Abstract
Background
About 50% of normal‐flow/low‐gradient patients (ie, low mean gradient [MG] or peak aortic jet velocity and small aortic valve area) have severe aortic valve calcification as measured by computed tomography. However, they are considered to have moderate aortic stenosis (AS) in current American College of Cardiology/American Heart Association guidelines. The objective was thus to evaluate the effect of hypertension and reduced arterial compliance (rAC) on MG and Vpeak measurements.
Methods and Results
Doppler‐echocardiography was performed in 4 sheep with experimentally induced severe and critical AS at: (1) normal aortic pressure, (2) during hypertension, and (3) with rAC. Hypertension and rAC induced a substantial decrease in MG/Vpeak compared with normal stage (both P≤0.03) despite a stable transvalvular flow (P>0.16). Hypertension and rAC resulted in a greater reduction of MG in critical (−42%) compared with severe (−35%) AS (P˂0.0001). Comprehensive Doppler‐echocardiography and computed tomography were performed in 220 AS patients (mean age: 69±13 years; MG 29±18 mm Hg) with normal flow. The population was divided in 3 groups according to the presence of hypertension and rAC. The slope of the linear association between MG/Vpeak and aortic valve calcification divided by the cross‐sectional area of the aortic annulus was significantly reduced in patients with hypertension and/or rAC compared with normotensive/normal AC patients (P<0.01). Accordingly, patients with normal‐flow/low‐gradient and severe aortic valve calcification density were more frequent in hypertension and rAC groups compared with the normotensive/normal‐AC group (16% and 12% compared with 2%; P=0.03).
Conclusions
Hypertension and rAC are associated with a substantial reduction in MG/Vpeak for similar aortic valve calcification (ie, similar AS anatomic severity), which may lead to underestimation of AS hemodynamic severity.
Keywords: aortic stenosis, arterial compliance, hypertension, normal flow‐low gradient
Subject Categories: Animal Models of Human Disease, Echocardiography, Computerized Tomography (CT)
Clinical Perspective
What Is New?
Previous studies had also reported that hypertension may cause a decrease in flow, and it was already well known that transvalvular gradients and velocities are highly flow dependent and may thus underestimate aortic stenosis (AS) severity.
The present study is the first to report that gradients and velocities may also be directly influenced (ie, blunted) by hypertension and reduced arterial compliance even in normal flow conditions.
Hence, gradients or velocity may underestimate AS severity in the presence of hypertension and reduced arterial compliance.
On the other hand, aortic valve area and Doppler velocity index appear to be less affected by hypertension or reduced arterial compliance and may thus be more reliable to assess AS severity in the presence of hypertension or reduced arterial compliance.
What Are the Clinical Implications?
Hypertension and/or reduced arterial compliance, which frequently coexist with AS, may result in a substantial reduction in peak aortic jet velocity and transvalvular pressure gradient and thus lead to a normal flow/small aortic valve area/low gradient pattern, despite the presence of a possible severe AS.
Hence, in a symptomatic patient with normal‐flow/low‐gradient AS, it is recommended to perform additional diagnostic tests such as quantitation of aortic valve calcification by computed tomography to confirm the stenosis severity.
Further studies are needed to assess the following: (1) elucidate the mechanisms that may explain the direct impact of hypertension and/or arterial compliance on peak aortic jet velocity and mean transvalvular gradient; and (2) assess the impact of aortic valve replacement on outcomes in patients with normal‐flow/low‐gradient AS.
Introduction
Calcified aortic stenosis (AS) is the most frequent valvular heart disease in developed countries and the second most common indication for cardiac surgery after coronary artery bypass grafting.1 The primary method to confirm the diagnosis and severity of AS is Doppler‐echocardiography.2 Nevertheless, assessment of AS severity is still challenging in some patients. According to the current American Heart Association/American College of Cardiology and European Society of Cardiology/European Association of Cardio‐thoracic Surgery guidelines,3, 4 cut‐off values for Doppler‐echocardiography evaluation of severe AS are defined by the following: peak transvalvular velocity (Vpeak) ≥4.0 m/s, mean gradient (MG) ≥40 mm Hg, aortic valve area (AVA) ≤1.0 cm2 and/or AVA indexed for body surface area ≤0.6 cm2/m2. Severe AS is a class I indication for aortic valve replacement if the patient has symptoms and/or left ventricular (LV) systolic dysfunction defined as LV ejection fraction ˂50%. However, discrepancies are frequently encountered between MG or Vpeak and AVA.5 The most frequent discordant situation is the combination of a small AVA (˂1.0 cm2), consistent with a severe AS, with a low MG or Vpeak (˂40 mm Hg or <4 m/s) rather consistent with the presence of a moderate AS. In daily practice, these discordant findings may lead to inadequate assessment of the actual severity of the disease and thus to an inappropriate therapeutic management, which may, in turn, have a negative impact on patient outcome.6, 7, 8
In patients with AVA ≤1.0 cm2 and preserved LV ejection fraction, 4 groups have been identified according to flow (indexed stroke volume ˂ or ≥35 mL/m2) and gradient (˂ or ≥40 mm Hg): (1) Normal‐Flow, High‐Gradient; (2) Normal‐Flow, Low‐Gradient; (3) Low‐Flow, High Gradient; and (4) Low‐Flow, Low‐Gradient.7 The Low‐Flow, Low‐Gradient pattern is observed in 5% to 20% of patients with AS and is characterized by the presence of pronounced LV concentric remodeling, advanced diastolic dysfunction, reduced LV longitudinal myocardial systolic function, and increased brain natriuretic peptide levels.6, 9, 10, 11 The Normal‐Flow, Low‐Gradient pattern is observed in up to 40% of patients and is not necessarily associated with the features of Low‐Flow, Low‐Gradient described above.12, 13 Among patients with discordant‐AS grading (ie, small AVA but low gradient: Low‐Flow, Low‐Gradient and Normal‐Flow, Low‐Gradient), 50% have severe aortic valve calcification as measured by multidetector computed tomography.14 Recent meta‐analyses reported that aortic valve replacement is associated with major survival benefit in both Low‐Flow, Low‐Gradient and Normal‐Flow, Low‐Gradient AS.15, 16 However, in current clinical guidelines, Normal‐Flow, Low‐Gradient patients are considered as moderate AS and there is no specific recommendation for referral to aortic valve replacement in these patients.17
Systemic hypertension is a common comorbidity in patients with AS, with a prevalence up to 75%.18, 19, 20 In patients with AS, reduced systemic arterial compliance (rAC) is the predominant cause of hypertension. We hypothesized that the severity of AS may be, at least in part, masked by the presence of coexisting hypertension/rAC and lead to a decrease in MG and Vpeak and thus to a higher rate of Normal‐Flow, Low‐Gradient pattern in severe AS.
The objective of this study was to evaluate the effect of hypertension and rAC on MG and Vpeak measured by Doppler‐echocardiography in normal flow state. To address this objective, we performed (1) an animal study to examine the impact of hypertension and rAC on aortic valve hemodynamics in the context of severe or critical AS; (2) a clinical study to evaluate the effect of hypertension and rAC on the measurement of MG or Vpeak in any stage of AS. AS severity was assessed by aortic valve calcification measured by multidetector computed tomography; and (3) In both clinical and experimental studies, we evaluated the interaction between AS severity and hypertension or rAC.
Material and Methods
Animal Study
Experimental conditions
Animal care and experiments were conducted in accordance with the guidelines of the Canadian Council on Animal Care. The protocol was approved by the institutional animal care committee of Laval University, Sainte‐Foy, Quebec, Canada.
Four sheep weighing >35 kg were anesthetized (intramuscular premedication with midazolam, induction by intravenous injection of ketamine [2.75 mg/kg] and diazepam [0.2 g/kg], and maintenance with inhalation of 2–3% isoflurane). A lateral right thoracotomy was performed and fourth and fifth right ribs were removed. In each sheep, a severe (AVA <0.6 cm2) and a critical (AVA <0.4 cm2) supravalvular AS were induced by a ligature around the aorta about 0.5 cm downstream to the aortic valve annulus.21, 22 Central arterial hypertension of 130 mm Hg of systolic blood pressure was induced by banding of the distal ascending aorta while normal systolic blood pressure was 90 mm Hg in anesthetized sheep. Central arterial compliance was reduced (rAC) by implanting a Dacron prosthesis (length: 35–50 mm) around the ascending aorta (Figure 1). Two types of severity of AS were tested in order to assess the possible interactions between hypertension or AC and AS severity. The order of realization of the different experimental conditions (n=6) was randomly selected to avoid measurement or time‐shift biases.
Figure 1.
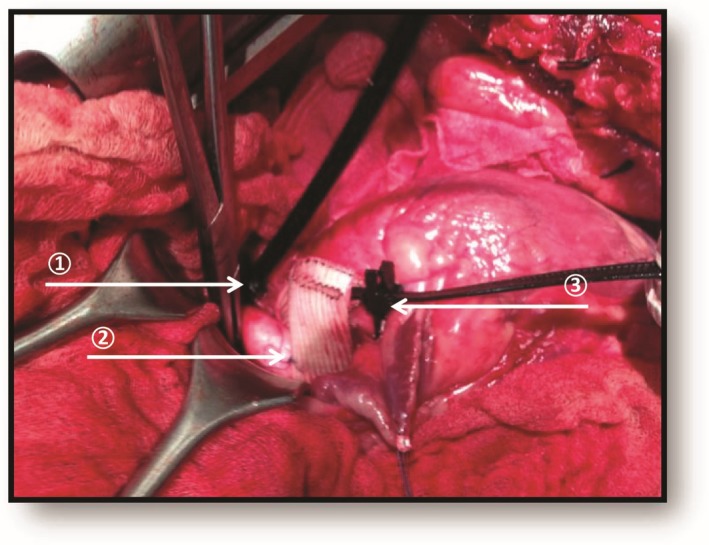
Intraoperative photography of surgical technique in sheep. (1) Distal aorta banding induced central hypertension. (2) Dacron prosthesis around aorta reduced central arterial compliance. (3) Proximal aorta banding induced severe or critical aortic stenosis.
Doppler echocardiography
The Doppler‐echocardiographic measurements described below were obtained in the 6 following experimental conditions: (1) severe AS; (2) severe AS+hypertension; (3) severe AS+rAC; (4) critical AS; (5) critical AS+hypertension; and (6) critical AS+rAC. Doppler echocardiograms were recorded with a Sonos 5500 (Philips Medical Systems, Andover, MA). The ultrasound probe (S3; Philips Medical Systems) was covered with a sheet and was introduced into the thoracic cavity and positioned at the level of the cardiac apex. This window allowed the visualization of high‐quality images in apical 3‐, 4‐, and 5‐chamber views and optimal recording of the LV outflow tract pulsed‐wave velocity and aortic jet continuous wave velocity. The Doppler derived mean and peak transvalvular pressure gradients were calculated with the modified Bernoulli formula, the AVA was calculated with the standard continuity equation, and the Doppler velocity index by dividing the time velocity integral in the LV outflow tract by the time velocity integral in the aorta. The Doppler‐derived mean systolic flow rate across the stenosis was calculated by dividing the measured stroke volume by LV ejection time obtained on the aortic jet velocity signal.23
Clinical Study
Patient population
We analyzed data from 220 adult AS patients with normal flow (stroke volume index >35 mL/m2) who underwent comprehensive Doppler‐echocardiography and multidetector computed tomography without contrast within 3 months. We excluded patients with rheumatic AS, infective endocarditis, and moderate or severe aortic regurgitation. The study was approved by the Ethics Committee of the Quebec Heart and Lung Institute, Quebec, Canada, who granted us a waiver of consent.
Clinical data
Clinical data included age, sex, height, weight, body surface area, systolic and diastolic blood pressure, documented diagnosis of traditional cardiovascular risk factors and comorbidities such as history of hypertension, diabetes mellitus, coronary artery disease (defined by history of myocardial infarction or ≥50% coronary artery stenosis on coronary angiography), and renal failure. Systolic and diastolic blood pressures were measured with an arm‐cuff sphygmomanometer on the right arm in supine position at the time of stroke volume measurement, thus after 30 minutes of rest in all patients.
Patients were separated into 3 groups according to systolic blood pressure and systemic AC: (1) Normotensive (systolic blood pressure <140 mm Hg) ‐normal compliance (systemic AC >0.8 mL/m2 per mm Hg; NTN‐nAC) composed of 54 patients (25%); (2) Hypertensive (systolic blood pressure >140 mm Hg; hypertension) composed of 93 patients (44%); and (3) normotensive‐reduced compliance (systolic blood pressure <140 mm Hg and systemic AC <0.8 mL/m2 per mm Hg; NTN‐rAC) composed of 73 patients (34%).
Doppler‐echocardiography
All patients underwent comprehensive echocardiography using commercially available echocardiographic systems. All Doppler‐echocardiographic examinations were performed and analyzed in the same laboratory by the same team of sonographers and cardiologists. Indexed stroke volume was calculated by multiplying the LV outflow tract area by the flow velocity–time integral and by indexing to body surface area. A normal flow was defined as indexed stroke volume >35 mL/m2.17 The echocardiographic indices of AS severity included VPeak, MG obtained with the use of the modified Bernoulli formula, and AVA calculated by the standard continuity equation and indexed to body surface area. LV ejection fraction was measured by the biplane Simpson method. Arterial compliance was calculated as the indexed stroke volume divided by the systemic pulse pressure (systolic blood pressure−diastolic blood pressure).24
Multidetector computed tomography
To assess AS severity by aortic valve calcification (AVC),14 multidetector computed tomography scans without contrast were performed using a 64‐slices helical scanner (Somaton Definition; Siemens AG Medical Solution, Germany) with a tube potential at 120 kV and a tube current‐time product at 60 to 80 mAs. Operators blinded to clinical and echocardiographic data performed all computed tomography examinations and analyses. The entire heart was assessed by 2.4‐ to 3‐mm‐thick transverse slices with a pitch of 0.15 to 0.25 mm during end‐inspiration breath‐hold. Acquisition was triggered by ECG at 60% to 70% of the R‐to‐R‐wave interval. Aortic valve calcification score was measured with the Agatston scoring method using commercially available and validated software (Aquarius iNtuition; TeraRecon Inc, San Mateo, CA) and all aortic valve calcification data were expressed in Agatston units (AU).25 Calcification was defined as 4 adjacent pixels with a density >130 Hounsfield units. The summation of per‐slice lesion scores was performed individually for every aortic valve calcification score. In order to take into account the interpatient variability in valve size, we used the aortic valve calcification density, where aortic valve calcification was divided by the cross‐sectional aortic annulus area (π×[Aortic annulus diameter/2]2) measured by echocardiography.14, 26, 27
Statistical Analysis
The continuous variables were tested for normality of distribution with the Shapiro–Wilk test. Continuous data were expressed as mean±SD and not normally distributed variables were presented as median [percentile 25–75]. Categorical data were expressed as percentage.
Animal study
Continuous variables measured during the 3 experimental stages (normal, hypertension, and rAC stages) were compared with the use of 2‐way ANOVAs for repeated measurements adjusted for the severity of the stenosis. The 2‐way ANOVAs allowed testing of the interaction between the experimental stages and stenosis severity. The ANOVAs were followed by post hoc Tukey tests for pairwise multiple comparisons (Figure 2).
Figure 2.
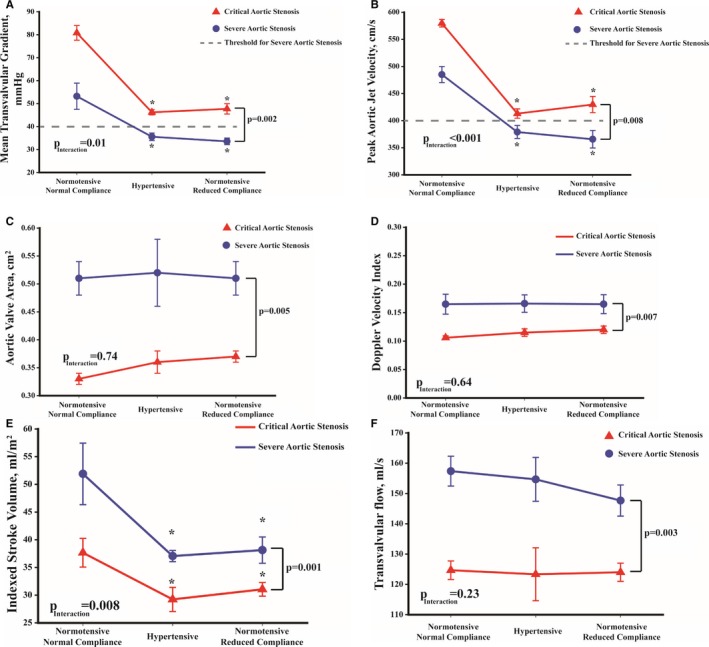
Evolution of hemodynamic variables of severe (blue line) and critical aortic stenosis (red line) in sheep with normal blood pressure, hypertension, and reduced systemic arterial compliance. (A) The evolution of mean transvalvular gradient, (B) peak aortic jet velocity, (C) aortic valve area, (D) Doppler velocity index, (E) stroke volume index, and (F) mean transvalvular flow rate. Note that in severe AS, peak aortic jet velocity and mean gradient are in the non‐severe range with concomitant hypertension or reduced systemic arterial compliance. P is P value of severe stenosis vs critical stenosis. P Interaction is the interaction between stage (ie, normotensive and normal compliance, hypertension or reduced systemic arterial compliance) and stenosis severity (ie, critical or severe). *P<0.05 compared with normotensive and normal compliance stage of the same stenosis severity group.
Clinical study
Continuous normal variables were compared between groups with the use of 1‐way ANOVA followed by post hoc Tukey tests for pairwise multiple comparisons. Aortic valve calcification and aortic valve calcification density were continuous non‐normal variables and were compared between groups with the use of Wilcoxon test and nominal variables with the use of χ2 or Fisher exact test when appropriate. Correlations were determined using Pearson's correlation coefficients or Spearman's correlation coefficients. To achieve normal distribution and linear association between aortic valve calcification, aortic valve calcification density, and hemodynamic markers of AS severity, aortic valve calcification variables were square‐root transformed. The transformation was used only in Figure 3.
Figure 3.
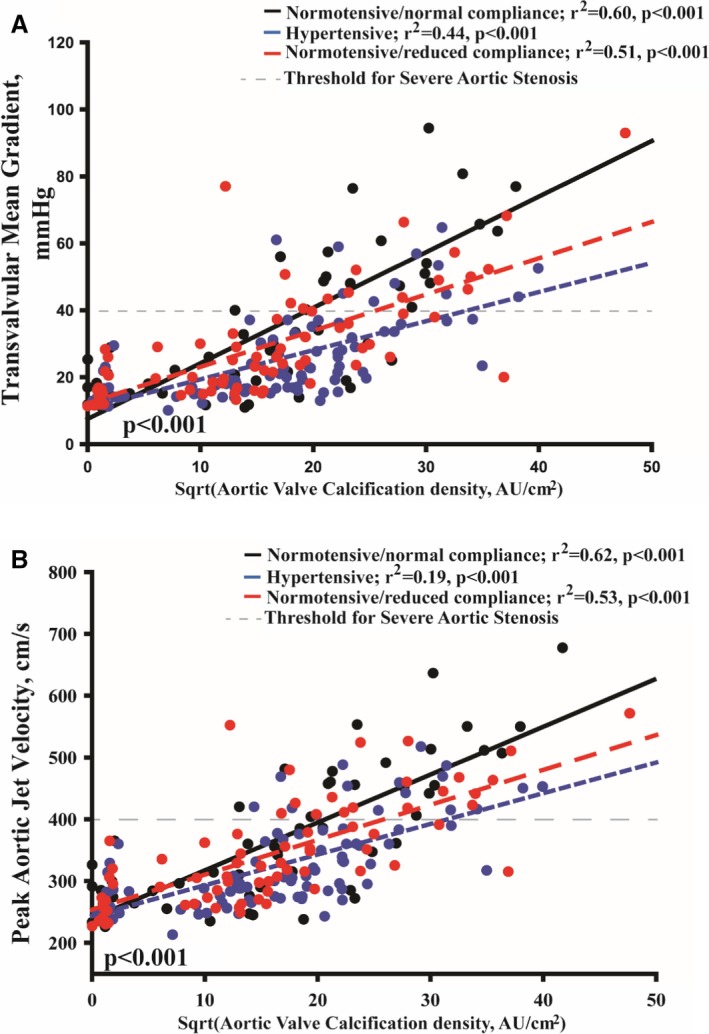
Correlation between hemodynamic and anatomic aortic stenosis severity according to blood pressure and systemic arterial compliance status. (A) The correlation between aortic valve calcification density (normalized by square root [Sqrt]) and mean gradient and (B) the correlation between aortic valve calcification density (normalized by square root [Sqrt]) and peak aortic jet velocity. Note that both peak aortic jet velocity and mean gradient are highly influenced by hypertension and reduced systemic arterial compliance compared with normotensive patients with normal arterial compliance. Moreover, the decrease in mean gradient and peak aortic jet velocity is more important with more severe AS.
A P˂0.05 was considered statistically significant. All statistics were performed with the use of JMP 12 and SPSS 20 softwares.
Results
Animal Study
Without AS, Vpeak was 1.1±0.1 m/s, MG 3±1 mm Hg, stroke volume index 29.3±6.2 mL/m2, transvalvular flow 122±2 mL/s, AVA 1.88±0.05 cm2, Doppler velocity index 0.62±0.05. In critical AS stage compared with severe AS stage, the Vpeak (4.7±0.8 m/s versus 4.1±5.8 m/s; P=0.04) and MG (58±17 mm Hg versus 41±10 mm Hg; P=0.001) were significantly increased, while stroke volume index (32.7±4.2 mL/m2 versus 42.4±7.8 mL/m2; P=0.005), transvalvular flow (124±5 mL/s versus 153±7 mL/s; P˂0.0001), AVA (0.35±0.02 cm2 versus 0.51±0.04 cm2; P˂0.0001), and Doppler velocity index (0.11±0.01 versus 0.17±0.01; P=0.007) were significantly reduced.
The high MG caused by the supravalvular ligature in the severe AS stage was substantially decreased with the induction of hypertension and rAC (53±1 mm Hg versus 36±1 mm Hg with hypertension and 34±2 mm Hg with rAC, respectively; P˂0.0001, Figure 2A). Similarly, the MG achieved with critical AS was significantly reduced with hypertension or rAC (81±1 mm Hg versus 46±1 and 48±2 mm Hg, respectively; P˂0.001, Figure 2A). Interestingly, there was an interaction (P=0.01) between AS severity and the vascular conditions (hypertension or rAC) (ie, the more severe the AS was, the higher the decrease in mean gradient). The Vpeak showed similar results as MG (all P˂0.001, Figure 2B), whereas AVA and Doppler velocity index remained stable (all P>0.55, Figure 2C and 2D). The stroke volume index decreased (all P<0.05, Figure 2E) while the transvalvular flow remained unchanged in animals that had severe AS without or with hypertension or rAC (all P>0.18) and critical AS without or with hypertension or rAC (all P>0.96) (Figure 2F). In each AS severity, the LV ejection time was decreased in hypertension and rAC compared with no hypertension–no rAC condition (0.308 s and 0.328 s versus 0.407 s, respectively; all P≤0.008).
Clinical Study
Patient characteristics
The baseline characteristics of the 220 patients included in this study are presented in the Table. Mean age was 69±13 years and 71% were men, with a mean body surface area of 1.86±0.21 m2. Seventy‐one percent of patients had a history of hypertension, 25% of diabetes mellitus, 45% had a history of coronary artery disease, and 12% of renal failure. Average systolic and diastolic blood pressures were 135±18 and 74±10 mm Hg, respectively. Regarding echocardiographic data, all patients had normal flow and average indexed stroke volume in the whole cohort was 44±7 mL/m2. The average values of the AS severity parameters were as follows: VPeak=3.4±0.9 m/s, MG=29±18 mm Hg, AVA=1.06±0.3 cm2, and AVA indexed for body surface area=0.57±0.16 cm2/m2 (Table). Systemic AC was 0.77±0.26 mL/m2 per mm Hg and LV ejection fraction was 65±6% (Table).
Table 1.
Baseline Characteristics of the Study Population
| Variables | Whole Population | NTN‐nAC (n=54, 25%) | HTN (n=93, 42%) | NTN‐rAC (n=73, 33%) | P Value |
|---|---|---|---|---|---|
| Clinical data | |||||
| Age, y | 69±13 | 59±14a, b | 73±10c | 72±9c | <0.0001 |
| Male sex, % | 71 | 62 | 76 | 70 | 0.20 |
| Body mass index, kg/m2 | 28±5 | 27±5 | 29±5 | 27±4 | 0.07 |
| Body surface area, m2 | 1.86±0.21 | 1.84±0.22 | 1.89±0.21 | 1.83±0.20 | 0.36 |
| Blood pressure: systolic, mm Hg | 135±18 | 114±10a, b | 152±11c, b | 129±7a, c | <0.0001 |
| Blood pressure: diastolic, mm Hg | 74±10 | 72±10a | 79±11c, b | 69±8a | <0.0001 |
| Coronary artery disease, % | 45 | 19a, b | 50 | 56 | <0.0001 |
| Diabetes mellitus, % | 25 | 21 | 28 | 22 | 0.52 |
| Renal failure, % | 12 | 8 | 14 | 12 | 0.58 |
| Echocardiographic data | |||||
| Peak aortic jet velocity, m/s | 3.42±0.88 | 3.72±1.12a, b | 3.24±0.70c | 3.43±0.85c | 0.006 |
| Mean gradient, mm Hg | 29±18 | 36±24a, b | 25±12c | 29±17c | 0.001 |
| Aortic valve area, cm2 | 1.06±0.30 | 1.07±0.33 | 1.12±0.29b | 1.08±0.27a | 0.005 |
| Indexed aortic valve area, cm2/m2 | 0.57±0.16 | 0.59±0.18 | 0.59±0.15b | 0.52±0.13a | 0.009 |
| Stroke volume, mL | 82±17 | 88±19b | 84±17b | 76±11a, c | 0.0001 |
| Indexed stroke volume, mL/m2 | 44±7 | 48±8a, b | 45±8c, b | 41±5a, c | <0.0001 |
| LV ejection fraction, % | 65±6 | 65±6 | 65±7 | 64±6 | 0.80 |
| Multidetector computed tomography data | |||||
| Aortic valve calcification, AU | 1677 [1466–1889] | 1795 [1364–2260] | 1665 [1340–1990] | 1607 [1240–1975] | 0.81d |
| Aortic valve calcification density, AU/cm2 | 374 [323–426] | 418 [313–524] | 354 [274–433] | 369 [279–458] | 0.62d |
AU indicates Agatston units; HTN, hypertensive group; LV, left ventricle; NTN‐nAC, nonhypertensive–normal arterial compliance group; NTN‐rAC, nonhypertensive–reduced arterial compliance group.
P<0.05 from hypertension patients.
P<0.05 from NTN‐rAC patients.
P<0.05 from NTN‐nAC patients.
Statistical analysis stratified for sex.
Fifty‐four patients (25%) were normotensive with normal systemic AC (NTN‐nAC), 73 (33%) were normotensive with rAC (NTN‐rAC), and 93 patients (42%) had a systolic blood pressure ≥140 mm Hg (hypertension) at the time of echocardiography. The baseline characteristics of the 3 groups of patients are presented in the Table. Briefly, patients with hypertension and NTN‐rAC were older and had more history of coronary artery disease (both P<0.0001).
Effect of Hypertension and rAC on the Relationship Between Anatomic Severity and Hemodynamic Severity of AS
Aortic valve calcification density was well correlated with MG and Vpeak in all groups (all P<0.001, Figure 3). Regression slopes were significantly different and decreased in hypertension and NTN‐rAC groups compared with NTN‐nAC (all P<0.01, Figure 3). After adjusting for age, sex, body mass index, coronary artery disease, stroke volume index, aortic valve area index, and aortic valve calcification density, the association between groups and MG or Vpeak remained significant (both P≤0.03). In patients with anatomically severe AS as documented by aortic valve calcification density (≥292 Agatston units /cm2 in women and ≥476 Agatston units/cm2 in men),14 Vpeak and MG were lower in hypertension and NTN‐rAC compared with NTN‐nAC (all P<0.05) and were below the severe cut point (<40 mm Hg or <4 m/s) in the vast majority of patients (Figure 4). However, indexed AVA was similar in hypertension and NTN‐rAC compared to NTN‐nAC (all P>0.15) (Figure 4). Accordingly, among patients with severe aortic valve calcification density, the proportion of Normal‐Flow, Low‐Gradient patients were more frequent in the hypertension and rAC groups compared with the NTN‐nAC group (68% and 46%, respectively, compared with 24%; P=0.006) (Figure 5). Despite remaining within normal range in all patients, indexed stroke volume was lower in hypertension and NTN‐rAC groups compared with the NTN‐nAC group (P<0.0001) (Figure 4).
Figure 4.
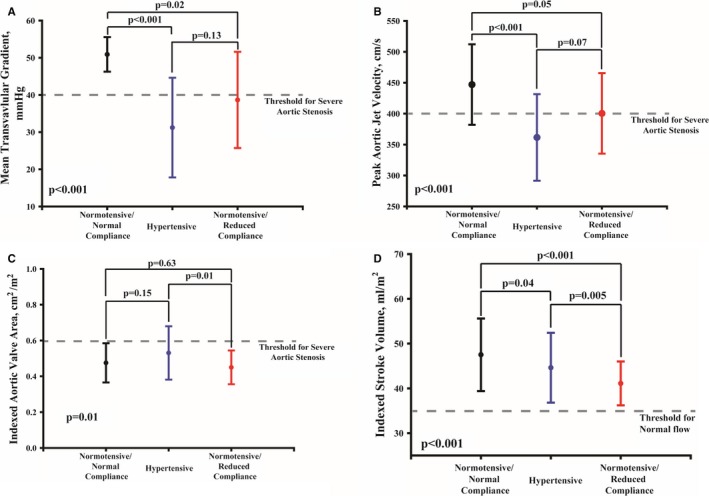
Impact of hypertension and reduced systemic arterial compliance on valvular hemodynamics and flow in patients with severe aortic stenosis. Graphs show the mean±SD of mean transvalvular gradient (A), peak aortic jet velocity (B), indexed aortic valve area (C), and stroke volume index (D). Severe AS was documented by aortic valve calcification density ≥292 Agatston units/cm2 in women and ≥476 Agatston units/cm2 in men.14
Figure 5.
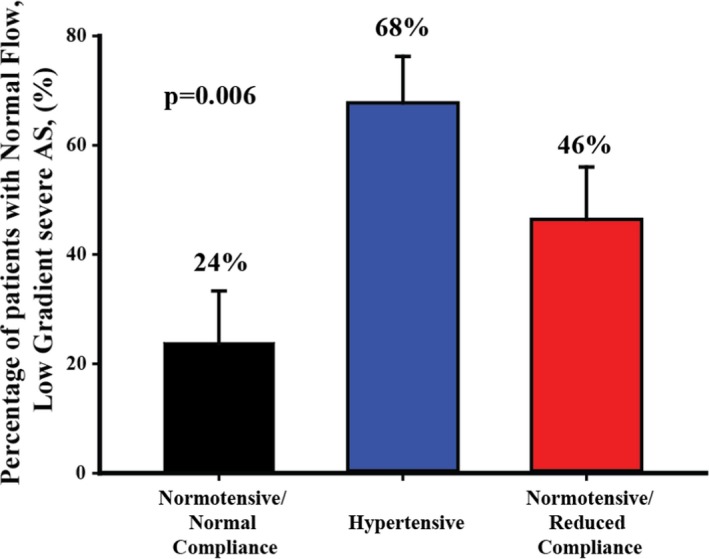
Impact of hypertension and reduced systemic arterial compliance on the percentage of patients with discordant normal‐flow, low‐gradient severe AS. Severe AS was documented by aortic valve calcification density ≥292 Agatston units/cm2 in women and ≥476 Agatston units/cm2 in men.14 Error bars represent SE. AS indicates aortic stenosis.
Discussion
The main findings of this study are that in an experimental model of AS as well as in patients with AS, (1) the occurrence of systemic hypertension or rAC results in an important reduction of MG and Vpeak, independently of flow state; (2) The relationship between MG or Vpeak and aortic valve calcification density is highly influenced by hypertension and rAC; (3) The effects of hypertension and rAC increase with the severity of the stenosis; and (4) The proportion of patients with severe AS and Normal‐Flow, Low‐Gradient is higher in patients with hypertension and rAC compared with normotensive patients with normal compliance.
Normal‐Flow, Low‐Gradient Pattern
Although flow and AVA are major determinants of MG and Vpeak, other factors such as AC and hypertension may have an effect on arterial hemodynamics and lead to Normal‐Flow, Low‐Gradient severe AS pattern. Fifty percent of patients with discordant (small AVA but low gradient) AS grading have severe aortic valve calcification as measured by computed tomography.14 The findings of the present study may provide some explanation for this intriguing combination of low gradient/velocity despite a normal flow and severe AS. The concomitant presence of increased blood pressure and/or rAC may directly reduce gradients and velocity even though flow is stable, and this may explain why some patients with normal flow may have severe AVA and severe aortic valve calcification density but nevertheless a low gradient.
Impact of Hypertension on AS Severity Grading
Hypertension is a well‐established risk factor for cardiovascular events in the general population. In studies involving young patients with AS, hypertension was present in 30% to 40%,18 whereas in recent series with older patients at high risk for aortic valve replacement, the prevalence of hypertension was 75% or higher.19, 20 Moreover, in recent series, hypertension was found in one third of patients presenting with symptomatic severe AS.28 There are several mechanisms that could explain the important reduction of MG in the presence of hypertension at any AS severity. First, hypertension induces an increased systemic arterial resistance and rAC that, in turn, result in an increase in LV afterload. The decrease in flow induced by hypertension leads to a reduction in transvalvular gradient and velocity. Kadem et al previously demonstrated in an animal model of supravalvular AS that acute hypertension induces a significant reduction in the peak and mean gradients as measured by catheter without significant decrease in mean transvalvular flow rate.22 In our animal study, the induction of hypertension resulted in a slight decrease in stroke volume but did not influence significantly the mean transvalvular flow rate. Despite stable flow rate conditions, the MG and Vpeak measured by Doppler‐echocardiography decreased markedly with hypertension. Thus, the presence of high pressure in the aorta could per se interfere with the aortic valve hemodynamics and thus with the Doppler‐echocardiographic evaluation of the severity of AS. As recommended in the current guidelines, hypertension, if any, should be controlled before evaluating AS severity by Doppler‐echocardiography or catheterization.17 However, it is not always feasible to normalize blood pressures, especially in patients with severe AS, and antihypertensive medications do not necessarily normalize arterial compliance either.
Effect of Reduced AC on AS Severity Assessment
Arterial stiffening has been associated with hypertension, dyslipidemia, diabetes mellitus, and atherosclerosis.29, 30, 31, 32, 33 The buffering action of the systemic arterial system is reduced with rAC. rAC is a major contributor to the development of systolic hypertension, contributing to LV afterload and decreased coronary flow during diastole, and it has been shown to be a strong predictor of LV dysfunction and adverse events.30, 31, 32, 33, 34 Patients with AS already have increased LV afterload because of valvular stenosis, and it has been demonstrated that rAC has a significant additive effect and contributes to LV dysfunction and occurrence of adverse outcomes.22, 35 In the present study, we showed that rAC induces a marked reduction in MG and Vpeak for any AS severity, even if flow is stable and even in the absence of hypertension. One can hypothesize that faster and earlier reflection of arterial wave from the periphery may blunt the transvalvular gradients and velocities. However, further studies are needed to elucidate the mechanisms underlying the effect of AC and hypertension on aortic valve hemodynamics.
Limitations of the Study
The supravalvular aortic banding used in our animal model does not represent the complex biomechanics and hemodynamics of AS in humans. Also, the model used to induce hypertension does not necessarily reflect the actual physiopathology of hypertension in AS patients. However, the experimental model was only used to assess the effect of central arterial hemodynamics on flow and AS hemodynamics and not to evaluate the impact on LV remodeling and hypertrophy. Moreover, the results obtained in this experimental model of supravalvular AS were replicated in a population of patients with severe AS and concomitant hypertension and rAC. Finally, further study on the reduction of systemic hypertension and compliance in AS patients would be important to confirm these results.
Conclusion
This study shows that hypertension and/or reduced arterial compliance, which are highly prevalent in patients with AS, may induce an important decrease in transvalvular gradient and velocity, independently of flow conditions. This phenomenon may lead to an underestimation of AS severity in the presence of concomitant hypertension or rAC. This study also provides an explanation for the intriguing finding of patients with Normal‐Flow, Low‐Gradient AS pattern at Doppler‐echocardiography and having nonetheless severe AS according to aortic valve calcification measured by computed tomography. Indeed, we showed that hypertension and/or rAC might be the cause of the blunting of gradient and thus the AVA‐gradient discordance in patients with severe AS and normal flow. Hence, patients with discordant findings at echocardiography and concomitant elevated blood pressure and reduced AC require special attention, especially if they are symptomatic, and they may need additional diagnostic tests to confirm stenosis severity. First, Doppler‐echocardiography should be repeated and parameters of stenosis severity reassessed once blood pressures and AC have ideally been normalized. However, this goal cannot always be achieved with pharmacotherapy, and quantitation of aortic valve calcification by multidetector computed tomography provides a valuable complementary diagnostic tool to assess anatomic severity of AS. Calcium scoring using multidetector computed tomography has the advantage of being a simple measurement, which is not influenced by flow or arterial hemodynamics.
Sources of Funding
Dr Clavel holds a young investigator grant from the Quebec Heart and Lung Institute Foundation (Fondation de l'institut universitaire de cardiologie et de pneumologie de Québec).
Disclosures
None.
Acknowledgments
We would like to express special thanks to Benoît Savard, Sébastien Poulin, Justin Robillard, François Dagenais, and Éric Dumont for their technical help throughout the study process.
(J Am Heart Assoc. 2017;6:e006276 DOI: 10.1161/JAHA.117.006276.)28687561
References
- 1. Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. [DOI] [PubMed] [Google Scholar]
- 2. Lancellotti P, Donal E, Magne J, Moonen M, O'Connor K, Daubert JC, Piérard LA. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364–1371. [DOI] [PubMed] [Google Scholar]
- 3. Bonow RO, Carabello BA, Kanu C, de Leon ACJ, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. [DOI] [PubMed] [Google Scholar]
- 4. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012): Joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42:S1–S44. [DOI] [PubMed] [Google Scholar]
- 5. Minners J, Allgeier M, Gohlke‐Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart. 2010;96:1463–1468. [DOI] [PubMed] [Google Scholar]
- 6. Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. [DOI] [PubMed] [Google Scholar]
- 7. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebo A, Pedersen TR, Skjaerpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke‐Barwolf C. Outcome of patients with low‐gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. [DOI] [PubMed] [Google Scholar]
- 9. Clavel MA, Magne J, Pibarot P. Low‐gradient aortic stenosis. Eur Heart J. 2016;37:2645–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area and low‐flow, low‐gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259–1267. [DOI] [PubMed] [Google Scholar]
- 11. Pibarot P, Dumesnil JG. Aortic stenosis: look globally, think globally. JACC Cardiovasc Imaging. 2009;2:400–403. [DOI] [PubMed] [Google Scholar]
- 12. Lancellotti P, Magne J, Donal E, Davin L, O'Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis. Insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235–243. [DOI] [PubMed] [Google Scholar]
- 13. Monin JL, Lancellotti P, Monchi M, Lim P, Weiss E, Piérard L, Guèret P. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120:69–75. [DOI] [PubMed] [Google Scholar]
- 14. Clavel MA, Messika‐Zeitoun D, Pibarot P, Aggarwal S, Malouf J, Araoz P, Michelena H, Cueff C, Larose É, Capoulade R, Vahanian A, Enriquez‐Sarano M. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler‐echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–2338. [DOI] [PubMed] [Google Scholar]
- 15. Dayan V, Vignolo G, Magne J, Clavel MA, Mohty D, Pibarot P. Outcome and impact of aortic valve replacement in patients with preserved LV ejection fraction and low gradient aortic stenosis: a meta‐analysis. J Am Coll Cardiol. 2015;66:2594–2603. [DOI] [PubMed] [Google Scholar]
- 16. Berthelot‐Richer M, Pibarot P, Capoulade R, Dumesnil JG, Dahou A, Thébault C, Le Ven F, Clavel MA. Discordant grading of aortic stenosis severity: echocardiographic predictors of survival benefit associated with aortic valve replacement. JACC Cardiovasc Imaging. 2016;9:797–805. [DOI] [PubMed] [Google Scholar]
- 17. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 18. Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft C, Miyake‐Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–2270. [DOI] [PubMed] [Google Scholar]
- 19. Rodés‐Cabau J, Webb JG, Cheung A, Ye J, Dumont É, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, Devarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochellière R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. [DOI] [PubMed] [Google Scholar]
- 20. Lindman BR, Arnold SV, Madrazo JA, Zajarias A, Johnson SN, Pérez JE, Mann DL. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. 2011;4:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith MD, Dawson PL, Elion JL, Booth DC, Handshoe R, Kwan OL, Earle GF, DeMaria AN. Correlation of continuous wave Doppler velocities with cardiac catheterization gradients: an experimental model of aortic stenosis. J Am Coll Cardiol. 1985;6:1306–1314. [DOI] [PubMed] [Google Scholar]
- 22. Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart. 2005;91:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kadem L, Pibarot P, Dumesnil JG, Mouret F, Garitey V, Durand LG, Rieu R. Independent contribution of the left ventricular ejection time to the mean gradient in aortic stenosis. J Heart Valve Dis. 2002;11:615–623. [PubMed] [Google Scholar]
- 24. Chemla D, Hébert J‐L, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume‐to‐aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. [DOI] [PubMed] [Google Scholar]
- 25. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 26. Clavel MA, Pibarot P, Messika‐Zeitoun D, Capoulade R, Malouf J, Aggarval S, Araoz PA, Michelena HI, Cueff C, Larose É, Miller JD, Vahanian A, Enriquez‐Sarano M. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aggarwal SR, Clavel MA, Messika‐Zeitoun D, Cueff C, Malouf J, Araoz PA, Mankad R, Michelena H, Vahanian A, Enriquez‐Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging. 2013;6:40–47. [DOI] [PubMed] [Google Scholar]
- 28. Antonini‐Canterin F, Huang G, Cervesato E, Faggiano P, Pavan D, Piazza R, Nicolosi GL. Symptomatic aortic stenosis: does systemic hypertension play an additional role? Hypertension. 2003;41:1268–1272. [DOI] [PubMed] [Google Scholar]
- 29. Sutton‐Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell GF. Pulse pressure, arterial compliance and cardiovascular morbidity and mortality. Curr Opin Nephrol Hypertens. 1999;8:335–342. [DOI] [PubMed] [Google Scholar]
- 31. Schram MT, Kostense PJ, Van Dijk RA, Dekker JM, Nijpels G, Bouter LM, Heine RJ, Stehouwer CD. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens. 2002;20:1743–1751. [DOI] [PubMed] [Google Scholar]
- 32. de Simone G, Roman MJ, Koren MJ, Mensah GA, Ganau A, Devereux RB. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999;33:800–805. [DOI] [PubMed] [Google Scholar]
- 33. O'Rourke MF, Staessen JA. Clinical applications of arterial stiffness; definitions and references values. Am J Hypertens. 2002;15:426–444. [DOI] [PubMed] [Google Scholar]
- 34. Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, Nieminen MS, Dahlof B, Devereux RB. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781–787. [DOI] [PubMed] [Google Scholar]
- 35. Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. [DOI] [PubMed] [Google Scholar]


