Abstract
Background
The original non–vitamin K antagonist oral anticoagulant (NOAC) trials in nonvalvular atrial fibrillation (AF) enrolled patients with native valve pathologies. The object of this study was to quantify the benefit–risk profiles of NOACs versus warfarin in AF patients with native valvular heart disease (VHD).
Methods and Results
Trials were identified by exhaustive literature search. Trial data were combined using inverse variance weighting to produce a meta‐analytic summary hazard ratio (HR) and 95% confidence interval (CI) of efficacy and safety of NOACs versus warfarin. Our final analysis included 4 randomized controlled trials that enrolled 71 526 participants, including 13 574 with VHD. Pooling results from included trials showed that NOACs versus warfarin reduced stroke or systemic embolism (HR: 0.70; 95% CI, 0.60–0.82) and intracranial hemorrhage (HR: 0.47; 95% CI, 0.24–0.92) in AF patients with VHD. However, risk reduction of major bleeding and intracranial hemorrhage was driven by apixaban, edoxaban, and dabigatran (HR for major bleeding: 0.79 [95% CI, 0.69–0.91]; HR for intracranial hemorrhage: 0.33 [95% CI, 0.25–0.45]) but not rivaroxaban (HR for major bleeding: 1.56 [95% CI, 1.20–2.04]; HR for intracranial hemorrhage: 1.27 [95% CI, 0.77–2.10]).
Conclusions
Among patients with AF and native VHD, NOACs reduce stroke and systemic embolism compared with warfarin. Evidence shows that apixaban, dabigatran, and edoxaban also reduce bleeding in this patient subgroup, whereas major bleeding (but not intracranial hemorrhage or mortality rate) is significantly increased in VHD patients treated with rivaroxaban. NOACs are a reasonable alternative to warfarin in AF patients with VHD.
Keywords: anticoagulant, atrial fibrillation, bleeding, stroke, valve
Subject Categories: Arrhythmias, Cerebrovascular Disease/Stroke, Anticoagulants, Valvular Heart Disease, Meta Analysis
Clinical Perspective
What Is New?
Non–vitamin K antagonist oral anticoagulants, compared with warfarin, reduced stroke or systemic embolism and intracranial hemorrhage in atrial fibrillation patients with or without valvular heart disease.
What Are the Clinical Implications?
Non–vitamin K antagonist oral anticoagulants are a reasonable alternative to warfarin in atrial fibrillation patients with native valvular heart disease.
Introduction
Patients with atrial fibrillation (AF) have a 4‐fold increased risk of ischemic stroke compared with patients with sinus rhythm,1, 2, 3 and the risk of stroke is up to 17‐fold higher in AF patients with rheumatic mitral stenosis.1 Oral anticoagulation with vitamin K antagonists, including warfarin, is indicated for AF patients with mitral stenosis or mechanical heart valve,4 and these 2 types of valvular heart disease (VHD) were generally excluded from randomized controlled trials (RCTs) that evaluated non–vitamin K antagonist oral anticoagulants (NOACs) versus warfarin in nonvalvular AF patients.5, 6, 7, 8 However, the aforementioned trials actually enrolled patients with other native valve pathologies,5, 6, 7, 8 rendering the term nonvalvular AF a misnomer. The term nonvalvular heart disease, used in the original NOAC trials, may cause some clinicians to hesitate before prescribing NOACs to AF patients with any form of VHD.
Several post hoc analyses evaluating the effect of NOACs in comparison with warfarin in AF patients with VHD have been published.9, 10, 11 A review of NOACs in AF patients with VHD, based on the aforementioned publications, suggested that little direct evidence exists to support treatment recommendations in clinical practice12; however, relevant data13 from the ENGAGE AF‐TIMI 48 (Effective Anticoagulation with factor Xa Next Generation in Atrial Fibrillation‐Thrombolysis In Myocardial Infarction 48) trial were not included. The objective of this study was to systematically review the totality of the published literature to qualitatively and quantitatively evaluate the overall efficacy and safety profiles (ie, stroke or systemic embolism, all‐cause mortality, major bleeding, and intracranial hemorrhage) of NOACs, compared with warfarin, in AF patients with and without VHD.
Methods
This study was performed in accordance with the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analysis) statement.14 This study was prospectively registered in PROSPERO (CRD42016052243). Regarding the Declaration of Helsinki, because this is a meta‐analysis of published articles, it is not necessary to obtain approval from the locally appointed ethics committee or informed consent from patients.
Data Sources and Searches
We searched PubMed (1966 to May 2017), Embase and Medline (1980 to May 2017), the Cochrane Central Register of Controlled Trials (CENTRAL), and presentations at major cardiology conferences within the past year (American Heart Association, American College of Cardiology, European Society of Cardiology) using the terms novel oral anticoagulants or non‐vitamin K antagonist oral anticoagulants or direct oral anticoagulants or dabigatran or rivaroxaban or apixaban or edoxaban AND stroke or systemic embolism or mortality or major bleeding or intracranial hemorrhage AND valvular heart disease or mitral regurgitation or aortic regurgitation or tricuspid regurgitation or mitral stenosis or aortic stenosis. There were no language restrictions. We also reviewed the Introduction and Discussion sections of retrieved trials and relevant review articles to identify additional trials.
Study Selection
Criteria for inclusion of a study were as follows: (1) The study design was an RCT; (2) the study included a comparison of a NOAC with warfarin; (3) quantitative estimates of the hazard ratio (HR) and 95% confidence interval (CI) were reported for stroke or systemic embolism with NOACs versus warfarin among AF patients with and without VHD. Studies were excluded if the outcome of stroke or systemic embolism was not either prespecified or adjudicated as a major (primary or secondary) end point. Participants of any age and of either sex were included. One investigator (M.L.) developed selection criteria and conducted literature searches. Another investigator (K.L.P.) assessed these criteria and independently checked the enrolled trials. Discrepancies were resolved by discussion with a third investigator (B.O.) and by referencing the original report.
Data Extraction
All data from eligible studies were extracted by 2 independent investigators according to a standard protocol. Discrepancies were resolved by discussion with a third investigator and by referencing the original report. Recorded data variables were trial name, eligibility criteria, types of VHD, mean age, proportion of women in the study, baseline characteristics, percentage of persistent or permanent AF, baseline CHADS2 scores, history of stroke, history of myocardial infarction, history of heart failure, percentage of prior warfarin use, renal status, and follow‐up duration.
Study Quality Assessment
All included studies were derived from RCTs. The risk of bias (eg, selection bias, performance bias, detection bias, attrition bias, and reporting bias) of the original included trials was assessed using the Cochrane risk‐of‐bias algorithm (http://www.cochrane.org/training/cochranehandbook).
Objectives
The objectives of these analyses were (1) to evaluate differences in baseline characteristics among AF patients with and without VHD; (2) to compare the rates of stroke or systemic embolism, all‐cause mortality, major bleeding, and intracranial hemorrhage in AF patients with and without VHD; and (3) to assess the efficacy (stroke or systemic embolism, all‐cause mortality) and safety (major bleeding, intracranial hemorrhage) of NOACs in comparison to warfarin in AF patients with and without VHD.
Data Synthesis and Analysis
Data on baseline characteristics are reported as median and interquartile range, mean and standard deviation, or number and percentage, as appropriate. To explore differences in baseline characteristics among AF patients with and without VHD, we pooled data across trials. Heterogeneity was considered significant when the P value of χ2 statistics was <0.05 or the I2 value exceeded 25%. Because the different types of NOACs may have somewhat different treatment effects, a random‐effects model was used. We used HRs with 95% CIs to compare the rates of stroke or systemic embolism, all‐cause mortality, major bleeding, and intracranial hemorrhage in AF patients with and without VHD and to assess the efficacy and safety of NOACs versus warfarin in AF patients with and without VHD. In each study, we converted these values to their natural logarithms, and we calculated the standard errors from these logarithmic numbers with their corresponding 95% CIs. For the statistical analysis, we combined log HRs and standard errors using the inverse variance approach. If 2 groups of active treatment existed in a trial, we pooled results only from the higher dose NOAC in a trial (eg, dabigatran 150 mg twice daily in the RE‐LY trial [Randomized Evaluation of Long‐Term Anticoagulant Therapy] and edoxaban 60 mg once daily in the ENGAGE ‐ AF‐TIMI 48 trial). The Cochrane Collaboration's Review Manager software package (RevMan 5.3) was used for this meta‐analysis.15
Results
Basic Characteristics of AF Patients With and Without VHD
The literature review identified 13 articles for detailed assessment, of which 8 were excluded for not reporting relevant data in patients with VHD and 1 was excluded because it was derived from the same study population as another report.16 Our final analysis included 4 RCTs that enrolled 71 526 patients (Figure 1).9, 10, 11, 13 The baseline characteristics of these RCTs based on VHD status are shown in Table 1. Of these patients, 13 574 (19%) had VHD at baseline. The majority of patients with VHD had mitral regurgitation; a smaller proportion had tricuspid regurgitation, aortic stenosis or regurgitation, mild mitral stenosis, or previous valve surgery. The prevalence of female patients, persistent or permanent AF, history of heart failure, history of myocardial infarction or coronary artery disease, and prior warfarin usage was higher in AF patients with than without VHD (Figure 2). AF patients with VHD, compared with no VHD, also had higher rates of moderate renal disease9, 10 and lower creatinine clearance.10, 11, 13 Patients in the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial, both with and without VHD, had substantially higher CHADS2 stroke risk scores. The median follow‐up duration ranged from 1.8 years9 to 2.8 years.13 The assessment of risk of bias in the original RCTs5, 6, 7, 8 is shown in Table 2, and all 4 relevant trials were of good quality with low risk of bias.
Figure 1.
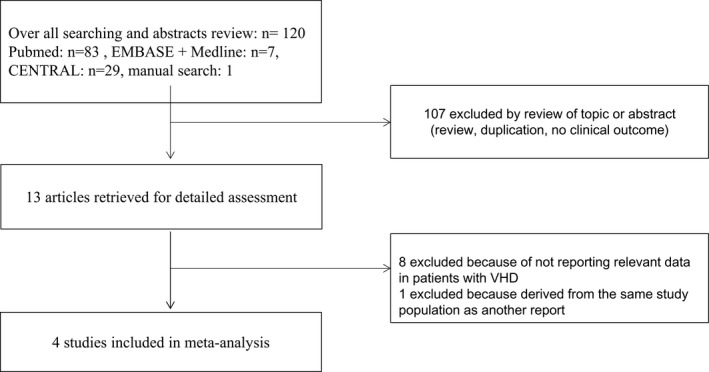
Flow chart of study selection. CENTRAL indicates Cochrane Central Register of Controlled Trials; VHD indicates valvular heart disease.
Table 1.
Baseline Characteristics by VHD Status in Patients With AF From Included Trials
| Characteristic | ARISTOTLE9 | ENGAGE AF13 | RE‐LY10 | ROCKET AF11 | ||||
|---|---|---|---|---|---|---|---|---|
| VHD (n=4808) | No VHD (n=13 389) | VHD (n=2824) | No VHD (n=18 222) | VHD (n=3950) | No VHD (n=14 162) | VHD (n=1992) | No VHD (n=12 179) | |
| Types of VHD, n (% in VHD category) |
MR 3526 (73.3) Mild MS 131 (2.7) AR 887 (18.4) AS 387 (8.0) TR 2124 (44.2) Previous valve surgery 251 (5.2) |
··· |
MR 2250 (79.6) AR 369 (13.0) AS 165 (5.8) Valve surgery 325 (11.5) |
··· |
MR 3101 (78.5) Mild MS 193 (4.9) AR 817 (20.7) AS 471 (11.9) TR 1179 (29.8) |
··· |
MR 1756 (89.6) AR 486 (24.8) AS 215 (11.0) Other 11 (0.6) |
··· |
| Age, y, median (25th, 75th) or mean (SD) | 71 (64, 77) | 69 (62, 76) | 71.8 (9.4) | 70.4 (9.4) | 74 (68, 79) | 72 (66, 77) | 75 (68, 79) | 72 (65, 78) |
| Female, n (%) | 1936 (40.3) | 4480 (33.5) | 1193 (42.2) | 6828 (37.5) | 1607 (40.7) | 4991 (35.2) | 785 (39.3) | 4280 (39.6) |
| Persistent or permanent AF | 4212 (87.6) | 1198 (83.6) | 2269 (80.3) | 13 416 (73.6) | 1341 (34.0) | 4448 (31.4) | 1653 (83.0) | 9832 (80.7) |
| CHADS2 score mean (SD) or medium (25th, 75th) | 2.2 (1.1) | 2.1 (1.1) | 2.9 (1.0) | 2.8 (1.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 3.5 (1.0) | 3.5 (0.9) |
| History of stroke, embolism or TIA, n (%) | 905 (18.8) | 2632 (19.7) | 668 (23.7) | 5290 (29.0) | 875 (22.2) | 3078 (21.7) | 961 (48.2) | 6806 (55.9) |
| History of heart failure, n (%) | 2337 (48.6) | 4113 (30.7) | 2082 (73.7) | 10 011 (54.9) | 1570 (39.7) | 4223 (29.8) | 1402 (70.4) | 7449 (61.2) |
| History of myocardial infarction or CAD | 837 (17.4) | 1748 (13.1) | 1122 (39.8) | 5822 (32.3) | 1285 (32.5) | 3749 (26.5) | 482 (24.2) | 1964 (16.1) |
| Hypertension | 4102 (85.3) | 11 811 (88.2) | 2629 (93.1) | 17 068 (93.7) | NA | NA | 1775 (89.1) | 11 049 (90.7) |
| Diabetes mellitus | 1086 (22.6) | 3460 (25.8) | 908 (32.2) | 6696 (36.7) | NA | NA | 798 (40.1) | 4849 (39.8) |
| Vitamin K antagonist experienced, n (%) | 2918 (62.0) | 7418 (55.4) | NA | NA | 2673 (67.7) | 8562 (60.1) | 1444 (72.5) | 7409 (60.8) |
| Renal function, CrCl (mL/min) medium (25th, 75th) or mean (SD) | 70.0 (29.6) | 77.2 (31.4) | 65.8 (51.0, 83.7) | 69.0 (54.0, 87.7) | 62 (49, 80) | 68 (53, 88) | ||
| Normal (>80 mL/min), n (%) | 1608 (33.6) | 5909 (44.3) | NA | NA | NA | NA | NA | NA |
| Mild impairment (>50–80 mL/min), n (%) | 2101 (43.8) | 5486 (41.2) | NA | NA | NA | NA | NA | NA |
| Moderate impairment (>30–50 mL/min), n (%) | 980 (20.5) | 1766 (13.3) | NA | NA | 863 (21.8) | 2480 (17.5) | NA | NA |
| Severe impairment (≤30 mL/min), n (%) | 103 (2.1) | 167 (1.3) | NA | NA | NA | NA | NA | NA |
| Median follow‐up duration, y | 1.8 | 1.8 | 2.8 | 2.8 | 2.0 | 2.0 | 1.9 | 1.9 |
AF indicates atrial fibrillation; AR, aortic regurgitation; ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AS, aortic stenosis; CAD, coronary artery disease; CrCl, creatinine clearance; ENGAGE AF, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; MR, mitral regurgitation; MS, mitral stenosis; NA, not available; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; TIA, transient ischemic attack; TR, tricuspid regurgitation; VHD, valvular heart disease.
Figure 2.
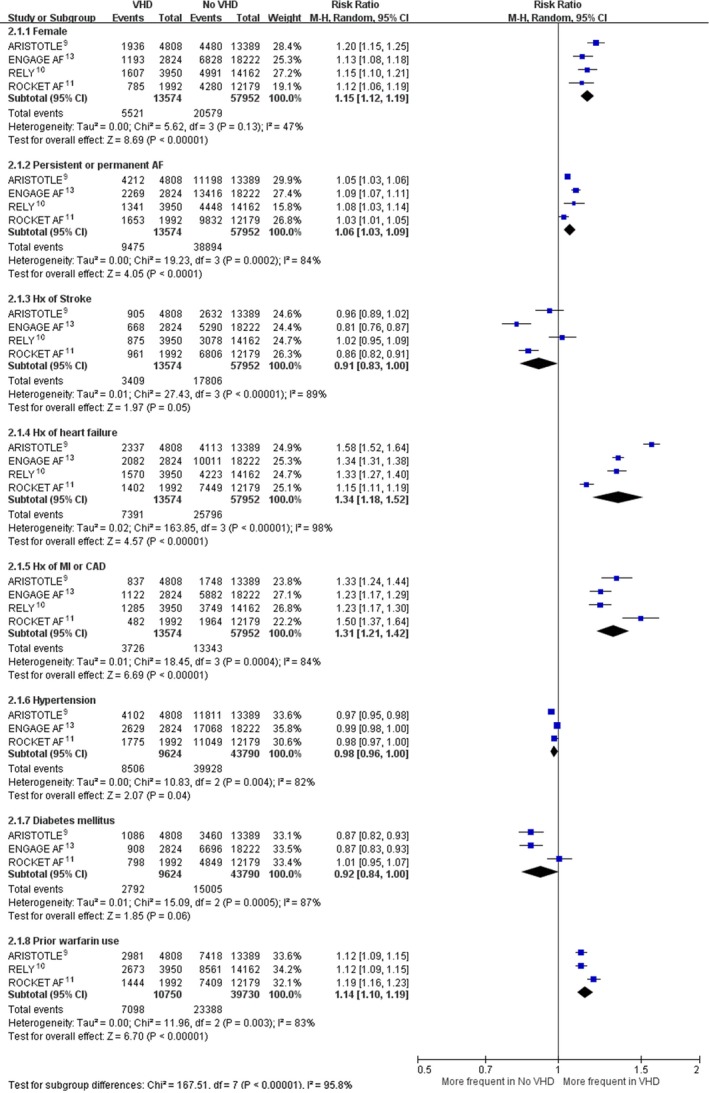
Prevalence of baseline characteristics of AF patients with and without valvular heart disease. AF indicates atrial fibrillation; ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; CAD, coronary artery disease; CI, confidence interval; ENGAGE AF, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; Hx, history; M‐H, Mantel–Haenszel; MI, myocardial infarction; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; VHD, valvular heart disease.
Table 2.
Risk‐of‐Bias Assessment of Original Randomized Controlled Trials
| Trial | ARISTOTLE7 | ENGAGE AF8 | RE‐LY5 | ROCKET AF6 |
|---|---|---|---|---|
| Random sequence generation (selection bias) |
Low risk Quote: “Each subject will be randomly assigned to 1 of 2 treatment groups in a 1:1 ratio by the Interactive Voice Response System (IVRS).” Comment: probably done |
Low risk Quote: “Patients were randomly assigned, in a 1:1:1 ratio. Randomization was performed with the use of a central, 24‐hour, interactive, computerized response system.” Comment: probably done |
Low risk Quote: “The patients were randomized by a central randomization service, through an interactive voice response system (IVRS).” Comment: probably done |
Low risk Quote: “Treatment allocation is randomized using a blinded, central telephonic Interactive Voice Response System.” Comment: probably done |
| Allocation concealment (selection bias) |
Low risk Quote: “Randomization schedules will be generated and kept by Bristol‐Myers Squibb (BMS)” Comment: probably done |
Low risk Quote: “Randomization was performed with the use of a central, 24‐hour, interactive, computerized response system” Comment: probably done |
Low risk Quote: “Randomization service located at the Coordinating Centre at Population Health Research Institute (PHRI) in Hamilton, Canada” Comment: probably done |
Low risk To effect concealment of randomization, treatment allocation is randomized using a blinded, central telephonic Interactive Voice Response System Comment: probably done |
| Blinding of participants and personnel (performance bias) |
Low risk Quote: “double‐blind, double‐dummy, parallel‐arm study” Comment: probably done |
Low risk Quote: “double‐blind, double‐dummy trial” Comment: probably done |
High risk Quote: “open‐label, randomized trial with blinded evaluation of all outcomes”Comment: probably done |
Low risk Quote: “double‐blind, double‐dummy, randomized trial” Comment: probably done |
| Blinding of outcome assessment (detection bias) |
Low risk Quote: “double blind” Comment: probably done |
Low risk Quote: “double‐blind, double‐dummy trial” Comment: probably done |
Low risk Quote: “open‐label, randomized trial with blinded evaluation of all outcomes” Comment: probably done |
Low risk Quote: “double‐blind, double‐dummy, randomized trial” Comment: probably done |
| Incomplete outcome data (attrition bias) |
Low risk Comment: <1% patients lost follow‐up |
Low risk Comment: 0.5% incomplete information on the primary end point |
Low risk Comment: 0.11% lost to follow‐up |
Low risk Comment: 0.22% lost to follow‐up |
| Selective reporting (reporting bias) |
Low risk Comment: study protocol is available, and all of the study's prespecified outcomes of interest in the review have been reported in the prespecified way |
Low risk Comment: study protocol is available, and all of the study's prespecified outcomes of interest in the review have been reported in the prespecified way |
Low risk Comment: study protocol is not available, but the published reports clearly include all expected outcomes, including those that were prespecified |
Low risk Comment: study protocol is not available, but the published reports clearly include all expected outcomes, including those that were prespecified |
| Other potential bias |
Low risk Comment: study seems to be free of other sources of bias |
Low risk Comment: study seems to be free of other sources of bias |
Low risk Comment: study seems to be free of other sources of bias |
Low risk Comment: study seems to be free of other sources of bias |
ARISTOTLE indicates Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; ENGAGE AF, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
Outcomes According to VHD Status
Pooling results from included trials, AF patients with versus without VHD had similar rates of stroke (ischemic or hemorrhagic) or systemic embolism (HR: 1.10; 95% CI, 0.95–1.28; P=0.04 for heterogeneity, I2=63%) and intracranial hemorrhage (HR: 1.15; 95% CI, 0.95–1.40; P=0.35 for heterogeneity, I2=4%). AF patients with VHD had higher rates of all‐cause mortality (HR: 1.26; 95% CI, 1.07–1.47; P<0.0001 for heterogeneity; I2=86%) and major bleeding (HR: 1.24; 95% CI, 1.14–1.34; P=0.25 for heterogeneity; I2=26%) than AF patients without VHD (Figure 3).
Figure 3.
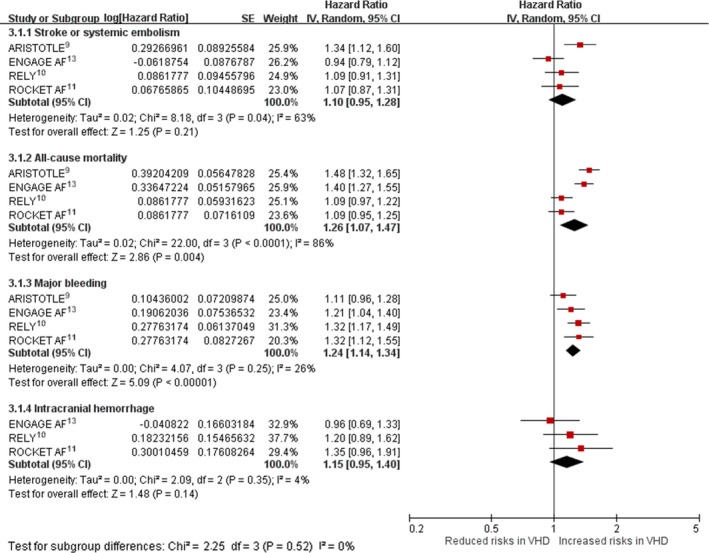
Hazard ratio with 95% confidence interval of outcomes, using overall patients enrolled in included trials, based on valvular status (VHD vs non‐VHD). ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; CI, confidence interval; ENGAGE AF, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; IV, instrumental variable; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; VHD indicates valvular heart disease.
Efficacy and Safety of NOACs and Warfarin in Patients With and Without VHD
Pooling results from the 4 included trials showed that the benefits of NOACs in comparison with warfarin in reducing stroke or systemic embolism were consistent in AF patients with VHD (HR: 0.70; 95% CI, 0.60–0.82; P=0.60 for heterogeneity; I2=0%) and without VHD (HR: 0.84; 95% CI, 0.74–0.94; P=0.13 for heterogeneity; I2=46%; Figure 4A). Pooling results from the 4 included trials showed that the NOACs in comparison with warfarin did not reduce the overall mortality rate in AF patients with VHD (HR: 1.01; 95% CI, 0.91–1.12; P=0.61 for heterogeneity; I2=0%) but reduced mortality in AF patients without VHD (HR: 0.88; 95% CI, 0.82–0.93; P=0.84 for heterogeneity; I2=0%). These differences in the HRs for the mortality rates (NOACs/warfarin) between the VHD and no‐VHD groups achieved statistical significance (P=0.03 for subgroup differences in HR; Figure 4B).
Figure 4.
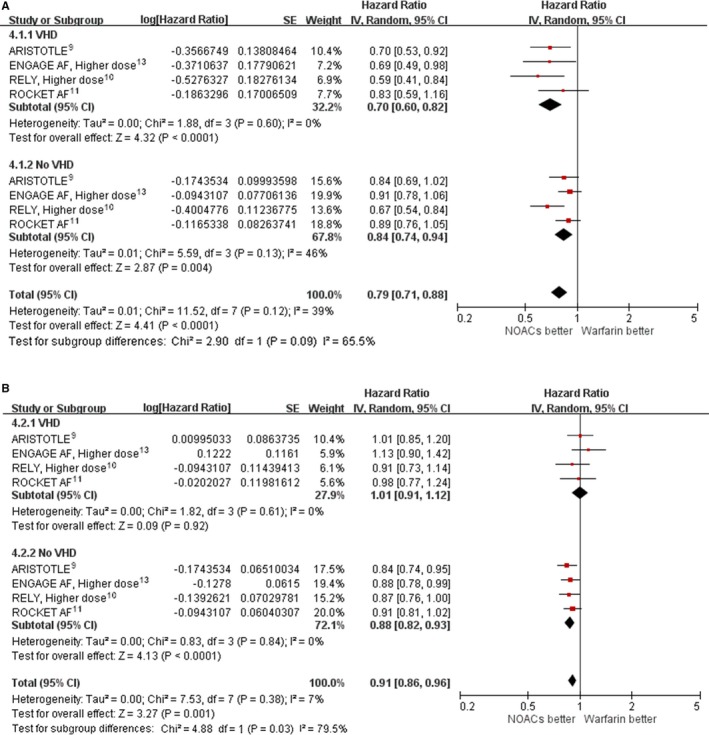
Hazard ratio with 95% confidence interval of efficacy outcomes (NOACs vs warfarin) in patients with and without VHD. A, Stroke or systemic embolism. B, Death. In ARISTOTLE, patients received apixaban 5 mg twice daily. ENGAGE AF used a higher dose, and patients received edoxaban 60 mg once daily. RE‐LY was higher dose, and patients received dabigatran 150 mg twice daily. In ROCKET AF, patients received rivaroxaban 20 or 15 mg once daily. ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; CI, confidence interval; ENGAGE AF, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; IV, instrumental variable; NOAC, non–vitamin K antagonist oral anticoagulant; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; VHD indicates valvular heart disease.
Pooling results from the 4 included trials showed that the NOACs in comparison with warfarin did not significantly reduce major bleeding in AF patients with VHD (HR: 0.93; 95% CI, 0.67–1.28; P=0.0002 for heterogeneity; I2=85%) or without VHD (HR: 0.85; 95% CI, 0.71–1.02; P=0.0003 for heterogeneity; I2=84%; Figure 5A). Pooling results from 4 included trials showed that the benefits of NOACs in comparison with warfarin in reducing intracranial hemorrhage were consistent in patients with VHD (HR: 0.47; 95% CI, 0.24–0.92; P=0.0001 for heterogeneity; I2=86%) and without VHD (HR: 0.49; 95% CI, 0.42–0.57; P=0.57 for heterogeneity; I2=0%; Figure 5B).
Figure 5.
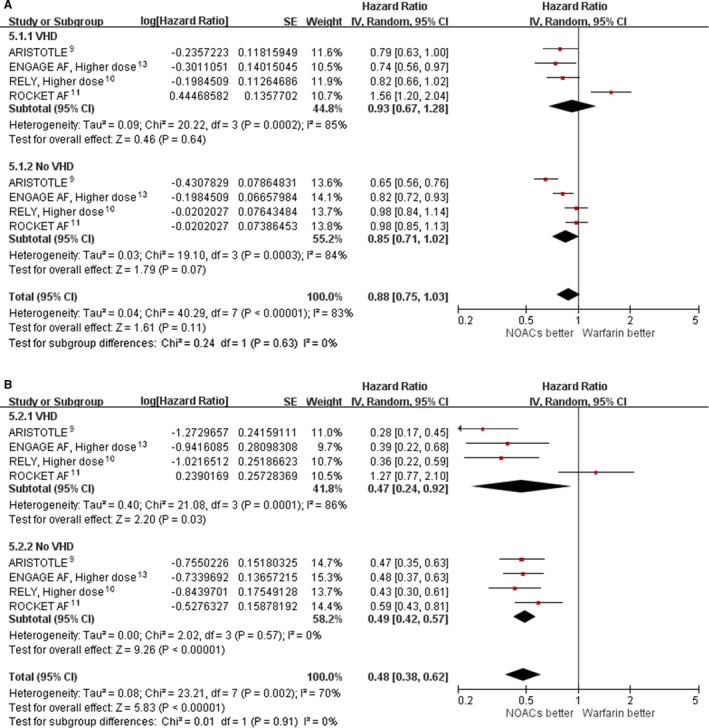
Hazard ratio with 95% confidence interval of safety outcomes (NOACs vs warfarin) in patients with and without VHD. A, Major bleeding. B, Intracranial hemorrhage. In ARISTOTLE, patients received apixaban 5 mg twice daily. ENGAGE AF used a higher dose, and patients received edoxaban 60 mg once daily. RE‐LY was higher dose, and patients received dabigatran 150 mg twice daily. In ROCKET AF, patients received rivaroxaban 20 or 15 mg once daily. ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; CI, confidence interval; ENGAGE AF, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; IV, instrumental variable; NOAC, non–vitamin K antagonist oral anticoagulant; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; VHD indicates valvular heart disease.
We conducted further analyses to explore substantial heterogeneity in major bleeding and intracranial hemorrhage end points among AF patients with VHD. Apixaban, edoxaban, and dabigatran versus warfarin reduced major bleeding (HR: 0.79; 95% CI, 0.69–0.91; P=0.85 for heterogeneity; I2=0%) among patients with VHD, whereas rivaroxaban versus warfarin increased major bleeding (HR: 1.56; 95% CI, 1.20–2.04; Figure 6A). In patients with VHD, apixaban, edoxaban, and dabigatran versus warfarin reduced intracranial hemorrhage (HR: 0.33; 95% CI, 0.25–0.45; P=0.63 for heterogeneity; I2=0%), whereas rivaroxaban versus warfarin did not show a difference in intracranial hemorrhage (HR: 1.27; 95% CI, 0.77–2.10; Figure 6B).
Figure 6.
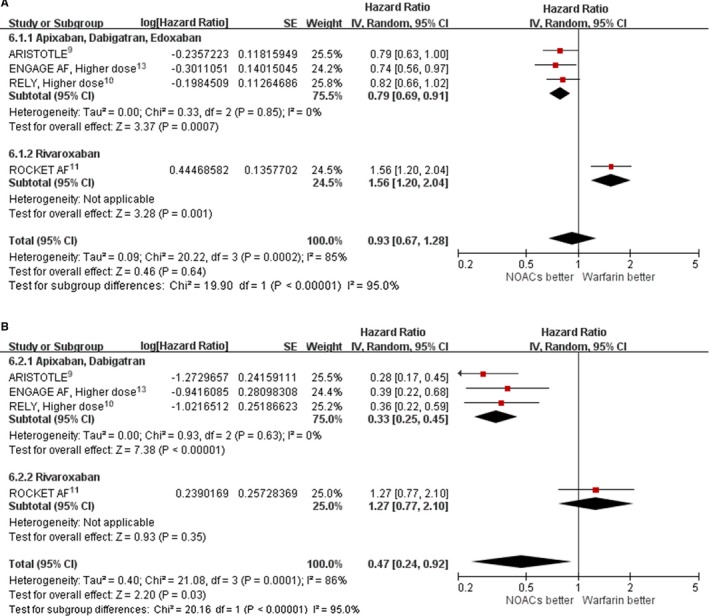
Effect of different non–vitamin K antagonist oral anticoagulants (dabigatran and apixaban vs rivaroxaban) in atrial fibrillation patients with valvular heart disease for safety end points. A, Major bleeding. B, Intracranial hemorrhage. In ARISTOTLE, patients received apixaban 5 mg twice daily. ENGAGE AF used a higher dose, and patients received edoxaban 60 mg once daily. RE‐LY was higher dose, and patients received dabigatran 150 mg twice daily. In ROCKET AF, patients received rivaroxaban 20 or 15 mg once daily. ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; CI, confidence interval; ENGAGE AF, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; IV, instrumental variable; NOAC, non–vitamin K antagonist oral anticoagulant; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
Discussion
In this meta‐analysis of 4 RCTs comparing NOACs and warfarin in >70 000 AF patients, we found that one‐fifth of patients with “nonvalvular” AF had moderate or severe VHD. AF patients with VHD were at modestly higher risk of all‐cause mortality and major bleeding than those without VHD. Importantly, the benefits of NOACs in comparison with warfarin in reducing stroke or systemic embolism were consistent in AF patients with or without VHD.
Prevention of ischemic stroke in patients with AF cannot be overemphasized. Traditionally, the vitamin K antagonists, notably warfarin, have been the cornerstone for stroke prevention in patients with AF17; however, warfarin has a narrow window of therapeutic benefit, marked variation in its effect in different patients, a need for a long‐term coagulation monitor, and increased risks of major bleeding, especially devastating intracranial hemorrhage.18 Although the original individual NOAC trials were designed as noninferiority trials and not superiority, this meta‐analysis of the pooled trials shows the NOACs, in aggregate, to be superior to warfarin for patients both with and without VHD. We observed substantial heterogeneity in major bleeding and intracranial hemorrhage end points among AF patients with VHD for which dabigatran, edoxaban, and apixaban reduced major bleeding and intracranial hemorrhage versus warfarin, whereas rivaroxaban appeared to increase major bleeding and did not reduce intracranial hemorrhage. Although rivaroxaban significantly reduced intracranial hemorrhage overall in the ROCKET trial, this effect was not seen in patients with VHD.16 Still, there was no increase in mortality risk in VHD patients treated with rivaroxaban versus warfarin. We noted that several recent observational analyses of real‐world outcomes with NOACs have been reported recently. Those results have been mixed regarding extracranial bleeding risk with rivaroxaban.
A meta‐analysis comprising the overall patient population of 4 large clinical trials showed that NOACs, compared with warfarin, further decreased the risk of stroke or systemic embolism, death, and intracranial hemorrhage in nonvalvular AF patients.19 Although experts may well understand that the term nonvalvular actually denotes that these trial patients do not have significant mitral valve stenosis or prosthetic heart valves, others may be hesitant to apply NOACs to AF patients with other types of VHD. The current study makes it clear that NOACs have similar benefits in terms of reducing stroke or systemic embolism as well as intracranial hemorrhage in AF patients both with and without VHD.
No dedicated clinical trial to date has compared NOACs with warfarin in AF patients with moderate or severe mitral valve stenosis, and so warfarin use in these patients is suggested.4 The use of a NOAC, dabigatran, in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin and thus is not justified for these patients.20
This study has limitations. First, a meta‐analysis is a retrospective approach, and subgroup analysis in patients with and without VHD was not prespecified in the original clinical trials. Second, AF patients with and without VHD had different baseline characteristics, as did patients enrolled in the 4 trials. Substantial bias might remain despite statistical adjustment. Third, the RE‐LY and ENGAGE AF trials used both higher and lower dose NOACs as active treatment agents, and their comparisons with warfarin were reported separately. Because this was a study‐level meta‐analysis, we were unable to combine groups of NOAC in a trial to create a single pairwise comparison. Consequently, only data from the higher dose NOACs versus warfarin were used in this meta‐analysis.
In conclusion, this meta‐analysis showed that AF patients with native VHD had a higher risk of all‐cause mortality and major bleeding. NOACs, compared with warfarin, reduced stroke or systemic embolism as well as intracranial hemorrhage in AF patients with or without VHD. Although evidence supported increased extracranial bleeding risk among VHD patients treated with rivaroxaban versus warfarin, there was no increase in mortality risk. Our results further establish that for AF patients with native VHD, NOACs are an attractive alternative to warfarin for stroke preventive therapy.
Sources of Funding
This study was funded by grants from Ministry of Science and Technology Taiwan (MOST104‐2314‐B‐182‐019 and MOST105‐2628‐B‐182‐008‐MY2) and Chang Gung Memorial Hospital (CORPG6D0103). The sponsors played no role in the study design, data collection and analysis, or decision to submit the article for publication.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005835 DOI: 10.1161/JAHA.117.005835.)28720644
References
- 1. Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham Study. Neurology. 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 2. Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30‐year follow‐up in the Framingham Study. JAMA. 1985;254:3449–3453. [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 4. European Heart Rhythm A, European Association for Cardio‐Thoracic S , Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 5. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; Committee R‐LS and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; Investigators RA . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 7. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; Committees A and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 8. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; Investigators EA‐T . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 9. Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M, Pais P, Erol C, Diaz R, Bahit MC, Bartunek J, De Caterina R, Goto S, Ruzyllo W, Zhu J, Granger CB, Alexander JH. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation. 2015;132:624–632. [DOI] [PubMed] [Google Scholar]
- 10. Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, Clemens A, Reilly PA, Connolly SJ, Yusuf S, Wallentin L. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease: the RE‐LY Trial (Randomized Evaluation of Long‐Term Anticoagulant Therapy). Circulation. 2016;134:589–598. [DOI] [PubMed] [Google Scholar]
- 11. Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Stevens SR, Lokhnygina Y, Patel MR, Halperin JL, Singer DE, Hankey GJ, Hacke W, Becker RC, Nessel CC, Mahaffey KW, Fox KA, Califf RM; Committee RAS and Investigators . Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non‐valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Biase L. Use of direct oral anticoagulants in patients with atrial fibrillation and valvular heart lesions. J Am Heart Assoc. 2016;5:e002776. doi: 10.1161/JAHA.115.002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Caterina R, Renda G, Carnicelli AP, Nordio F, Trevisan M, Mercuri MF, Ruff CT, Antman EM, Braunwald E, Giugliano RP. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF‐TIMI 48 trial. J Am Coll Cardiol. 2017;69:1372–1382. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. West Sussex: The Cochrane Collaboration; 2011. [Google Scholar]
- 16. Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Lokhnygina Y, Halperin JL, Singer DE, Hankey GJ, Hacke W, Becker RC, Nessel CC, Mahaffey KW, Califf RM, Fox KA, Patel MR; Committee RAS and Investigators . Native valve disease in patients with non‐valvular atrial fibrillation on warfarin or rivaroxaban. Heart. 2016;102:1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501. [DOI] [PubMed] [Google Scholar]
- 18. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest P . Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133:160S–198S. [DOI] [PubMed] [Google Scholar]
- 19. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 20. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de Werf F; Investigators R‐A . Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. [DOI] [PubMed] [Google Scholar]


