Abstract
Background
Bradycardia on presentation is frequently observed in patients with right coronary artery ST‐segment elevation myocardial infarction, but it is largely unknown whether it predicts poor angiographic or clinical outcomes in that patient population. We sought to determine the prognostic implications of admission heart rate (AHR) in patients with ST‐segment elevation myocardial infarction and a right coronary artery culprit lesion.
Methods and Results
We analyzed 1460 patients with ST‐segment elevation myocardial infarction and a right coronary artery culprit lesion enrolled in the randomized HORIZONS‐AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial who underwent primary percutaneous coronary intervention. Patients presenting with high‐grade atrioventricular block were excluded. Outcomes were examined according to AHR range (AHR <60, 61–79, 80–99, and ≥100 beats per minute). Baseline and procedural characteristics did not vary significantly with AHR except for a more frequent history of diabetes mellitus, longer symptom‐to‐balloon time, more frequent cardiogenic shock, and less frequent restoration of thrombolysis in myocardial infarction 3 flow in patients with admission tachycardia (AHR >100 beats per minute). Angiographic analysis showed no significant association between AHR and lesion location or complexity. On multivariate analysis, admission bradycardia (AHR <60 beats per minute) was not associated with increased 1‐year mortality (hazard ratio 1.33; 95% CI 0.41–4.34, P=0.64) or major adverse cardiac events (hazard ratio 1.08; 95% CI 0.62–1.88, P=0.78), whereas admission tachycardia was a strong independent predictor of mortality (hazard ratio 5.02; 95% CI 1.95–12.88, P=0.0008) and major adverse cardiac events (hazard ratio 2.20; 95% CI 1.29–3.75, P=0.0004).
Conclusions
In patients with ST‐segment elevation myocardial infarction and a right coronary artery culprit lesion undergoing primary percutaneous coronary intervention, admission bradycardia was not associated with increased mortality or major adverse cardiac events at 1 year.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00433966.
Keywords: bradycardia, coronary artery disease, electrocardiogram, heart rate, infarct‐related artery, inferior myocardial infarction, ST‐segment elevation myocardial infarction
Subject Categories: Myocardial Infarction, Percutaneous Coronary Intervention, Stent
Clinical Perspective
What Is New?
In patients with right coronary artery ST‐segment elevation myocardial infarction, sinus or junctional bradycardia on admission does not portend poor long‐term prognosis and is not a reliable predictor of angiographic lesion location or complexity.
In patients with right coronary artery ST‐segment elevation myocardial infarction, sinus or junctional tachycardia on admission is associated with increased mortality and major adverse cardiac events at 1 year.
What Are the Clinical Implications?
In patients with right coronary artery ST‐segment elevation myocardial infarction, bradycardia on admission is not a marker of lesion complexity or location; however, tachycardia on admission indicates a severe ischemic substrate and should be approached as rigorously as in patients with anterior infarcts, with aggressive medical therapy and rapid revascularization.
Introduction
Resting heart rate has been identified as a modifiable risk factor of cardiovascular outcomes in patients with coronary artery disease.1, 2, 3, 4, 5, 6 During the acute phase of ST‐segment elevation myocardial infarction (STEMI), elevated admission heart rate (AHR) portends a poor short‐ and long‐term prognosis compared with a lower AHR in patients managed with thrombolytic therapy or percutaneous coronary intervention (PCI).7, 8 Elevated AHR is often associated with anterior infarcts8; however, it is unknown whether the frequently observed sinus or junctional bradycardic response in the setting of inferior STEMI confers clinical benefit or risk in that patient population. An early report indicated that 75% of patients with inferior myocardial infarction present with sinus bradycardia within 15 minutes of infarction, as opposed to 15% of patients with anterior myocardial infarction.6 Recently, a registry‐based study has demonstrated an association between AHR and poor cardiovascular outcomes in patients with STEMI,8 but the nature of the study did not allow for angiographic correlation and determination of the prognostic implications of AHR depending on the infarct‐related artery (IRA). Therefore, the aim of this study was to analyze the relationship between AHR, angiographic correlates, and cardiovascular outcomes in patients with a right coronary artery (RCA) culprit lesion undergoing primary PCI in the setting of acute STEMI.
Methods
Study Population
Details of the rationale, design, and results of the HORIZONS‐AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial have been previously published.9, 10, 11 In brief, HORIZONS‐AMI was a prospective, dual‐arm, open‐label, randomized, multicenter trial in patients with STEMI who were undergoing primary PCI that compared bivalirudin alone with heparin plus a glycoprotein IIb/IIIa inhibitor (primary randomization) followed by a secondary stratified randomization in a 3:1 fashion to either paclitaxel‐eluting stents (TAXUS Express, Boston Scientific, Marlborough, MA) or uncoated, bare metal stents that were identical in appearance (Express, Boston Scientific). The study was approved by the institutional review board or ethics committee at each participating site. All subjects provided informed consent. Consecutive patients 18 years of age or older who presented within 12 hours after the onset of symptoms and who had ST‐segment elevation of ≥1 mm in ≥2 contiguous leads, new left bundle‐branch block, or true posterior myocardial infarction were considered for enrollment. The principal exclusion criteria were contraindications to the study medications. A total of 3602 patients were enrolled at 123 international centers. Emergency coronary angiography with left ventriculography was performed after randomization, followed by triage, at the discretion of the physician, to PCI, coronary artery bypass grafting, or medical management, as described previously.12 Two primary end points were prespecified: major bleeding (not related to coronary artery bypass grafting) and net adverse clinical events, defined as the combination of major bleeding and major adverse cardiac events (MACE; death, re‐infarction, target‐vessel revascularization for ischemia, and stroke). All primary end point events as well as stent thrombosis and stent failure/malfunction were centrally adjudicated by a clinical events committee blinded to treatment assignment using strict protocol‐described definitions.
The present analysis was not prespecified in the HORIZONS‐AMI trial. Formal analysis was limited to patients who had an angiographically documented RCA culprit lesion and who underwent primary PCI and serial ECGs as per protocol. AHR was based on the baseline ECG performed on each subject. Patients were categorized according to AHR in 4 groups: <60, 61 to 79, 80 to 99, and >100 beats per minute (bpm). Patients with acute high‐grade atrioventricular block (AVB; Mobitz II, III, and 2:1 AVB) were excluded. Patients with left anterior descending artery (LAD) IRA and left circumflex IRA were also analyzed according to AHR range for comparison for MACE and mortality at 1 year.
Statistical Analysis
Continuous variables were expressed as median and interquartile range and were compared using the Wilcoxon rank sum test. Categorical variables were compared using the χ2 test or Fisher exact text (when the expected cell count was <5). Survival analysis for clinical outcomes was performed using Kaplan–Meier curves and the log‐rank test. Multivariate Cox proportional hazard models were used to derive the adjusted hazard ratio (HR) and 95% CI of AHR on mortality and MACE to ascertain the independent prognostic significance of AHR on these events. The following variables were included in this multivariable analysis: age, previous myocardial infarction, diabetes mellitus, heart failure, left ventricular ejection fraction <40%, cardiogenic shock, AHR range, and β‐blocker use on admission. To test the assumption of proportional hazards, an interaction term of each covariate by time was created and tested, along with the main effect of the covariate, in a proportional hazards model for both time to death and time to MACE. For any interaction terms that were significant, Kaplan–Meier survival curves were examined across strata of the covariate to ascertain whether the interaction was disordinal (ie, whether the survival curves crossed). Using this method, the proportional hazards assumption was found to be tenable for all covariates. To account for missing values for left ventricular ejection fraction, additional sensitivity analysis was performed using a Markov Chain Monte Carlo method to impute 20 data sets. All tests were 2‐tailed, and P<0.05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
The RCA was the IRA in 1460 patients (75.5% men; mean age 59.6 years). Conversely, the LAD was the IRA in 1403 patients and the left circumflex was the IRA in 550 patients. Baseline and procedural characteristics of patients with an RCA culprit lesion are presented in Table 1. Patients with AHR ≥100 bpm had more frequent clinical evidence of cardiogenic shock on presentation compared with lower AHR. Left ventricular ejection fraction <40% was also more frequently observed with increasing AHR. Symptoms onset‐to‐balloon time increased linearly across AHR, with the longest delay observed in the AHR ≥100 bpm group. Utilization of β‐blockers and calcium channel blockers at baseline did not differ significantly across AHR groups.
Table 1.
Baseline and Procedural Characteristics According to AHR in Patients With a RCA Culprit Lesion
| AHR <60 (n=233) | 60 ≤ AHR ≤79 (n=682) | 80 ≤ AHR ≤99 (n=422) | AHR ≥100 (n=123) | P Value (All) | |
|---|---|---|---|---|---|
| Age, y | 61.3 (54.1–70.1) | 59.9 (52.4–69.2) | 58.4 (51.7–67.8) | 60.9 (52.6–68.6) | 0.051 |
| Male | 76.8% (179/233) | 74.6% (509/682) | 77.3% (326/422) | 72.4% (89/123) | 0.60 |
| Hypertension | 53.6% (125/233) | 49.4% (337/682) | 55.7% (235/422) | 60.2% (74/123) | 0.06 |
| Hyperlipidemia | 45.5% (106/233) | 42.8% (292/682) | 44.5% (188/422) | 40.7% (50/123) | 0.78 |
| Smoking | 64.5% (149/231) | 69.4% (471/679) | 70.6% (296/419) | 65.9% (81/123) | 0.36 |
| Diabetes mellitus | 16.3% (38/233) | 10.9% (74/682) | 17.5% (74/422) | 23.6% (29/123) | <0.001 |
| Prior myocardial infarction | 11.2% (26/233) | 11.3% (77/682) | 11.4% (48/422) | 13.8% (17/123) | 0.87 |
| Prior percutaneous coronary intervention | 12.4% (29/233) | 11.1% (76/682) | 10.0% (42/422) | 9.8% (12/123) | 0.76 |
| Prior coronary artery bypass grafting | 1.7% (4/233) | 2.9% (20/682) | 1.2% (5/422) | 1.6% (2/123) | 0.23 |
| History of congestive heart failure | 1.7% (4/233) | 2.8% (19/682) | 3.8% (16/422) | 2.4% (3/123) | 0.48 |
| Peripheral vascular disease | 4.7% (11/233) | 4.8% (33/682) | 4.5% (19/421) | 7.3% (9/123) | 0.64 |
| History of renal insufficiency | 2.1% (5/233) | 3.2% (22/682) | 2.4% (10/421) | 4.1% (5/123) | 0.63 |
| Atrial fibrillation or flutter | 1.7% (4/233) | 1.3% (9/682) | 1.4% (6/422) | 4.1% (5/123) | 0.17 |
| Baseline medications | |||||
| β‐Blockers | 22.0% (51/232) | 21.2% (144/680) | 20.1% (85/422) | 17.1% (21/123) | 0.71 |
| Calcium channel blockers | 7.8% (18/232) | 12.1% (82/680) | 10.0% (42/422) | 13.8% (17/123) | 0.19 |
| Symptom onset to balloon, min | 200.0 (139.0–286.0) | 221.0 (157.0–308.0) | 230.0 (158.0–332.0) | 300.0 (189.0–454.0) | <0.0001 |
| Cardiogenic shock | 1.3% (3/233) | 0.4% (3/682) | 0.0% (0/422) | 4.1% (5/123) | <0.0001 |
| Left ventricular ejection fraction <40% | 3.6% (7/194) | 3.5% (21/592) | 6.1% (22/361) | 15.5% (16/103) | <0.0001 |
Values are shown as % (n/N) or median (interquartile range). AHR indicates admission heart rate; RCA, right coronary artery.
Angiographic Correlates
Angiographic correlates of AHR in patients with an RCA culprit lesion are shown in Table 2. Patients with admission tachycardia (AHR ≥100 bpm) had more frequent severe calcification compared with all other AHR groups; thrombolysis in myocardial infarction 0/1 and myocardial blush 0/1 were observed more frequently with increasing AHR. Intracoronary thrombus was observed with similar frequency among patients with low (<60 bpm) and high (≥100 bpm) AHR. There was no evidence of an association between AHR and lesion location or complexity.
Table 2.
Angiographic Characteristics According to AHR in Patients With a RCA Culprit Lesion
| AHR <60 bpm | 60 ≤ AHR ≤79 bpm | 80 ≤ AHR ≤99 bpm | AHR ≥100 bpm | P Value (All) | |
|---|---|---|---|---|---|
| TIMI flow | |||||
| 0/1 | 1.2% (2/162) | 7.1% (34/482) | 8.5% (26/307) | 12.5% (12/96) | 0.003 |
| 2 | 8.6% (14/162) | 8.1% (39/482) | 7.2% (22/307) | 10.4% (10/96) | 0.77 |
| 3 | 90.1% (146/162) | 84.9% (409/482) | 84.4% (259/307) | 77.1% (74/96) | 0.05 |
| Myocardial blush grade | |||||
| 0/1 | 1.4% (2/146) | 7.8% (35/450) | 9.1% (26/286) | 14.3% (12/84) | 0.003 |
| 2 | 7.5% (11/146) | 9.3% (42/450) | 7.3% (21/286) | 11.9% (10/84) | 0.53 |
| 3 | 91.1% (133/146) | 82.9% (373/450) | 83.6% (239/286) | 73.8% (62/84) | 0.007 |
| Lesion morphology | |||||
| Location | |||||
| Ostial | 0.9% (2/225) | 2.5% (17/670) | 2.2% (9/413) | 3.4% (4/119) | 0.42 |
| Proximal | 36.9% (83/225) | 30.3% (203/670) | 33.7% (139/413) | 34.5% (41/119) | 0.27 |
| Thrombus | 85.8% (193/225) | 77.8% (521/670) | 81.3% (335/412) | 84.0% (100/119) | 0.04 |
| Calcification (moderate/severe) | 43.6% (98/225) | 35.7% (238/666) | 39.2% (161/411) | 44.5% (53/119) | 0.09 |
| Angulation >90° | 1.4% (3/222) | 0.8% (5/658) | 0.7% (3/404) | 0.8% (1/118) | 0.86 |
| Eccentric | 5.3% (12/225) | 6.0% (40/669) | 8.2% (34/413) | 4.2% (5/119) | 0.27 |
| Tortuosity (moderate/severe) | 2.2% (5/225) | 2.2% (15/669) | 1.9% (8/412) | 0.0% (0/119) | 0.44 |
| Lesion class ACC/AHA >B1 | 92.4% (208/225) | 90.0% (603/670) | 91.3% (377/413) | 92.4% (110/119) | 0.63 |
Values are shown as % (n/N). ACC indicates American College of Cardiology; AHA, American Heart Association; AHR, admission heart rate; bpm, beats per minute; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
Clinical Outcomes
Table 3 summarizes 30‐day and 1‐year death, cardiac death, net adverse clinical events, MACE, reinfarction, and major bleeding (non–coronary artery bypass graft related) in patients with an RCA culprit lesion. RCA IRA and admission bradycardia had similar net adverse clinical events, MACE, major bleeding, and death rates compared with an AHR range 60 to 99 bpm; however, elevated AHR (≥100 bpm) resulted in significantly worse clinical outcomes at 30 days and 1 year compared with all other groups. Patients with admission bradycardia had nominally higher crude death rates at 1 year compared with the reference group, but this was not statistically significant. Unadjusted analyses according to culprit vessel indicated that admission tachycardia was associated with increased death and MACE across the spectrum of culprit vessels (Figures 1 and 2). After multivariable adjustment, admission tachycardia (≥100 bpm) in patients with RCA IRA remained an independent predictor of both early (30 days) and late (1 year) MACE (HR 3.51, 95% CI 1.57–7.86; P=0.023 and HR 2.20; 95% CI 1.29–3.75; P=0.0036, respectively) and mortality (HR 11.22; 95% CI 2.10–59.95; P=0.047 and HR 5.02, 95% CI 1.95–12.88; P=0.0008, respectively) (Table 4). In contrast, admission bradycardia (<60 bpm) was not an independent predictor of MACE or mortality. Sensitivity analysis using imputation methods to account for missing left ventricular ejection fraction values showed that AHR retained the same statistical significance for MACE (AHR <60 bpm, P=0.286; AHR 80–99 bpm, P=0.860; and AHR >100 bpm, P=0.0029) and death (AHR <60 bpm, P=0.243; AHR 80–99 bpm, P=0.434; and AHR >100 bpm P=0.001). Similarly, following adjustment, admission tachycardia remained an independent predictor of mortality in patients with an LAD culprit lesion (HR 2.49 95% CI 1.29–4.81, P=0.0067) but not MACE.
Table 3.
Clinical Outcomes at 30 Days and 1 Year According to AHR in Patients With a RCA Culprit Lesion
| AHR <60 bpm | 60 ≤ AHR ≤79 bpm | 80 ≤ AHR ≤99 bpm | AHR ≥100 bpm | P Value (All) | |
|---|---|---|---|---|---|
| 30 d | |||||
| Net adverse clinical events | 10.3% (24) | 9.6% (65) | 6.6% (28) | 22.0% (27) | <0.0001 |
| Major adverse cardiac events | 5.6% (13) | 2.8% (19) | 4.0% (17) | 12.2% (15) | <0.0001 |
| All‐cause death | 1.7% (4) | 0.6% (4) | 1.7% (7) | 8.2% (10) | <0.0001 |
| Cardiac death | 1.3% (3) | 0.6% (4) | 1.4% (6) | 8.2% (10) | <0.0001 |
| Reinfarction | 3.0% (7) | 1.0% (7) | 1.4% (6) | 1.5% (22) | 0.19 |
| Major bleeding | 6.5% (15) | 8.1% (55) | 4.0% (17) | 15% (18) | 0.0004 |
| 1 y | |||||
| Net adverse clinical events | 16.9% (39) | 14.6% (99) | 11.0% (46) | 29.5% (36) | <0.0001 |
| Major adverse cardiac events | 10.9% (25) | 8.6% (58) | 8.6% (36) | 21.4% (26) | <0.0001 |
| All‐cause death | 3.0% (7) | 1.6% (11) | 2.6% (11) | 11.5% (14) | <0.0001 |
| Cardiac death | 1.3% (3) | 0.9% (6) | 2.1% (9) | 10.7% (13) | <0.0001 |
| Reinfarction | 4.8% (11) | 2.8% (19) | 2.2% (9) | 6.3% (7) | 0.07 |
| Major bleeding | 7.8% (18) | 8.2% (56) | 4.0% (17) | 7.5% | 0.0005 |
Values are shown as % (n). AHR indicates admission heart rate; bpm, beats per minute; RCA, right coronary artery.
Figure 1.
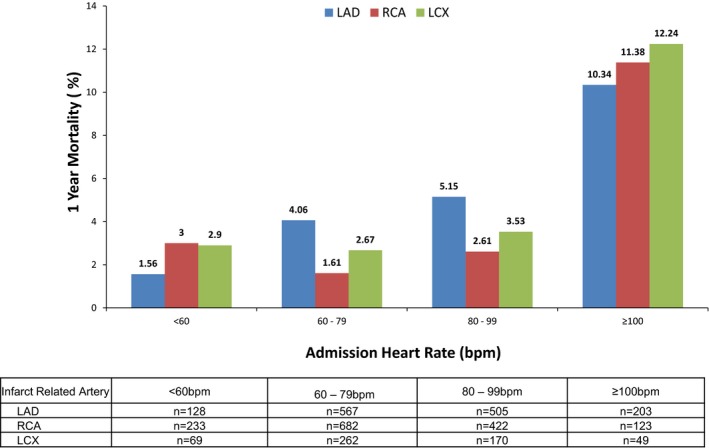
Unadjusted 1‐year mortality in patients with right coronary artery, left anterior descending coronary artery, or left circumflex infarct–related artery according to admission heart rate. bpm indicates beats per minute; LAD, left anterior descending coronary artery; LCX, left circumflex; RCA, right coronary artery.
Figure 2.
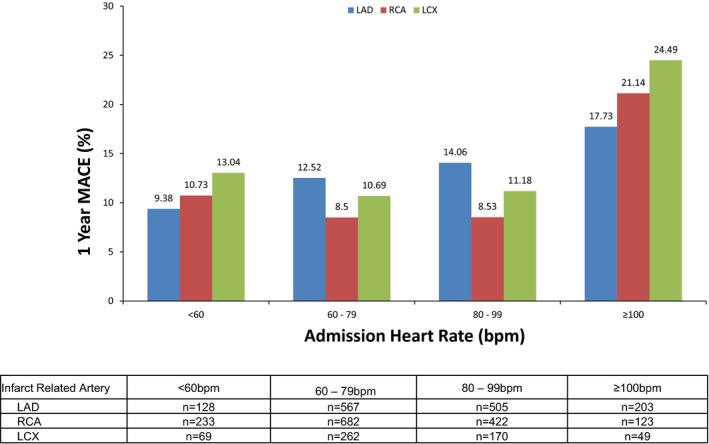
Unadjusted 1‐year major adverse cardiac events in patients with right coronary artery, left anterior descending coronary artery, or left circumflex infarct–related artery according to admission heart rate. bpm indicates beats per minute; LAD, left anterior descending coronary artery; LCX, left circumflex; MACE, major adverse cardiac events; RCA, right coronary artery.
Table 4.
Multivariable Predictors of Mortality and Major Adverse Cardiac Events at 1 Year in Patients With a RCA Culprit Lesion
| Characteristic | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| 1‐y mortalitya | |||
| Age | 1.06 | 1.02 to 1.09 | 0.001 |
| AHR <60 bpm vs 60 to 79 bpm | 1.33 | 0.41 to 4.34 | 0.64 |
| AHR 80 to 99 bpm vs 60 to 79 bpm | 1.69 | 0.67 to 4.28 | 0.27 |
| AHR ≥100 bpm vs 60 to 79 bpm | 5.02 | 1.95 to 12.88 | 0.0008 |
| Left ventricular ejection fraction <40% | 5.61 | 2.54 to 12.40 | <0.0001 |
| Cardiogenic shock | 6.33 | 1.84 to 21.72 | 0.003 |
| 1‐y major adverse cardiac eventsa | |||
| History of congestive heart failure | 2.61 | 1.21 to 5.63 | 0.01 |
| AHR <60 bpm vs 60 to 79 bpm | 1.08 | 0.62 to 1.88 | 0.78 |
| AHR 80 to 99 bpm vs 60 to 79 bpm | 0.87 | 0.54 to 1.40 | 0.57 |
| AHR ≥100 bpm vs 60 to 79 bpm | 2.20 | 1.29 to 3.75] | 0.004 |
| Left ventricular ejection fraction <40% | 2.50 | 1.43 to 4.37 | 0.0013 |
| Cardiogenic shock | 7.13 | 2.71 to 18.75 | <0.0001 |
The following covariates were included in the multivariable analyses: age, previous myocardial infarction, diabetes mellitus, congestive heart failure, left ventricular ejection fraction <40%, cardiogenic shock, AHR range, and β‐blocker use on admission. Covariates not presented in the final model were non–statistically significant. AHR indicates admission heart rate; bpm, beats per minute; RCA, right coronary artery.
AHR 60 to 79 bpm was used as reference.
Discussion
In the present analysis of AHR in patients with STEMI treated with PCI, we demonstrated that (1) sinus or junctional bradycardia (AHR <60 bpm) did not portend a worse prognosis for patients with RCA IRA compared with patients with normal heart rate on admission; (2) admission tachycardia (AHR >100 bpm) was an ominous prognostic factor irrespective of IRA, with a pronounced effect on outcomes in patients with RCA IRA, similar to LAD and left circumflex culprit vessels; and (3) AHR was not a reliable predictor of angiographic lesion localization or complexity in the RCA.
Several epidemiologic studies have suggested a near linear relationship between AHR and cardiovascular outcomes in patients with or without preexistent cardiac disease.3, 4 Early analyses of patients presenting with acute coronary syndromes in the prethrombolytic era demonstrated a linear relationship between admission AHR and early mortality.13 While the negative influence of increased AHR in patients with acute coronary syndromes has been confirmed in the thrombolytic and PCI eras,8, 14 there is still conflicting evidence regarding the impact of admission bradycardia that is predominantly noted in patients with inferior infarcts. Our findings indicate that the association of AHR with mortality followed a J‐shaped curve in patients with an RCA IRA and were in agreement with previously published data on patients with non‐STEMI1, 2, 5 as well as STEMI.7, 8 An association between low AHR and mortality was previously observed by Parodi and colleagues8 in a large single‐center registry of patients with STEMI undergoing primary PCI; the authors commented on the potential association between lower AHR (<60 bpm) and pre‐agonic states or life‐threatening arrhythmias; however, consistent with our findings, admission bradycardia was not an independent predictor of mortality at 6 months. Further, Salwa and colleagues15 presented a retrospective analysis of patients with STEMI and reported a U‐shaped relationship between AHR and mortality; however, the treatment modality was not indicated. Our study expands these observations to demonstrate a significant association between admission tachycardia and 1‐year clinical outcomes while affirming the lack of adverse prognostic significance of bradycardia on presentation in a STEMI population with RCA disease treated with primary PCI.
Qualitative and quantitative analysis in the current study demonstrated a trend toward increasing lesion complexity with increasing AHR in patients with an RCA culprit lesion, with a predominance of complex angiographic features in the tachycardia group, such as thrombolysis in myocardial infarction 0/1, myocardial blush grade 0/1, and severe calcification. In contrast, bradycardia was not associated with lesion complexity or localization within the proximal or ostial RCA, contrary to prior findings by Serrano and colleagues,16 which indicated a strong association between ostial/proximal RCA lesions and AHR. This discrepancy may be explained by a number of factors. First, our results reflect the current era population presenting with STEMI; as such, we did not exclude patients previously treated with β‐blockers or calcium channel blockers, potentially affecting the observed results. Further, our analysis excluded patients with acute AVB, a phenomenon typically associated with ostial or proximal RCA lesions or very extensive LAD disease; however, acute AVB was noted in 1.5% of the HORIZONS AMI population (2.2% in the RCA IRA population), and it was unlikely that inclusion of patients with AVB would have a significant impact on the present analysis. A separate HORIZONS‐AMI analysis of patients with high‐grade AVB during the acute phase of STEMI indicated a direct association with long‐term mortality17 likely explained by a direct ischemic insult on the atrioventricular conduction system rather than a heightened parasympathetic drive that leads to sinus or junctional bradycardia and does not correlate with increasing size of myocardium at risk.
Our analyses provides further insight into the old question of whether heart rate is an independent factor or a prognostic marker of increased risk in patients with STEMI, as we clearly demonstrated that increased AHR was an ominous prognostic predictor of mortality irrespective of the affected coronary vessel and further showed that slow heart rate was neither a predominant RCA IRA phenomenon nor was it associated with worse outcomes in that patient population. Collectively, our results indicate that admission tachycardia in patients with RCA IRA indicates a severe ischemic substrate and should be approached as rigorously as in patients with anterior infarcts using aggressive β blockade and rapid revascularization.
Limitations
Several limitations must be noted. First, this analysis was not prespecified in the HORIZONS‐AMI protocol, and the methods utilized were not conceptualized for assessment of the impact of AHR on clinical outcomes. Second, the sample size on each AHR group defined for the purpose of this analysis did not allow for complex statistical modeling, and some important parameters may have been overlooked. Further, patients with advanced AV nodal disease were excluded; this may have impacted our results in patients with RCA infarcts, given the more frequent occurrence of advanced AVB in that population. However, the overall incidence of acute advanced AVB in the HORIZONS‐AMI cohort was low and as such, it is unlikely that our results would have been significantly different with the inclusion of patients with AVB. Third, electrocardiographic assessment (with right‐sided leads) or imaging for right ventricular infarction was not specified in the HORIZONS AMI protocol and our results might not be generalized to patients with hemodynamically significant right ventricular infarction. Last, a significant number of patients were on β‐blockers or calcium channel blockers at the time of presentation and admission, potentially affecting the number of patients presenting with STEMI and bradycardia; however, β‐blockers and calcium channel blockers were equally utilized across all AHR groups in patients with an RCA IRA. Further, our population reflected the current standard of care.
Conclusions
In patients with STEMI and an RCA IRA, admission tachycardia correlated with complex angiographic findings and portended a significant risk for MACE and mortality at 1 year. In contrast, admission bradycardia, although frequently observed, was not associated with proximal/ostial lesion localization or complexity and was not an independent predictor of mortality or adverse cardiac outcomes.
Disclosures
Mehran discloses receiving Institutional research grant support from Eli Lilly/Daiichi‐Sankyo, Inc, Bristol‐Myers Squibb, AstraZeneca, The Medicines Company, OrbusNeich, Bayer, CSL Behring, Abbott Laboratories, Watermark Research Partners, Novartis Pharmaceuticals, Medtronic, AUM Cardiovascular, Inc., Beth Israel Deaconess Medical Center, participation in executive committees at Janssen Pharmaceuticals, Osprey Medical Inc, membership at data safety monitoring boards at Watermark Research Partners; consulting—Medscape, The Medicines Company, Boston Scientific, Merck & Company, Cardiovascular Systems, Inc (CSI); Sanofi USA, LLC, Shanghai BraccoSine Pharmaceutical Corp; AstraZeneca and equity at Claret Medical Inc and Elixir Medical Corporation. Mintz discloses receiving consultation fees from Boston Scientific and ACIST, fellowship/grant support from Volcano, Boston Scientific, InfraReDx and honoraria from Boston Scientific and ACIST. The rest of the authors have no relevant conflicts to disclose.
(J Am Heart Assoc. 2017;6:e006181 DOI: 10.1161/JAHA.117.006181.)28724652
References
- 1. Asaad N, El‐Menyar A, AlHabib KF, Shabana A, Alsheikh‐Ali AA, Almahmeed W, Al Faleh H, Hersi A, Al Saif S, Al‐Motarreb A, Sulaiman K, Al Nemer K, Amin H, Al Suwaidi J. Initial heart rate and cardiovascular outcomes in patients presenting with acute coronary syndrome. Acute Card Care. 2014;16:49–56. [DOI] [PubMed] [Google Scholar]
- 2. Bangalore S, Messerli FH, Ou FS, Tamis‐Holland J, Palazzo A, Roe MT, Hong MK, Peterson ED; CRUSADE Investigators . The association of admission heart rate and in‐hospital cardiovascular events in patients with non‐ST‐segment elevation acute coronary syndromes: results from 135 164 patients in the CRUSADE quality improvement initiative. Eur Heart J. 2010;31:552–560. [DOI] [PubMed] [Google Scholar]
- 3. Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. [DOI] [PubMed] [Google Scholar]
- 4. Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. [DOI] [PubMed] [Google Scholar]
- 5. Perne A, Schmidt FP, Hochadel M, Giannitsis E, Darius H, Maier LS, Schmitt C, Heusch G, Voigtlander T, Mudra H, Gori T, Senges J, Munzel T; German Chest Pain Unit Registry . Admission heart rate in relation to presentation and prognosis in patients with acute myocardial infarction. Treatment regimens in German chest pain units. Herz. 2016;41:233–240. [DOI] [PubMed] [Google Scholar]
- 6. Zipes DP. The clinical significance of bradycardic rhythms in acute myocardial infarction. Am J Cardiol. 1969;24:814–825. [DOI] [PubMed] [Google Scholar]
- 7. Noman A, Balasubramaniam K, Das R, Ang D, Kunadian V, Ivanauskiene T, Zaman AG. Admission heart rate predicts mortality following primary percutaneous coronary intervention for ST‐elevation myocardial infarction: an observational study. Cardiovasc Ther. 2013;31:363–369. [DOI] [PubMed] [Google Scholar]
- 8. Parodi G, Bellandi B, Valenti R, Memisha G, Giuliani G, Velluzzi S, Migliorini A, Carrabba N, Antoniucci D. Heart rate as an independent prognostic risk factor in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Atherosclerosis. 2010;211:255–259. [DOI] [PubMed] [Google Scholar]
- 9. Mehran R, Brodie B, Cox DA, Grines CL, Rutherford B, Bhatt DL, Dangas G, Feit F, Ohman EM, Parise H, Fahy M, Lansky AJ, Stone GW. The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) Trial: study design and rationale. Am Heart J. 2008;156:44–56. [DOI] [PubMed] [Google Scholar]
- 10. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R; Investigators H‐AT . Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. [DOI] [PubMed] [Google Scholar]
- 11. Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Ochala A, Kellock A, Parise H, Mehran R. Paclitaxel‐eluting stents versus bare‐metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946–1959. [DOI] [PubMed] [Google Scholar]
- 12. Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Carroll JD, Rutherford BD, Lansky AJ. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346:957–966. [DOI] [PubMed] [Google Scholar]
- 13. Hjalmarson A, Gilpin EA, Kjekshus J, Schieman G, Nicod P, Henning H, Ross J Jr. Influence of heart rate on mortality after acute myocardial infarction. Am J Cardiol. 1990;65:547–553. [DOI] [PubMed] [Google Scholar]
- 14. Kovar D, Cannon CP, Bentley JH, Charlesworth A, Rogers WJ. Does initial and delayed heart rate predict mortality in patients with acute coronary syndromes? Clin Cardiol. 2004;27:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salwa P, Gorczyca‐Michta I, Wozakowska‐Kaplon B. The relationship between admission heart rate and early prognosis in patients with ST‐elevation myocardial infarction. Kardiol Pol. 2015;73:177–182. [DOI] [PubMed] [Google Scholar]
- 16. Serrano CV Jr, Bortolotto LA, Cesar LA, Solimene MC, Mansur AP, Nicolau JC, Ramires JA. Sinus bradycardia as a predictor of right coronary artery occlusion in patients with inferior myocardial infarction. Int J Cardiol. 1999;68:75–82. [DOI] [PubMed] [Google Scholar]
- 17. Kosmidou I, Redfors B, Dordi R, Dizon JM, McAndrew T, Mehran R, Ben‐Yehuda O, Mintz GS, Stone GW. Incidence, predictors, and outcomes of high‐grade atrioventricular block in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (from the HORIZONS‐AMI trial). Am J Cardiol. 2017;119:1295–1301. [DOI] [PubMed] [Google Scholar]


