Abstract
Background
Digoxin is widely used in patients with atrial fibrillation despite the lack of randomized controlled trials. Observational studies report conflicting results regarding its association with mortality, perhaps because of residual confounding by the presence of heart failure (HF).
Methods and Results
In the ENGAGE AF‐TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation‐Thrombolysis in Myocardial Infarction 48) trial, clinical outcomes of patients with atrial fibrillation with and without HF were examined by baseline digoxin use during a median follow‐up of 2.8 years. HF was defined at baseline as prior or current clinical stage C or D HF. Of 21 105 patients enrolled, 6327 (30%) were treated with digoxin at baseline. Among patients without HF (n=8981), digoxin use (20%) was independently associated with sudden cardiac death (adjusted hazard ratio, 1.51; 95% CI, 1.10–2.08), with no significant interaction by age, sex, left ventricular ejection fraction, renal function, or concomitant medications (P>0.05 for each). Consistent results were observed using propensity matching (adjusted hazard ratio for sudden cardiac death, 1.90; 95% CI, 1.36–2.65). Among patients with HF (n=12 124), digoxin use (37%) was associated with an increase in all‐cause death, cardiovascular death, sudden cardiac death, and death caused by HF/cardiogenic shock (P<0.01 for each), but not with noncardiovascular death, stroke/systemic embolism, or myocardial infarction.
Conclusions
In this observational analysis of patients with atrial fibrillation without investigator‐reported HF, digoxin use was significantly associated with sudden cardiac death. While residual confounding cannot be excluded, the association between digoxin use and worse clinical outcomes highlights the need to examine digoxin use, particularly when prescribed to control heart rate in patients with atrial fibrillation in a randomized trial.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00781391.
Keywords: atrial fibrillation, digoxin, heart failure, mortality, sudden cardiac death
Subject Categories: Arrhythmias, Heart Failure, Sudden Cardiac Death
Clinical Perspective
What is New?
In this observational analysis of patients with atrial fibrillation with or without heart failure (HF), digoxin use, after adjusting for multiple confounders, was associated with worse clinical outcomes. In patients with HF, it was associated with all‐cause death, cardiovascular death, sudden cardiac death, HF death, and HF hospitalizations. In patients without HF, it was significantly associated with sudden cardiac death.
While the results in patients with HF might be caused by residual confounding, the study highlights the need to examine digoxin use in patients with atrial fibrillation without HF in a randomized controlled trial.
What are the Clinical Implications?
Given the availability of other rate‐control agents, until the safety of digoxin in patients with atrial fibrillation without HF has been established, the use of the drug in this population should be undertaken with great care, if at all.
Introduction
Digoxin, a positive inotropic agent that blocks atrioventricular conduction, is widely used in patients with atrial fibrillation (AF), despite the lack of randomized clinical trials in this population.1 In patients with AF and concomitant heart failure (HF), digoxin may be prescribed for both of these indications, while in patients without HF, it is used mainly as a rate‐control therapy.2, 3 The 2014 AF guidelines of the American Heart Association/American College of Cardiology/Heart Rhythm Society recommend using digoxin to control resting heart rate in patients with reduced left ventricular ejection fraction (LVEF) (class I, level of evidence C), and also in combination with β‐blockers or nondihydropyridine calcium channel blockers, regardless of HF status (class IIa, level of evidence B).4, 5 The 2016 European Society of Cardiology AF guidelines recommend using digoxin in the long‐term management of AF patients with LVEF above or below 40% (class I, level of evidence B).3
Conflicting data exist regarding adverse outcomes that are associated with digoxin use in patients with AF.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 While several retrospective studies and meta‐analyses have demonstrated an association between digoxin use and all‐cause mortality, cardiovascular death, and sudden cardiac death (SCD),6, 7, 8, 9, 10, 11, 12, 13 other studies have not found a compelling association.14, 15, 16 These contradictory results can be attributed to the nature of observational studies and to digoxin prescription biases. It remains unclear whether the association between digoxin and worse clinical outcome is causal or may be the result of residual confounding, mainly dominated by the presence of HF.
Thus, we aimed to examine the association between digoxin use and clinical outcomes (fatal and nonfatal events) in a large contemporary cohort of patients with AF both with and without HF.
Methods
This study represents an analysis from the ENGAGE AF‐TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation‐Thrombolysis in Myocardial Infarction 48) trial. The ENGAGE AF‐TIMI 48 trial was a 3‐group, randomized, double‐blind, double‐dummy multinational trial that compared 2 dosing regimens of the oral factor Xa inhibitor edoxaban with warfarin in 21 105 patients with AF (NCT00781391).17 A detailed description of the study population, study design, and results of the trial have been published.17, 18 In brief, eligible patients were 21 years and older with ECG evidence of AF within 12 months before randomization and had a CHADS2 score ≥2 and planned anticoagulation. Key exclusion criteria were AF attributable to a reversible cause, presence of a mechanical valve or moderate‐severe mitral stenosis, increased risk of bleeding, recent acute coronary syndrome or stroke within 30 days before randomization, severe renal insufficiency (creatinine clearance <30 mL/min), and life expectancy <12 months. Patients with any class of HF could be enrolled provided they were clinically stable at the time of randomization. The protocol and amendments were approved by the ethics committee at each participating center. All patients provided written informed consent.
Digoxin Use and HF Definition
Digoxin use was captured by the treating physician in the concomitant medication case report form at baseline before enrollment. HF was defined as the presence or history of clinical stage C or D HF according to the American College of Cardiology/American Heart Association criteria.19 This includes structural heart disease with prior or current symptoms of HF, such as shortness of breath, fatigue, and decreased exercise tolerance (stage C), or refractory HF requiring specialized interventions (stage D). Investigators classified HF at baseline as “systolic,” “diastolic,” or “both,” provided the New York Heart Association functional class, and when available, reported the most recent LVEF before randomization.
Clinical End Points
End points of this analysis were as defined in the trial protocol17, 18 and examined during a median follow‐up of 2.8 years. Deaths in the ENGAGE AF‐TIMI 48 trial were adjudicated by an independent clinical end point committee without knowledge of randomized anticoagulant treatment assignment. Deaths were categorized as cardiovascular or noncardiovascular. Cardiovascular death included SCD, defined as an unexpected death that was either witnessed (occurring within 60 minutes from the onset of new symptoms, in the absence of a clear cause other than cardiovascular) or unwitnessed (within 24 hours of being observed alive, in the absence of preexisting progressive circulatory failure or other noncardiovascular causes of death), death caused by HF or cardiogenic shock, or other cardiovascular causes (eg, systemic embolic event, pulmonary embolism, atherosclerotic vascular disease excluding coronary, related to coronary revascularization, or other).17, 18 Nonfatal events included stroke or systemic embolism, HF hospitalization, or myocardial infarction (MI).
Statistical Analysis
Baseline characteristics of patients by digoxin treatment and HF status were compared between groups using chi‐square tests for categorical variables and Kruskal–Wallis tests for continuous variables. Three methods were used to examine the association between digoxin use and clinical outcomes. The first used the Cox proportional hazards model. Clinical outcomes were examined in the intention‐to‐treat population, including all events between randomization and the end of the study treatment period, whether occurring on or off study drug. Variables in the multivariable models were based on the baseline characteristics, with a P<0.1 in the univariate model, omitting colinear variables and adding variables that were judged to be of clinical importance. All multivariable analyses were adjusted for the following baseline characteristics: age; sex; weight; region; race; creatinine; pattern of AF (persistent/permanent versus paroxysmal); hypertension; diabetes mellitus; prior stroke/transient ischemic attack; coronary artery disease; prior MI; peripheral arterial disease; smoking (current or past); increased risk of falling; LVEF; HF (when appropriate); mitral valve disease; aortic valve disease; prior electrical cardioversion; left ventricular hypertrophy on ECG; use of a vitamin K antagonist ≥60 consecutive days at any time before enrollment; lipid‐lowering drugs; class I, class II (β‐blockers), or class III antiarrhythmics; renin‐angiotensin‐aldosterone system inhibitors; randomization group; heart rate; chronic obstructive pulmonary disease; and diuretic use. Clinical outcomes were examined separately in patients with or without HF. Among patients with HF, clinical outcomes were further examined in patients with systolic HF, diastolic HF, or both.
The second method was propensity matching, which was performed on key baseline characteristics to balance digoxin use at baseline in patients with or without HF. Matching was performed using a 1:1 nearest neighbor approach without replacement with a caliper of 0.1 of the SD. In a sensitivity analysis of the propensity model, we explored a trimmed model eliminating patients with extreme propensity scores (<5th or >95th percentile) to reduce the potential impact of unmeasured confounders.
The third method utilized inverse probability treatment weighting (IPTW), which uses stablized weights calculated with the inverse value of the propensity scores.
A sensitivity analysis using digoxin as a time‐varying covariate examined the association between digoxin use during the trial and clinical outcomes in patients without HF. Digoxin use was reported at each study visit (every 3 months) and was regarded as a time‐dependent variable using 2 methods. In the first method, if digoxin was not present at baseline but was started during follow‐up, the patient was regarded as taking digoxin throughout the trial (digoxin “on”), while in the second method, the patient was regarded as taking or not taking digoxin per the status of digoxin use every 3 months (digoxin “on/off”).
Results are presented as hazard ratios (HRs) and 95% CIs, with P<0.05 considered significant. Propensity score matching was performed using R Studio. All other analyses were performed in SAS Enterprise Guide 7.1 (SAS Institute Inc).
Results
Overall Population
Of the 21 105 patients who were enrolled in the ENGAGE AF‐TIMI 48 trial, 6327 patients (30.0%) were treated with digoxin at baseline. Compared with patients not treated with digoxin, patients taking digoxin were slightly younger, more often female, and were more likely to have persistent/permanent AF, HF at baseline, and lower LVEF (P<0.001 for each; other baseline variables that differed between the 2 groups are shown in Table 1).20, 21, 22
Table 1.
Baseline Characteristics by Digoxin Use at Baseline
| Characteristic, No. (%) | Digoxin n=6327 (30%) | No Digoxin n=14 778 (70%) | P Value | Total (N=21 105) |
|---|---|---|---|---|
| Age, median (IQR), y | 71 (63–77) | 72 (65–78) | <0.001 | 72 (64–78) |
| Female sex | 2556 (40.4) | 5484 (37.1) | <0.001 | 8040 (38.1) |
| Weight, mean (SD), kg | 82.0 (20.6) | 84.7 (20.0) | <0.001 | 83.9 (20.2) |
| Regiona | <0.001 | |||
| North America | 1154 (18.2) | 3527 (23.9) | 4681 (22.2) | |
| Latin America | 977 (15.4) | 1684 (11.4) | 2661 (12.6) | |
| Western Europe | 733 (11.6) | 2503 (16.9) | 3236 (15.3) | |
| Eastern Europe | 2315 (36.6) | 4829 (32.7) | 7144 (33.8) | |
| Asia‐Pacific and South Africa | 1148 (18.1) | 2235 (15.1) | 3383 (16.0) | |
| White race (n=21 104) | 4892 (77.3) | 12 175 (82.4) | <0.001 | 17 067 (80.9) |
| Type of atrial fibrillation (n=21 099) | <0.001 | |||
| Paroxysmal | 713 (11.3) | 4653 (31.5) | 5366 (25.4) | |
| Persistent | 1424 (22.5) | 3444 (23.3) | 4868 (23.1) | |
| Permanent | 4189 (66.2) | 6676 (45.2) | 10 865 (51.5) | |
| Qualifying risk factor | ||||
| Congestive heart failure | 4502 (71.2) | 7622 (51.6) | <0.001 | 12 124 (57.4) |
| Hypertension requiring treatment | 5853 (92.5) | 13 901 (94.1) | <0.001 | 19 754 (93.6) |
| Age ≥75 y | 2279 (36.0) | 6195 (41.9) | <0.001 | 8474 (40.2) |
| Diabetes mellitus | 2329 (36.8) | 5295 (35.8) | 0.17 | 7624 (36.1) |
| Prior stroke or transient ischemic attack | 1706 (27.0) | 4267 (28.9) | 0.005 | 5973 (28.3) |
| CHADS2 scoreb | ||||
| Mean (SD) | 2.9 (1.0) | 2.8 (1.0) | <0.001 | 2.8 (1.0) |
| 4 to 6 | 1531 (24.2) | 3237 (21.9) | <0.001 | 4768 (22.6) |
| CHA2DS2‐VASc scoreb | ||||
| Mean (SD) | 4.4 (1.4) | 4.3 (1.4) | 0.06 | 4.3 (1.4) |
| 4 to 9 | 4471 (70.7) | 10 448 (70.7) | 0.96 | 14 919 (70.7) |
| HAS‐BLED scoreb | ||||
| Mean (SD) | 2.4 (0.9) | 2.6 (1.0) | <0.001 | 2.5 (1.0) |
| ≥3 | 2643 (41.8) | 7159 (48.4) | <0.001 | 9802 (46.4) |
| Coronary artery disease (n=21 102) | 2004 (31.7) | 5019 (34.0) | 0.001 | 7023 (33.3) |
| Prior myocardial infarction | 704 (11.1) | 1729 (11.7) | 0.23 | 2433 (11.5) |
| Ejection fractionc | <0.001 | |||
| <30% | 398 (8.6) | 384 (3.5) | 782 (5.0) | |
| 30% to 39% | 673 (14.6) | 848 (7.7) | 1521 (9.8) | |
| 40% to 49% | 1058 (22.9) | 1810 (16.5) | 2868 (18.4) | |
| ≥50% | 2488 (53.9) | 7905 (72.2) | 10 393 (66.8) | |
| NYHA III or IVd (n=11 988) | 1220 (27.4) | 1415 (18.8) | <0.001 | 2635 (22.0) |
| Peripheral arterial disease (n=21 096) | 211 (3.3) | 630 (4.3) | 0.002 | 841 (4.0) |
| Former/current smoker (n=21 098) | 532 (8.4) | 1020 (6.9) | <0.001 | 1552 (7.4) |
| COPD | 626 (9.9) | 1155 (7.8) | <0.001 | 1781 (8.4) |
| Mitral valve disease (n=20 983) | 2276 (36.2) | 4972 (33.8) | 0.001 | 7248 (34.5) |
| Aortic valve disease (n=21 014) | 907 (14.4) | 2227 (15.1) | 0.17 | 3134 (14.9) |
| Prior electrical cardioversion for AF (n=21 104) | 995 (15.7) | 2796 (18.9) | <0.001 | 3791 (18.0) |
| Charlson comorbidity index, mean (SD) | 2.9 (1.1) | 2.8 (1.1) | <0.001 | 2.8 (1.1) |
| Heart rate, mean (SD), bpm (n=21 074) | 77.1 (14.2) | 73.0 (13.8) | <0.001 | 74.3 (14.0) |
| Hypertrophy per ECG (n=20 950) | 1311 (20.8) | 2126 (14.5) | <0.001 | 3437 (16.4) |
| Vitamin K antagonist experiencede (n=21 104) | 3918 (61.9) | 8523 (57.7) | <0.001 | 12 441 (59.0) |
| Medication at randomization | ||||
| Aspirin (n=21 101) | 1630 (25.8) | 4550 (30.8) | <0.001 | 6180 (29.3) |
| Lipid‐lowering | 2558 (40.4) | 7524 (50.9) | <0.001 | 10 082 (47.8) |
| Antiarrhythmics | ||||
| Class I | 95 (1.5) | 798 (5.4) | <0.001 | 893 (4.2) |
| Class II (β‐blockers) | 4119 (65.1) | 9865 (66.8) | 0.02 | 13 984 (66.3) |
| Class III | 588 (9.3) | 2546 (17.2) | <0.001 | 3134 (14.8) |
| Class IV | 629 (9.9) | 1276 (8.6) | 0.002 | 1905 (9.0) |
| Diuretics | 4445 (70.3) | 8211 (55.6) | <0.001 | 12 656 (60.0) |
| RAAS inhibitor | 4313 (68.2) | 9593 (64.9) | <0.001 | 13 906 (65.9) |
| CrCl, median (IQR), mL/min | 70.4 (53.5–93.0) | 70.3 (53.9–91.4) | 0.37 | 70.3 (53.8–91.9) |
| Dose reduction at randomization | 1779 (28.1) | 3577 (24.2) | <0.001 | 5356 (25.4) |
| Randomized treatment | ||||
| High‐dose edoxaban | 2078 (32.8) | 4957 (33.5) | 0.10 | 7035 (33.3) |
| Low‐dose edoxaban | 2073 (32.8) | 4961 (33.6) | 7034 (33.3) | |
| Warfarin | 2176 (34.4) | 4860 (32.9) | 7036 (33.3) | |
Data are expressed as number (percentage) unless otherwise indicated. bpm indicates beats per minute; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; IQR, interquartile range; RAAS, renin‐angiotensin‐aldosterone system.
Percentages are for each region except for the Total column.
Ejection fraction was unknown for 5541 patients (digoxin, 1710 patients; no digoxin, 3831 patients).
New York Heart Association (NYHA) functional class at baseline was reported only in patients with heart failure.
Vitamin K antagonist experienced denotes ≥60 consecutive days of treatment with a vitamin K antagonist at any time before enrollment.
Digoxin use was associated with all‐cause mortality (annual rate, 5.2% versus 3.6%; adjusted HR, 1.22 [95% CI, 1.12–1.34]), cardiovascular death, SCD, death caused by HF or cardiogenic shock, and HF hospitalizations (Table 2). No significant association was observed between digoxin use at baseline and noncardiovascular death, stroke or systemic embolism, or MI (Table 2). There was no effect of randomized treatment on the relationship between digoxin use and clinical outcomes (data not shown).
Table 2.
Clinical Outcomes by Digoxin Use and HF Status at Baseline
| End Point, No. (%/y) | Total (N=21 105) | HF (n=12 124) | No HF (n=8981) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Digoxin (n=6327) | No Digoxin (n=14 778) | Adjusted HRa (95% CI) | Digoxin (n=4502) | No Digoxin (n=7622) | Adjusted HRa (95% CI) | Digoxin (n=1825) | No Digoxin (n=7156) | Adjusted HRa (95% CI) | |
| All‐cause death | 899 (5.2) | 1450 (3.6) | 1.22 (1.12–1.34) | 714 (5.9) | 870 (4.2) | 1.27 (1.14–1.41) | 185 (3.6) | 580 (2.9) | 1.10 (0.92–1.31) |
| Cardiovascular death | 673 (3.9) | 995 (2.4) | 1.27 (1.14–1.41) | 555 (4.6) | 633 (3.0) | 1.32 (1.16–1.49) | 118 (2.3) | 362 (1.8) | 1.13 (0.91–1.41) |
| SCD | 333 (1.9) | 416 (1.0) | 1.49 (1.27–1.74) | 273 (2.3) | 273 (1.3) | 1.45 (1.21–1.74) | 60 (1.2) | 143 (0.7) | 1.51 (1.10–2.08) |
| HF/cardiogenic shock death | 173 (1.0) | 217 (0.5) | 1.41 (1.14–1.76) | 152 (1.3) | 162 (0.8) | 1.44 (1.13–1.83) | 21 (0.4) | 55 (0.3) | 1.34 (0.77–2.33) |
| Noncardiovascular death | 226 (1.3) | 455 (1.1) | 1.10 (0.92–1.31) | 159 (1.3) | 237 (1.1) | 1.12 (0.90–1.40) | 67 (1.3) | 218 (1.1) | 1.04 (0.77–1.40) |
| Stroke/SSE | 317 (1.9) | 699 (1.8) | 1.00 (0.87–1.16) | 218 (1.9) | 348 (1.7) | 0.99 (0.83–1.18) | 99 (2.0) | 351 (1.8) | 1.06 (0.84–1.33) |
| HF hospitalizations | 688 (4.3) | 1073 (2.8) | 1.22 (1.10–1.36) | 608 (5.5) | 786 (4.0) | 1.21 (1.08–1.36) | 80 (1.6) | 287 (1.5) | 1.11 (0.85–1.45) |
| MI | 124 (0.7) | 319 (0.8) | 0.91 (0.73–1.14) | 86 (0.7) | 175 (0.9) | 0.85 (0.64–1.13) | 38 (0.8) | 144 (0.7) | 1.08 (0.74–1.58) |
SCD indicates sudden cardiac death; SSE, stroke or systemic embolism.
Hazard ratios (HRs) are adjusted for age; weight; sex; region; race; creatinine; atrial fibrillation type; heart failure (HF; for total HRs only); hypertension; diabetes mellitus; history of stroke or transient ischemic attack; history of coronary artery disease; prior myocardial infarction (MI); history of peripheral artery disease; smoking status; increased risk of falling; left ventricular ejection fraction; mitral valve disease; aortic valve disease; prior electrical cardioversion; left ventricular hypertrophy; previous use of vitamin K antagonists ≥60 days before randomization; lipid‐lowering medications; class I, II, or III antiarrhythmics; renin‐angiotensin‐aldosterone inhibitors; randomized treatment group; heart rate; history of chronic obstructive pulmonary disease; and diuretic use at randomization.
Patients With HF at Baseline
A total of 12 124 patients (57.4%) had HF reported at baseline. Of these, 4502 patients (37.1%) were treated with digoxin at baseline. Compared with patients not treated with digoxin, patients treated with digoxin were younger and more likely to have permanent AF, lower LVEF, worse New York Heart Association class, and higher heart rate and be treated with diuretics (P<0.001 for each, Table S1).
In patients with HF, digoxin use was associated with an increase in all‐cause mortality (adjusted HR, 1.27; 95% CI, 1.14–1.41), cardiovascular death (adjusted HR, 1.32; 95% CI, 1.16–1.49), SCD (adjusted HR, 1.45; 95% CI, 1.21–1.74), death caused by HF or cardiogenic shock (adjusted HR, 1.44; 95% CI, 1.13–1.83), and HF hospitalizations (adjusted HR, 1.21; 95% CI, 1.08–1.36) (Table 2). There was no significant association between digoxin use at baseline and noncardiovascular death, stroke or systemic embolism, or MI (Table 2). Consistent qualitatively similar results were demonstrated using propensity matching and IPTW for all end points except for noncardiovascular death (matched HR, 1.21; 95% CI, 1.00–1.46) and MI (matched HR, 0.75; 95% CI, 0.59–0.95) (Table 3).
Table 3.
Digoxin Use in Patients With or Without HF at Baseline Using Propensity Matching and Inverse Probability Weighting
| End Point | HF | No HF | ||
|---|---|---|---|---|
| Propensity Matchinga HR (95% CI) | Inverse Probability Weighting HR (95% CI) | Propensity Matchingb HR (95% CI) | Inverse Probability Weighting HR (95% CI) | |
| All‐cause death | 1.31 (1.19–1.43) | 1.29 (1.15–1.44) | 1.16 (0.98–1.36) | 1.13 (0.92–1.38) |
| Cardiovascular death | 1.34 (1.20–1.48) | 1.33 (1.17–1.51) | 1.22 (0.99–1.50) | 1.11 (0.87–1.41) |
| SCD | 1.58 (1.36–1.85) | 1.46 (1.21–1.75) | 1.90 (1.36–2.65) | 1.47 (1.02–2.10) |
| HF/cardiogenic shock death | 1.49 (1.21–1.84) | 1.46 (1.14–1.86) | 1.40 (0.78–2.50) | 1.34 (0.75–2.38) |
| Noncardiovascular death | 1.21 (1.00–1.46) | 1.19 (0.93–1.51) | 1.06 (0.81–1.38) | 1.17 (0.82–1.65) |
| Stroke/SSE | 0.87 (0.75–1.01) | 1.00 (0.83–1.21) | 0.84 (0.69–1.03) | 0.97 (0.75–1.25) |
| HF hospitalizations | 1.12 (1.02–1.24) | 1.27 (1.12–1.43) | 1.18 (0.91–1.51) | 1.20 (0.89–1.63) |
| MI | 0.75 (0.59–0.95) | 0.95 (0.70–1.28) | 0.83 (0.59–1.18) | 1.08 (0.71–1.65) |
HR indicates hazard ratio; MI, myocardial infarction; SCD, sudden cardiac death; SSE, stroke or systemic embolism.
Included 4051 matched pairs based on age; sex; region; race; weight; atrial fibrillation type; hypertension; diabetes mellitus; history of stroke or transient ischemic attack; history of coronary artery disease; history of peripheral artery disease; increased risk of falling; left ventricular ejection fraction; prior electrical cardioversion; hypertrophy per ECG; pervious use of vitamin K agonists for ≥60 days before randomization; lipid‐lowering medications; class I, II, III, or IV antiarrhythmics; renin‐angiotensin‐aldosterone inhibitors; randomized treatment group; diuretic use; aspirin use; dose adjustment; serum creatinine; heart rate; Charlson comorbidity index; and heart failure (HF) type.
Included 1817 matched pairs based on age; sex; region; race; weight; atrial fibrillation type; hypertension; diabetes mellitus; history of stroke or transient ischemic attack; history of coronary artery disease; history of peripheral artery disease; increased risk of falling; left ventricular ejection fraction; prior electrical cardioversion; hypertrophy per ECG; pervious use of vitamin K agonists for ≥60 days before randomization; lipid‐lowering medications; class I, II, III, or IV antiarrhythmics; renin‐angiotensin‐aldosterone inhibitors; randomized treatment group; diuretic use; aspirin use; dose adjustment; serum creatinine; heart rate; and Charlson comorbidity index.
Among patients with systolic HF (n=5027), including 2875 patients with isolated systolic and 2152 patients with both systolic and diastolic HF, 34.3% were treated with digoxin at baseline. Among patients with isolated diastolic HF (n=4150), 41.3% were treated with digoxin at baseline. In patients with any systolic HF, digoxin use was associated with all‐cause death, cardiovascular death, SCD, and death caused by HF or cardiogenic shock, with the latter being the strongest association (adjusted HR, 2.20; 95% CI, 1.48–3.29) (Table S2). In patients with isolated diastolic HF, a similar qualitative association between digoxin use and worse outcomes was observed, with the exception of death caused by HF or cardiogenic shock (adjusted HR, 1.17; 95% CI, 0.79–1.72 [P interaction by HF type=0.01]) (Table S2).
Patients Without HF at Baseline
A total of 8981 patients (42.6%) did not have HF at baseline. Of these, 1825 patients (20%) were treated with digoxin at baseline. Compared with patients not treated with digoxin, patients treated with digoxin were of similar age (median 75 years), were more likely to have permanent AF and higher heart rate, and were less likely to be treated with beta blockers (Table S1).
In patients without HF, digoxin use was significantly associated with SCD (adjusted HR, 1.51; 95% CI, 1.10–2.08 [P=0.01]) but not all‐cause mortality, other causes of death, or nonfatal events (Table 2; Figure 1). Qualitatively similar results were demonstrated using IPTW (HR for SCD, 1.47; 95% CI, 1.02–2.10 [P=0.04]), propensity matching (HR for SCD, 1.90; 95% CI, 1.36–2.65 [P<0.001]) (Table 3), and a trimmed propensity score (1.37; 95% CI, 0.96–1.95 [P=0.08]).
Figure 1.
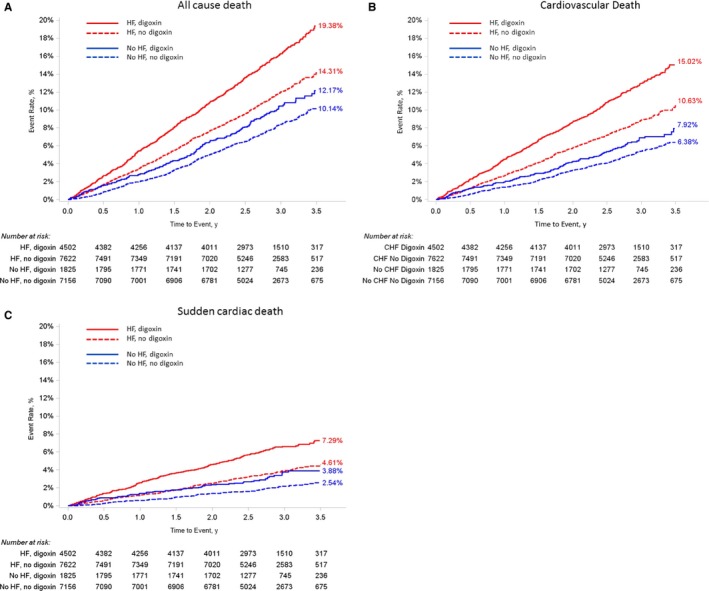
Kaplan–Meier curves of (A) all‐cause death, (B) cardiovascular death, and (C) sudden cardiac death, by digoxin use at baseline and heart failure (HF) status. P interaction between digoxin use and HF status is 0.16 (A), 0.14 (B), and 0.75 (C). CHF indicates congestive heart failure.
There was no significant heterogeneity in the association between digoxin use and SCD across subgroups including age, sex, region, weight, renal function, β‐blocker use, and heart rate (Table 4). In addition, in patients without HF with LVEF ≥50% (n=5525), digoxin use was associated with SCD (adjusted HR, 1.58; 95% CI, 1.03–2.43) but not other causes of death including death caused by HF or cardiogenic shock (adjusted HR, 0.85; 95% CI, 0.35–2.06 [Figure 2]).
Table 4.
SCD by Digoxin Use in Subgroups of Patients Without Heart Failure
| Subgroup | Patients, No. | Digoxin SCD, No. (%/y) | No Digoxin SCD, No. (%/y) | Adjusted HRa (95% CI) | P Interaction |
|---|---|---|---|---|---|
| Age, y | 0.36 | ||||
| <65 | 1665 | 7 (0.8) | 24 (0.6) | 1.11 (0.47–2.61) | |
| 65–74 | 2657 | 17 (1.0) | 27 (0.5) | 2.21 (1.17–4.15) | |
| ≥75 | 4659 | 36 (1.4) | 92 (0.9) | 1.39 (0.92–2.11) | |
| Sex | 0.85 | ||||
| Male | 5517 | 38 (1.3) | 97 (0.8) | 1.55 (1.04–2.29) | |
| Female | 3464 | 22 (1.0) | 46 (0.6) | 1.46 (0.86–2.45) | |
| Race | 0.98 | ||||
| White | 7115 | 43 (1.1) | 108 (0.7) | 1.52 (1.05–2.21) | |
| Nonwhite | 1865 | 17 (1.5) | 35 (0.9) | 1.51 (0.83–2.73) | |
| Region | 0.33 | ||||
| North America | 2814 | 17 (1.0) | 36 (0.6) | 1.73 (0.95–3.15) | |
| Latin America | 975 | 10 (1.7) | 37 (1.9) | 0.77 (0.37–1.62) | |
| Western Europe | 1808 | 11 (1.2) | 29 (0.7) | 1.72 (0.85–3.49) | |
| Eastern Europe | 1719 | 9 (0.9) | 19 (0.5) | 1.83 (0.80–4.18) | |
| Asia‐pacific and South Africa | 1665 | 13 (1.3) | 22 (0.6) | 2.11 (1.06–4.20) | |
| Weight, kg | 0.81 | ||||
| ≤60 | 956 | 14 (1.9) | 21 (1.1) | 1.64 (0.83–3.25) | |
| >60 | 8025 | 46 (1.0) | 122 (0.7) | 1.50 (1.04–2.14) | |
| Type of AF | 0.03 | ||||
| Paroxysmal | 2928 | 2 (0.2) | 44 (0.6) | 0.21 (0.03–1.45) | |
| Persistent/permanent | 6048 | 58 (1.3) | 99 (0.8) | 1.77 (1.25–2.50) | |
| LV ejection fraction | 0.33 | ||||
| <50% | 597 | 10 (2.5) | 16 (1.3) | 2.48 (1.06–5.81) | |
| ≥50% | 5525 | 31 (1.1) | 82 (0.6) | 1.55 (1.01–2.37) | |
| Charlson comorbidity index | 0.45 | ||||
| ≤Mean | 5227 | 32 (1.1) | 75 (0.6) | 1.64 (1.07–2.52) | |
| >Mean | 3754 | 28 (1.2) | 68 (0.8) | 1.30 (0.82–2.05) | |
| LV hypertrophy per ECG | 0.55 | ||||
| Yes | 737 | 9 (1.8) | 22 (1.5) | 1.23 (0.54–2.80) | |
| No | 8164 | 50 (1.1) | 118 (0.6) | 1.61 (1.15–2.25) | |
| Heart rate at baseline, bpm | 0.40 | ||||
| <80 | 6365 | 30 (0.9) | 92 (0.6) | 1.35 (0.89–2.05) | |
| ≥80 | 2596 | 30 (1.7) | 49 (0.9) | 1.77 (1.09–2.88) | |
| β‐Blockers at baseline | 0.35 | ||||
| Yes | 5313 | 36 (1.3) | 87 (0.7) | 1.72 (1.15–2.58) | |
| No | 3668 | 24 (1.1) | 56 (0.7) | 1.26 (0.77–2.08) | |
| Diuretic use at baseline | 0.38 | ||||
| Yes | 3918 | 33 (1.3) | 63 (0.7) | 1.74 (1.12–2.71) | |
| No | 5063 | 27 (1.0) | 80 (0.7) | 1.31 (0.83–2.07) | |
| CrCl, mL/min | 0.96 | ||||
| ≤60 | 3356 | 33 (1.7) | 78 (1.1) | 1.51 (0.98–2.33) | |
| >60 | 5625 | 27 (0.8) | 65 (0.5) | 1.48 (0.94–2.35) | |
| Randomized treatment | 0.59 | ||||
| High‐dose edoxaban | 2938 | 20 (1.2) | 45 (0.7) | 1.70 (1.00–2.90) | |
| Low‐dose edoxaban | 3055 | 12 (0.7) | 44 (0.6) | 1.13 (0.59–2.17) | |
| Warfarin | 2988 | 28 (1.6) | 54 (0.8) | 1.64 (1.02–2.64) |
AF indicates atrial fibrillation; bpm, beats per minute; CrCl, creatinine clearance; SCD, sudden cardiac death.
Hazard ratios (HRs) are adjusted for age; weight; sex; region; race; creatinine; atrial fibrillation type; hypertension; diabetes mellitus; history of stroke or transient ischemic attack; history of coronary artery disease; prior myocardial infarction; history of peripheral artery disease; smoking status; increased risk of falling; left ventricular (LV) ejection fraction; mitral valve disease; aortic valve disease; prior electrical cardioversion; LV hypertrophy; previous use of vitamin K antagonists ≥60 days before randomization; lipid‐lowering medications; class I, II, or III antiarrhythmics; renin‐angiotensin‐aldosterone inhibitors; randomized treatment group; heart rate; history of chronic obstructive pulmonary disease; and diuretic use at randomization.
Figure 2.
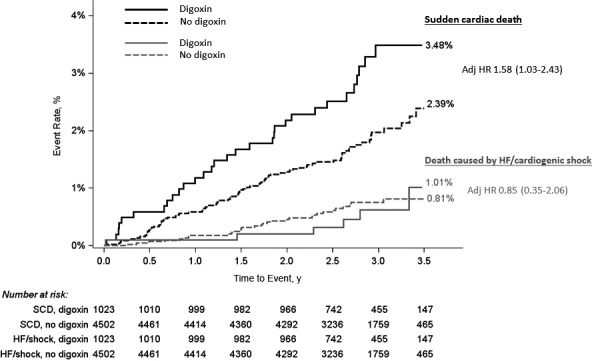
Kaplan–Meier curve of sudden cardiac death (SCD) and death caused by heart failure (HF) or cardiogenic shock, by digoxin use in patients without HF and left ventricular ejection fraction (LVEF) ≥50% (n=5525). Log‐rank P value for SCD=0.02; log‐rank P value for death caused by HF or cardiogenic shock=0.95. HR indicates hazard ratio.
In a sensitivity analysis using digoxin as a time‐variant covariate, digoxin use remained a significant predictor of SCD (HR, 5.53; 95% CI, 1.66–18.41 in the digoxin “on” analysis and HR, 4.45; 95% CI, 1.37–14.44 in the digoxin “on/off” analysis) (Table S3). Digoxin was not independently associated with all‐cause death, all cardiovascular death (including both SCD and non‐SCD), or noncardiovascular death in either analysis (Table S3).
Discussion
In the current analysis from a large international cohort of patients with AF, treatment with digoxin was common, with more than a third of the patients with concomitant HF and a fifth of the patients without HF taking the agent. In the trial as a whole, digoxin use was associated with increased all‐cause mortality, cardiovascular death, SCD, death caused by HF or cardiogenic shock, and HF hospitalization. This association was evident particularly among patients with HF. Importantly, in patients without HF, digoxin use was significantly associated with SCD. While the association between digoxin use and clinical outcomes may be a result of residual confounding by differences in comorbidities and treatment in patients with HF, it is less likely to be influenced by these factors in patients without HF.
Digoxin is widely used in patients with AF for several indications. While in patients with concomitant HF it is used both as a positive inotropic agent and as rate‐control therapy, in patients with AF without HF it is prescribed primarily to achieve better rate control. As demonstrated in this study and others,1, 9, 14 in patients with HF, digoxin is prescribed to patients with more advanced HF, lower LVEF, worse New York Heart Association functional class, and greater burden of other comorbidities. As such, the association between digoxin use and clinical outcomes in patients with HF may be biased by residual confounding caused by the baseline medical condition and concomitant medications.15 Adjusting for multiple confounders, propensity matching, and IPTW may not fully account for this difference. Thus, our results in patients with HF should be interpreted with caution. In contrast, in patients without HF who are presumably treated with digoxin as a rate‐control therapy, residual confounding may play a much smaller role. In these patients, the association between digoxin use and SCD, which was also demonstrated in propensity matching and IPTW, was robust. Furthermore, given the possibility that among patients who were categorized as not having HF at baseline there were patients with undiagnosed HF, the association between digoxin use and clinical outcomes was also examined in a more homogenous group of patients without HF and LVEF ≥50%. In these patients, not only did the association between digoxin use and SCD remain significant, but the adjusted HRs were qualitatively similar. Furthermore, these results were strengthened by the lack of association between digoxin use and death caused by HF or cardiogenic shock (Figure 2).
SCD is the single most common cause of death among patients with AF, accounting for about a third of all deaths.23, 24, 25, 26 In a recent analysis from the ENGAGE AF‐TIMI 48 trial, the cause for SCD was unknown in most cases and an arrhythmia was documented in only a minority of SCD events (11.5%).25 Digoxin is an important factor associated with SCD in this population.25 The association between digoxin use and SCD in patients with AF appears to be more dominant in individuals without HF, yet the causality is not clear and seems complex. Digoxin has a narrow therapeutic range and is influenced by drug‐to‐drug interactions, serum electrolyte concentrations, and renal function.3 In patients with AF, the majority of whom are elderly with concomitant medically treated conditions, digoxin concentration may fluctuate. In this study, patients without HF who were treated with digoxin were elderly (more than half were ≥75 years) with a high burden of comorbidities including diabetes mellitus and reduced renal function. These characteristics may have predisposed them to abnormal digoxin concentration, yet our results did not show any heterogeneity for the risk of SCD by any of these factors, perhaps owing to low statistical power. Whether the concomitant use of β‐blockers with digoxin in patients without HF has a protective effect against SCD is not known and it cannot be established from this observational study. In addition, whether digoxin's inotropic activity in patients without HF can be harmful deserves further research. Thus, revealing additional mechanisms of the association between digoxin use and SCD in patients with AF without HF is of interest.
This study has several clinical implications, mainly in patients with AF without HF, in whom randomized studies with digoxin are absent. In these patients, digoxin is usually not prescribed as a first‐line therapy, but rather as an add‐on treatment with another rate‐control agent. Indeed, in our study, about 70% of the patients without HF treated with digoxin were also treated with β‐blockers or nondihydropyridine calcium channel blockers. However, prescribing digoxin as a single rate‐control agent in patients with AF without HF is not rare and may be the result of an adverse event, such as bradycardia experienced with the other rate‐control therapies. Regardless of the reason for prescribing digoxin in patients with AF without HF, our study emphasizes the concern with the use of therapy, which is not supported by adequately sized randomized clinical trials. Consistent with prior data,1, 27 our findings highlight the complex intersection between digoxin use, HF, and worse clinical outcomes, and raise a concern of a possible association between digoxin use and SCD. This study highlights the unmet need for examining digoxin use in patients with AF with, but particularly without, HF in a randomized trial.
Limitations
This study is an observational post hoc analysis of a randomized trial and should be considered hypothesis generating. Dose and drug concentrations of digoxin were not available during the study. Digoxin use was considered at baseline and data regarding its use during the trial were available only every 3 months in the study visit. Nevertheless, using digoxin as a time‐variant covariate did not change the key results. In addition, both methods of analyzing a concomitant medication have their limitations.28 Given the low rates of some end points, the multivariable models may be overspecified. Nevertheless, to be consistent, similar multivariate models were used throughout. With regard to SCD, several important factors were not available in this study including documentation of arrhythmia, family history of SCD, and genetic tests; however, all SCD events were adjudicated by an independent committee. The ENGAGE AF‐TIMI 48 trial included patients with AF who were at moderate‐high risk, and our results may not be generalized to low‐risk patients with AF. Nevertheless, this study is based on the largest randomized controlled trial in patients with AF. Finally, the association between digoxin and worse clinical outcomes as demonstrated in the current analysis was not demonstrated in the only prospective trial with digoxin in patients with HF.29 However, this trial was limited to patients in sinus rhythm, and no conclusions can be made with regards to patients with AF.
Conclusions
In this observational study in patients with AF, digoxin use was associated with worse clinical outcomes. In patients with HF, digoxin use was associated with all‐cause death, cardiovascular death, SCD, HF death, and HF hospitalizations. In patients without HF, digoxin use was significantly associated with SCD. While the results in patients with HF might be caused by residual confounding, our results highlight the need to examine digoxin use in patients with AF without HF, in a randomized controlled trial. Given the availability of other rate‐control agents, until the safety of digoxin in patients with AF without HF has been established, the use of the drug in this population should be undertaken with great care, if at all.
Disclosures
Eisen discloses a significant research grant from Daiichi Sankyo to TIMI; honoraria for consulting: Daiichi Sankyo; and honoraria, modest; AstraZeneca. Ruff discloses a significant research grant from AstraZeneca, Eisai, and Intarcia; honoraria, significant: Boehringer Ingelheim and Daiichi Sankyo; consultant/advisory board, significant: Boehringer Ingelheim and Daiichi Sankyo. Braunwald discloses a modest research grant from Astra Zeneca, Johnson & Johnson, Bristol Myers Squibb, Merck & Co, Daiichi Sankyo, Glaxo Smith Kline, Sanofi Aventis, Duke University, and Novartis; and honoraria, modest: Merck & Co (no compensation), The Medicines Co, Medscape, Bayer, Daiichi Sankyo, Menarini International, Sanofi, and Novartis (uncompensated). Hamershock discloses a significant research grant from Daiichi Sankyo to TIMI. Lewis discloses a significant research grant from Pfizer, BMS, Bayer, and MSD; consultant/advisory board, significant: Pfizer, BMS, Bayer, and MSD. Hassager discloses modest honoraria from Novartis, TEVA, and Orion; and an investigator on the ENGAGE AF‐TIMI 48 trial. Chao is an investigator on the ENGAGE AF‐TIMI 48 trial. Le Heuzey is a consultant/advisory board, modest; Bayer, Boehringer Ingelheim, BMS/Pfizer, Daiichi Sankyo, Sanofi, Servier, Astra Zeneca, Meda, and Novartis. Mercuri is employed by Daiichi Sankyo Inc. Rutman is employed by Daiichi Sankyo Inc. Antman discloses significant research grants from Astra Zeneca, Daiichi Sankyo, and Eli Lilly and Company. Giugliano discloses significant research grants from Daiichi Sankyo; honoraria, significant; honoraria for CME programs: Daiichi‐Sankyo and American College of Cardiology; consultant/advisory board, modest: honoraria for consulting: Boehringer Ingelheim, Bristol Myers Squibb, Merck, Portola, and Pfizer; consultant/advisory board, significant; honoraria for consultant: Daiichi Sankyo.
Supporting information
Table S1. Baseline Characteristics by Baseline HF Status and Digoxin Use
Table S2. Clinical Outcomes by Digoxin Use and Type of HF at Baseline
Table S3. Digoxin Use in Patients Without HF at Baseline Using Digoxin as a Time‐Variant Covariate (Digoxin “on” or Digoxin “on/off” Methods*)
(J Am Heart Assoc. 2017;6:e006035 DOI: 10.1161/JAHA.117.006035.)28666993
References
- 1. Washam JB, Stevens SR, Lokhnygina Y, Halperin JL, Breithardt G, Singer DE, Mahaffey KW, Hankey GJ, Berkowitz SD, Nessel CC, Fox KA, Califf RM, Piccini JP, Patel MR; ROCKET AF Steering Committee and Investigators . Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Lancet. 2015;385:2363–2370. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 3. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J. 2016;37:2893–2962.27567408 [Google Scholar]
- 4. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 5. Camm AJ, Savelieva I, Lip GY. Rate control in the medical management of atrial fibrillation. Heart. 2007;93:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chao TF, Liu CJ, Tuan TC, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Chen TJ, Chiang CE, Chen SA. Rate‐control treatment and mortality in atrial fibrillation. Circulation. 2015;132:1604–1612. [DOI] [PubMed] [Google Scholar]
- 7. Hallberg P, Lindback J, Lindahl B, Stenestrand U, Melhus H. Digoxin and mortality in atrial fibrillation: a prospective cohort study. Eur J Clin Pharmacol. 2007;63:959–971. [DOI] [PubMed] [Google Scholar]
- 8. Friberg L, Hammar N, Rosenqvist M. Digoxin in atrial fibrillation: report from the Stockholm Cohort study of Atrial Fibrillation (SCAF). Heart. 2010;96:275–280. [DOI] [PubMed] [Google Scholar]
- 9. Whitbeck MG, Charnigo RJ, Khairy P, Ziada K, Bailey AL, Zegarra MM, Shah J, Morales G, Macaulay T, Sorrell VL, Campbell CL, Gurley J, Anaya P, Nasr H, Bai R, Di Biase L, Booth DC, Jondeau G, Natale A, Roy D, Smyth S, Moliterno DJ, Elayi CS. Increased mortality among patients taking digoxin—analysis from the AFFIRM study. Eur Heart J. 2013;34:1481–1488. [DOI] [PubMed] [Google Scholar]
- 10. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Behlouli H, Pilote L. Relation of digoxin use in atrial fibrillation and the risk of all‐cause mortality in patients ≥65 years of age with versus without heart failure. Am J Cardiol. 2014;114:401–446. [DOI] [PubMed] [Google Scholar]
- 11. Mulder BA, Van Veldhuisen DJ, Crijns HJ, Tijssen JG, Hillege HL, Alings M, Rienstra M, Van den Berg MP, Van Gelder IC; RACE Investigators II . Digoxin in patients with permanent atrial fibrillation: data from the RACE II study. Heart Rhythm. 2014;11:1543–1550. [DOI] [PubMed] [Google Scholar]
- 12. Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–888. [DOI] [PubMed] [Google Scholar]
- 13. Vamos M, Erath JW, Hohnloser SH. Digoxin‐associated mortality: a systematic review and meta‐analysis of the literature. Eur Heart J. 2015;36:1831–1838. [DOI] [PubMed] [Google Scholar]
- 14. Gheorghiade M, Fonarow GC, van Veldhuisen DJ, Cleland JG, Butler J, Epstein AE, Patel K, Aban IB, Aronow WS, Anker SD, Ahmed A. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity‐matched analysis of the AFFIRM trial. Eur Heart J. 2013;34:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY, Steeds RP, Townend J, Kotecha D. Safety and efficacy of digoxin: systematic review and meta‐analysis of observational and controlled trial data. BMJ. 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen LA, Fonarow GC, Simon DN, Thomas LE, Marzec LN, Pokorney SD, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Chang P, Peterson ED, Piccini JP; ORBIT‐AF Investigators . Digoxin use and subsequent outcomes among patients in a contemporary atrial fibrillation cohort. J Am Coll Cardiol. 2015;65:2691–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 18. Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D, McCabe CH, Braunwald E. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation‐Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF‐TIMI 48). Am Heart J. 2010;160:635–641. [DOI] [PubMed] [Google Scholar]
- 19. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 20. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 21. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 22. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. [DOI] [PubMed] [Google Scholar]
- 23. Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. JAMA Intern Med. 2013;173:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen LY, Benditt DG, Alonso A. Atrial fibrillation and its association with sudden cardiac death. Circ J. 2014;78:2588–2593. [DOI] [PubMed] [Google Scholar]
- 25. Eisen A, Ruff CT, Braunwald E, Nordio F, Corbalán R, Dalby A, Dorobantu M, Mercuri M, Lanz H, Rutman H, Wiviott SD, Antman EM, Giugliano RP. Sudden cardiac death in patients with atrial fibrillation: insights from the ENGAGE AF‐TIMI 48 Trial. J Am Heart Assoc. 2016;5:e003735 DOI: 10.1161/JAHA.116.003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S; RELY Investigators . Causes of death and influencing factors in patients with atrial fibrillation: a competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013;128:2192–2201. [DOI] [PubMed] [Google Scholar]
- 27. Reinier K, Marijon E, Uy‐Evanado A, Teodorescu C, Narayanan K, Chugh H, Gunson K, Jui J, Chugh SS. The association between atrial fibrillation and sudden cardiac death: the relevance of heart failure. JACC Heart Fail. 2014;2:221–227. [DOI] [PubMed] [Google Scholar]
- 28. Murphy SA. When ‘digoxin use’ is not the same as ‘digoxin use’: lessons from the AFFIRM trial. Eur Heart J. 2013;34:1465–1467. [DOI] [PubMed] [Google Scholar]
- 29. Digitalis Investigation Group . The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics by Baseline HF Status and Digoxin Use
Table S2. Clinical Outcomes by Digoxin Use and Type of HF at Baseline
Table S3. Digoxin Use in Patients Without HF at Baseline Using Digoxin as a Time‐Variant Covariate (Digoxin “on” or Digoxin “on/off” Methods*)


