Abstract
Background
Atrial flutter (AFL) has been identified to be equivalent to atrial fibrillation (AF) in terms of preventing ischemic stroke, although differences exist in atrial rate, substrate, and electrophysiological mechanisms. This study aimed to investigate differences in clinical outcomes between nonvalvular AF and AFL.
Methods and Results
AF and AFL patients without any prescribed anticoagulation were enrolled from a 13‐year national cohort database. Under series exclusion criteria, ischemic stroke, heart failure hospitalization, and all‐cause mortality were compared between the groups in real‐world conditions and after propensity score matching. We identified 175 420 patients in the AF cohort and 6239 patients in the AFL cohort, and the prevalence of most comorbidities and frequency of medications were significantly higher in the AF group than the AFL group. In the real‐world setting the AF patients had higher incidence rates of ischemic stroke, heart failure hospitalization, and all‐cause mortality than the AFL patients (all P<0.001). After propensity score matching, the incidence rate of ischemic stroke in the AF cohort was 1.63‐fold higher than in the AFL cohort (P<0.001), the incidence rate of heart failure hospitalization in the AF cohort was 1.70‐fold higher than in the AFL cohort (P<0.001), and the incidence rate of all‐cause mortality in the AF cohort was 1.08‐fold higher than in the AFL cohort (P=0.002).
Conclusions
There were differences between AF and AFL in comorbidities and prognosis with regard to ischemic stroke, heart failure hospitalization, and all‐cause mortality.
Keywords: atrial fibrillation, atrial flutter, heart failure, ischemic, mortality, stroke
Subject Categories: Arrhythmias
Clinical Perspective
What Is New?
Atrial fibrillation and atrial flutter patients without anticoagulation therapy were different in their incidence of ischemic stroke, heart failure, and all‐cause mortality, and these differences persisted after propensity score matching.
What Are the Clinical Implications?
Atrial flutter and atrial fibrillation should be considered to be different in terms of stroke prevention, and the currently recommended level of CHA2DS2‐VASc score for risk stratification in preventing ischemic stroke in patients with atrial flutter should be prospectively reevaluated.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. AF is characterized by an irregularly irregular atrial rhythm with a rate of more than 350 beats per minute and electrophysiological mechanisms involving rapidly firing foci and random multiple micro‐ or macro‐reentrant activation wavelets.1 The number of patients with AF worldwide was reportedly 33.5 million in 2010,2 and it will be expected to double by 2050.3 The Framingham heart study reported that AF is associated with an ≈5‐fold increased risk of embolic stroke, 2‐fold increased risk of heart failure, and 2‐fold higher risk of mortality.4 Therefore, public awareness of AF and its related complications is an urgent and important issue.5, 6
Atrial flutter (AFL) is a macro‐reentrant atrial tachycardia characterized by an organized atrial rhythm with a rate usually between 250 and 350 beats per minute.7 The prevalence of AFL is around 200 000 in the United States, and AFL has been reported to increase the risk of heart failure, stroke, and mortality.8, 9 AF and AFL have been observed to switch back and forth to one another clinically.10 Therefore, AF and AFL are conceptually recognized to be the same risk factor in terms of preventing complications related to atrial tachyarrhythmias, and it is generally recommended according to guidelines and expert opinions that AFL patients should be risk stratified and treated in the same manner as AF patients.5, 6
Although AF and AFL share several common risk factors with regard to occurrence,11, 12 and both have been reported to contribute to heart failure, mortality, and stroke,8, 9, 13, 14, 15 they are fundamentally different in terms of atrial rate, substrate, and electrophysiological mechanisms, and consequently, they differ in the degree of atrial myopathy and neurohumoral activation. Therefore, it is reasonable to conduct a study to investigate the differential impact of AFL and AF on the development of heart failure, ischemic stroke, and mortality.
Accordingly, we conducted this study to investigate the difference in the impact of solitary nonvalvular AF versus AFL on the development of heart failure, ischemic stroke, and all‐cause mortality in a large‐population national database.
Methods
Data Sources
Data for this national cohort study were retrieved from the Taiwan National Health Insurance Research Database (NHIRD) released by the Taiwan National Health Research Institutes. The National Health Insurance system is a mandatory universal health insurance program that offers comprehensive medical care coverage to all Taiwan residents, and the NHIRD contains healthcare information of more than 99% of the Taiwanese population since the inception of the program in 1997.16 In the NHIRD, the patients’ original identification numbers are encrypted to protect their privacy, and the encrypting procedure is consistent, so that linking claims belonging to the same enrollee is feasible and can be followed longitudinally. The healthcare information includes complete outpatient visits, hospitalization, any drug prescription, vital status and diseases, which were registered using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes. Patients whose AF or AFL was confirmed more than twice in outpatient visits or in a discharge diagnosis were included in this study, which was approved by the Institutional Review Board of Chang Gung Memorial Hospital (104‐7401B). The need for written informed consent from all patients was granted an exemption from Institutional Review Board of Chang Gung Memorial Hospital because this study included only encrypted noninvasive data analysis.
AF and AFL Cohorts
Two cohorts were evaluated in this study, those newly diagnosed with AF and those newly diagnosed with AFL from January 1, 2001 to December 31, 2013. After excluding those who were ever diagnosed with AFL from the AF cohort, and those with AF from the AFL cohort during this observation period, we identified 320 769 patients in the solitary AF cohort and 10 617 patients in the solitary AFL cohort (Figure 1). The exclusion criteria were an age younger than 20 years and those with rheumatic heart disease, surgery for valvular heart disease, those with reversible causes of AF and AFL such as hyperthyroidism and coexisting sepsis or heart surgery when AF/AFL was diagnosed in the same hospitalization. In addition, those who received radiofrequency catheter ablation for AF/AFL and received any anticoagulant therapy in the observation period were excluded. In order to exclude the possible impact of prior history of stroke or heart failure on study outcomes, patients with history of stroke or heart failure were excluded. Finally, 175 420 patients were enrolled in solitary nonvalvular AF cohort, while 6239 patients were in solitary nonvalvular AFL cohort.
Figure 1.
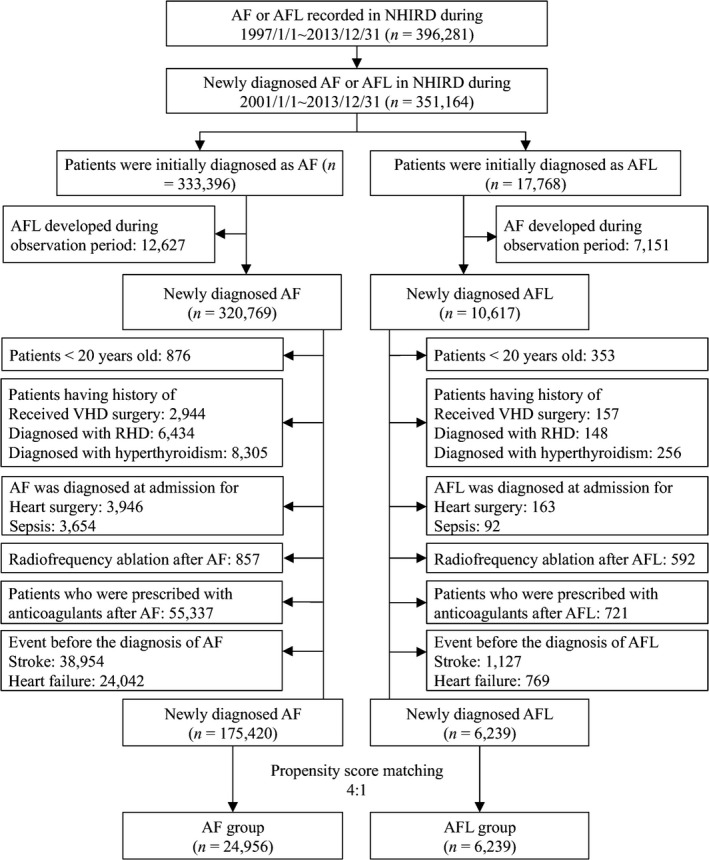
Flow chart of the study design. AF indicates atrial fibrillation; AFL, atrial flutter; NHIRD, National Health Insurance Research Database; RHD, rheumatic heart disease; VHD, valvular heart disease.
Outcomes Assessment and Propensity Score Matching Analysis
The index date was defined as the date when AF or AFL was first diagnosed, and the patients were followed until a defined clinical outcome occurred or until December 31, 2013. The 3 clinical outcomes assessed in this study were heart failure hospitalization, ischemic stroke, and all‐cause mortality. Heart failure hospitalization and ischemic stroke were defined according to the primary diagnosis of admission. All‐cause mortality was defined as withdrawal of the patient from the NHI program17 (http://www.nhi.gov.tw/webdata/webdata.aspx?menu=19&menu_id=774&webdata_id=3466). Differences in the 3 clinical outcomes between the 2 cohorts were assessed in real‐world conditions without any adjustment, and analysis was done after propensity score matching of the patients with AF and AFL.
We used propensity score matching analysis to reduce potential confounding factors and selection bias due to differences in baseline characteristics and medications in the study cohorts. Each patient in the AFL cohort was matched with four patients in the AF cohort using probability calculated by logistic regression based on the following confounding variables: age, sex, comorbidities, medications (listed in Table 1), and year of index date. After the propensity score matching, the clinical outcomes between the 2 cohorts were analyzed again.
Table 1.
Baseline Characteristics of the AF and AFL Groups Before and After Propensity Score Matching
| Variables | All Patients | Propensity Score Matched | |||
|---|---|---|---|---|---|
| AFL (n=6239) | AF (n=175 420) | P Value | AF (n=24 956) | P Value | |
| Age (y), mean±SD | 66.4±16.6 | 71.8±13.9 | <0.001 | ||
| Age group | <0.001 | 0.786 | |||
| <65 y | 2512 (40.3) | 47 862 (27.3) | 10 038 (40.2) | ||
| 65 to 74 y | 1505 (24.1) | 42 912 (24.5) | 6117 (24.5) | ||
| ≥75 y | 2222 (35.6) | 84 646 (48.3) | 8801 (35.3) | ||
| Sex | <0.001 | 0.917 | |||
| Male | 3747 (60.1) | 98 830 (56.3) | 14 970 (60.0) | ||
| Female | 2492 (39.9) | 76 590 (43.7) | 9986 (40.0) | ||
| Comorbidities | |||||
| Hypertension | 3140 (50.3) | 96 575 (55.1) | <0.001 | 12 462 (49.9) | 0.579 |
| Diabetes mellitus | 970 (15.5) | 28 655 (16.3) | 0.098 | 3804 (15.2) | 0.550 |
| Ischemic heart disease | 1786 (28.6) | 56 742 (32.3) | <0.001 | 7002 (28.1) | 0.372 |
| Dyslipidemia | 736 (11.8) | 19 230 (11.0) | 0.038 | 2960 (11.9) | 0.889 |
| Gout | 546 (8.8) | 15 973 (9.1) | 0.339 | 2212 (8.9) | 0.780 |
| Chronic obstructive pulmonary disease | 985 (15.8) | 31 787 (18.1) | <0.001 | 3858 (15.5) | 0.522 |
| Peripheral arterial disease | 149 (2.4) | 4072 (2.3) | 0.730 | 602 (2.4) | 0.912 |
| Renal status | 0.357 | 0.725 | |||
| Nonchronic kidney disease | 5487 (87.9) | 154 347 (88.0) | 21 987 (88.1) | ||
| Chronic kidney disease without dialysis | 597 (9.6) | 17 165 (9.8) | 2321 (9.3) | ||
| Chronic kidney disease with dialysis | 155 (2.5) | 3908 (2.2) | 648 (2.6) | ||
| Immune disease | 117 (1.9) | 3006 (1.7) | 0.334 | 478 (1.9) | 0.836 |
| Abnormal liver function | 740 (11.9) | 19 376 (11.0) | 0.044 | 2872 (11.5) | 0.436 |
| Malignancy | 543 (8.7) | 14 109 (8.0) | 0.060 | 2117 (8.5) | 0.577 |
| History of disease | |||||
| Myocardial infarction | 172 (2.8) | 4241 (2.4) | 0.087 | 667 (2.7) | 0.713 |
| Medications | |||||
| Angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers | 1855 (29.7) | 63 709 (36.3) | <0.001 | 7281 (29.2) | 0.387 |
| Calcium channel blockers | 1333 (21.4) | 41 953 (23.9) | <0.001 | 5360 (21.5) | 0.847 |
| β‐Blockers | 2170 (34.8) | 57 140 (32.6) | <0.001 | 8790 (35.2) | 0.514 |
| Digoxin | 584 (9.4) | 28 882 (16.5) | <0.001 | 2427 (9.7) | 0.383 |
| Diuretics | 736 (11.8) | 28 786 (16.4) | <0.001 | 3022 (12.1) | 0.498 |
| Spironolactone | 193 (3.1) | 7985 (4.6) | <0.001 | 822 (3.3) | 0.425 |
| Dipeptidyl peptidase 4 inhibitors | 106 (1.7) | 3190 (1.8) | 0.487 | 423 (1.7) | 0.983 |
| Statins | 657 (10.5) | 18 124 (10.3) | 0.612 | 2640 (10.6) | 0.912 |
| Biguanides | 535 (8.6) | 15 610 (8.9) | 0.377 | 2047 (8.2) | 0.339 |
| Sulfonylurea | 562 (9.0) | 16 416 (9.4) | 0.350 | 2164 (8.7) | 0.400 |
| Thiazolidinedione | 88 (1.4) | 2176 (1.2) | 0.234 | 322 (1.3) | 0.456 |
| Insulin | 128 (2.1) | 4195 (2.4) | 0.084 | 511 (2.0) | 0.984 |
| Antiarrhythmic drugs | |||||
| Amiodarone/dronedarone | 871 (14.0) | 27 592 (15.7) | <0.001 | 3486 (14.0) | 0.987 |
| Propafenone | 385 (6.2) | 11 518 (6.6) | 0.215 | 1530 (6.1) | 0.906 |
| Sotalol | 17 (0.3) | 219 (0.1) | 0.001 | 59 (0.2) | 0.605 |
| Flecainide | 15 (0.2) | 204 (0.1) | 0.005 | 62 (0.2) | 0.909 |
| Antiplatelet medication | 1867 (29.9) | 74 750 (42.6) | <0.001 | 7477 (30.0) | 0.956 |
| Clinical outcomes at the end of follow‐up | |||||
| Ischemic stroke | 242 (3.9) | 13 231 (7.5) | <0.001 | 1541 (6.2) | |
| Heart failure hospitalization | 193 (3.1) | 12 073 (6.9) | <0.001 | 1285 (5.1) | |
| All‐cause mortality | 2182 (35.0) | 74 278 (42.3) | <0.001 | 9319 (37.3) | |
Data are presented as mean±SD or number (percentage). AF indicates atrial fibrillation; AFL, atrial flutter.
Ascertainment of AF and AFL, Comorbidities and Outcomes
AF, AFL, and all comorbidities were defined according to the diagnoses made during hospitalization or in at least 2 consecutive clinic visits (AF: ICD‐9‐CM code 427.31; AFL: ICD‐9‐CM code 427.32). The high accuracy of the diagnosis of AF based on ICD‐9‐CM coding in the NHIRD has been confirmed in a previous study.18 A validation study for AFL was conducted in 1 medical center using ICD‐9‐CM code 427.32 after randomly sampling the records of 100 hospitalized patients with AFL and 100 patients with AFL in outpatient clinics. After an experienced physician (Y.‐S. Lin) reviewed the medical records and all ECGs, the positive predictive values were determined to be 97.5%. The major comorbidities as reported in the literature were also validated.19, 20 In addition, hypertension, diabetes mellitus, and dyslipidemia were diagnosed according to the ICD‐9‐CM codes combined with the use of related medications to increase the diagnostic accuracy. The definitions of the diagnoses and anatomical therapeutic chemical codes of the study medications are listed in Tables S1 and S2. Validation of the diagnosis of ischemic stroke has previously been reported, with an accuracy of around 94%.21 Because the validation of heart failure hospitalization and ischemic stroke in patients with AF/AFL has not previously been reported, we conducted a validation study at 1 center. After review of the medical records and imaging studies of hospitalized patients with AF/AFL whose primary diagnosis in the index hospitalization was ischemic stroke (ICD‐9‐CM 433‐437) or heart failure (ICD‐9‐CM 428) by physicians Y.‐S. L., Y.‐L. C., and G.‐H. L., the positive predicted value of ischemic stroke was 94.2% based on 500 randomly selected hospitalizations for ischemic stroke, and the positive predicted value of heart failure hospitalization was 97.6% based on 500 randomly selected hospitalizations for heart failure.
Statistical Analysis
The patients’ clinical characteristics (ie, age, sex, baseline comorbidities, and medications) were compared between the 2 study cohorts (AF versus AFL) using the independent‐sample t test for continuous variables or chi‐squared test for categorical variables. The risk of clinical outcomes (ischemic stroke, heart failure hospitalization, and all‐cause mortality) was expressed as incidence density (number of events per 100 person‐years). We further compared the risk of time‐to‐event outcomes between the cohorts in the real‐world setting without any adjustments and after matching using the Cox proportional hazards model with adjustments for propensity score.
The propensity score was calculated according to age, sex, baseline comorbidities (hypertension, diabetes mellitus, ischemic heart disease, heart failure, dyslipidemia, gout, chronic obstructive pulmonary disease, peripheral artery disease, chronic kidney disease, immune disease, abnormal liver function, and malignancy), history of disease (old myocardial infarction), medications (angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, calcium channel blockers, β‐blockers, digoxin, diuretics, spironolactone, dipeptidyl peptidase 4 inhibitors, statins, biguanides, sulfonylurea, thiazolidinedione, insulin, amiodarone/dronedarone, propafenone, sotalol, flecainide, and antiplatelet agents), and index year of enrollment. The matching algorithm was based on the nearest‐neighbor method (known as Greedy matching), and the tolerance level for the maximum distance was set as 0.2 times the standard deviation of the propensity score (caliper radius=0.2). SAS software for Windows (version 9.4, Cary, NC) was used for all statistical analyses.
Results
Baseline Characteristics and Clinical Outcomes of the AF and AFL Groups Under Real‐World Conditions Without Any Adjustment
During the 13‐year observation period, 396 281 patients were diagnosed with AF or AFL, for a prevalence rate of 1.72% of the Taiwanese population. Of these patients, 91.34% were diagnosed with solitary AF, and 3.02% were diagnosed with solitary AFL. The annual rate of new solitary AF was around 107.3/100 000 person‐years, and the annual rate of new solitary AFL was around 3.6/100 000 person‐years. The incidence increased with age in the AFL cohort, as it did in the AF cohort (Figure 2). After the exclusion criteria were applied, 175 420 patients with a mean age of 71.8±13.9 years were included in the AF cohort, and 6239 patients with a mean age of 66.4±16.6 years in the AFL cohort (Figure 1; left columns of Table 1). The AF group was significantly older than the AFL group, and the prevalence of most comorbidities and frequency of medications were significantly higher in the AF group than the AFL group, especially in terms of hypertension, ischemic heart disease, and chronic obstructive pulmonary disease (Table 1).
Figure 2.
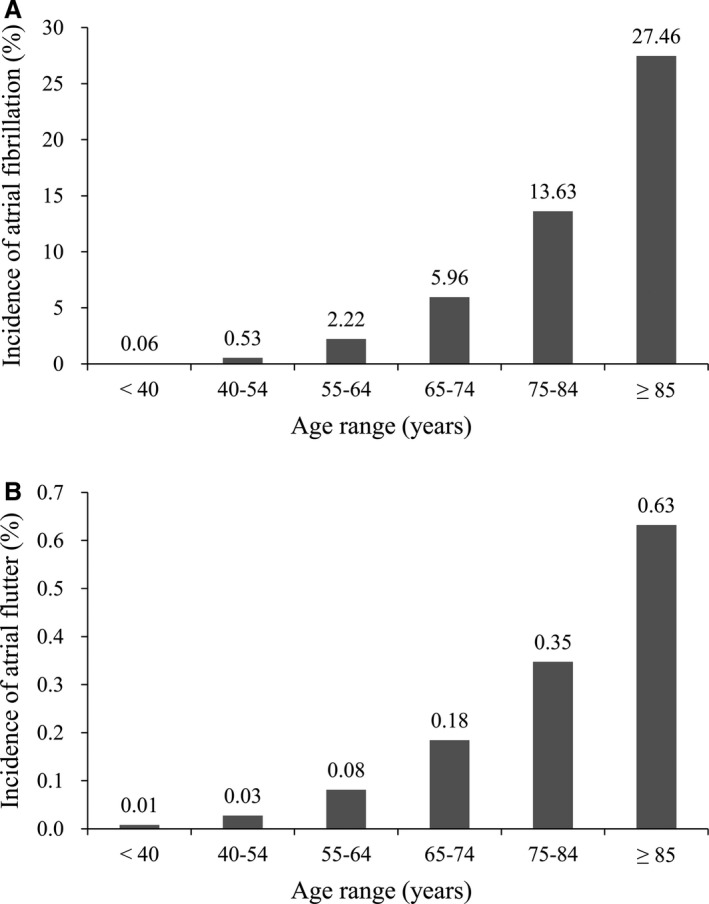
The incidence of atrial fibrillation and atrial flutter stratified by age group of Taiwan population. A, The incidence of atrial fibrillation by age. B, The incidence of atrial flutter by age.
At the end of the 13‐year observational period, the event rates of ischemic stroke (7.5% in the AF cohort versus 3.9% in the AFL cohort), heart failure hospitalization (6.9% versus 3.1%), and all‐cause mortality (42.3% versus 35.0%) were significantly higher in the AF cohort than in AFL cohort (all P<0.001). In terms of the incidence density of clinical outcomes, the incidence of ischemic stroke (2.5 versus 1.1 events per 100 person‐years in the AF and AFL groups, respectively), heart failure hospitalization (2.2 versus 0.9 events per 100 person‐years) and all‐cause mortality (13.2 versus 9.8 events per 100 person‐years) were still significantly higher in the AF cohort compared with the AFL cohort (Figure 3A through 3C).
Figure 3.
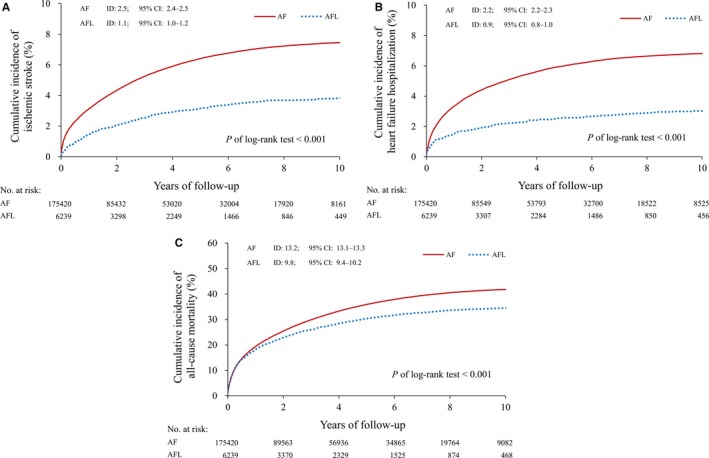
Kaplan‐Meier curves for ischemic stroke, heart failure hospitalization, and all‐cause mortality in AF and AFL cohorts in a real‐world condition without any adjustment. A, The cumulative incidence of ischemic stroke. B, The cumulative incidence of heart failure hospitalization. C, The cumulative incidence of all‐cause mortality. AF indicates atrial fibrillation; AFL, atrial flutter.
Clinical Outcomes Between AF and AFL Patients After Propensity Score Matching
All baseline characteristics listed in Table 1 between AFL and AF groups were balanced by 1:4 matching (right column of Table 1: 6239 patients in the AFL cohort and 24 956 patients in the AF cohort). Patients in both cohorts were followed for a mean of 3.3±3.3 years. No group differences were observed in age, sex, comorbidities, or medications after matching. However, the incidence of ischemic stroke was 1.63‐fold higher in the AF cohort than in the AFL cohort (1.8 versus 1.1 events per 100 person‐years; hazard ratio 1.63; 95%CI 1.42‐1.87), the incidence of heart failure hospitalization was 1.70‐fold higher in the AF cohort than that in the AFL cohort (1.5 versus 0.9 events per 100 person‐years; hazard ratio 1.70; 95%CI 1.46‐1.97), and the incidence of all‐cause mortality was 1.08‐fold higher in the AF cohort than that in the AFL cohort (10.6 versus 9.8 events per 100 person‐years; hazard ratio 1.08; 95%CI 1.03‐1.13) (Figure 4A through 4C and Table 2).
Figure 4.
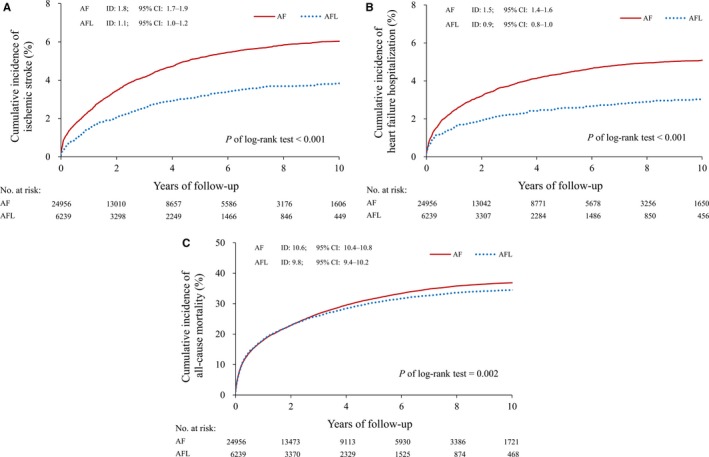
Kaplan‐Meier curves for ischemic stroke, heart failure hospitalization, and all‐cause mortality in AF and AFL cohorts after 1:4 propensity score matching. A, The cumulative incidence of ischemic stroke. B, The cumulative incidence of heart failure hospitalization. C, The cumulative incidence of all‐cause mortality. AF indicates atrial fibrillation; AFL, atrial flutter.
Table 2.
Clinical Outcomes Between the Patients With AF and AFL After Propensity Score Matching
| Variable | AF (n=24 956) | AFL (n=6239) | P Value |
|---|---|---|---|
| Ischemic stroke | |||
| Number of events, n (%) | 1541 (6.17) | 242 (3.88) | |
| Incidence densitya | 1.8 (1.7‐1.9) | 1.1 (1.0‐1.2) | |
| Hazard ratio (95%CI) | 1.63 (1.42‐1.87) | Reference | <0.001 |
| Heart failure hospitalization | |||
| Number of events, n (%) | 1285 (5.15) | 193 (3.09) | |
| Incidence densitya | 1.5 (1.4‐1.6) | 0.9 (0.8‐1.0) | |
| Hazard ratio (95%CI) | 1.70 (1.46‐1.97) | Reference | <0.001 |
| All‐cause mortality | |||
| Number of events, n (%) | 9319 (37.34) | 2182 (34.97) | |
| Incidence densitya | 10.6 (10.4‐10.8) | 9.8 (9.4‐10.2) | |
| Hazard ratio (95%CI) | 1.08 (1.03‐1.13) | Reference | 0.002 |
AF indicates atrial fibrillation; AFL, atrial flutter.
Incidence density indicates number of events per 100 person‐years.
Discussion
This is the first observation cohort study to evaluate the different impacts on ischemic stroke, heart failure hospitalization, and mortality between AF and AFL from real‐world observation and propensity score matching for all baseline characteristics including medications. The results showed that AF had a more significant impact on ischemic stroke, heart failure hospitalization, and all‐cause mortality than AFL.
Real World Characteristics Between AF and AFL
A previous study reported an AF and/or AFL incidence of 1.12% in patients in the MarketScan database, of whom 91.6% were diagnosed with AF only, and 2% were diagnosed with AFL only.22 This is consistent with the current study in which the incidence of AF and/or AFL in patients from the NHIRD was 1.72%, of whom 91.3% were diagnosed with AF only and 3.0% were diagnosed with AFL only. In clinical practice, patients with AFL are stratified with regard to risk and treated in the same manner as patients with AF, as more than 3 of 4 patients with AFL also have episodes of AF. We also found that around twice the number of patients were diagnosed with coexisting AFL and AF (n=19 778) compared with those diagnosed with AFL only (n=10 617). In terms of incidence of AFL, our result (0.049%) is consistent with 2 small population‐based studies, MESA (the Marshfield Epidemiological Study Area), which reported an incidence of 0.088%,8 and the Framingham heart study, which reported an incidence of 0.036%.13 In terms of comorbidities there existed significant differences in comorbidities between AF and AFL, especially hypertension, in the real‐world data (MESA, Framingham, and MarketScan databases),8, 13, 22 and these differences in the distribution of comorbidities were also consistent with our findings.
Clinical Outcomes of the Patients With AF and AFL
No prior studies have reported the impact of AF/AFL on clinical outcomes without the interference of confounding factors. In this study we used propensity score matching to reduce potential confounding factors and selection bias, and our results showed that AF patients had a significantly higher incidence of ischemic stroke, heart failure hospitalization, and all‐cause mortality compared with AFL patients. Recently, the burden of AF was reported to be an independent predictor of ischemic stroke after adjustment for CHADS2 score in the SOS AF (Stroke Prevention Strategies based on AF) project,23 and an echocardiographic substudy from ENGAGE AF‐TIMI 48 reported that left atrial structure and function were increasingly abnormal with a greater electrical burden of AF and higher risk of stroke.24 These findings indicate a parallel correlation between AF burden and the severity of left atrial remodeling and the risk of ischemic stroke. Based on the differences in atrial rate, substrate, and electrophysiological mechanisms and consequently the degree of atrial remodeling in patients with AFL and AF, AF and AFL may develop different degrees of endocardial remodeling and neurohumoral activation that result in different clinical outcomes. Moreover, the differences in the incidence density of clinical outcomes between AF and AFL in the “real world” condition without any adjustment (Figure 3) were more prominent than those in the propensity score–matching setting (Figure 4 and Table 2). These differences in the incidence density of clinical outcomes between the real‐world condition and propensity score matching setting may be driven by the “additive impacts” of the differences in the baseline risk characteristics between AF and AFL patients.
Differences in Clinical Results Between Our Study and Others
Few studies have investigated differences in clinical outcomes between AF and AFL in a real‐world setting. The Canadian Registry of Atrial Fibrillation reported incident stroke rates were 1.33/100 person‐years in AF patients and 1.24/100 person‐years in AFL patients,25 whereas incident stroke rates were 3.2/100 person‐years in AF patients and 2.8/100 person‐years in AFL patients in the Framingham study.13 Although a meta‐analysis reported embolic events in patients with AFL after cardioversion,9 and the Framingham study reported that AFL significantly contributed to stroke,13 they both concluded that the impact of AFL and AF on stroke was similar, which is different from our findings. This may be due to several reasons. First, those 2 studies included a small number of patients with AFL (<150 cases) compared with >5000 patients with AFL and >100 000 patients with AF in our study with a longer observation period. Second, we excluded patients receiving anticoagulant therapy, whereas the other 2 studies did not. Anticoagulation therapy is known to reduce the risk of stroke.5, 6 Third, ischemic stroke but not hemorrhagic stroke is the major type of stroke related to AF, and previous studies did not specialize in ischemic stroke as we did in the current study.13, 25 Although the Framingham study reported no differences in heart failure and mortality between patients with AF and AFL in age‐ and sex‐adjusted analysis, the authors still suggested that future studies with a larger number of cases were needed to confirm their results.13 We included a large number of patients with AF and AFL in this study and found significant differences in the incident heart failure and mortality between AF and AFL in the real‐world setting and after propensity score matching.
Study Limitations
This retrospective case‐control database study has inherent limitations. First, the lack of echocardiographic data and some clinical presentations is the major limitation. Several studies24, 26, 27 have reported that left atrial size is a predictor of clinical outcomes in patients with AF; however, no studies have reported a correlation between left atrial size and clinical outcomes in patients with AFL. Second, we were unable to adjust for the effects of tobacco use, alcohol consumption, and physical status because such data are unavailable in the NHIRD. Third, the accuracy of the diagnoses and clinical outcomes based on an insurance database is controversial. The diagnosis of cardiovascular disease according to ICD‐9‐CM coding has been validated,16 and we also increased the accuracy by including relevant examinations and medications related to the diagnosis in addition to the ICD‐9‐CM code in the analysis. We also validated clinical outcomes at 1 study center and found a high positive predictive value. In addition, from a statistical viewpoint,19, 20 the accuracy was high enough to reach a conclusion. Finally, subclassification of AF such as paroxysmal AF, persistent AF, chronic AF, and typical and atypical types of AFL are not available in the NHIRD. Although different types of AF and AFL may have had different impacts on clinical outcomes, further studies are needed for clarification.
Conclusions
This case‐control cohort study included a large number of cases and showed different prognoses between AFL and AF. In a real‐world setting without any preventative ischemic stroke strategy, AF and AFL were associated with different comorbidities and incidences of ischemic stroke, heart failure hospitalization, and all‐cause mortality. These differences in the incidences of ischemic stroke, heart failure hospitalization, and all‐cause mortality still existed after propensity score matching.
Sources of Funding
This work was supported by grants from the Chang Gung Memorial Hospital, Taiwan (grants CMRPG6F0031). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
Supporting information
Table S1. Diagnoses Used to Define the Cohorts, Comorbidities, and Outcomes
Table S2. Anatomical Therapeutic Chemical Codes of the Study Medications
(J Am Heart Assoc. 2017;6:e006406 DOI: 10.1161/JAHA.117.006406.)28733435
References
- 1. Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–216. [DOI] [PubMed] [Google Scholar]
- 2. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 3. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 4. Lin HJ, Wolf PA, Kelly‐Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 6. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 7. Blomström‐Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW, Stevenson WG, Tomaselli GF, Antman EM, Smith SC Jr, Alpert JS, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ; European Society of Cardiology Committee, NASPE‐Heart Rhythm Society . ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE‐Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493–1531. [DOI] [PubMed] [Google Scholar]
- 8. Granada J, Uribe W, Chyou PH, Maassen K, Vierkant R, Smith PN, Hayes J, Eaker E, Vidaillet H. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–2246. [DOI] [PubMed] [Google Scholar]
- 9. Ghali WA, Wasil BI, Brant R, Exner DV, Cornuz J. Atrial flutter and the risk of thromboembolism: a systematic review and meta‐analysis. Am J Med. 2005;118:101–107. [DOI] [PubMed] [Google Scholar]
- 10. Estes NA III, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJ, Waldo AL, Wyse DG, Bonow RO, DeLong E, Goff DC Jr, Grady K, Green LA, Hiniker A, Linderbaum JA, Masoudi FA, Piña IL, Pressler S, Radford MJ, Rumsfeld JS; American College of Cardiology; American Heart Association Task Force on Performance Measures; Physician Consortium for Performance Improvement . ACC/AHA/Physician Consortium 2008 Clinical Performance Measures for Adults with Nonvalvular Atrial Fibrillation or Atrial Flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation) Developed in Collaboration with the Heart Rhythm Society. J Am Coll Cardiol. 2008;51:865–884. [DOI] [PubMed] [Google Scholar]
- 11. Anumonwo JM, Kalifa J. Risk factors and genetics of atrial fibrillation. Heart Fail Clin. 2016;12:157–166. [DOI] [PubMed] [Google Scholar]
- 12. Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315–318. [DOI] [PubMed] [Google Scholar]
- 13. Rahman F, Wang N, Yin X, Ellinor PT, Lubitz SA, LeLorier PA, McManus DD, Sullivan LM, Seshadri S, Vasan RS, Benjamin EJ, Magnani JW. Atrial flutter: clinical risk factors and adverse outcomes in the Framingham Heart Study. Heart Rhythm. 2016;13:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vidaillet H, Granada JF, Chyou P, Maassen K, Ortiz M, Pulido JN, Sharma P, Smith PN, Hayes J. A population‐based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113:365–370. [DOI] [PubMed] [Google Scholar]
- 15. Biblo LA, Yuan Z, Quan KJ, Mackall JA, Rimm AA. Risk of stroke in patients with atrial flutter. Am J Cardiol. 2001;87:346–349. [DOI] [PubMed] [Google Scholar]
- 16. Wu TY, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. London J Prim Care (Abingdon). 2010;3:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus‐related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. [DOI] [PubMed] [Google Scholar]
- 18. Chang CH, Lee YC, Tsai CT, Chang SN, Chung YH, Lin MS, Lin JW, Lai MS. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis. 2014;232:224–230. [DOI] [PubMed] [Google Scholar]
- 19. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self‐reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9:e112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sung SF, Hsieh CY, Lin HJ, Chen YW, Yang YH, Li CY. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int J Cardiol. 2016;215:277–282. [DOI] [PubMed] [Google Scholar]
- 21. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–242. [DOI] [PubMed] [Google Scholar]
- 22. Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. [DOI] [PubMed] [Google Scholar]
- 23. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE. Device‐detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta DK, Shah AM, Giugliano RP, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman E, Patel I, Shi M, Mercuri M, Mitrovic V, Braunwald E, Solomon SD; Effective aNticoaGulation with factor xA next GEneration in AF‐Thrombolysis In Myocardial Infarction 48 Echocardiographic Study Investigators . Left atrial structure and function in atrial fibrillation: ENGAGE AF‐TIMI 48. Eur Heart J. 2014;35:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lelorier P, Humphries KH, Krahn A, Connolly SJ, Talajic M, Green M, Sheldon R, Dorian P, Newman D, Kerr CR, Yee R, Klein GJ. Prognostic differences between atrial fibrillation and atrial flutter. Am J Cardiol. 2004;93:647–649. [DOI] [PubMed] [Google Scholar]
- 26. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:6–12. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe T, Iwai‐Takano M, Oikawa M, Yamaki T, Yaoita H, Maruyama Y. Optimal noninvasive assessment of diastolic heart failure in patients with atrial fibrillation: comparison of tissue Doppler echocardiography, left atrium size, and brain natriuretic peptide. J Am Soc Echocardiogr. 2008;21:689–696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Diagnoses Used to Define the Cohorts, Comorbidities, and Outcomes
Table S2. Anatomical Therapeutic Chemical Codes of the Study Medications


