indications for OAC of which 48.5% were women (Figure 1). Women were older, had lower body mass index, and had a lower prevalence of coronary artery disease and higher ejection fraction compared to men. Men had a higher prevalence of many of the CHA2DS2‐VASc risk factors (Table).
Table 1.
Baseline Characteristics by Sex
| Characteristic | Women (n=335 756) | Men (n=356 150) | P Value |
|---|---|---|---|
| Demographicsa | |||
| Agea, y | 75.4±11.0 | 73.9±10.2 | <0.001 |
| Race | <0.001 | ||
| White | 64.4% | 66.1% | |
| Black | 3.4% | 2.6% | |
| Asian | 0.6% | 0.6% | |
| American Indian/Alaskan Native | 0.3% | 0.4% | |
| Native Hawaiian/Pacific Islander | 0.1% | 0.1% | |
| Mixed | 0.2% | 0.2% | |
| Missing | 31.1% | 30.1% | |
| Insurance | <0.001 | ||
| Private | 45.1% | 47.6% | |
| Military | 2.0% | 2.3% | |
| Medicare | 60.3% | 59.0% | |
| Medicaid | 4.1% | 2.5% | |
| Other | 2.0% | 2.1% | |
| None | 5.0% | 5.4% | |
| Missing | 21.4% | 21.3% | |
| Clinical characteristicsa | |||
| CHA2DS2‐VASc, median (IQR) | 4.0 (3.0‐5.0) | 3.0 (2.0‐4.0) | <0.001 |
| CHA2DS2‐VASc Score | <0.001 | ||
| 2 | 11.6% | 27.5% | |
| 3 | 21.3% | 31.5% | |
| 4 | 31.3% | 22.5% | |
| 5 | 19.9% | 11.6% | |
| 6+ | 15.8% | 6.8% | |
| Thromboembolic risk factorsa | |||
| CHF | 23.3% | 30.5% | <0.001 |
| Hypertension | 80.3% | 81.4% | <0.001 |
| Age 65 to 74 y | 27.7% | 33.7% | <0.001 |
| Age ≥75 y | 57.7% | 51.3% | <0.001 |
| Diabetes mellitus | 20.3% | 27.8% | <0.001 |
| Ischemic stroke | 1.1% | 1.2% | <0.001 |
| TIA | 1.6% | 1.6% | 0.48 |
| CVA | 10.2% | 11.3% | <0.001 |
| CAD | 39.0% | 59.9% | <0.001 |
| PAD | 6.8% | 10.6% | <0.001 |
| Bleeding risk factorsa | |||
| mHAS‐BLED Score, median (IQR) | 2.0 (2.0‐3.0) | 2.0 (2.0‐3.0) | <0.001 |
| Renal dysfunction | 0.3% | 0.5% | <0.001 |
| Bleeding history | 1.4% | 1.6% | <0.001 |
| Antiplatelet or NSAID drug use | 51.4% | 59.9% | <0.001 |
| Age ≥65 y | 85.4% | 83.1% | <0.001 |
| Heavy ETOH use | 0.1% | 0.3% | <0.001 |
| Hemorrhagic stroke | 0.09% | 0.12% | <0.001 |
| Intracranial hemorrhage | 1.1% | 1.3% | <0.001 |
| Vascular complications | 0.8% | 1.1% | <0.001 |
| Other clinical characteristicsa | |||
| BMI | 28.9±6.9 | 29.7±5.8 | <0.001 |
| LVEF | 57.4±12.1 | 50.9±13.9 | <0.001 |
| Hyperlipidemia | 55.3% | 65.9% | <0.001 |
| History of tobacco use | 46.7% | 66.4% | <0.001 |
| Practice site variablesa | |||
| Practice size (number of providers) | 25.1±19.5 | 24.7±19.1 | <0.001 |
| Provider type | <0.001 | ||
| Physician | 89.4% | 89.8% | |
| Other | 10.6% | 10.2% | |
BMI indicates body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular attack; Heavy ETOH, alcohol use defined at >8 drinks/day; IQR, interquartile range; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti‐inflammatory medication; PAD, peripheral arterial disease; TIA, transient ischemic attack.
Continuous variables reported as mean±SD, except where noted as Median (IQR), all categorical variables presented as percentage.
The median estimated thromboembolic risk was higher in women than in men (median CHA2DS2‐VASc score 4.0; IQR, 3.0‐5.0, versus 3.0; IQR, 2.0‐4.0, respectively, P<0.001), and a larger proportion of women than men were in the higher risk CHA2DS2‐VASc strata (Table). The estimated bleeding risk (mHAS‐BLED score) was slightly lower in women compared to men (median score 2; IQR, 2.0‐3.0, versus 2; IQR, 2.0‐3.0, P<0.001).
Association of Sex and CHA2DS2‐VASc Components With OAC Use
Overall, 59.1% of the study cohort with an indication for OAC was prescribed OAC; women were significantly less likely to receive OAC compared to men (56.7% versus 61.3%, unadjusted RR 0.92, 95%CI 0.92‐0.93) (Figure 2). Among individual components of CHA2DS2‐VASc risk score, female sex and vascular disease were associated with significantly decreased OAC prescriptions (adjusted RR 0.90, 95%CI 0.90‐0.91 and RR 0.97, 95%CI 0.96‐0.98, respectively). Factors associated with increased OAC prescription were HTN (RR 1.52, 95%CI 1.50‐1.54), age 65 to 74 (RR 1.68, 95%CI 1.66‐1.71), and age ≥75 years (RR 1.70, 95%CI 1.67‐1.72) (Figure 3).
Figure 2.
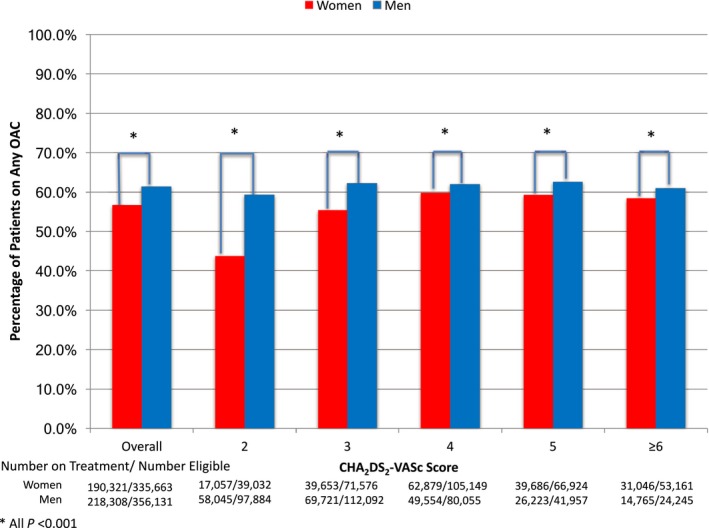
Unadjusted rates of oral anticoagulant use for nonvalvular atrial fibrillation in women and men by CHA 2 DS 2‐VASc Score. OAC indicates oral anticoagulants.
Figure 3.
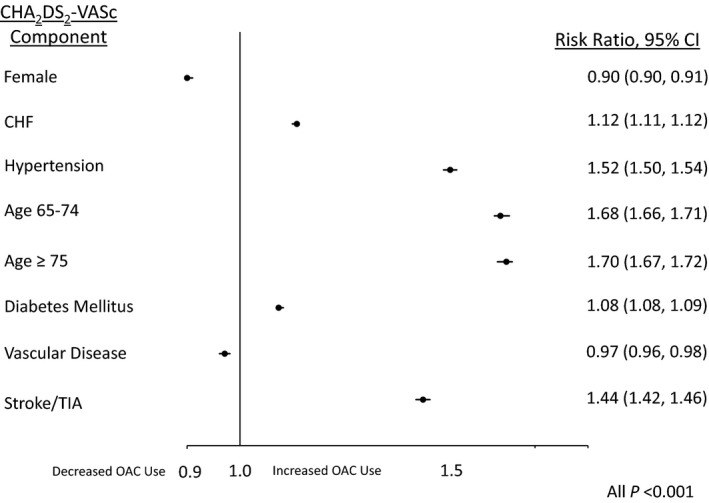
Adjusted association between individual CHA 2 DS 2‐VASc factors and OAC use among those with CHA 2 DS 2‐VASc ≥2. Analyses were adjusted for other CHA 2 DS 2‐VASc variables and race, insurance (private vs nonprivate), mHAS‐BLED score, rhythm control therapy, total number of physicians at site, provider type (physician vs other provider), proportion of female patients at site, and clustering by practice and provider. CHF indicates congestive heart failure; OAC, oral anticoagulant; TIA, transient ischemic attack. Vascular Disease indicates peripheral vascular disease, history of myocardial infarction, prior coronary artery bypass, or prior percutaneous coronary intervention.
OAC Use in Women and Men by CHA2DS2‐VASc Score
In analysis stratified by CHA2DS2‐VASc score, women had significantly lower rates of OAC use compared to men at all strata (adjusted RR 9% to 33% lower, all P<0.001) (Figure 4). For example, in the fully adjusted models, women with a CHA2DS2‐VASc score=5 were 12% less likely to have OAC prescribed than men with CHA2DS2‐VASc score=5 (adjusted RR 0.88, 95%CI 0.87‐0.89). In sensitivity analysis these relationships persisted when stratifying by the CHADS2 score (Figure 5). Similarly, removal of the mHAS‐BLED score from the multivariable models did not significantly change the results (not shown).
Figure 4.
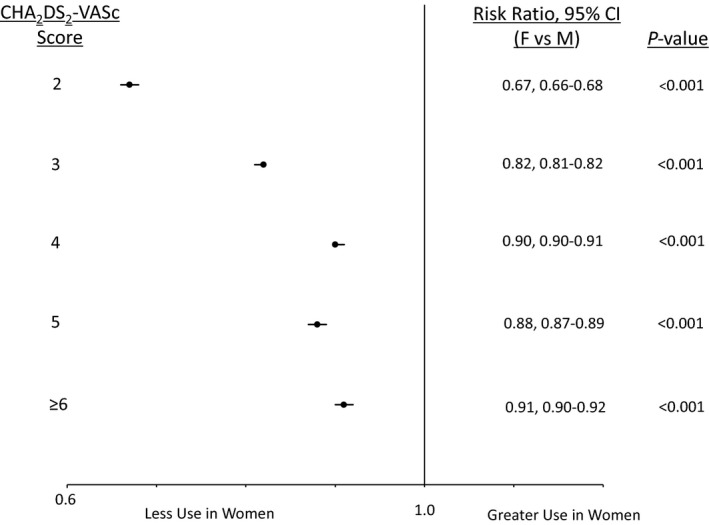
Adjusted rates of oral anticoagulant use for nonvalvular atrial fibrillation in women and men stratified by CHA 2 DS 2‐VASc score. Analyses were adjusted for race, insurance type (private vs nonprivate), modified HAS‐BLED score, rhythm control therapy, number of physicians at site, number of physician vs other providers, proportion of female patients at site, and clustering by practice and provider. P value for sex CHA2DS2‐VASc interaction < 0.001.
Figure 5.
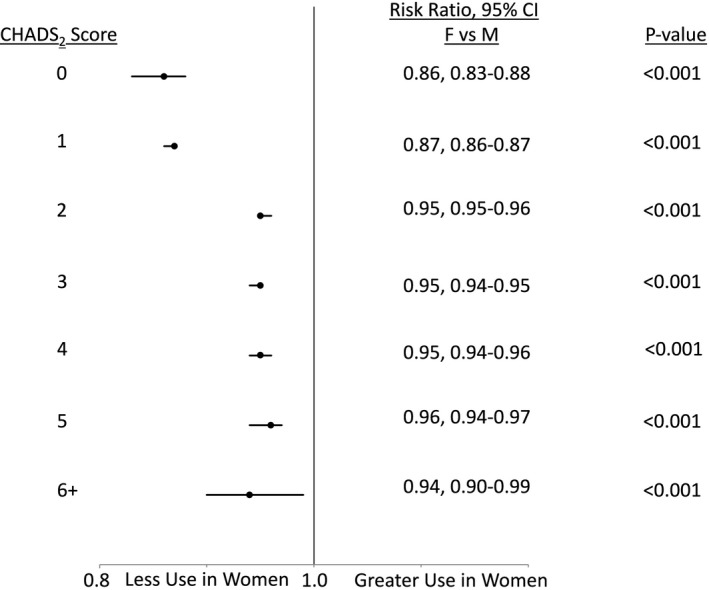
Adjusted rates of oral anticoagulant use for nonvalvular atrial fibrillation in women and men stratified by CHADS 2 score. Analyses adjusted for race, insurance (private vs nonprivate), mHAS‐BLED score, rhythm control therapy, total number of physicians at site, provider type (physician vs other provider), proportion of female patients at site. P value for sex×CHADS 2 interaction <0.001.
Temporal Trends in OAC Use
Over the study, there was a similar increase in overall OAC use in both women and men (women 3.0% increase per year, 95%CI 2.5% to 3.5%; and men 2.8% increase per year, 95%CI 2.3% to 3.3%; P‐value for time‐by‐sex interaction 0.12) (Figure 6). Women remained significantly less likely to receive any OAC compared to men at all time points (all P<0.001). There was no significant change in overall OAC use after adoption of CHA2DS2‐VASc into European Society of Cardiology or American Heart Association/American College of Cardiology guidelines (all P>0.05).
Figure 6.
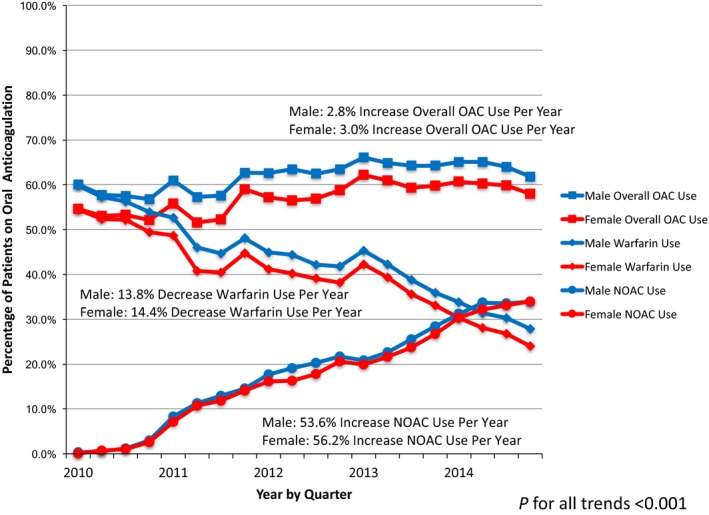
Trends in oral anticoagulant use from 2010 to 2014 by anticoagulant type in women and men. There was no significant change in OAC use for women or men following introduction of European Society of Cardiology guidelines in 2010 or American Heart Association/American College of Cardiology guidelines in 2014 (all P<0.05). Analyses were adjusted for: race, insurance type (private vs nonprivate), CHA 2 DS 2‐VASc score, modified HAS‐BLED score, rhythm control therapy, total number of physicians at site, provider type (physician vs other provider), proportion of female subjects at site, and clustering by provider and practice. NOAC indicates non–vitamin K oral anticoagulant; OAC, oral anticoagulant.
Beginning in 2010, NOAC use increased at a slightly higher rate in women (56.2% increase per year, 95%CI 54.6% to 57.9%) compared to men (53.6% increase per year, 95%CI 52.0% to 55.2%; P‐value for time‐by‐sex interaction <0.001) (Figure 6). By the second quarter of 2014, NOAC use surpassed the use of warfarin in both women and men. Over the same timeframe, warfarin use declined at a slightly higher rate in women (14.4% decrease per year, 95%CI 13.8% to 15%) compared to men (13.8% decrease per year, 95%CI 13.1% to 14.4%; P‐value for time by sex interaction 0.003).
Discussion
In this contemporary cohort of US patients with AF and indications for OAC, female sex was associated with significantly less OAC use compared to male sex across the spectrum of thromboembolic risk. Over the past decade, OAC use has gradually increased each year for both women and men. Warfarin use has been declining and NOAC use increasing; these changes have been slightly more pronounced in women compared to men. Even with these shifts in therapy type, women remained significantly less likely than men to receive OAC at all time points. Despite the introduction of the CHA2DS2‐VASc score and NOACs, a risk treatment paradox for OAC use in eligible women with AF persists.
Our study suggests that female sex is underemphasized as a thromboembolic risk factor. Compared to other thromboembolic risk factors in the CHA2DS2‐VASc score (ie, HTN or age), female sex was associated with relatively lower use of OAC. Further, women were significantly less likely than men to receive guideline‐concordant OAC at all levels of estimated thromboembolic risk. In the CHA2DS2‐VASc scoring system, women previously viewed as intermediate risk (CHADS2=1) are now categorized as high risk (CHA2DS2‐VASc 2 or more).34, 35, 36 In our study, differences in OAC use were most pronounced in lower CHA2DS2‐VASc scores (ie, CHA2DS2‐VASc=2), which suggests that female sex as a thromboembolic risk factor has less weight on OAC use compared to other factors. However, in our sensitivity analysis stratified by CHADS2 scores (sex not included as an independent risk factor), we found lower rates of OAC use in women across the spectrum of estimated thromboembolic risk. Taken together, women were consistently less likely to receive OAC compared to men independent of level or method of estimating thromboembolic risk. Therefore, our findings suggest factors beyond thromboembolic risk alone contribute to lower rates of OAC use in women.
We also examined whether expanded treatment options, specifically NOACs, have affected sex differences in OAC use over time. Compared to warfarin therapy, NOAC therapy offers potential benefits including standardized dosing regimens, lack of intensive laboratory monitoring, and lower rates of major bleeding.17, 37 Our findings suggest that sex differences in OAC use may be primarily due to differences in the use of warfarin. Over the past 5 years, warfarin use has gradually decreased, and NOAC use has increased by as much as 50% per year in both women and men with a slightly greater rate of increase for women. Prior studies assessing sex differences in OAC have been unable to assess the impact of NOACs by sex due to low rates of NOAC use.38, 39, 40 As of 2014, 1 in 3 people with AF were prescribed a NOAC, representing >50% of those receiving some form of OAC for AF. Therefore, it is possible that if NOAC use continues to increase over time, sex differences in overall OAC use may decrease and eventually be eliminated.
Our findings differ from other AF specific registries that found no significant sex differences in OAC use.38, 39, 40 The ORBIT (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation), EORP (Euro Observational Research Program), and GARFIELD (Global Anticoagulant Registry in the FIELD‐Atrial Fibrillation) registries prospectively enroll patients based on specific inclusion and exclusion criteria, introducing the possibility of selection bias. In contrast, PINNACLE is a quality‐improvement program that captures data on all patients with a diagnosis of AF and may more closely reflect broad clinical practice. Another important difference between our study and the GARFIELD and EORP registries is that these studies were largely international cohorts. European Society of Cardiology guidelines included the CHA2DS2‐VASc score in 2010, whereas the American Heart Association/American College of Cardiology guidelines included the score in 2014.14, 15 Earlier diffusion of evidence in these countries supporting female sex as an independent risk factor for thromboembolic events may contribute to these differences.
In contemporary general US cardiology practices, our study provides evidence for sex differences in OAC use among eligible patients with AF that are independent of thromboembolic risk and the introduction of NOACs. A potential reason for these observed sex differences might be differences in patient or provider preferences. For example, women may be more likely to decline OAC therapy, particularly warfarin, due to concerns for bleeding, inconvenience, or lack of social support (ie, transportation for international normalized ratio check).9 Additionally, providers may perceive increased frailty or bleeding risk in women compared to men because women have been shown to have higher rates of bleeding while on warfarin and after cardiac interventions.6, 11, 16, 41, 42, 43 Our finding of greater increases in NOAC use in women compared to men may support the notion that higher bleeding risk contributed to past sex differences in OAC use when therapy options were more limited. However, in our study, sex differences persisted across our entire study timeframe after controlling for estimated bleeding risk and allowing for the introduction of NOACs. Finally, US cardiologists may apply clinical guidelines at lower rates in women compared to men, suggesting a bias in how care is delivered.44, 45, 46 Future studies should examine these potential causes in order to understand and work to eliminate sex differences in OAC use.
Certain limitations must be considered when interpreting our study. First, whether the demonstrated statistically significant differences in OAC use correspond to clinically significant differences in patient outcomes was not investigated and warrants further evaluation. However, prior studies have demonstrated that sex‐related differences in the risk of stroke decrease when OAC are used.9 Also, sex differences were observed at the highest level of estimated thromboembolic risk (CHA2DS2‐VASc ≥6), suggesting that even small absolute differences in OAC use may translate into significant sex differences in clinical outcomes. Second, we were unable to determine whether all potential contraindications or provider or patient preferences regarding OAC use potentially differed by patient sex. However, we excluded patients with a documented contraindication for OAC use, either for personal preference or medical reasons, and we saw no sex differences in this exclusion (49.5% female versus 50.5% male, P>0.05). Third, the CHA2DS2‐VASc score was only incorporated into US clinical guidelines in 2014; thus, many clinicians in the United States may not have been using the CHA2DS2‐VASc score for risk stratification during the cohort period. However, our findings were unchanged in sensitivity analysis stratified by CHADS2 score, the previous guideline‐recommended risk stratification tool. Finally, reported OAC use may be lower than actual use due to underreporting in the PINNACLE registry. We would not expect underreporting to occur differentially according to patient sex. Further, we allowed 1 year of follow‐up for OAC use to be documented, increasing capture of OAC use, and our observed rates of OAC use were similar to what has been seen in previous clinical cohorts.40, 47, 48
Conclusions
In this contemporary cohort of cardiology patients in the United States with AF and indications for anticoagulation, women were 9% to 33% less likely than men to receive OAC at all levels of thromboembolic risk. Despite the introduction of NOACs and their rapidly increased use over time, women remained significantly less likely to receive OAC at all time points. Underrecognition of female sex as a thromboembolic risk factor does not fully explain these differences, suggesting that clinical guidelines may be applied differently in women and men. Further studies are needed to understand whether lower rates of OAC use in women are associated with differences in clinical outcomes, and if so, action is needed to eliminate unnecessary differences in OAC use by sex.
Sources of Funding
Dr Thompson is supported by NIH/NCATS Colorado CTSI grant number UL1 TR001082 . Dr Daugherty is supported by award number R01 HL133343 from the National Heart, Lung, and Blood Institute. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Disclosures
Dr Masoudi has a contract with the American College of Cardiology for his role as Chief Medical Officer of the NCDR®. The remaining authors have no disclosures to report.
Acknowledgments
Lei, Song, Dr Thompson, and Dr Daugherty had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2017;6:e005801 DOI: 10.1161/JAHA.117.005801.)28724655
This article was handled independently by N.A. Mark Estes III, MD, as a guest editor. The editors had no role in the evaluation of this manuscript or the decision about its acceptance.
References
- 1. Fang MC, Singer DE, Chang Y, Hylek EM, Henault LE, Jensvold NG, Go AS. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Gender differences in stroke risk of atrial fibrillation patients on oral anticoagulant treatment. Thromb Haemost. 2009;101:938–942. [PubMed] [Google Scholar]
- 3. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta‐analysis of cohort studies. BMJ. 2016;352:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lip GY, Eikelboom J, Yusuf S, Shestakovska O, Hart RG, Connolly S. Modification of outcomes with aspirin or apixaban in relation to female and male sex in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Stroke. 2014;45:2127–2130. [DOI] [PubMed] [Google Scholar]
- 5. Agarwal S, Bennett D, Smith DJ. Predictors of warfarin use in atrial fibrillation patients in the inpatient setting. Am J Cardiovasc Drugs. 2010;10:37–48. [DOI] [PubMed] [Google Scholar]
- 6. Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New‐onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. [DOI] [PubMed] [Google Scholar]
- 7. Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, Spertus JA. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation and Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race‐ and sex‐related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shantsila E, Wolff A, Lip G, Lane D. Gender differences in stroke prevention in atrial fibrillation in general practice: using the GRASP‐AF audit tool. Int J Clin Pract. 2015;69:840–845. [DOI] [PubMed] [Google Scholar]
- 10. Jönsson AC, Ek J, Kremer C. Outcome of men and women after atrial fibrillation and stroke. Acta Neurol Scand. 2015;132:125–131. [DOI] [PubMed] [Google Scholar]
- 11. Cosma Rochat M, Waeber G, Wasserfallen JB, Nakov K, Aujesky D. Hospitalized women experiencing an episode of excessive oral anticoagulation had a higher bleeding risk than men. J Womens Health. 2009;18:321–326. [DOI] [PubMed] [Google Scholar]
- 12. Lane DA, Lip G. Female gender is a risk factor for stroke and thromboembolism in atrial fibrillation patients. Thromb Haemost. 2009;101:802–805. [PubMed] [Google Scholar]
- 13. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 14. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey J‐Y, Ponikowski P, Rutten FH, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck‐Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Vardas PE, Agladze V, Aliot E, Balabanski T, Blomstrom‐Lundqvist C, Capucci A, Crijns H, Dahlöf B, Folliguet T, Glikson M, Goethals M, Gulba DC, Ho SY, Klautz RJM, Kose S, McMurray J, Perrone Filardi P, Raatikainen P, Salvador MJ, Schalij MJ, Shpektor A, Sousa J, Stepinska J, Uuetoa H, Zamorano JL, Zupan I. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 15. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 16. Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Pilote L. Sex differences in dabigatran use, safety, and effectiveness in a population‐based cohort of patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:593–599. [DOI] [PubMed] [Google Scholar]
- 17. Patel KK, Mehdirad AA, Lim MJ, Ferreira SW, Mikolajczak PC, Stolker JM. Beyond warfarin: a patient‐centered approach to selecting novel oral anticoagulants for stroke prevention in atrial fibrillation. J Hosp Med. 2014;9:400–406. [DOI] [PubMed] [Google Scholar]
- 18. Pancholy SB, Sharma PS, Pancholy DS, Patel TM, Callans DJ, Marchlinski FE. Meta‐analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol. 2014;113:485–490. [DOI] [PubMed] [Google Scholar]
- 19. Dentali F, Riva N, Crowther M, Turpie AG, Lip GY, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta‐analysis of the literature. Circulation. 2012;126:2381–2391. [DOI] [PubMed] [Google Scholar]
- 20. Romanelli RJ, Nolting L, Dolginsky M, Kym E, Orrico KB. Dabigatran versus warfarin for atrial fibrillation in real‐world clinical practice. Circ Cardiovasc Qual Outcomes. 2016;9:126–134. [DOI] [PubMed] [Google Scholar]
- 21. NCDR® PINNACLE Registry® v1.3 full data dictionary. 2014.
- 22. Chan PS, Oetgen WJ, Spertus JA. The improving continuous cardiac care (IC3) program and outpatient quality improvement. Am J Med. 2010;123:217–219. [DOI] [PubMed] [Google Scholar]
- 23. Masoudi FA, Ponirakis A, Yeh RW, Maddox TM, Beachy J, Casale PN, Curtis JP, De Lemos J, Fonarow G, Heidenreich P, Koutras C, Kremers M, Messenger J, Moussa I, Oetgen WJ, Roe MT, Rosenfield K, Shields TP Jr, Spertus JA, Wei J, White C, Young CH, Rumsfeld JS. Cardiovascular care facts: a report from the National Cardiovascular Data Registry: 2011. J Am Coll Cardiol. 2013;62:1931–1947. [DOI] [PubMed] [Google Scholar]
- 24. Maddox TM, Borden WB, Tang F, Virani SS, Oetgen WJ, Mullen JB, Chan PS, Casale PN, Douglas PS, Masoudi FA, Farmer SA, Rumsfeld JS. Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;64:2183–2192. [DOI] [PubMed] [Google Scholar]
- 25. Messenger JC, Ho KKL, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA. The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. [DOI] [PubMed] [Google Scholar]
- 26. NCDR PINNACLE Registry v1.3 full data dictionary. 2015.
- 27. Puurunen MK, Kiviniemi T, Schlitt A, Rubboli A, Dietrich B, Karjalainen P, Nyman K, Niemelä M, Lip GYH, Airaksinen KEJ. CHADS2, CHA2DS2‐VASc and HAS‐BLED as predictors of outcome in patients with atrial fibrillation undergoing percutaneous coronary intervention. Thromb Res. 2014;133:560–566. [DOI] [PubMed] [Google Scholar]
- 28. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey J‐Y, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S; ACC/AHA Task Force Members , Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B; ESC Committee for Practice Guidelines , Priori SG, Blanc J‐J, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 29. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 30. Greenland S. Model‐based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case‐control studies. Am J Epidemiol. 2004;160:301–305. [DOI] [PubMed] [Google Scholar]
- 31. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 32. Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174:984–992. [DOI] [PubMed] [Google Scholar]
- 33. Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–670. [DOI] [PubMed] [Google Scholar]
- 34. Piyaskulkaew C, Singh T, Szpunar S, Saravolatz L II, Rosman H. CHA(2)DS(2)‐VASc versus CHADS(2) for stroke risk assessment in low‐risk patients with atrial fibrillation: a pilot study from a single center of the NCDR‐PINNACLE registry. J Thromb Thrombolysis. 2014;37:400–403. [DOI] [PubMed] [Google Scholar]
- 35. Marzec LN, Katz DF, Maddox TM, Turakhia MP, Gehi AK, O'Brien EC, Lubitz SA, Varosy PD, Hsu JC. Effects of guideline recommended change in use of the CHADS2 to the CHA2DS2‐VASc score for the assessment of thromboembolic risk in atrial fibrillation patients at low to moderate risk of stroke: An analysis from the NCDR PINNACLE AF Registry. HRS 2015 Abstract Presentation 2015.
- 36. Mason PK, Lake DE, DiMarco JP, Ferguson JD, Mangrum JM, Bilchick K, Moorman LP, Moorman JR. Impact of the CHA2DS2‐VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012;125:603.e601–603.e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Renda G, di Nicola M, De Caterina R. Net clinical benefit of non‐vitamin K antagonist oral anticoagulants versus warfarin in phase III atrial fibrillation trials. Am J Med. 2015;9:1007–1014. [DOI] [PubMed] [Google Scholar]
- 38. Piccini JP, Simon DN, Steinberg BA, Thomas L, Allen LA, Fonarow GC, Gersh B, Hylek E, Kowey PR, Reiffel JA, Naccarelli GV, Chan PS, Spertus JA, Peterson ED. Differences in clinical and functional outcomes of atrial fibrillation in women and men: two‐year results from the ORBIT‐AF registry. JAMA Cardiol. 2016;1:282–291. [DOI] [PubMed] [Google Scholar]
- 39. Lip GYH, Laroche C, Boriani G, Cimaglia P, Dan G‐A, Santini M, Kalarus Z, Rasmussen LH, Popescu MI, Tica O, Hellum CF, Mortensen B, Tavazzi L, Maggioni AP. Sex‐related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot Survey on Atrial Fibrillation. Europace. 2015;17:24–31. [DOI] [PubMed] [Google Scholar]
- 40. Lip GY, Rushton‐Smith SK, Goldhaber SZ, Fitzmaurice DA, Mantovani LG, Goto S, Haas S, Bassand JP, Camm AJ, Ambrosio G, Jansky P, Al Mahmeed W, Oh S, van Eickels M, Raatikainen P, Steffel J, Oto A, Kayani G, Accetta G, Kakkar AK. Does sex affect anticoagulant use for stroke prevention in nonvalvular atrial fibrillation? The prospective global anticoagulant registry in the FIELD‐atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:S12–S20. [DOI] [PubMed] [Google Scholar]
- 41. Volgman AS, Manankil MF, Mookherjee D, Trohman RG. Women with atrial fibrillation: greater risk, less attention. Gend Med. 2009;6:419–432. [DOI] [PubMed] [Google Scholar]
- 42. Decker C, Garavalia L, Garavalia B, Simon T, Loeb M, Spertus JA, Daniel WC. Exploring barriers to optimal anticoagulation for atrial fibrillation: interviews with clinicians. J Multidiscip Healthc. 2012;5:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daugherty SL, Thompson LE, Kim S, Rao SV, Subherwal S, Tsai TT, Messenger JC, Masoudi FA. Patterns of use and comparative effectiveness of bleeding avoidance strategies in men and women following percutaneous coronary interventions: an observational study from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2013;61:2070–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Daugherty SL, Magid DJ. Do sex differences exist in patient preferences for cardiovascular testing? Ann Emerg Med. 2011;57:561–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, Fletcher GF, Gulati M, Mehta LS, Pettey C, Reckelhoff JF. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation. 2016;133:1302–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalra A, Pokharel Y, Glusenkamp N, Wei J, Kerkar PG, Oetgen WJ, Virani SS. Gender disparities in cardiovascular care access and delivery in India: insights from the American College of Cardiology's PINNACLE India Quality Improvement Program (PIQIP). Int J Cardiol. 2016;215:248–251. [DOI] [PubMed] [Google Scholar]
- 47. Kalra L, Yu G, Perez I, Lakhani A, Donaldson N. Prospective cohort study to determine if trial efficacy of anticoagulation for stroke prevention in atrial fibrillation translates into clinical effectiveness. BMJ. 2000;320:1236–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Samsa GP, Matchar DB, Goldstein LB, Bonito AJ, Lux LJ, Witter DM, Bian J. Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. Arch Intern Med. 2000;160:967–973. [DOI] [PubMed] [Google Scholar]


