Abstract
Background
The ABC‐stroke score (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) was proposed to predict stroke in atrial fibrillation (AF). This score was derived/validated in 2 clinical trial cohorts in which patients with AF were highly selected and carefully followed‐up. However, the median follow‐up was 1.9 years in the trial cohort; therefore, its long‐term predictive performance remains uncertain. This study aimed to compare the long‐term predictive performances of the ABC‐stroke and CHA 2 DS 2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) scores in a cohort of anticoagulated patients with AF.
Methods and Results
We recruited 1125 consecutive patients with AF who were stable on vitamin K antagonists and followed‐up for a median of 6.5 years. ABC‐stroke and CHA 2 DS 2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) scores were calculated and compared. Median CHA 2 DS 2‐VASc and ABC‐stroke scores were 4 (interquartile range 3–5) and 9.1 (interquartile range 7.3–11.3), respectively. There were 114 ischemic strokes (1.55% per year) at 6.5 years. The C‐index of ABC‐stroke at 3.5 years was significantly higher than CHA 2 DS 2‐VASc (0.663 versus 0.600, P=0.046), but both C‐indexes were nonsignificantly different at 6.5 years. Integrated discrimination improvement showed a small improvement (<2%) in sensitivity at 3.5 and 6.5 years with ABC‐stroke. For ABC‐stroke, net reclassification improvement was nonsignificantly different at 3.5 years, and showed a negative reclassification at 6.5 years compared with CHA 2 DS 2‐VASc. Decision curve analyses did not show a marked improvement in clinical usefulness of the ABC‐stroke score over the CHA 2 DS 2‐VASc score.
Conclusions
In anticoagulated patients with AF followed‐up over a long‐term period, the novel ABC‐stroke score does not offer significantly better predictive performance compared with the CHA 2 DS 2‐VASc score.
Keywords: anticoagulants, atrial fibrillation, biomarkers, risk prediction, stroke
Subject Categories: Arrhythmias, Ischemic Stroke, Biomarkers, Clinical Studies
Clinical Perspective
What Is New?
Recent guidelines suggest the use of biomarkers for risk stratification in atrial fibrillation.
In this first study comparing the ABC‐stroke (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) and CHA2DS2‐VASc scores in a “real‐world” cohort of patients with atrial fibrillation, the biomarker‐based ABC‐stroke score loses much of its prognostic value in the long term (by 6.5 years).
Hence, there is little difference in the clinical utility of the ABC‐stroke score compared with the clinical risk factor–based CHA2DS2‐VASc score.
What Are the Clinical Implications?
Although measuring biomarkers may help improve prediction of patients at high risk (at least statistically), this is at the cost of adding substantial complexity, expense, and lack of practicality.
CHA2DS2‐VASc score has the advantage of simplicity and performed better than the ABC‐stroke score in categorizing patients at low risk, thus allowing clinicians to rapidly estimate whether oral anticoagulation is indicated.
Introduction
Stroke risk is increased in patients with atrial fibrillation, but this risk is not homogeneous and depends on the presence of various risk factors. The more common stroke risk factors have been used to formulate risk scores, such as the CHA2DS2‐VASc score, to help select patients who need oral anticoagulant therapy.1 The CHA2DS2‐VASc score has been widely validated and its use for stroke risk prediction is recommended by current guidelines for the management of AF.2, 3, 4
For many years, the role of biomarkers in predicting adverse events and improving risk stratification in cardiovascular disease has been investigated.5 Biomarkers have been proposed to help refine stroke risk stratification in AF for over a decade,6 and recent guidelines have proposed consideration of biomarkers to aid risk stratification.2 More recently, the ABC‐stroke score (age, cardiac biomarkers [NT‐proBNP (N‐terminal fragment B‐type natriuretic peptide)], high‐sensitivity cardiac troponin), and clinical history [prior stroke/transient ischemic attack]) has been proposed to predict stroke in patients with AF.7 The ABC‐stroke score was derived and validated in 2 clinical trial cohorts in which patients with AF are often highly selected and carefully followed‐up. “Real‐world” patients tend to be older, with associated comorbidities and polypharmacy. The median follow‐up was 1.9 years in the trial cohort, therefore the long‐term predictive performance of ABC‐stroke is uncertain.
In the present study, we compared the long‐term predictive performance of the ABC‐stroke score with CHA2DS2‐VASc in a real‐world cohort of anticoagulated patients with AF.
Methods
We recruited consecutive patients with paroxysmal, persistent, or permanent nonvalvular AF who were stable on vitamin K antagonist (international normalized ratio [INR] 2.0–3.0) for at least the previous 6 months in our single anticoagulation center in a tertiary hospital in Murcia (Southeast Spain), from May 1, 2007, to December 1, 2007. At entry, all patients were receiving anticoagulation therapy with acenocoumarol (the most common vitamin K agonist used in Spain) and consistently achieved an INR between 2.0 and 3.0 during the previous 6 months (to ensure baseline homogeneity avoiding any influence of fluctuant INR in the value of biomarkers). We excluded patients with rheumatic mitral valves or prosthetic heart valves and those with any acute coronary syndrome, stroke, hemodynamic instability, hospital admissions or surgical interventions in the preceding 6 months.
At baseline, a complete medical history was recorded and stroke risk (CHA2DS2‐VASc) and bleeding risk (HAS‐BLED [hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly (>65 years), drugs/alcohol concomitantly]) were calculated. The time in therapeutic range was calculated at 6 months after entry by the linear interpolation method of Rosendaal. The ABC‐stroke score was calculated using an Excel‐based calculator that performed the results according to the nomogram proposed by Hijazi et al.7 This calculator provided the total score and the corresponding predicted 1‐ and 3‐year risk of stroke. In the present study, we used the “Troponin T” version of the nomogram, provided by Hijazi et al in their supplementary material online.7
Blood Samples and Laboratory Analysis
At entry, blood samples were drawn atraumatically and without stasis into syringes preloaded with trisodium citrate (0.011 mol/L). Platelet‐poor plasma fractions were obtained by centrifugation at 4°C for 20 minutes at 2200g. Aliquots were stored at −80°C to allow batch analysis. High‐sensitivity troponin T and NT‐proBNP levels were assessed at the time of patient inclusion by electrochemiluminescence in an automated analyzer (Cobas e 601, Roche Diagnostica). The intra‐assay variation coefficient was 5.6% and the lower limits of detection of these assays were 3.0 pg/mL for high‐sensitivity troponin T and 5.0 pg/mL for NT‐proBNP.
Study Outcomes
To investigate clinical outcomes at ≈3 and 6 years, we analyzed stroke events at a median of 3.5 years (interquartile range [IQR] 3.1–3.6) of follow‐up, and a final analysis at a median of 6.5 years (IQR 4.3–7.9). Follow‐up was performed by personal interview at each visit to the anticoagulation clinic and through medical records, and no patient was lost to follow‐up. The primary end point for the present analysis was ischemic stroke and was defined as the sudden onset of a focal neurological deficit in a location consistent with the territory of a major cerebral artery resulted of an obstruction documented by imaging, surgery, or autopsy. Other adverse events were recorded, such as major bleeding (based on 2005 International Society on Thrombosis and Haemostasis criteria8) and all‐cause deaths. The investigators identified, confirmed, and recorded all adverse events, as well as other clinical outcomes.
The study protocol was approved by the ethics committee from University Hospital Morales Meseguer and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients gave informed consent to participate in the study.
Statistical Analysis
Categorical variables were expressed as frequencies and percentages. Continuous variables were tested for normality by the Kolmogorov–Smirnov test and presented as mean±SD or median and IQR, as appropriate.
The Pearson chi‐square test was used to compare proportions. Cox proportional hazard regression models were performed to determine the association between higher values of the ABC‐stroke and CHA2DS2‐VASc scores and ischemic stroke. Differences in event‐free survival between patients with different risk categories of ABC‐stroke and CHA2DS2‐VASc were reflected by Kaplan–Meier curves. Correlation between ABC‐stroke and CHA2DS2‐VASc scores were performed using the Spearman's Rho.
Receiver operating characteristic curves were applied to evaluate the predictive ability (expressed as C‐indexes) of the CHA2DS2‐VASc and ABC‐stroke scores. Comparisons of receiver operating characteristic curves were performed by the DeLong et al method.9 Discrimination and reclassification performance of the 2 scores were evaluated by calculating the integrated discrimination improvement and the net reclassification improvement, as described by Pencina et al.10 We also estimated the clinical usefulness and net benefit of the ABC‐stroke score in comparison to CHA2DS2‐VASc using the decision curve analysis, according to the method proposed by Vickers et al.11, 12
A P<0.05 was accepted as statistically significant. Statistical analyses were performed using SPSS version 19.0 (SPSS Inc), MedCalc version 16.4.3 (MedCalc Software bvba), and STATA version 12.0 (Stata Corp, College Station, TX) for Windows.
Results
We included 1125 patients (49.7% men) with a median age of 76 years (IQR 71–81). A summary of baseline clinical characteristics is shown in Table 1. Median CHA2DS2‐VASc and ABC‐stroke scores were 4 (IQR 3–5) and 9.1 (IQR 7.3–11.3), respectively. The median time in the therapeutic range at 6 months after entry was 80% (IQR 66–100) and the median HAS‐BLED score was 2 (IQR 2–3). During 6.5 years (IQR 4.3–7.9) of follow‐up, there were 203 major bleeding events (18%, 2.77% per year) and 450 patients died (40%, 6.15% per year).
Table 1.
Baseline Clinical Characteristics
| N=1125 | CHA2DS2‐VASc Score | P Value | ABC‐Stroke Score (at 3 y) | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Low Risk | Medium Risk | High Risk | Low Risk | Medium Risk | High Risk | |||
| n=13 | n=50 | n=1062 | n=53 | n=489 | n=583 | |||
| Male sex, No. (%) | 13 (100.0) | 44 (88.0) | 502 (47.3) | <0.001 | 32 (60.4) | 253 (51.7) | 274 (47.0) | 0.085 |
| Age, median (IQR), y | 61 (55.5–63.5) | 63 (57.7–71.0) | 77 (72–81) | <0.001 | 67 (59–73) | 73 (67–78) | 79 (75–83) | <0.001 |
| Comorbidities, No. (%) | ||||||||
| Hypertension | 0 (0.0) | 16 (32.0) | 906 (85.3) | <0.001 | 33 (62.3) | 406 (83.0) | 483 (82.8) | 0.001 |
| Diabetes mellitus | 0 (0.0) | 3 (6.0) | 296 (27.9) | <0.001 | 12 (22.6) | 129 (26.4) | 158 (27.1) | 0.774 |
| Heart failure | 0 (0.0) | 3 (6.0) | 334 (31.5) | <0.001 | 7 (13.2) | 126 (25.8) | 204 (35.0) | <0.001 |
| History of stroke/TIA | 0 (0.0) | 0 (0.0) | 212 (20.0) | <0.001 | 0 (0.0) | 16 (3.3) | 196 (33.6) | <0.001 |
| Renal impairment | 1 (7.7) | 3 (6.0) | 113 (10.6) | 0.547 | 0 (0.0) | 30 (6.1) | 87 (14.9) | <0.001 |
| Coronary artery disease | 0 (0.0) | 3 (6.0) | 218 (20.5) | 0.008 | 11 (20.8) | 84 (17.2) | 126 (21.6) | 0.187 |
| Current smoking | 5 (38.5) | 7 (14.0) | 173 (16.3) | 0.090 | 12 (22.6) | 81 (16.6) | 92 (15.8) | 0.433 |
| Concomitant antiplatelet treatment | 1 (7.7) | 7 (14.0) | 200 (18.8) | 0.415 | 13 (24.5) | 64 (13.1) | 131 (22.5) | <0.001 |
| High‐sensitivity troponin T, ng/mL | 6.8 (4.9–10.5) | 9.2 (5.4–12.6) | 12.3 (8.1–18.6) | 0.005 | 5.1 (3.8–7.3) | 9.2 (6.6–13.0) | 16.0 (11.5–22.8) | <0.001 |
| NT‐proBNP, ng/mL | 339.4 (205.9–561.9) | 369.5 (158.2–552.9) | 635.4 (329.9–1074.5) | 0.084 | 62.8 (40.0–102.6) | 408.1 (233.1–584.0) | 946.6 (643.6–1464.0) | <0.001 |
| TTR at 6 mo, median (IQR), % | 80 (73–100) | 80 (60–100) | 80 (66–100) | 0.377 | 80 (60–83) | 80 (66–100) | 80 (60–100) | 0.001 |
| TTR <65% at 6 mo, No. (%) | 2 (15.4) | 13 (26.0) | 258 (24.3) | 0.726 | 15 (28.3) | 105 (21.5) | 153 (26.2) | 0.151 |
ABC‐stroke indicates age, biomarkers (N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin), and clinical history (prior stroke/transient ischemic attack); CHA2DS2‐VASc, cardiac failure or dysfunction, hypertension, age ≥75 (doubled), diabetes mellitus, stroke (doubled)—vascular disease, age 65 to 74 years and sex category (female); IQR, interquartile range; NT‐proBNP, N‐terminal fragment B‐type natriuretic peptide; TIA, transient ischemic attack; TTR, time in therapeutic range.
At the interim 3.5 years (IQR 3.1–3.6) of follow‐up, 58 patients experienced an ischemic stroke (5.2%, an annual rate of 1.50% per year). Of these, 98.3% were “high risk” by CHA2DS2‐VASc category (ie, score ≥2) and 70.7% were high risk by ABC‐stroke category (ie, predicted event rate >2%). At the end of follow‐up, ie, at 6.5 years (IQR 4.3–7.9), 114 patients experienced an ischemic stroke (10.1%, annual rate of 1.55% per year). Of these, 99.1% were high risk by CHA2DS2‐VASc category and 68.4% by ABC‐stroke (Table 2).
Table 2.
Distribution of Ischemic Strokes According to the Stroke Risk Scores Categories
| Risk Categories | CHA2DS2‐VASc Score | ABC‐Stroke Score | ||
|---|---|---|---|---|
| No. (%) | Annual Rate (% per y) | No. (%) | Annual Rate (% per y) | |
| At 3.5 y | Ischemic strokes (n=58) | |||
| Low risk | 0 (0.0) | 0 | 1 (1.7) | 0.54 |
| Medium risk | 1 (1.7) | 0.58 | 16 (27.6) | 0.96 |
| High risk | 57 (98.3) | 1.55 | 41 (70.7) | 2.03 |
| At 6.5 y | Ischemic strokes (n=114) | |||
| Low risk | 0 (0.0) | 0 | 1 (0.9) | 0.30 |
| Medium risk | 1 (0.9) | 0.31 | 35 (30.7) | 1.10 |
| High risk | 113 (99.1) | 1.64 | 78 (68.4) | 2.06 |
ABC‐stroke indicates age, biomarkers (N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin), and clinical history (prior stroke/transient ischemic attack); CHA2DS2‐VASc, cardiac failure or dysfunction, hypertension, age ≥75 (doubled), diabetes mellitus, stroke (doubled)—vascular disease, age 65 to 74 years and sex category (female).
Based on Cox regression analyses, the overall risk at 3.5 years for each score point was 1.28 (95% CI, 1.10–1.60; P=0.003) for CHA2DS2‐VASc and 1.22 (95% CI, 1.13–1.31; P<0.001) for ABC‐stroke. At 6.5 years, corresponding figures were 1.40 (95% CI, 1.25–1.57; P<0.001) for CHA2DS2‐VASc and 1.24 (95% CI, 1.17–1.30; P<0.001) for ABC‐stroke (Table S1).
Survival analyses by the Kaplan–Meier curves within different risk categories of ABC‐stroke and CHA2DS2‐VASc are shown in Figure 1. The Kaplan–Meier curves show that the “low‐risk” category as defined by CHA2DS2‐VASc were clearly low risk, with virtually no events at 3.5 and 6.5 years. In contrast, the low‐risk group defined by ABC‐stroke experienced 17 and 36 ischemic stroke events at 3.5 and 6.5 years, respectively (annual rates of 0.43% per year and 0.50% per year). Kaplan–Meir analysis of the low‐moderate risk category defined by CHA2DS2‐VASc and ABC‐stroke scores also show a significant difference at 6.5 years (CHA2DS2‐VASc: log‐rank test 7.31, P=0.007; ABC‐stroke: log‐rank test 27.08, P<0.001) (Figure S1).
Figure 1.
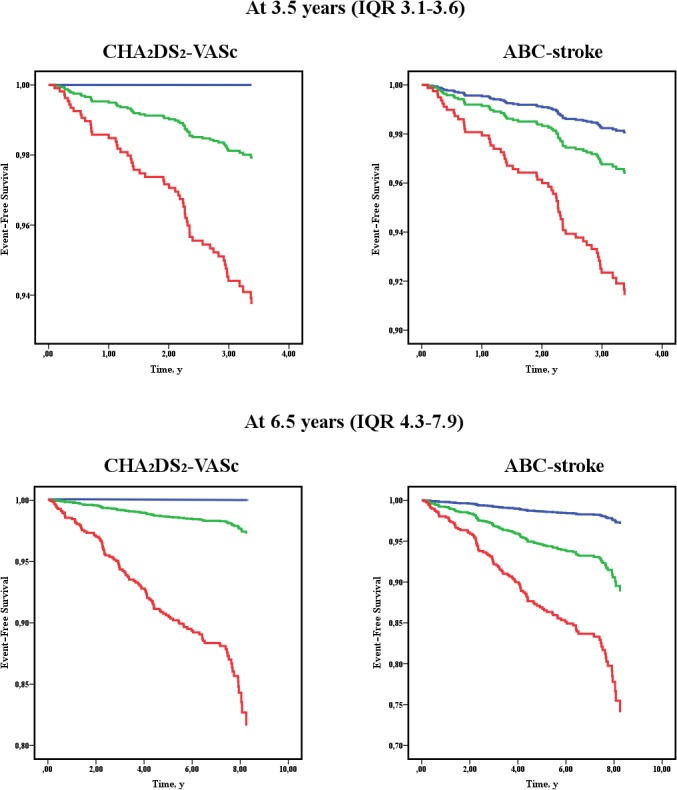
Event‐free survival for ischemic stroke according to the risk categories of each score. Blue line=low risk; green line=medium risk; red line=high risk. CHA 2 DS 2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) categories were defined as low risk (score=0), medium risk (score=1), and high risk (score ≥2). ABC‐stroke (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) categories were defined as low risk (<1% predicted 1‐year risk of stroke), medium risk (1–2% predicted 1‐year risk of stroke), and high risk (>2% predicted 1‐year risk of stroke). IQR indicates interquartile range.
Prediction of Patients at High Risk Who Sustain Strokes
With regard to predictive performance, the C‐index of the ABC‐stroke at 3.5 years was higher from that of CHA2DS2‐VASc which was statistically significant (0.663 versus 0.600, P=0.046) (Table 3). At 6.5 years, the C‐index of the ABC‐stroke was 0.662, while the C‐index of CHA2DS2‐VASc was 0.620, and nonsignificantly different (Table 3, Figure S2). Based on the integrated discrimination improvement, the ABC‐stroke score had a poor improvement (<2%) in sensitivity at 3.5 and 6.5 years. Net reclassification improvement analyses demonstrated a nonsignificant improvement in reclassification at 3.5 years and a (significant) negative reclassification at 6.5 years with the ABC‐stroke score compared with CHA2DS2‐VASc (Table 3).
Table 3.
C‐Indexes of the ROC Curves, ROC Curves Comparison, IDI, and NRI of the ABC‐Stroke Score in Comparison With CHA2DS2‐VASc Score at 3.5 and 6.5 Years
| C‐Index | 95% CI | P Value | z Statistica | P Valuea | IDI | P Value | NRI | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| At 3.5 y | |||||||||
| ABC‐stroke score | 0.663 | 0.634 to 0.690 | <0.001 | 1.998 | 0.046 | 0.019 | 0.002 | 0.002 | 0.903 |
| CHA2DS2‐VASc score | 0.600 | 0.567 to 0.625 | <0.001 | ||||||
| At 6.5 y | |||||||||
| ABC‐stroke score | 0.662 | 0.633 to 0.690 | <0.001 | 1.574 | 0.116 | 0.019 | 0.002 | −0.053 | <0.001 |
| CHA2DS2‐VASc score | 0.620 | 0.590 to 0.648 | <0.001 | ||||||
ABC‐stroke indicates age, biomarkers (N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin), and clinical history (prior stroke/transient ischemic attack); CHA2DS2‐VASc, cardiac failure or dysfunction, hypertension, age ≥75 (doubled), diabetes mellitus, stroke (doubled)—vascular disease, age 65 to 74 years and sex category (female); IDI, integrated discriminatory improvement; NRI, net reclassification index; ROC, receiver operating characteristic.
For C‐index comparison.
When we plotted decision curve analyses to assess the clinical usefulness in real practice, use of the ABC‐stroke score showed an approximate net benefit of 0.5% at 3.5 years and 1% at 6.5 years, over the CHA2DS2‐VASc score (Figure 2).
Figure 2.
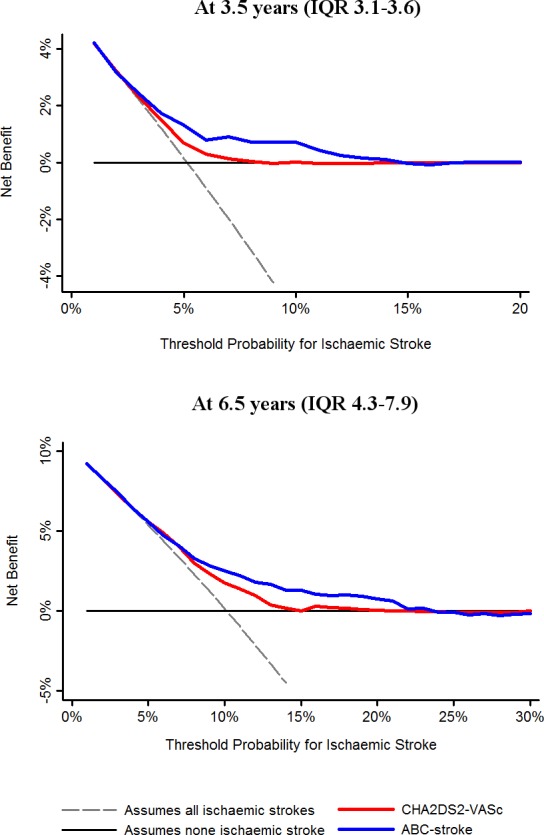
Decision curves for the ABC‐stroke (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) and CHA 2 DS 2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) scores. This analysis shows the clinical usefulness of each score based on a continuum of potential thresholds for ischemic stroke (x axis) and the net benefit of using the model to stratify patients at risk (y axis) relative to assuming that no patient will have an ischemic stroke. IQR indicates interquartile range.
Prediction of Low Risk Patients
The proportion of patients categorized as low‐medium risk by the CHA2DS2‐VASc and ABC‐stroke scores were 5.6% and 48.2%, respectively. Importantly, a high proportion (89.3%) of patients classified as low‐medium risk according to the ABC‐stroke score could be categorized as high risk (score ≥2) with the CHA2DS2‐VASc score (Figure S3). As expected, the ABC‐stroke score and CHA2DS2‐VASc score showed a moderate correlation (Spearman's rho: 0.539; 95% CI, 0.496–0.580 [P<0.001]).
Only 1 of the patients categorized as having low‐moderate risk by CHA2DS2‐VASc score experienced an ischemic stroke at 6.5 years (0.31% per year); however, with the ABC‐stroke score, patients in the low‐moderate risk category had 36 strokes at 6.5 years (1.1% per year). This means that at 6.5 years, 6.64% of patients categorized as having low‐medium risk with the ABC‐stroke score experienced an ischemic stroke, while only 1.59% of patients categorized as having low‐medium risk with the CHA2DS2‐VASc score experienced an ischemic stroke. Patients at low‐medium risk in the ABC‐stroke group still had a median CHA2DS2‐VASc score of 3 (IQR 2–4) and a high risk of stroke per every CHA2DS2‐VASc score point (hazard ratio, 1.3; 95% CI, 1.09–1.70; P=0.007).
Discussion
In this analysis of anticoagulated patients with AF, our principal finding was that the ABC‐stroke score did not provide better predictive accuracy for stroke in patients with AF followed‐up over a long‐term period, in comparison to the CHA2DS2‐VASc score. Second, the CHA2DS2‐VASc score performed well in identifying patients at “low risk,” better than ABC‐stroke.
The role of biomarkers in the prediction of adverse outcomes in patients with AF has been extensively investigated. These include some biomarkers related to hemostasis (fibrin D‐dimer, plasminogen activator inhibitor, tissue factor, and P‐selectin), inflammation (C‐reactive protein, interleukin 6, galectin‐3, tumor necrosis factor‐α), myocardial stress or injury (cardiac troponins and natriuretic peptides), endothelial damage or dysfunction (thrombomodulin, E‐selectin, and von Willebrand factor), fibrosis and extracellular matrix turnover (transforming growth factor‐β, myeloperoxidase, and metallopeptidases and their inhibitors), renal function (Cystatin C), or genetic factors (micro‐RNA and single‐nucleotide polymorphisms).13, 14 Indeed, inflammatory and hemostatic markers such us plasminogen activator inhibitor‐1, thrombin‐antithrombin, and D‐dimer have all been shown to be associated with stroke and thromboembolic events.15 Similarly, interleukin 6 has been demonstrated to be related to mortality, thromboembolic events, and major bleeding in patients with AF, while C‐reactive protein was associated with myocardial infarction.16 In the ARISTOTLE biomarker substudy (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation), a high level of growth differentiation factor 15, a member of the transforming growth factor‐β cytokine family, was an independent risk factor for major bleeding, mortality, and stroke in patients with AF.13, 17, 18
One of the most well‐studied biomarkers in AF is the von Willebrand factor, which is a marker of endothelial damage/dysfunction.5 In 2006, the von Willebrand factor was first reported to refine clinical stroke risk stratification using the CHADS2 and Birmingham (the precursor of CHA2DS2‐VASc) scores.6 More recently, we confirmed the prognostic value of the von Willebrand factor in a contemporary cohort of patients with AF and how its addition to the CHA2DS2‐VASc and HAS‐BLED scores improved the prediction of cardiovascular events (including cardiovascular mortality), stroke, and major bleeding; however, the clinical utility of adding this biomarker to clinical scores remains limited given the marginal net benefit, especially after a long‐term follow‐up.19
Renewed interest into biomarkers is consequent upon publication of substudies from the large randomized trials of anticoagulation in AF. For example, Hijazi et al20, 21, 22 determined that high‐sensitivity cardiac troponins I and T, as well as NT‐proBNP, provided important prognostic information in patients with AF. In a subanalysis of the ENGAGE AF‐TIMI 48 trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction Study 48), adding troponin I, NT‐proBNP, and D‐dimer levels to the CHA2DS2‐VASc score incrementally enhanced risk assessment for stroke, systemic embolism, and death compared with traditional clinical risk stratification.23 In smaller real‐world series, Providência et al24 demonstrated that the addition of troponin to clinical risk scores helped identify patients at greater risk of intracardiac thrombi, while Roldán et al25 demonstrated that increased blood concentrations of NT‐proBNP identified patients with AF at risk for stroke and provided complementary prognostic information to the CHA2DS2‐VASc score. Another biomarker, B‐type natriuretic peptide, has been shown to be a predictor of incident AF and improved risk stratification in the Framingham cohort.26 In contrast, Potpara et al27 showed that adding troponin I, fibrinogen, and C‐reactive protein to the CHA2DS2‐VASc score did not significantly increase the predictive ability of the risk model.
Despite the increasing number of studies investigating biomarkers in AF, their value in the everyday real‐world clinical evaluation of patients with AF is debated. For example, the ABC‐stroke score was developed using data from patients with AF who had biomarkers measured at entry into the ARISTOTLE trial. This risk score demonstrated improved predictive performance (at least statistically) compared with CHA2DS2‐VASc, but the follow‐up was only to 3 years in the external validation cohort.7 In clinical trials, patients are often carefully selected with specific inclusion/exclusion criteria and followed‐up in a protocol‐based manner, whereas patients with AF in real‐world clinical practice tend to be older, with many associated comorbidities and polypharmacy, and have variable treatment adherence and follow‐up.28 In addition, many biomarkers are concurrently predictive of ischemic stroke, bleeding, myocardial infarction, venous thromboembolism, heart failure, and death, which may lead to uncertainty among clinicians over which end point should be the priority. Biomarkers are also subject to laboratory assay variability and some have a diurnal variation in levels. All of these factors may make accurate estimation of stroke and bleeding risk more difficult and less practical with a biomarker‐based strategy.
Recently, the validation of risk scores in other populations who the score was not created for has been criticized, as validation studies are only valid for the data source in which they are performed29; however, surprisingly, the recent European Society of Cardiology guidelines suggest the use of biomarkers based on the ABC scores.7, 30 Hence, it would be highly relevant to see how this score performed in real‐world patients in everyday clinical practice. Our study shows that in the long term (particularly from 3.5 years of follow‐up) the biomarker‐based ABC‐stroke score loses much of its prognostic value and the clinical usefulness does not differ much from a simple clinical risk score, CHA2DS2‐VASc. Indeed, improvement of the net benefit was marginal (≤1%) and measurement of (multiple) biomarkers may help improve the prediction of patients at high risk; however, this would be at the cost of adding substantial complexity, expense, and lack of practicality.14
One of the advantages of the CHA2DS2‐VASc score is its relative simplicity, which allows clinicians to rapidly estimate the risk of stroke in patients with AF and whether oral anticoagulant therapy is indicated. Indeed, the default should be to offer stroke prevention (ie, oral anticoagulants) unless the patient is initially shown to be at low risk and CHA2DS2‐VASc performs well in categorizing those at low risk (stroke <1% per year).
Limitations
This study is limited by its recruitment of a white‐based patient population and by its single‐center design. At the beginning of the study, all patients were stable with a vitamin K agonist (INR 2.0–3.0) during the previous 6 months to ensure homogeneity, thus future studies are needed to validate our results in a population taking other oral anticoagulation therapies. The strict selection criteria (all INRs between 2 and 3 at 6 months before entry) may not reflect “typical” clinical practice, but the long follow‐up and standard care received make this cohort suitable. Although our data set was collected prospectively, biomarker determinations and all statistical analyses were performed retrospectively. Indeed, patients with recent acute coronary syndrome, stroke, unstable chest pain, or any hemodynamic instability, as well as patients with hospital admission or surgical intervention in the past 6 months were excluded, since biomarkers would be expected to be abnormally elevated in these patients. We consider that a 6‐month period would be enough to stabilize biomarkers values and—most importantly—ensured homogeneity at baseline, avoiding the bias produced by high biomarkers at entry. We also measured biomarkers at baseline, but we acknowledge that levels may fluctuate over time and during the acute phase of adverse events. Indeed, most of the studies on biomarkers and AF are based on a single unique blood sample determination at baseline. Of note, participants were carefully followed‐up and all events (even early ones) were recorded.
Conclusions
In anticoagulated patients with AF, the novel ABC‐stroke score does not offer significantly better predictive performance compared with the CHA2DS2‐VASc score over a long‐term period of follow‐up (median 6.5 years). Importantly, the CHA2DS2‐VASc score performed well in identifying patients at low risk better than the ABC‐stroke score.
Sources of Funding
This work was supported by Instituto de Salud Carlos III (ISCIII), Fondo Europeo de Desarrollo Regional (FEDER) (Research projects: PI13/00513 and P14/00253), Fundación Séneca (grant number: 19245/PI/14), RD12/0042/0050, and Instituto Murciano de Investigación Biosanitaria (IMIB16/AP/01/06). Rivera‐Caravaca has received a grant from Sociedad Española de Trombosis y Hemostasia (grant for short international training stays 2016).
Disclosures
Dr Lip is a consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife, and Daiichi‐Sankyo. He is a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi‐Sankyo. No fees are received personally. The other authors have nothing to disclose.
Supporting information
Table S1. Univariate Cox Regression Analysis Between Stroke Risk Scores and Ischemic Stroke
Figure S1. Kaplan–Meier analysis for ischemic stroke according to the low‐risk categories of each score.
Figure S2. Comparison of the receiver operating characteristic (ROC) curves of the ABC‐stroke (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) and CHA2DS2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) scores.
Figure S3. Correlation of the ABC‐stroke (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) and CHA2DS2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) scores. Focus on patients at low‐moderate risk with the ABC‐stroke score.
(J Am Heart Assoc. 2017;6:e006490 DOI: 10.1161/JAHA.117.006490.)28729407
References
- 1. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National‐Institute‐for‐Health‐and‐Care‐Excellence . Atrial fibrillation: the management of atrial fibrillation. (clinical guideline 180.). 2014. Available at: http://guidance.Nice.Org.Uk/cg180. Accessed February 9, 2017.
- 5. Thomas MR, Lip GY. Novel risk markers and risk assessments for cardiovascular disease. Circ Res. 2017;120:133–149. [DOI] [PubMed] [Google Scholar]
- 6. Lip GY, Lane D, Van Walraven C, Hart RG. Additive role of plasma von Willebrand factor levels to clinical factors for risk stratification of patients with atrial fibrillation. Stroke. 2006;37:2294–2300. [DOI] [PubMed] [Google Scholar]
- 7. Hijazi Z, Lindbäck J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RAH, White HD, Granger CB, Wallentin L. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker‐based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 9. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 10. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 11. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hijazi Z, Oldgren J, Siegbahn A, Wallentin L. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem. 2017;63:152–164. [DOI] [PubMed] [Google Scholar]
- 14. Szymanski FM, Lip GY, Filipiak KJ, Platek AE, Hrynkiewicz‐Szymanska A, Opolski G. Stroke risk factors beyond the CHA(2)DS(2)‐VASc score: can we improve our identification of “high stroke risk” patients with atrial fibrillation? Am J Cardiol. 2015;116:1781–1788. [DOI] [PubMed] [Google Scholar]
- 15. Wu N, Chen X, Cai T, Wu L, Xiang Y, Zhang M, Li Y, Song Z, Zhong L. Association of inflammatory and hemostatic markers with stroke and thromboembolic events in atrial fibrillation: a systematic review and meta‐analysis. Can J Cardiol. 2015;31:278–286. [DOI] [PubMed] [Google Scholar]
- 16. Aulin J, Siegbahn A, Hijazi Z, Ezekowitz MD, Andersson U, Connolly SJ, Huber K, Reilly PA, Wallentin L, Oldgren J. Interleukin‐6 and C‐reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am Heart J. 2015;170:1151–1160. [DOI] [PubMed] [Google Scholar]
- 17. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Asberg S, Granger CB, Siegbahn A. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847–1858. [DOI] [PubMed] [Google Scholar]
- 18. Marin F, Roldan V. Biomarkers: GDF‐15 and risk stratification in atrial fibrillation. Nat Rev Cardiol. 2015;12:8–9. [DOI] [PubMed] [Google Scholar]
- 19. García‐Fernández A, Roldán V, Rivera‐Caravaca JM, Hernández‐Romero D, Valdés M, Vicente V, Lip GY, Marín F. Does von Willebrand factor improve the predictive ability of current risk stratification scores in patients with atrial fibrillation? Sci Rep. 2017;7:41565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hijazi Z, Siegbahn A, Andersson U, Granger CB, Alexander JH, Atar D, Gersh BJ, Mohan P, Harjola VP, Horowitz J, Husted S, Hylek EM, Lopes RD, McMurray JJ, Wallentin L. High‐sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;129:625–634. [DOI] [PubMed] [Google Scholar]
- 21. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Alexander JH, Atar D, Gersh BJ, Hanna M, Harjola VP, Horowitz JD, Husted S, Hylek EM, Lopes RD, McMurray JJ, Granger CB. High‐sensitivity troponin T and risk stratification in patients with atrial fibrillation during treatment with apixaban or warfarin. J Am Coll Cardiol. 2014;63:52–61. [DOI] [PubMed] [Google Scholar]
- 22. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ, Granger CB. N‐terminal pro‐B‐type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol. 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 23. Ruff CT, Giugliano RP, Braunwald E, Murphy SA, Brown K, Jarolim P, Mercuri M, Antman EM, Morrow DA. Cardiovascular biomarker score and clinical outcomes in patients with atrial fibrillation: a subanalysis of the ENGAGE AF‐TIMI 48 randomized clinical trial. JAMA Cardiol. 2016;1:999–1006. [DOI] [PubMed] [Google Scholar]
- 24. Providencia R, Paiva L, Faustino A, Botelho A, Trigo J, Casalta‐Lopes J, Nascimento J, Leitao‐Marques AM. Cardiac troponin I: prothrombotic risk marker in non‐valvular atrial fibrillation. Int J Cardiol. 2013;167:877–882. [DOI] [PubMed] [Google Scholar]
- 25. Roldan V, Vilchez JA, Manzano‐Fernandez S, Jover E, Galvez J, Puche CM, Valdes M, Vicente V, Lip GY, Marin F. Usefulness of N‐terminal pro‐B‐type natriuretic peptide levels for stroke risk prediction in anticoagulated patients with atrial fibrillation. Stroke. 2014;45:696–701. [DOI] [PubMed] [Google Scholar]
- 26. Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA, Magnani JW, Ellinor PT, Wang TJ, Levy D, Vasan RS, Benjamin EJ. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potpara TS, Polovina MM, Djikic D, Marinkovic JM, Kocev N, Lip GY. The association of CHA2DS2‐VASc score and blood biomarkers with ischemic stroke outcomes: the Belgrade stroke study. PLoS One. 2014;9:e106439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freedman B, Lip GY. “Unreal world” or “real world” data in oral anticoagulant treatment of atrial fibrillation. Thromb Haemost. 2016;116:587–589. [DOI] [PubMed] [Google Scholar]
- 29. Friberg L, Skeppholm M. Usefulness of Health Registers for detection of bleeding events in outcome studies. Thromb Haemost. 2016;116:1131–1139. [DOI] [PubMed] [Google Scholar]
- 30. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Granger CB, Wallentin L; Investigators AaR‐L . The novel biomarker‐based ABC (age, biomarkers, clinical history)‐bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387:2302–2311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Cox Regression Analysis Between Stroke Risk Scores and Ischemic Stroke
Figure S1. Kaplan–Meier analysis for ischemic stroke according to the low‐risk categories of each score.
Figure S2. Comparison of the receiver operating characteristic (ROC) curves of the ABC‐stroke (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) and CHA2DS2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) scores.
Figure S3. Correlation of the ABC‐stroke (age, biomarkers [N‐terminal fragment B‐type natriuretic peptide, high‐sensitivity troponin], and clinical history [prior stroke/transient ischemic attack]) and CHA2DS2‐VASc (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled]—vascular disease, age 65 to 74 years and sex category [female]) scores. Focus on patients at low‐moderate risk with the ABC‐stroke score.


