Abstract
Background
The contemporary role of prophylactic anticoagulation following extensive anterior wall ST‐segment myocardial infarction (STEMI) is unclear.
Methods and Results
We evaluated anterior STEMI patients with left ventricle dysfunction (left ventricular ejection fraction ≤40%) (“high risk”), categorized by prophylactic warfarin use, within a regional STEMI. Patients with pre‐existing atrial fibrillation were excluded. The primary outcome was an adjusted (for Global Registry of Acute Coronary Events risk score) 1‐year composite of recurrent ischemia, stroke/transient ischemic attack/systemic embolism, or all‐cause death. Of the 2032 STEMI admissions, 436 (21.5%) were high risk. After excluding 19 (4.4%) patients with definite left ventricle thrombus and 21 (4.8%) in‐hospital deaths (2 had left ventricle thrombus), prophylactic warfarin was utilized in 236/398 (59.3%) high‐risk survivors. Prescriptions were comparable across sex, but recipients were on average younger (58.5 years versus 64.0 years, P<0.001) and lower risk (Global Registry of Acute Coronary Events risk: 163 versus 181, P<0.001). No association on the adjusted ischemic composite (23.3% versus 25.3%, odds ratio 0.96, 95% CI 0.60–1.55) or thromboembolic events (2.1% versus 1.2%, odds ratio 1.99, 95% CI 0.38–10.51) was observed, but reduced 1‐year all‐cause mortality was noted (2.5% versus 8.6%, odds ratio 0.30, 95% CI 0.11–0.81); numerically higher major bleeding was observed at 1 year (2.5% versus 1.2%, odds ratio 2.17, 95% CI 0.43–10.96).
Conclusions
A high utilization of prophylactic warfarin occurs in anterior STEMI patients with left ventricle dysfunction, yet appears to provide no additional benefit on the ischemic composite. The association with lower all‐cause mortality, but higher bleeding, calls for an improved understanding of its role in high‐risk STEMI.
Keywords: anticoagulant, stroke prevention, systolic dysfunction
Subject Categories: Acute Coronary Syndromes, Coronary Artery Disease, Myocardial Infarction
Clinical Perspective
What Is New?
Prophylactic warfarin does not mitigate the ischemic composite, and in particular, the intended thromboembolic risk, in left ventricular dysfunction following anterior ST‐segment myocardial infarction.
In this high‐risk group, an interesting association between the use of oral anticoagulation and reduction in 1‐year all‐cause mortality is noted.
What Are the Clinical Implications?
Revision of the guideline recommendations on prophylactic anticoagulation in this high‐risk group needs to be considered.
An improved understanding of the role of oral anticoagulants, specifically at lower intensity, on cardiovascular mortality in high‐risk ST‐segment myocardial infarction is required.
Introduction
Current guidelines in the management of ST‐segment elevation myocardial infarction (STEMI) provide a recommendation for prophylactic oral anticoagulation (OAC) in patients with anterior wall myocardial infarction with associated wall motion abnormalities (Class IIb, Level of Evidence: C).1, 2 The basis for this recommendation, however, stems from evidence obtained in a relatively historical time frame before contemporary expedited reperfusion strategies with primary percutaneous coronary intervention or a fibrinolysis pharmacoinvasive strategy delivered within STEMI systems of care. Currently with more effective and rapid reperfusion therapies, the frequency of left ventricle (LV) thrombus has reduced,3, 4, 5, 6, 7 and combined with the aggressive use of secondary prevention therapies, improved outcomes of anterior STEMI with LV dysfunction (LVd) are expected in the present day.
The recognition of the prognostic relevance of major bleeding,8, 9, 10 particularly in patients with atrial fibrillation (AF) on “triple therapy” (combination dual antiplatelet therapy and OAC),11, 12 has called into question the suggestion of prophylactic anticoagulation in patients with apical wall motion abnormalities following an acute anterior STEMI. In fact, the European Society of Cardiology Working Group on Thrombosis recognizes the absence of high‐quality evidence in support of prophylactic warfarin in this patient subgroup.13 Furthermore, the uncertainty associated with prophylactic anticoagulation in day‐to‐day clinical practice is illustrated within The National Survey of Canadian Practice Patterns on prophylactic anticoagulation prescriptions following an anterior wall STEMI.14
In the absence of randomized data and given the paucity of observational data evaluating the contemporary role of prophylactic anticoagulation in patients with apical wall motion abnormalities, we sought to (1) compare outcomes of patients with anterior STEMI and LVd (left ventricular ejection fraction [LVEF] ≤40%) (high‐risk) to an “all‐comer” STEMI population (low risk) and (2) evaluate the prognostic impact of prophylactic OAC in this high‐risk group, within a regional comprehensive STEMI reperfusion network (The Vital Heart Response Registry).
Methods
Vital Heart Response Registry
The Vital Heart Response (VHR) is a regional reperfusion network of care, developed in 2005, to implement timely and evidence‐based reperfusion therapies to maximize the outcomes of STEMI patients in Central and Northern Alberta. In brief, a 24‐hour on‐call VHR interventional cardiologist coordinates care between the Emergency Medical Services or non–percutaneous coronary intervention–capable hospital emergency rooms, and based on the clinical scenario, an electronically transmitted, and estimated timings of transfer, decides on 1 of the 2 reperfusion options (pharmacoinvasive or primary percutaneous coronary intervention).
Consecutive STEMI hospitalizations (including cardiac arrests and cardiogenic shock) between October 2006 and March 2011 were recorded as part of a comprehensive and inclusive VHR registry. A standard definition of STEMI was utilized15 and determined by adjudication of the ECG by VHR interventional cardiologists. VHR contains detailed clinical information obtained by chart review including patient demographics, medical history, hospitalization characteristics, in‐hospital procedures and pharmacotherapy, and in‐hospital clinical events.
Administrative Databases
Patients in the VHR registry were linked to the administrative Discharge Abstract Database and the National Ambulatory Care Reporting System using unique patient identifiers. The Discharge Abstract Database contains all acute care hospitalizations in Alberta and includes admission and discharge dates, discharge disposition, and relevant diagnostic details including a most responsible diagnosis and up to 24 other diagnosis fields. National Ambulatory Care Reporting System contains details pertaining to in‐hospital clinic and emergency department visits in Alberta and contains arrival and departure dates, discharge disposition, a most responsible diagnosis, and up to 9 other diagnosis fields. Diagnosis fields in Discharge Abstract Database and National Ambulatory Care Reporting System are abstracted from patient charts by trained health record nosologists according to the International Statistical Classification of Diseases and Health Related Interventions, 10th revision, Canadian Enhancement (ICD‐10‐CA) and following national standards developed by the Canadian Institute for Health Information (http://www.cihi.ca). Dates of death for patients in the cohort were obtained from the Alberta Health Care Insurance Plan Registry, which tracks the vital status of Alberta residents. Ethics approval for the study was obtained from the Health Research Ethics Board of the University of Alberta. Since data were collected from a clinical registry, the need for informed consent from participants was waived.
Study Population
Patients in the VHR registry were linked to the administrative databases, with patients unable to be linked excluded from the current analysis. After selecting the earliest hospitalization for each patient, we excluded patients who had a diagnosis of AF (ICD‐10 I48.x) any time before the index hospitalization, as coded in any diagnosis field of the administrative inpatient (Discharge Abstract Database) or outpatient (National Ambulatory Care Reporting System) data. Lastly, patients with missing values for ejection fraction or first‐diagnosis ECG were excluded from analysis. All LV imaging was performed by transthoracic echocardiography and interpreted by cardiologists with level 3 certification within the index hospitalization. LVEF was recorded as a percentage (over a 5% category range) across the study period, and where indicated, echo contrast was utilized to enhance myocardial opacification and aid LV thrombus detection. Regional LV wall scores were obtained for all high‐risk patients using the 16‐segment model as recommended by the American Society of Echocardiography, and scored in a standard fashion from 1 to 5.16 LV apical scores were then generated by summing the 4 individual apical segments.
STEMI patients were classified as high risk if both of the following criteria were met: (1) ejection fraction ≤40%, and (2) anterior STEMI/left bundle branch block on the first diagnostic ECG. All patients not meeting these criteria were considered low risk. Additionally, each of the 2 groups was subdivided according to warfarin use at discharge. The decision to use prophylactic warfarin was at the discretion of treating physicians, and likely based on the combination of echocardiographic parameters and bleeding risk. Prophylactic warfarin is prescribed for 3 to 6 months per clinical practice guidelines. The Global Registry of Acute Coronary Events Risk Score was calculated using clinical variables from VHR including age, history of hypertension, diabetes mellitus or angina, systolic blood pressure, heart rate, Killip class, weight, anterior or left bundle branch block ECG, and time to treatment. Mean imputation was used for missing values in variables required for the risk score calculations.
Outcome Definitions
Our primary outcome was the composite of recurrent myocardial ischemia, stroke/transient ischemic attack/systemic embolism or all‐cause death within 1 year, of patients discharged alive from their index STEMI hospitalization. We evaluated a secondary outcome of major bleeding requiring hospitalization within 1 year of discharge, as well each component of the primary outcome separately. The primary and secondary outcomes are described according to the risk groups, and by warfarin status at discharge. Additionally, we provide both the ischemic and bleeding outcomes at 6 months to limit the bias on the 1‐year outcomes associated with discontinuation of prophylactic OAC at the 3‐ to 6‐month period. The components of the primary outcome were defined using the administrative data by a hospitalization or emergency department visit having one of the corresponding ICD‐10 codes as the most responsible diagnosis within 1 year of discharge (recurrent ischemia [I20–I25], ischemic stroke [I63–I64], transient ischemic attack [G45], systemic embolism [I74]). Bleeding requiring hospitalization was defined by a hospitalization having one of the bleeding ICD‐10 codes, as has been previously defined17 in either of the first 2 diagnosis fields ([H43.1, I85.x1, K22.11, K22.6, K25.0, K25.2, K25.4, K25.6, K26.0, K26.2, K26.4, K26.6, K27.0, K27.2, K27.4, K27.6, K28.0, K28.2, K28.4, K28.6, K29.x1, K31.811, K31.82, K55.21, K62.5, K66.1, K92.0‐ K92.2, N02, R31, R58, R04.x {x=0, 1, 2, 8, 9}, H35.6, M25.0xx]).
Statistical Analysis
Patient characteristics were summarized using proportions, means, and medians as appropriate and compared across the high‐risk and low‐risk groups using the χ2 test, t test, or Mann–Whitney test, respectively. Within each of the risk groups, characteristics among warfarin users compared with non‐warfarin users were similarly compared.
Logistic regression models were used to compare outcomes between the high‐ and low‐risk groups, and between warfarin status within the high‐risk subgroup. Adjusted odds ratios (ORs) (for Global Registry of Acute Coronary Events risk scores) and corresponding 95% CIs were calculated, and the Hosmer–Lemeshow statistic was used to confirm adequate calibration for all logistic regression models.
As a sensitivity analysis, we conducted a propensity‐matched analysis for comparing warfarin status within the high‐risk subgroup. The propensity scores were calculated using a logistic regression model with warfarin status as the response variable and using stepwise selection with χ2 P value entry criterion 0.3 and retention criterion 0.2 to select statistically significant covariates from the following candidates: age, sex, time to first medical contact, reperfusion type, systolic blood pressure, heart rate, comorbidities, in‐hospital events, in‐hospital medications, and postdischarge medications. Warfarin and nonwarfarin patients were then matched on their propensity scores with a maximum allowable difference between pairs of 0.10 to ensure balanced matching. Logistic regression was then used to calculate ORs and corresponding 95% CIs to compare outcomes among the matched groups. SAS version 9.4 (SAS Institute, Cary, NC) was used for all statistical analysis.
Results
The derivation of the study cohort is described in Figure 1. Of the 2032 non‐AF STEMI patients admitted, 436 (21.5%) comprised the high‐risk group, with the remainder as low‐risk group (n=1596). Both risk groups had comparable demographics and cardiovascular profiles at baseline; however, high‐risk patients presented later from symptom onset, were more likely to be treated with primary percutaneous coronary intervention, and had a higher predicted risk of in‐hospital mortality (Table 1). As anticipated, a substantially greater adverse in‐hospital event rate is noted within the high‐risk group, and importantly, severalfold higher incidence of heart failure/cardiogenic shock and all‐cause mortality observed compared with low‐risk patients (Table 1).
Figure 1.
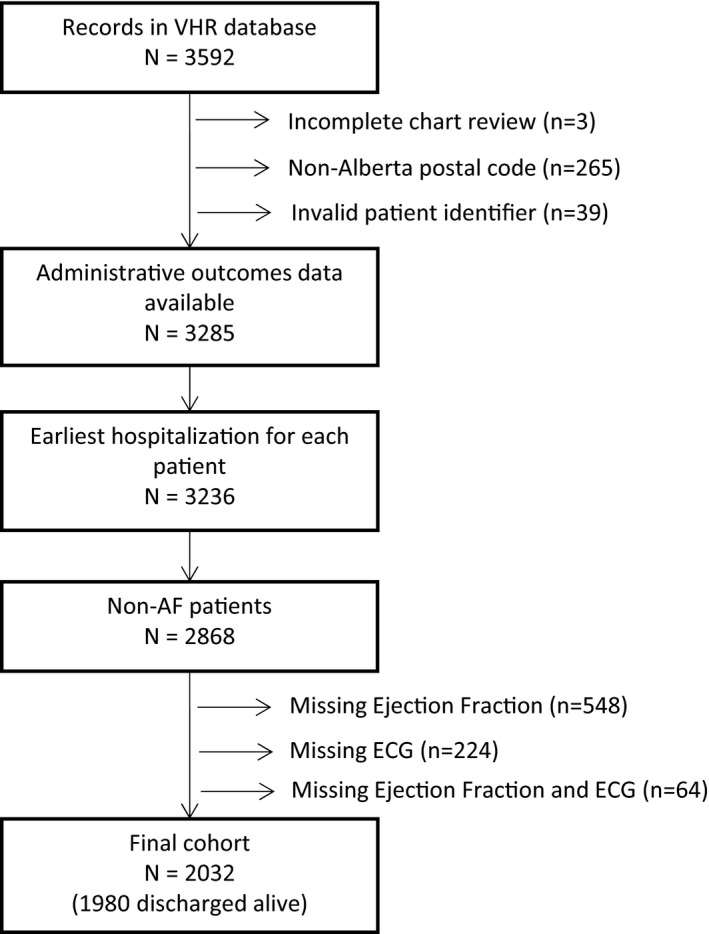
Patient identification and selection. AF indicates atrial fibrillation; VHR, vital heart registry.
Table 1.
Baseline Characteristics of STEMI Patients at Index Hospitalization
| High Risk (n=436) | Low Risk (n=1596) | P Value | |
|---|---|---|---|
| Age (y), mean (SD) | 60.8 (13.7) | 59.6 (12.7) | 0.09 |
| Female | 109 (25) | 369 (23.1) | 0.41 |
| GRACE risk score median (IQR) | 169 (148, 199) | 163 (144, 187) | <0.001 |
| Creatinine (μmol/L), mean (SD) | 95.9 (36.6) | 94.2 (43.1) | 0.44 |
| Medical history | |||
| Hypertension | 206 (47.2) | 736 (46.1) | 0.67 |
| Diabetes mellitus | 80 (18.3) | 252 (15.8) | 0.20 |
| Dyslipidemia | 178 (40.8) | 674 (42.2) | 0.60 |
| Prior MI | 74 (17.0) | 249 (15.6) | 0.49 |
| Family history premature CAD | 85 (19.5) | 417 (26.1) | 0.004 |
| Symptom onset to first medical contact (min), median (IQR) | 137 (69, 485) | 117 (62, 300) | 0.01 |
| Reperfusion modality | <0.001 | ||
| Primary PCI | 225 (51.6) | 682 (42.7) | |
| Fibrinolysis | 147 (33.7) | 700 (43.9) | |
| None | 64 (14.7) | 214 (13.4) | |
| Length of hospital stay (days), median (IQR) | 6 (5, 10) | 4 (3, 6) | <0.001 |
| In‐hospital events | |||
| Death | 21 (4.8) | 31 (1.9) | <0.001 |
| Re‐MI | 4 (0.9) | 4 (0.3) | 0.048 |
| Cardiogenic shock or heart failure | 102 (23.4) | 116 (7.3) | <0.001 |
| Cardiac arrest | 53 (12.2) | 126 (7.9) | 0.005 |
| Non‐ICH major bleeding | 28 (6.4) | 101 (6.3) | 0.94 |
| ICH | 1 (0.2) | 3 (0.2) | 0.86 |
| Ischemic stroke | 1 (0.2) | 2 (0.1) | 0.62 |
| In‐hospital medications | |||
| Dual anti‐platelet therapy | 417 (95.6) | 1550 (97.1) | 0.12 |
| ACE‐I/ARB | 412 (94.5) | 1506 (94.4) | 0.91 |
| β‐Blocker | 425 (97.5) | 1532 (96.0) | 0.14 |
| Cholesterol lowering | 414 (95.0) | 1499 (93.9) | 0.42 |
Numbers are n (%) unless specified otherwise; P values calculated by χ2 test (proportions), t test (means) or Wilcoxon‐Mann–Whitney test (medians). ACE‐I indicates angiotensin‐converting enzyme‐inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; GRACE, Global Registry of Acute Coronary Events; ICH, intracranial hemorrhage; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment myocardial infarction.
Of the 436 high‐risk patients admitted, the definite presence of LV thrombus was detected in 19 patients (4.4%), within a median duration of 2 days (interquartile range 1.5, 3) from the index infarct (all 19 patients received anticoagulation). As described within Table S1, high‐risk patients diagnosed with and without LV thrombus had comparable baseline cardiovascular risk profiles, total ischemic times, and proportion receiving revascularization. While 1 patient in the LV thrombus group developed an in‐hospital ischemic stroke and 2 died, similar in‐hospital and 1‐year outcomes are observed in the high‐risk subgroups with and without LV thrombus (Table S1).
Prophylactic warfarin was utilized in 59.3% (n=236/398) in the high‐risk patients at hospital discharge (after excluding 21 in‐hospital deaths and 19 patients with definite LV thrombus [2 of whom died]) and in 10.2% (n=161/1565) in the low‐risk group (after excluding 31 in‐hospital deaths). Table 2 describes the baseline characteristics of high‐risk patients discharged or not on prophylactic OAC (before and after propensity‐score matching). High‐risk warfarin‐treated patients were significantly younger, and with lower Global Registry of Acute Coronary Events risk scores at admission. No difference in the median LV apical scores (available for 144 of 436 patients) was noted in patients discharged or not on warfarin (warfarin median 12 [interquartile range 12–12], range [4–16], and no warfarin median 12 [interquartile range 11–12], range [6–20]). Additionally, no interaction for warfarin prescription versus not was observed across a range of LVEF (LVEF <20%: 5.2% versus 3.8%, 20–30%: 27.4% versus 21.4% and 30–40%: 67.3% versus 74.7%, P (interaction)=0.25).
Table 2.
Baseline Characteristics of High‐Risk Group by Warfarin Status (Before and After Matching)
| Before Matching | P Value | After Matching | P Value | Standardized Difference | |||
|---|---|---|---|---|---|---|---|
| Warfarin (n=252) | No Warfarin (n=184) | Warfarin (n=126) | No Warfarin (n=126) | ||||
| Age (y), mean (SD) | 58.5 (12.4) | 64.0 (14.8) | <0.001 | 61.9 (12.4) | 61.5 (14.3) | 0.81 | 0.03 |
| Female | 57 (22.6) | 52 (28.3) | 0.18 | 34 (27.0) | 29 (23.0) | 0.47 | 0.09 |
| GRACE risk score, median (IQR) | 163 (142, 189) | 181 (152, 213) | <0.001 | 167 (148, 197) | 169 (144, 199) | 0.56 | 0.06 |
| Creatinine (μmol/L), mean (SD) | 91.3 (24.4) | 102.2 (47.9) | 0.002 | 90.5 (25.6) | 95.1 (39.8) | 0.28 | −0.14 |
| Medical history | |||||||
| Hypertension | 111 (44.0) | 95 (51.6) | 0.12 | 61 (48.4) | 61 (48.4) | 0.99 | 0.00 |
| Diabetes mellitus | 47 (18.7) | 33 (17.9) | 0.85 | 25 (19.8) | 19 (15.1) | 0.32 | 0.13 |
| Dyslipidemia | 101 (40.1) | 77 (41.8) | 0.71 | 54 (42.9) | 51 (40.5) | 0.70 | 0.05 |
| Prior MI | 37 (14.7) | 37 (20.1) | 0.14 | 24 (19.0) | 22 (17.5) | 0.74 | 0.04 |
| Family history premature CAD | 46 (18.3) | 39 (21.2) | 0.44 | 22 (17.5) | 30 (23.8) | 0.21 | −0.16 |
| Symptom onset to first medical contact (min), median (IQR) | 120 (66, 400) | 169 (71, 593) | 0.25 | 140 (65, 567) | 157 (70, 398) | 0.87 | 0.10 |
| Reperfusion modality | |||||||
| Primary PCI | 138 (54.8) | 87 (47.3) | <0.001 | 63 (50.0) | 61 (48.4) | 0.96 | 0.03 |
| Fibrinolysis | 92 (36.5) | 55 (29.9) | 47 (37.3) | 48 (38.1) | … | … | |
| None | 22 (8.7) | 42 (22.8) | 16 (12.7) | 17 (13.5) | … | … | |
| Length of hospital stay (days), median (IQR) | 7 (5, 9) | 6 (4, 10) | 0.003 | 7 (5, 10) | 5 (4, 9) | 0.004 | −0.05 |
| In‐hospital medications | |||||||
| Dual anti‐platelet therapy | 245 (97.2) | 172 (93.5) | 0.059 | 119 (94.4) | 119 (94.4) | 0.99 | 0.00 |
| ACE‐I/ARB | 247 (98.0) | 165 (89.7) | <0.001 | 123 (97.6) | 124 (98.4) | 0.65 | −0.06 |
| β‐Blocker | 251 (99.6) | 174 (94.6) | <0.001 | 126 (100.0) | 125 (99.2) | 0.32 | 0.13 |
| Cholesterol lowering | 242 (96.0) | 172 (93.5) | 0.23 | 121 (96.0) | 122 (96.8) | 0.73 | −0.04 |
Numbers are n (%) unless specified otherwise; P values calculated by χ2 test (proportions), t test (means), or Wilcoxon‐Mann–Whitney test (medians). Matched groups exclude patients who died during index hospitalization and those with left ventricular thrombus. ACE‐I indicates angiotensin‐converting enzyme‐inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; GRACE, Global Registry of Acute Coronary Events; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Baseline characteristics of low‐risk patients are described within Table S2, and comparable demographics, cardiovascular risk profiles, and predicted risk of in‐hospital mortality are noted.
Clinical Outcomes for High‐Risk Versus Low‐Risk STEMI
As described in Table 1, high‐risk patients had significantly increased in‐hospital mortality (4.8% versus 1.9%, P<0.001), recurrent myocardial ischemia (0.9% versus 0.3%, P=0.048), and heart failure/cardiogenic shock (23.4% versus 7.3%, P<0.001), while in‐hospital ischemic stroke (0.2% versus 0.1%, P=0.62) and major bleeding (6.4% versus 6.3%, P=0.94) were comparable across both risk groups.
However, while worse adverse in‐hospital outcomes are anticipated in the high‐risk group, survivors to hospital discharge continue to have a significantly increased 1‐year risk of an adverse ischemic cardiovascular composite (recurrent cardiac ischemia/stroke/transient ischemic attack/systemic embolism or death) (24.1% versus 16.8%, adjusted OR 1.50, 95% CI 1.15–1.96), predominantly driven by all‐cause mortality and recurrent myocardial ischemia (Figure 2). Additionally, compared with low‐risk patients, a trend to increased bleeding requiring hospitalization at 1 year is observed within the high‐risk group (2.0% versus 0.8%, adjusted OR 2.26, 95% CI 0.92–5.53).
Figure 2.
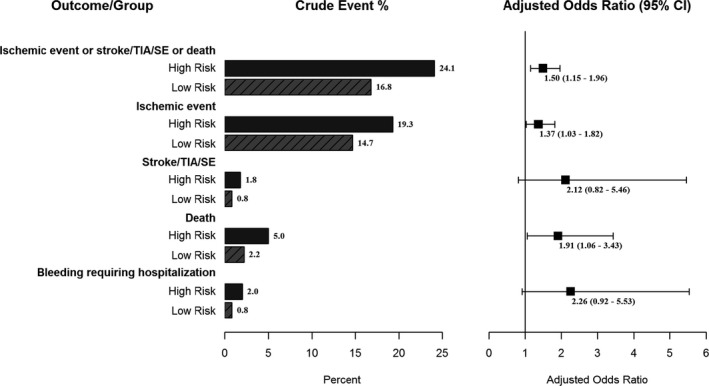
One‐year outcomes (adjusted for GRACE risk) in high‐risk vs low‐risk STEMI. GRACE indicates Global Registry of Acute Coronary Events; SE, systemic embolism; STEMI, ST‐segment myocardial infarction; TIA, transient ischemic attack.
One‐Year Outcomes for High‐Risk and Low‐Risk Patients by Warfarin Status
As depicted in Figure 3, no difference in the 1‐year ischemic composite (23.3% versus 25.3%, adjusted OR 0.96, 95% CI 0.60–1.55), or the individual thromboembolic components (including stroke/transient ischemic attack/systemic embolism) of this composite is noted in high‐risk STEMI patients discharged on prophylactic warfarin. Importantly, however, prophylactic OAC appears to be associated with a significant reduction in all‐cause death at 1‐year in high‐risk STEMI patients, but (limited by the small event rate, hence the wide confidence intervals) was associated with numerically increased bleeding requiring hospitalization (2.5% versus 1.2%, adjusted OR 2.17, 95% CI 0.43–10.96). Similar findings are noted within the propensity‐matched sensitivity analysis with the exception of a difference in mortality reduction with warfarin (Table S3).
Figure 3.
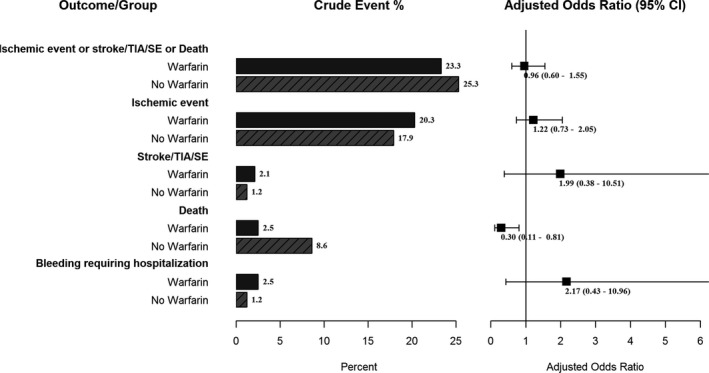
One‐year outcomes (adjusted for GRACE risk) in high‐risk STEMI on prophylactic warfarin vs not. GRACE indicates Global Registry of Acute Coronary Events; SE, systemic embolism; STEMI, ST‐segment myocardial infarction; TIA transient ischemic attack.
Comparable to the absence of benefit associated with warfarin in the high‐risk group, prophylactic warfarin in the low‐risk group was in fact associated with higher overall rates of the 1‐year adverse ischemic composite, driven by higher rates of recurrent myocardial ischemia and thromboembolic events; no significant differences in major bleeding were noted (Figure S1).
Six‐Month Outcomes by Risk Groups and Warfarin Status
Analysis of the 6‐month ischemic composite demonstrates a similar trend (albeit limited by the small event rates) with a higher overall adverse event rate in the high‐risk compared with the low‐risk group (Table S4). Furthermore, analysis of the ischemic and bleeding 6‐month outcomes categorized by warfarin status demonstrates outcomes comparable to the 1‐year observations, suggestive that the primary 1‐year outcomes are likely not biased by discontinuation of prophylactic anticoagulation at 3 to 6 months (Table S4).
Discussion
In this inclusive STEMI network of care registry evaluating 1‐year outcomes following anterior wall STEMI and LVd, and the prognostic utility of prophylactic warfarin in this high‐risk population, 2 key findings emerge. First, despite the high utilization of secondary prevention therapies at discharge, anterior STEMI patients with LVd continue to have higher odds of adverse cardiovascular outcomes out to 1 year. Second, despite incident LV thrombus occurring relatively infrequently, prophylactic anticoagulation is frequently utilized in this high‐risk group, yet it appears not to mitigate the risk associated with systemic emboli/recurrent ischemia; though associated with a reduction in all‐cause mortality, a trend to increased bleeding necessitating hospitalization at 1 year is noted in this high‐risk group.
The integration of rapid prehospital STEMI diagnosis, expedited reperfusion strategies, and potent post‐acute coronary syndrome (ACS) pharmacotherapies has translated into substantial reductions in both in‐hospital and long‐term STEMI mortality.18 However, the presence of symptomatic heart failure or systolic dysfunction (LVEF <40%) following an anterior wall myocardial infarction has been independently associated with adverse outcomes.19, 20, 21, 22 The results of this study continue to demonstrate that despite the substantial advances made in integrating STEMI care, the presence of LVd following anterior STEMI continues to be associated with substantially higher in‐hospital mortality. Worse still, survivors of this high‐risk STEMI subgroup continue to demonstrate a trend to recurrent myocardial ischemia and increased all‐cause mortality over the first year in spite of >95% utilization of dual antiplatelet and other key evidence‐based secondary prevention therapies. Similar observations have been described in anterior STEMI patients with a mean LVEF of 40% in a substudy of the Intracoronary Abciximab Infusion and Aspiration Thrombectomy in patients Undergoing Percutaneous Coronary Intervention for Anterior ST Segment Elevation Myocardial Infarction trial (INFUSE‐AMI) trial, where doubling of the 30‐day mortality is seen at 1 year, despite the high utilization of secondary prevention ACS therapies.23 These sobering results continue to highlight the fact that anterior STEMI patients with LVd remain a very high‐risk subgroup—one in which the in‐hospital mortality has remained persistently elevated, and in hospital survivors, the risk of adverse outcomes continues to increase in the long term.
Not surprisingly, LVd following an anterior wall infarct also forms the subgroup at highest risk of development of LV thrombus.24 While there has been a certain decrease in incident LV thrombus formation compared with the prethrombolytic reperfusion era,4, 7, 25, 26 contemporary data are highly variable in regard to the accurate incidence of LV thrombus formation following anterior wall infarction. This variability is predominantly attributable to the heterogeneity in the definition of LV thrombus (definite versus probable/possible), the timing of LV imaging relative to the infarct, and the utilization of superior imaging (cardiac magnetic resonance imaging) modalities in LV thrombus detection. Aligned with the results of this study, existing literature is consistent in reporting rates of definite LV thrombus between 3% and 9%,4, 7, 27, 28, 29 with a time‐dependent relationship in the development and less likelihood of detection if imaging is performed within 5 days of the infarct.30 With echocardiograms in the current study performed within a median duration of 2 days of the infarct, the possibility that LV thrombi occurring later were missed, or that high‐risk patients not discharged on prophylactic OAC could have developed LV thrombi during follow‐up exists; nevertheless, the low clinical event rates during follow‐up suggest that this possibility is low. The results of the current study also highlight that LV thrombus formation occurs independently of the baseline cardiac risk profile or total ischemic time and that regardless of the presence of LV thrombus, the 1‐year ischemic composite in high‐risk patients remains substantially elevated.
Despite the weak support (Class IIb, Level of Evidence: C) and evidence base, prophylactic anticoagulation still appears to be frequently utilized in clinical practice, with nearly two thirds of high‐risk patients in this study being discharged on prophylactic warfarin. The decision on using prophylactic warfarin in high‐risk patients with apical infarcts appears to be independent of the ejection fraction and the apical wall motion, as suggested by the significant overlap in the apical wall motion scores between the groups receiving prophylactic warfarin versus not. It is likely that both measured (younger age) and unmeasured variables supportive of a lower bleeding risk patient profile contribute in large part to prophylactic oral anticoagulant prescriptions in this high‐risk patient group. Regardless, as seen in this study and the study by Le May et al, prophylactic OAC consistently appears to show no benefit on the ischemic composite and a trend to major bleeding continues to persist with this strategy.5, 6 Interestingly, however, our results suggest an association between warfarin use and reduced all‐cause mortality in high‐risk patients after adjusting for the Global Registry of Acute Coronary Events Risk score (a finding not noted in the propensity sensitivity analysis). While provocative, these results are not entirely surprising, as a heightened pro‐thrombotic state is known to persist following the index ACS event, and clinical trials evaluating a dual‐pathway strategy (secondary prevention with a single antiplatelet and anticoagulant) have since reported significant reductions in major adverse events but with an increase in major bleeding.31, 32, 33 Patients at highest risk stand to derive the most benefit with an intervention that has common efficacy across both groups, potentially explaining the observed mortality benefit associated with prophylactic warfarin limited to the high‐risk group only in this study. It must also be acknowledged in this observational study that these findings could be related to unmeasured confounders.
We observe a much lower bleeding rate in patients discharged on prophylactic OAC than would be anticipated in comparison to clinical trials evaluating the use of “triple therapy.”11, 12, 14 This is likely because of a combination of factors including the definition of major bleeding, nonadjudication of bleeding events, lack of information regarding the duration of prophylactic anticoagulation, and the intensity of anticoagulation in our study. Knowledge of anticoagulation intensity in our study would have been particularly relevant in light of the reduced mortality associated with warfarin use in the high‐risk subgroup; this is particularly important, given that it has also been reported that lower doses of non‐vitamin K antagonists have resulted in a reduction in cardiovascular deaths, yet increased major bleeding. 32, 33
Other limitations within this study include the nonrandomized nature of the data, and although an attempt to mitigate this using a propensity‐matched analysis was made, the possibility of unidentified confounders cannot be excluded. While we excluded all patients with pre‐existing AF, we were unable to exclude STEMI patients developing new‐onset in‐hospital AF as an indication for OAC. Also, although all patients were treated per best practice guidelines, detailed information regarding the utilization and impact of resynchronization and implantable defibrillator therapies is not available for this study.
In conclusion, within a contemporary STEMI network of care, we continue to demonstrate that patients with LVd following an anterior STEMI remain a very high‐risk subgroup. Prophylactic anticoagulation, while frequently utilized, does not appear to mitigate the intended thromboembolic risk and calls this therapeutic indication into question. Further validation of the dual pathway strategy is required to accurately determine its relationship with secondary prevention of recurrent ischemia and all‐cause mortality.
Disclosures
Welsh has received research grants from Abbot Vascular, Astra Zeneca, Bayer, Bristol Myers‐Squibb, Boehringer Ingelheim, Canadian Institutes of Health Research, CSL Behring LLC, Edwards Lifesciences, Eli Lily, Jansen, Johnson and Johnson, Pfizer, Population Health Research Institute, University of Alberta Foundation and received consulting fees/honoraria from AstraZeneca, Bristol Myers‐Squibb, The Canadian Cardiovascular Society, Amgen, and Bayer. All other authors have declared no conflicts.
Supporting information
Table S1. Characteristics and Outcomes of High‐Risk Patients by Presence of Left Ventricular Thrombus
Table S2. Baseline Characteristics of Low‐Risk Group by Warfarin Status at Discharge
Table S3. Propensity Score Matched 1‐Year Outcomes Among High‐Risk Patients Who Survived Index Hospitalization Stratified by Warfarin Status
Table S4. Six‐Month Outcomes by Risk Group and Categorized by Warfarin Status
Figure S1. One‐year outcomes in low‐risk STEMI by prophylactic warfarin use. SE indicates systemic embolism; TIA, transient ischemic attack.
(J Am Heart Assoc. 2017;6:e006054 DOI: 10.1161/JAHA.117.006054.)28673899
References
- 1. American College of Emergency P, Society for Cardiovascular A, Interventions , O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 2. Task Force on the management of ST segment elevation acute myocardial infarction of the European Society of Cardiology , Steg PG, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ‘t Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 3. Pizzetti G, Belotti G, Margonato A, Carlino M, Gerosa S, Carandente O, Chierchia SL. Thrombolytic therapy reduces the incidence of left ventricular thrombus after anterior myocardial infarction. Relationship to vessel patency and infarct size. Eur Heart J. 1996;17:421–428. [DOI] [PubMed] [Google Scholar]
- 4. Osherov AB, Borovik‐Raz M, Aronson D, Agmon Y, Kapeliovich M, Kerner A, Grenadier E, Hammerman H, Nikolsky E, Roguin A. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J. 2009;157:1074–1080. [DOI] [PubMed] [Google Scholar]
- 5. Buss NI, Friedman SE, Andrus BW, DeVries JT. Warfarin for stroke prevention following anterior ST‐elevation myocardial infarction. Coron Artery Dis. 2013;24:636–641. [DOI] [PubMed] [Google Scholar]
- 6. Le May MR, Acharya S, Wells GA, Burwash I, Chong AY, So DY, Glover CA, Froeschl MP, Hibbert B, Marquis JF, Dick A, Blondeau M, Bernick J, Labinaz M. Prophylactic warfarin therapy after primary percutaneous coronary intervention for anterior ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:155–162. [DOI] [PubMed] [Google Scholar]
- 7. Poss J, Desch S, Eitel C, de Waha S, Thiele H, Eitel I. Left ventricular thrombus formation after ST‐segment‐elevation myocardial infarction: insights from a cardiac magnetic resonance multicenter study. Circ Cardiovasc Imaging. 2015;8:e003417. [DOI] [PubMed] [Google Scholar]
- 8. Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol. 2009;53:2019–2027. [DOI] [PubMed] [Google Scholar]
- 9. Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. [DOI] [PubMed] [Google Scholar]
- 10. Chhatriwalla AK, Amin AP, Kennedy KF, House JA, Cohen DJ, Rao SV, Messenger JC, Marso SP; National Cardiovascular Data R . Association between bleeding events and in‐hospital mortality after percutaneous coronary intervention. JAMA. 2013;309:1022–1029. [DOI] [PubMed] [Google Scholar]
- 11. Hess CN, Peterson ED, Peng SA, de Lemos JA, Fosbol EL, Thomas L, Bhatt DL, Saucedo JF, Wang TY. Use and outcomes of triple therapy among older patients with acute myocardial infarction and atrial fibrillation. J Am Coll Cardiol. 2015;66:616–627. [DOI] [PubMed] [Google Scholar]
- 12. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van ‘t Hof AW, ten Berg JM; Investigators Ws . Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
- 13. Lip GY, Piotrponikowski P, Andreotti F, Anker SD, Filippatos G, Homma S, Morais J, Pullicino P, Rasmussen LH, Marin F, Lane DA; Heart Failure Association of the European Society of C, the ESCWGoT . Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost. 2012;108:1009–1022. [DOI] [PubMed] [Google Scholar]
- 14. Schwalm JD, Ahmad M, Eikelboom JW, Natarajan MK. A national survey of Canadian practice patterns of warfarin after anterior wall myocardial infarction in the current era of dual antiplatelet therapy. Am J Cardiol. 2010;105:1844. [DOI] [PubMed] [Google Scholar]
- 15. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 17. Labos C, Dasgupta K, Nedjar H, Turecki G, Rahme E. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ. 2011;183:1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rouleau JL, Talajic M, Sussex B, Potvin L, Warnica W, Davies RF, Gardner M, Stewart D, Plante S, Dupuis R, Lauzon C, Ferguson J, Mikes E, Balnozan V, Savard P. Myocardial infarction patients in the 1990s—their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol. 1996;27:1119–1127. [DOI] [PubMed] [Google Scholar]
- 19. Risk stratification and survival after myocardial infarction. N Engl J Med. 1983;309:331–336. [DOI] [PubMed] [Google Scholar]
- 20. Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in‐hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 21. Stone PH, Raabe DS, Jaffe AS, Gustafson N, Muller JE, Turi ZG, Rutherford JD, Poole WK, Passamani E, Willerson JT, Sobel BE, Robertson T, Braunwald E. Prognostic significance of location and type of myocardial infarction: independent adverse outcome associated with anterior location. J Am Coll Cardiol. 1988;11:453–463. [DOI] [PubMed] [Google Scholar]
- 22. Hands ME, Lloyd BL, Robinson JS, de Klerk N, Thompson PL. Prognostic significance of electrocardiographic site of infarction after correction for enzymatic size of infarction. Circulation. 1986;73:885–891. [DOI] [PubMed] [Google Scholar]
- 23. Stone GW, Witzenbichler B, Godlewski J, Dambrink JH, Ochala A, Chowdhary S, El‐Omar M, Neunteufl T, Metzger DC, Dizon JM, Wolff SD, Brener SJ, Mehran R, Maehara A, Gibson CM. Intralesional abciximab and thrombus aspiration in patients with large anterior myocardial infarction: one‐year results from the INFUSE‐AMI trial. Circ Cardiovasc Interv. 2013;6:527–534. [DOI] [PubMed] [Google Scholar]
- 24. Garber AM, Mentz RJ, Al‐Khalidi HR, Shaw LK, Fiuzat M, O'Connor CM, Velazquez EJ. Clinical predictors and outcomes of patients with left ventricular thrombus following ST‐segment elevation myocardial infarction. J Thromb Thrombolysis. 2015;41:365–373. [DOI] [PubMed] [Google Scholar]
- 25. Jugdutt BI, Sivaram CA. Prospective two‐dimensional echocardiographic evaluation of left ventricular thrombus and embolism after acute myocardial infarction. J Am Coll Cardiol. 1989;13:554–564. [DOI] [PubMed] [Google Scholar]
- 26. Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left‐ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two‐dimensional echocardiography. N Engl J Med. 1981;305:297–302. [DOI] [PubMed] [Google Scholar]
- 27. Delewi R, Nijveldt R, Hirsch A, Marcu CB, Robbers L, Hassell ME, de Bruin RH, Vleugels J, van der Laan AM, Bouma BJ, Tio RA, Tijssen JG, van Rossum AC, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol. 2012;81:3900–3904. [DOI] [PubMed] [Google Scholar]
- 28. Driesman A, Hyder O, Lang C, Stockwell P, Poppas A, Abbott JD. Incidence and predictors of left ventricular thrombus after primary percutaneous coronary intervention for anterior ST‐segment elevation myocardial infarction. Clin Cardiol. 2015;38:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci. 2008;335:171–176. [DOI] [PubMed] [Google Scholar]
- 30. Gellen B, Biere L, Logeart D, Lairez O, Vicaut E, Furber A, Mercadier J‐J, Sirol M. Timing of cardiac magnetic resonance imaging impacts on the detection rate of left ventricular thrombus after myocardial infarction. JACC Cardiovasc Imaging. February 9, 2017. doi: 10.1016/j.jcmg.2016.12.006. Available at http://www.sciencedirect.com/science/article/pii/S1936878X16310208. Accessed June 25, 2017. [DOI] [PubMed] [Google Scholar]
- 31. Ardissino D, Merlini PA, Bauer KA, Galvani M, Ottani F, Franchi F, Bertocchi F, Rosenberg RD, Mannucci PM. Coagulation activation and long‐term outcome in acute coronary syndromes. Blood. 2003;102:2731–2735. [DOI] [PubMed] [Google Scholar]
- 32. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook‐Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 33. Hess CN, James S, Lopes RD, Wojdyla DM, Neely ML, Liaw D, Hagstrom E, Bhatt DL, Husted S, Goodman SG, Lewis BS, Verheugt FW, De Caterina R, Ogawa H, Wallentin L, Alexander JH. Apixaban plus mono versus dual antiplatelet therapy in acute coronary syndromes: insights from the APPRAISE‐2 trial. J Am Coll Cardiol. 2015;66:777–787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics and Outcomes of High‐Risk Patients by Presence of Left Ventricular Thrombus
Table S2. Baseline Characteristics of Low‐Risk Group by Warfarin Status at Discharge
Table S3. Propensity Score Matched 1‐Year Outcomes Among High‐Risk Patients Who Survived Index Hospitalization Stratified by Warfarin Status
Table S4. Six‐Month Outcomes by Risk Group and Categorized by Warfarin Status
Figure S1. One‐year outcomes in low‐risk STEMI by prophylactic warfarin use. SE indicates systemic embolism; TIA, transient ischemic attack.


