Abstract
Background
Obstructive sleep apnea (OSA) is an independent risk factor for many cardiovascular conditions such as coronary artery disease, myocardial infarction, systemic hypertension, pulmonary hypertension, and stroke. However, the association of OSA with outcomes in patients hospitalized for ST‐elevation myocardial infarction remains controversial.
Methods and Results
We used the nation‐wide inpatient sample between 2003 and 2011 to identify patients with a primary discharge diagnosis of ST‐elevation myocardial infarction and then used the International Classification of Diseases, Clinical Modification code 327.23 to identify a group of patients with OSA. The primary outcome of interest was in‐hospital mortality, and secondary outcomes were in‐hospital cardiac arrest, length of stay and hospital charges. Our cohort included 1 850 625 patients with ST‐elevation myocardial infarction, of which 1.3% (24 623) had documented OSA. OSA patients were younger and more likely to be male, smokers, and have chronic pulmonary disease, depression, hypertension, known history of coronary artery disease, dyslipidemia, obesity, and renal failure (P<0.001 for all). Patients with OSA had significantly decreased in‐hospital mortality (adjusted odds ratio, 0.78 [95% CI, 0.73–0.84]), longer hospital stay (5.00±4.68 versus 4.85±5.96 days), and incurred greater hospital charges ($79 460.12±70 621.91 versus $62 889.91±69 124.15). There was no difference in incidence of in‐hospital cardiac arrest (adjusted odds ratio, 0.93 [95% CI, 0.84–1.03]) between these 2 groups.
Conclusion
ST‐elevation myocardial infarction patients with recognized OSA had significantly decreased mortality compared with patients without OSA. Although patients with OSA had longer hospital stays and incurred greater hospital charges, there was no difference in incidence of in‐hospital cardiac arrest.
Keywords: myocardial infarction, obstructive sleep apnea, outcomes research
Subject Categories: Quality and Outcomes
Clinical Perspective
What Is New?
This is the largest study examining the association of recognized sleep apnea with immediate post‐ST‐elevation myocardial infarction outcomes
We show that patients with recognized sleep apnea have increased resource utilization and receive more‐aggressive treatment than those without sleep apnea.
What Are the Clinical Implications?
Patients with recognized sleep apnea have decreased in‐hospital mortality after ST‐elevation myocardial infarction, but have similar incidence of in‐hospital cardiac arrest.
Introduction
Obstructive sleep apnea (OSA) is a disease characterized by repeated collapse and obstruction of the muscular upper airways during sleep, resulting in significant hypopnea, apnea, and oxygen desaturation.1 OSA is an independent risk factor for many cardiovascular conditions, such as coronary artery disease (CAD), myocardial infarction (MI), systemic hypertension, pulmonary hypertension, and stroke.2, 3, 4, 5, 6 Also, increasing severity of OSA has been shown to have a positive correlation with the occurrence of CAD.2 Although the association with cardiovascular diseases is well established, the impact of OSA on immediate acute coronary syndrome outcomes remains controversial. Although some studies have associated OSA with increased rates of cardiovascular events and decreased long‐term survival post‐MI, others have shown that OSA is not a risk factor for adverse outcomes.7, 8 These studies are limited by small sample sizes, selection bias, and low event rates for immediate post‐MI outcomes in patients with and without OSA. Understanding the impact of OSA on these immediate outcomes is necessary to better risk stratify patients with known OSA and provide more‐aggressive treatment, such as positive airway pressure therapy, to these patients.8 Additionally, several human and animal studies have shown that OSA or chronic intermittent hypoxia (which is associated with OSA) is associated with less‐severe cardiac injury after an MI.9, 10 Several studies on OSA in the elderly and in other conditions, such as pneumonia, and those hospitalized for bariatric procedures have shown a survival benefit associated with OSA.1, 11, 12 In fact, the presence of OSA has been used to explain the “obesity paradox” wherein obese patients with established cardiovascular disease have better outcomes than their leaner counterparts.13, 14 We used the nation‐wide inpatient database to compare in‐hospital outcomes of patients with and without recognized OSA with a primary discharge diagnosis of ST‐elevation MI (STEMI). We also studied the comparative resource utilization, including in‐hospital procedures, hospital charges, and hospital length of stay, in these patients.
Methods
Data Source
We queried the nation‐wide inpatient database between the years 2003 and 2011. The nation‐wide inpatient database is sponsored by the Agency for Healthcare Research and Quality as a part of the Healthcare Cost and Utilization Project and includes data from over 8 million hospital stays from ≈1000 hospitals, approximating a 20% stratified sample of discharges from US hospitals. It is the largest all‐payer inpatient database that is publicly available. Criteria used for stratified sampling of the hospitals include bed size, teaching status, urban or rural location, geographical region, and ownership/control. Discharge weights are included for each patient record and can be used to obtain national estimates. We used the International Classification of Diseases, Ninth Edition, Clinical Modification (410.01–410.61 and 410.81) to identify patients with a primary discharge diagnosis of STEMI (n=1 850 634). We then used the International Classification of Diseases, Ninth Edition code of 327.23 to identify patients with OSA. This methodology has been previously utilized to identify patients with OSA in previous studies using administrative data.1
Baseline Characteristics
We included age, sex, median household income, all Elixhauser comorbidities, other clinically relevant comorbidities (smoking, dyslipidemia, and history of CAD), and location of MI (anterior, inferior, and others) as baseline patient characteristics. A list of International Classification of Diseases, Ninth Edition, Clinical Modification and Clinical Classification Software codes used to identify comorbidities and inpatient procedures is provided in Table S1. Hospital characteristics that were used included geographical region (Northeast, Midwest, South, and West), number of beds (small, medium, and large), location (rural and urban), and teaching status.
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included length of stay, hospital charges, and incidence of in‐hospital cardiac arrest (IHCA). We also looked at comparative rates of coronary artery bypass grafting (CABG), percutaneous intervention (PCI), and thrombolytic use in these patients.
Statistical Analysis
All data were weighted by discharge weight. For descriptive analyses, patient and hospital characteristics were compared between patients with and without OSA using the Pearson chi‐square test for categorical variables and the independent‐samples t test for continuous variables. Categorical variables are expressed as percentages and continuous variables as mean±SD. We used multivariable logistic regression (for dichotomous variables) and linear regression (for continuous variables) to study the association of OSA with primary and secondary outcomes post‐STEMI and to calculate adjusted odds ratios (aORs) for the same. The primary regression model was adjusted for demographics (age and sex), primary expected payer, median household income, all Elixhauser comorbidities, other clinically relevant comorbidities (smoking, dyslipidemia, and known history of CAD), and location of MI and hospital characteristics. To further explore the role of revascularization in affecting in‐hospital mortality among patients with OSA, we created a secondary regression model that included the primary model and all revascularization procedures (PCI+CABG+thrombolytics). Race was excluded from all regression analysis because there were 24% missing data for this variable. Log transformation of length of stay and hospital charge were used given that these variables were positively skewed. A 2‐tailed P<0.05 was used to denote statistical significance. SPSS software (version 20; IBM Corp., Armonk, NY) was used for all statistical analyses.
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. Our cohort included 1 850 625 (weighted) patients with STEMI, of which 1.3% (24 623) had documented OSA. Patients with OSA were generally young, male, white, smokers and were more likely to have chronic pulmonary disease, depression, diabetes mellitus (with and without complications), hypertension, known history of CAD, dyslipidemia, obesity, and renal failure (P<0.001 for all). Whereas patients with OSA had a greater prevalence of alcohol abuse (P=0.001), prevalence of drug abuse was similar between both groups. Patients with OSA were more likely to have higher socioeconomic status and be admitted to large, urban, and teaching hospitals (P<0.001 for all).
Table 1.
Baseline Characteristics of Patients With and Without OSA Admitted to the Hospital With STEMI
| Variable | Patients With OSA (n=24 623) | Patients Without OSA (n=1 826 001) | P Value |
|---|---|---|---|
| Age in years, mean±SD | 59.35±11.49 | 63.87±14.26 | <0.001 |
| Male | 79.8% | 66.0% | <0.001 |
| Location of MI | <0.001 | ||
| Anterior | 36.6% | 37.9% | |
| Inferior | 54.7% | 51.5% | |
| Other | 8.7% | 10.6% | |
| Race | <0.001 | ||
| White | 83.9% | 79.2% | |
| Black | 7.1% | 7.3% | |
| Hispanic | 4.9% | 7.3% | |
| Asian or Pacific Islander | 1.0% | 2.2% | |
| Native American | 0.5% | 0.5% | |
| Other | 2.6% | 3.5% | |
| Primary expected payer | <0.001 | ||
| Medicare | 35.7% | 45.3% | |
| Medicaid | 6.1% | 5.8% | |
| Private insurance | 46.9% | 37.2% | |
| Self‐pay | 6.8% | 7.6% | |
| No charge | 0.5% | 0.7% | |
| Other | 3.9% | 3.4% | |
| Median household income | <0.001 | ||
| 0 to 25th percentile | 23.6% | 26.4% | |
| 26th to 50th percentile | 25.8% | 27.3% | |
| 51st to 75th percentile | 27.1% | 24.8% | |
| 76th to 100th percentile | 23.4% | 21.5% | |
| Mean hospital charges | 79 460.12±70 621.91 | 62 889.91±69 124.15 | <0.001 |
| Hospital bed capacity | <0.001 | ||
| Small | 7.4% | 8.4% | |
| Medium | 22.7% | 22.4% | |
| Large | 69.9% | 69.3% | |
| Urban location | 94.8% | 90.3% | <0.001 |
| Teaching hospital | 53.2% | 48.2% | <0.001 |
| Comorbidities | |||
| Dyslipidemia | 66.2% | 49.0% | <0.001 |
| Known coronary artery disease | 86.5% | 76.7% | <0.001 |
| Smoking | 30.1% | 28.4% | <0.001 |
| Acquired immune deficiency syndrome | <0.1% | 0.1% | <0.001 |
| Alcohol abuse | 3.1% | 2.8% | 0.012 |
| Deficiency anemia | 10.5% | 9.0% | <0.001 |
| Rheumatoid arthritis/collagen vascular diseases | 2.2% | 1.7% | <0.001 |
| Chronic blood loss anemia | 0.7% | 0.9% | <0.001 |
| Congestive heart failure | 0.5% | 0.5% | 0.059 |
| Chronic pulmonary disease | 27.8% | 15.3% | <0.001 |
| Coagulopathy | 3.1% | 3.4% | 0.066 |
| Depression | 8.9% | 4.3% | <0.001 |
| Diabetes mellitus (uncomplicated) | 37.7% | 22.3% | <0.001 |
| Diabetes mellitus (complicated) | 6.4% | 2.9% | <0.001 |
| Drug abuse | 2.0% | 1.8% | 0.077 |
| Hypertension | 72.0% | 56.1% | <0.001 |
| Hypothyroidism | 8.3% | 6.4% | <0.001 |
| Liver disease | 1.3% | 0.8% | <0.001 |
| Lymphoma | 0.3% | 0.3% | 0.169 |
| Fluid and electrolyte disorder | 15.4% | 14.0% | <0.001 |
| Metastatic cancer | 0.1% | 0.7% | <0.001 |
| Other neurological disorders | 4.8% | 4.1% | <0.001 |
| Obesity | 45.2% | 8.7% | <0.001 |
| Paralysis | 1.1% | 1.1% | 0.990 |
| Peripheral vascular disease | 6.8% | 6.9% | 0.577 |
| Psychoses | 2.0% | 1.4% | <0.001 |
| Pulmonary circulation disorders | <0.1% | <0.1% | 0.862 |
| Renal failure (chronic) | 11.5% | 7.0% | <0.001 |
| Solid tumor without metastasis | 0.8% | 1.1% | <0.001 |
| Peptic ulcer (nonbleeding) | <0.1% | <0.1% | 0.288 |
| Valvular disease | <0.1% | 0.2% | <0.001 |
| Weight loss | 1.2% | 1.3% | 0.133 |
MI indicates myocardial infarction; OSA, obstructive sleep apnea; STEMI, ST‐elevation myocardial infarction.
In‐Hospital Procedures and Outcomes
The data related to in‐hospital procedures and outcomes are shown in Tables 2 and 3. Patients with OSA were more likely to receive thrombolytics, undergo diagnostic cardiac catheterization, and were also more likely to undergo PCI for STEMI (P<0.001 for all). Eleven percent of STEMI patients with OSA underwent CABG during the admission as compared with only 9.8% of the non‐OSA group (P<0.001). On multivariable logistic regression analysis, after controlling for baseline patient characteristics and hospital characteristics, patients with OSA had significantly reduced in‐hospital mortality (aOR, 0.83 [95% CI, 0.81–0.84]; P<0.001). Analysis of the secondary regression models revealed that after additional adjustment for revascularization, patients with OSA continued to have significantly reduced in‐hospital mortality (P<0.001 for all). We further stratified our population by age (<65 and ≥65 years), sex, race, obesity, diabetes mellitus, hypertension, and previous history of CAD to examine the effect of these variables on in‐hospital mortality. Whereas patients aged ≥65 years had greater mortality than those who were younger (aOR, 2.05 [2.00–2.09]; P<0.001), those with OSA (in both strata) had markedly lower mortality than their non‐OSA counterparts (P<0.001 for both). Although men with OSA had decreased in‐hospital mortality as compared with men without OSA (P<0.001), the mortality differences between women with and without OSA were only of borderline significance (P=0.086). aORs for other subgroups are displayed in Figure. On an additional multivariable logistic regression model, after controlling for baseline patient characteristics and hospital characteristics, incidence of IHCA (aOR, 0.93 [95% CI, 0.84–1.03]; P=0.285) was similar in the 2 groups. We also observed that on linear regression analysis, patients with OSA had a longer hospital stay than those without OSA (5.00±4.68 versus 4.85±5.96 days; P<0.001) and incurred greater hospital charges ($79 460.12±70 621.91 versus $62 889.91±69 124.15; P<0.001).
Table 2.
Resource Utilization in Patients With and Without OSA Admitted to the Hospital With STEMI
| Procedure/Outcome | Patients Without OSA (n=1 826 001) | Patients With OSA (n=24 623) | P Valuea |
|---|---|---|---|
| Diagnostic angiography | 85.8% | 90.9% | <0.001 |
| PCI | 63.3% | 75.5% | <0.001 |
| CABG | 9.8% | 11.0% | <0.001 |
| Thromobolytics | 1.0% | 1.5% | <0.001 |
| Total charges, mean±SD | $62 889.91±69 124.15 | $79 460.12±70 621.91 | <0.001 |
CABG indicates coronary artery bypass surgery; OSA, obstructive sleep apnea; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
Unadjusted P values derived from chi‐square test.
Table 3.
In‐Hospital Outcomes of Patients With STEMI With and Without OSA
| Outcomes | Patients Without OSA (n=1 826 001) | Patients With OSA (n=24 623) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Valuea |
|---|---|---|---|---|---|
| Categorical variables | |||||
| Mortality | 7.4% | 3.7% | 0.47 (0.44–0.51) | 0.78 (0.73–0.84) | <0.001 |
| IHCA | 2.0% | 1.7% | 0.84 (0.76–0.92) | 0.93 (0.84–1.03) | 0.285 |
| Continuous variables | |||||
| Length of stay, d | 4.85±5.96 | 5.00±4.68 | <0.001 | ||
| Hospital charges | 62 889.91±69 124.15 | 79 460.12±70 621.91 | <0.001 | ||
IHCA indicates in‐hospital cardiac arrest; OR, odds ratio; OSA, obstructive sleep apnea; STEMI, ST‐elevation myocardial infarction.
P value is adjusted demographics (age and sex), primary expected payer, all Elixhauser comorbidities, other clinically relevant comorbidities (smoking, dyslipidemia, known history of coronary artery disease), location of myocardial infarction, and hospital characteristics.
Figure 1.
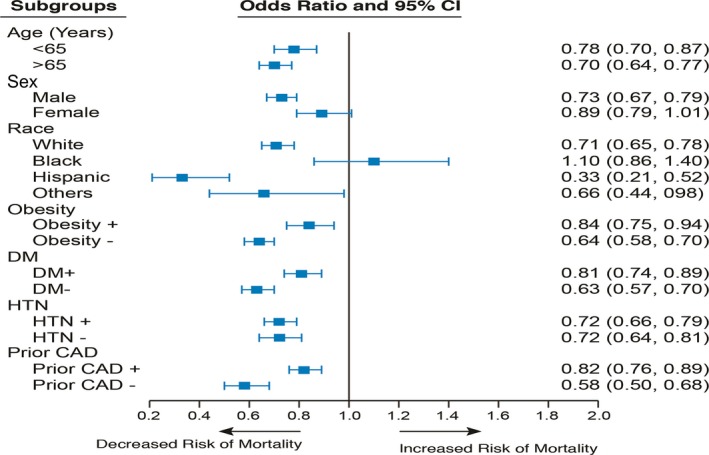
Forest plot displaying adjusted odds ratio for in‐hospital mortality among patients with OSA divided by subgroups. Odds ratio are adjusted for demographics (other than race), insurance status, socioeconomic status, comorbidities, location of myocardial infarction, and hospital characteristics. The subgroup race‐others includes Asians, Pacific Islander, and others. CAD indicates coronary artery disease; DM, diabetes mellitus; HTN, hypertension.
Discussion
Our study of over 1.8 million patients with STEMI revealed that STEMI patients with recognized OSA were younger, male smokers with a greater burden of cardiovascular risk factors than those without OSA. These patients were more likely to undergo diagnostic and therapeutic procedures (thrombolysis, diagnostic angiography, PCI, and CABG), have longer hospital stays, and incur greater hospital costs. Whereas the incidence of IHCA was similar, STEMI patients with recognized OSA had significantly reduced in‐hospital mortality. Secondary analyses also confirmed that after additional adjustment for PCI, CABG, or all revascularization, patients with OSA continued to have significantly reduced in‐hospital mortality. This effect was of greatest significance in the elderly and was of borderline significance in women. We also found that patients with OSA had longer hospital stays and incurred greater hospital charges, were of higher socioeconomic class, and were treated more often in larger teaching hospitals.
Many studies have reported the association of OSA with cardiovascular conditions such as CAD, MI, systemic hypertension, pulmonary hypertension, and stroke.2, 3, 4, 5, 6 Our results were consistent with these studies’ findings. At the time of hospitalization for STEMI, patients with OSA were younger and were more likely to have a history of dyslipidemia, obesity, known CAD, congestive heart failure, systemic hypertension, chronic pulmonary disease, and renal failure. We can postulate that the comparatively younger age and significantly greater comorbidity profile of OSA patients may explain the greater likelihood of these patients receiving procedures such as thrombolysis, PCI, and CABG. Another explanation for the observed survival benefit may be that patients with OSA were more likely to be taken to larger, urban teaching hospitals that might be better equipped to manage complex patients with significant obesity. Although we did not find any association of OSA on incidence of IHCA, our adjusted models revealed significantly decreased in‐hospital mortality in these patients.
Although it is possible that this improved survival represents the presence of unmeasured confounding factors, our study is in line with a growing body of evidence that “recognized” OSA confers a survival benefit during inpatient stays for a number of conditions. A recent study of over 250 000 patients with pneumonia showed that even though patients with OSA had a significant burden of comorbidities and used greater hospital resources than those without OSA (similar to our findings), they had modestly lower inpatient mortality.1 In another large cohort study of over 1 million patients undergoing elective surgery, patients with sleep‐disordered breathing had significantly decreased in‐hospital mortality after cardiovascular surgery (OR, 0.54 [95% CI, 0.40–0.73]).15 In a large study of patients undergoing bariatric surgeries, those with OSA had improved postoperative survival. A similar survival benefit has also been noted with elderly patients (a group in which OSA has high prevalence). Lavie and Lavie studied around 600 elderly patients with a mean age of 70 years who were referred to a sleep laboratory for suspected OSA. Over a mean follow‐up of around 5 years, patients with OSA had significantly improved survival as compared with an age‐, sex‐, and ethnicity‐matched cohort.12 These findings are similar to those from our cohort, where we observed that the greatest survival benefit of OSA was present in elderly patients (aged >65 years; aOR, 0.70 [95% CI, 0.63–0.77]).
There are several potential explanations for our findings. Given that 45.2% of the patients with OSA were also obese, it is possible that the “obesity paradox” may be contributory. The “obesity paradox” refers to a phenomenon wherein obese patients (defined by body mass index) with established cardiovascular disease may have better outcomes than their leaner counterparts.13, 14 Explanations for this include an earlier onset of symptoms from cardiovascular disease, more‐aggressive optimization of preventive medical treatment, and certain neurohormonal alterations, such as increased soluble tumor necrosis factor‐alpha receptors in obese patients.16, 17, 18
Another potential explanation for the survival benefit of OSA is “ischemic preconditioning” through chronic intermittent hypoxia, which is characteristic of OSA.19 This is a process by which repeated episodes of sublethal ischemia confer protection from infarction, arrhythmias, and further ischemic insults.12, 20 A study on rats showed that exposure to chronic intermittent hypoxia results in a decreased infarct size after occlusion of the left anterior descending coronary artery. The researchers also noted that rats exposed to chronic intermittent hypoxia had decreased incidence of arrhythmias, including ventricular tachycardia.9 In another study on rat models, researchers found that intermittent hypoxia was associated with greater improvement in post‐MI ejection fraction and smaller infarct size at 21 and 35 days after an MI.21 Shah et al studied over 100 patients with acute MI and found that patients with OSA had lower peak troponin T values. This effect was most significant for patients with severe OSA.10 The paradoxically decreased infarct size and improved cardiac function can be partly explained by neovascularization and increased coronary collateralization. In rat models, hypoxia resulted in an increased capillary density of up to 60% in the peri‐infarct zone. This was accompanied by a 134% increase in vascular endothelial growth factor production.21 These findings have been corroborated by human studies. Steiner et al reported that patients with OSA and chronic total occlusion have greater coronary collateral vessel development than those without OSA.22
Although our results are thought provoking, there are several limitations to our analysis. First, we used International Classification of Diseases, Ninth Edition, Clinical Modification codes to identify OSA rather than PSG. This almost certainly led to underestimation of the actual prevalence of OSA in our population. The prevalence of 1.3% observed in our cohort is markedly less than the 45% to 65% noted in studies where PSG was used.23, 24 However, our estimates are similar to other studies using administrative data.1, 11 Inclusion of potentially undiagnosed patients in our cohort may have pushed the effect toward null. Second, we cannot exclude the possibility of selection bias, wherein only patients with severe symptomatic OSA were captured in our database. We also lack data regarding the treatment status or severity of OSA in our population, both of which might impact our reported outcomes. Last, though we have carried out extensive multivariable adjustment for demographics and comorbid conditions, there is a possibility of unmeasured confounding variables. However, the large size of the data may compensate partly for some of the above‐mentioned limitations.
Conclusion
STEMI patients with recognized OSA are more likely to undergo life‐saving procedures despite having greater comorbidity burden and are more likely to survive a hospitalization for STEMI. Although patients with OSA had longer hospital stays and incurred greater hospital charges, there was no difference in incidence of IHCA.
Disclosures
Dr Desai is supported by the Haslam Family Endowed Chair in Cardiovascular Medicine. Dr Griffin is supported by the Brown family Endowed Chair in Cardiovascular Medicine. None of the authors in this manuscript have any conflict of interests pertaining to this document. Dr Desai is a consultant for Myocardia, Inc.
Supporting information
Table S1. International Classification of Diseases, Ninth Edition, Clinical Modification and Clinical Classifications Software Codes Used to Identify ST‐Elevation Myocardial Infarction, Comorbidities, Procedures, and Outcomes
(J Am Heart Assoc. 2017;6:e006133 DOI: 10.1161/JAHA.117.006133.)28729411
References
- 1. Lindenauer PK, Stefan MS, Johnson KG, Priya A, Pekow PS, Rothberg MB. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest. 2014;145:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92:79–84. [DOI] [PubMed] [Google Scholar]
- 3. Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–184. [DOI] [PubMed] [Google Scholar]
- 4. Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkilä K, Sarna S. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J (Clin Res Ed). 1987;294:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep‐disordered breathing, sleep apnea, and hypertension in a large community‐based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 6. Prisco DL, Sica AL, Talwar A, Narasimhan M, Omonuwa K, Hakimisefat B, Dedopoulos S, Shakir N, Greenberg H. Correlation of pulmonary hypertension severity with metrics of comorbid sleep‐disordered breathing. Sleep Breath. 2011;15:633–639. [DOI] [PubMed] [Google Scholar]
- 7. Lee CH, Khoo SM, Chan MY, Wong HB, Low AF, Phua QH, Richards AM, Tan HC, Yeo TC. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehra R, Principe‐Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6‐month outcome. Sleep Med. 2006;7:521–528. [DOI] [PubMed] [Google Scholar]
- 9. Neckar J, Ostadal B, Kolar F. Myocardial infarct size‐limiting effect of chronic hypoxia persists for five weeks of normoxic recovery. Physiol Res. 2004;53:621–628. [PubMed] [Google Scholar]
- 10. Shah N, Redline S, Yaggi HK, Wu R, Zhao CG, Ostfeld R, Menegus M, Tracy D, Brush E, Appel WD, Kaplan RC. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2013;17:819–826. [DOI] [PubMed] [Google Scholar]
- 11. Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep‐disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg. 2013;23:1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18:397–403. [DOI] [PubMed] [Google Scholar]
- 13. Ozeke O, Ozer C, Gungor M, Celenk MK, Dincer H, Ilicin G. Chronic intermittent hypoxia caused by obstructive sleep apnea may play an important role in explaining the morbidity‐mortality paradox of obesity. Med Hypotheses. 2011;76:61–63. [DOI] [PubMed] [Google Scholar]
- 14. Gupta T, Kolte D, Mohananey D, Khera S, Goel K, Mondal P, Aronow WS, Jain D, Cooper HA, Iwai S, Frishman WH, Bhatt DL, Fonarow GC, Panza JA. Relation of obesity to survival after in‐hospital cardiac arrest. Am J Cardiol. 2016;118:662–667. [DOI] [PubMed] [Google Scholar]
- 15. Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep‐disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. [DOI] [PubMed] [Google Scholar]
- 17. Hansel B, Roussel R, Elbez Y, Marre M, Krempf M, Ikeda Y, Eagle KA, Elisaf M, Bhatt DL, Steg PG; Investigators RR . Cardiovascular risk in relation to body mass index and use of evidence‐based preventive medications in patients with or at risk of atherothrombosis. Eur Heart J. 2015;36:2716–2728. [DOI] [PubMed] [Google Scholar]
- 18. Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537–544. [DOI] [PubMed] [Google Scholar]
- 19. Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (Auckl). 2016;4:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. [DOI] [PubMed] [Google Scholar]
- 21. Xu WQ, Yu Z, Xie Y, Huang GQ, Shu XH, Zhu Y, Zhou ZN, Yang HT. Therapeutic effect of intermittent hypobaric hypoxia on myocardial infarction in rats. Basic Res Cardiol. 2011;106:329–342. [DOI] [PubMed] [Google Scholar]
- 22. Steiner S, Schueller PO, Schulze V, Strauer BE. Occurrence of coronary collateral vessels in patients with sleep apnea and total coronary occlusion. Chest. 2010;137:516–520. [DOI] [PubMed] [Google Scholar]
- 23. Liu B, Guo R, Zhou S, Xie S, Wang K, Xu Y. Effects of obstructive sleep apnea on cardiac function and clinical outcomes in Chinese patients with ST‐elevation myocardial infarction. ScientificWorldJournal. 2014;2014:908582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee CH, Khoo SM, Tai BC, Chong EY, Lau C, Than Y, Shi DX, Lee LC, Kailasam A, Low AF, Teo SG, Tan HC. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–1495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, Ninth Edition, Clinical Modification and Clinical Classifications Software Codes Used to Identify ST‐Elevation Myocardial Infarction, Comorbidities, Procedures, and Outcomes


