ABSTRACT
Most floating aquatic plants have stomata on their upper leaf surfaces, and usually their stomata are permanently open. We previously identified 3 distinct crystallinity patterns in stomatal cell walls, with angiosperm kidney-shaped stomata having the highest crystallinity in the polar end walls as well as the adjacent polar regions of the guard cells. A numerical bio-mechanical model suggested that the high crystallinity areas are localized to regions where the highest stress is imposed. Here, stomatal cell wall crystallinity was examined in 4 floating plants from 2 different taxa: basal angiosperms from the ANITA grade and monocots. It appears that the non-functional stomata of floating plants display reduced crystallinity in the polar regions as compared with high crystallinity of the ventral (inner) walls. Thus their guard cells are both less flexible and less stress resistant. Our findings suggest that the pattern of cellulose crystallinity in stomata of floating plants from different families was altered as a consequence of similar evolutionary pressures.
KEYWORDS: Aquatic plants, cell wall, cellulose crystallinity, evolution, polarized-light microscopy, stomata
Stomata are crucial for plant functioning because of their fundamental role in the regulation of gas exchange between the plant and its surrounding environment. It is evident that the morphology, distribution, orientation and development of stomata have diversified since they first evolved ∼400 million years ago.1,2 Stomatal cell walls are uniquely strong and flexible, enabling repeated opening and closing of the stomatal pore multiple times every day; interestingly the triggers for stomatal opening (i.e. light and CO2) appear to be similar among the different plant groups whereas those for stomatal closing differ.3 There is significant ongoing debate regarding stomatal evolution, the differences in stomatal function between taxonomic groups, and how they impact plant performance, partially driven by disparities between evidence from different sources, for example ABA-responsiveness and the presence and localization of ABA-signaling pathway components.4-8 Although relatively few studies focus on the effect of cell wall composition and structure on stomatal function, wall properties are known to directly affect the structure and mechanical properties of guard cell walls and therefore stomatal function.1,9-11 In our previous work12 we found 3 distinct, taxonomic group-dependent crystallinity patterns in stomatal cell walls, with angiosperm kidney-shaped stomata having the highest crystallinity in the polar end walls and in the adjacent polar regions of the guard cells (Fig. 1A–C). Our Finite Elements model indicated that the highly crystalline areas might serve a biomechanical purpose by strengthening the cell wall in areas of high stress.12 However, this work omitted to investigate the numerous different highly-modified types and behaviors of stomata that exist within extant plant groups.3 For instance, there are several examples of non-functional stomata, i.e., those that do not have the ability to open and close13 such as the stomata present in parasitic plants, flowers or fruits. While probably the most interesting example of non-functional stomata are the permanently open stomata of aquatic plants, or macrophytes. Non-functional stomata are considered to be an advanced character of aquatic plants, while the ability to open (and close) the stomata is a vestigial trait of the terrestrial ancestry.14
Figure 1.
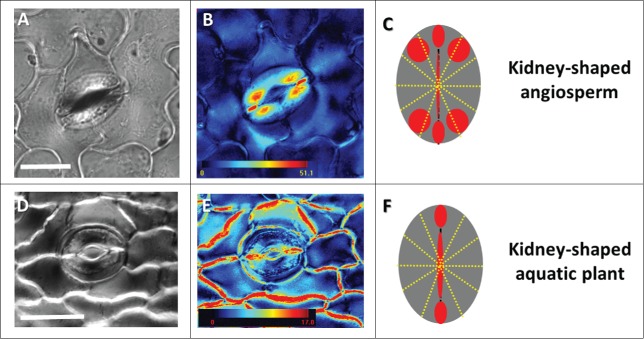
Floating aquatic plants have stomata with an altered pattern of cellulose crystallinity. Crystallinity patterns in the land plant Cyclamen persicum (A,B); and aquatic plant Nuphar lutea (D,E) shown by liquid crystal polarized light microscopy (LC-PolScope). A schematic representation of the regular crystallinity pattern in angiosperm kidney-shaped stomata (C) and the altered pattern in the stomata of floating plants (F). The retardance color scale bar codes the retardance range. Scale: 25 µm.
Floating aquatic plants have independently evolved multiple times in ferns, liverworts and angiosperms and can be found in most freshwater habitats globally.15 They appear to have evolved once in liverworts (Ricciaceae) and at least twice in ferns (Ceratopteris in the Pteridaceae and Azolla and Salvinia in the Salviniaceae). In angiosperms, several families contain aquatic plants with floating leaves. It is likely that floating plants arose independently at least 13 times in angiosperms and they are found in the ANITA grade (Nymphaeaceae), eudicots (Polygonaceae, Lythraceae, Plantaginaceae, Ranunculaceae, Gentianaceae), and monocots (Butomaceae, Hydrocharitaceae, Potamogetonaceae, Araceae, Aponogetonaceae, Typhaceae, Gramineae).15 Floating plants often exhibit similar adaptations to the aquatic habitat, providing an interesting example of convergent evolution. They remain buoyant on water by the means of large air spaces15,16 in their floating leaf blades, petioles or roots. Floating leaves have their lower (abaxial) surface completely submerged in water, while the upper (adaxial) surface is exposed to the atmosphere. Unsurprisingly, floating plants usually have epistomatous leaves (in which only the upper surface has stomata).14,15 Therefore, gas exchange occurs mainly from the adaxial leaf surface. Often aquatic plants have non-functional i.e., permanently open stomata that cannot regulate water loss.14 In aquatic plants, CO2-exchange is not limited by water availability. Thus, as a floating leaf has no need to conserve water, closing the stomatal pore is not necessary and losing the ability to do so would likely have no associated negative selection pressure(s). Indeed, many floating plants have relatively high photosynthetic capacities.17 Furthermore, large-scale deletion of genes from the stomata developmental pathway has been seen to accompany the loss of stomata in marine angiosperms.18
Only a few works discuss the subject of non-functional stomata mechanics. In Salvinia herzogii it was proposed that the guard cells are physically unable to close because of peculiar, probably cuticular, extensions near the pore.19 In Lemna minor the guard cells are apparently dead, and thus unable to move.14 In Nymphaea and Nuphar the guard cells are intact, and Ziegler suggested that there is no substomatal cavity, which possibly prevents the guard cells movement14; although, the stomata of Nyphaea violacea and several other members of the genus have since clearly been shown to possess substomatal cavities.20,21 It is interesting that the reasons considered to be involved in control of stomatal closure and therefore the generation of non-functional stomata are largely thought to be anatomic in light of discussions that stomatal closure may be controlled by different triggers in non-vascular compared with vascular plants.3 Plant cell wall composition is also known to differ between different plant groups22 and it is now known that cell wall composition contributes to stomatal function.3,4,6 However, as far as we are aware, there are no studies that have investigated the cell wall composition and structure of the non-functional stomata of floating plants.
In the current study, stomatal cell wall crystallinity was investigated in 4 floating plants: Nymphaea alba and Nuphar lutea (Nymphaeaceae, ANITA grade; one of the earliest diverging lineages of Angiosperms), Alisma plantago-aquatica and Limnobium laevigatum (Alismataceae, monocots). Alisma apparently has partially functional stomata,23 while in Nymphaea,14 Nuphar,14 and probably Limnobium, stomata are completely non-functional. We focused our study on angiosperms to reduce the effects of differences that may exist in stomatal function due to, for example, differences in ABA-responsiveness between ferns and angiosperms.4
It appears that for the aquatic plants investigated, although belonging to different families and taxonomic groups, cell wall crystallinity pattern in the guard cells is similarly altered, as compared with the pattern typically observed for angiosperm kidney-shaped stomata (Fig. 1). All the plants examined had high crystallinity near the pore. Nymphaea, Nuphar and Limnobium seem to share a very similar crystallinity pattern having high crystallinity in the polar end walls and lacking crystalline areas in the adjacent polar regions (Figs. 2A, B and C). In contrast, Alisma (having partially functional stomata), possess crystallinity pattern in the polar end walls and adjacent polar-regions, similar to that previously observed for angiosperm kidney-shaped stomata (Fig. 2D). All 4 species displayed similar patterns of cellulose orientation in their stomata, identical to all kidney-shaped stomata (data not shown). It is important to mention, that usually the stomata close after the epidermis is peeled. However, non-functional stomata are unable to do so and hence remaine open; potentially impacting stomatal geometry.
Figure 2.
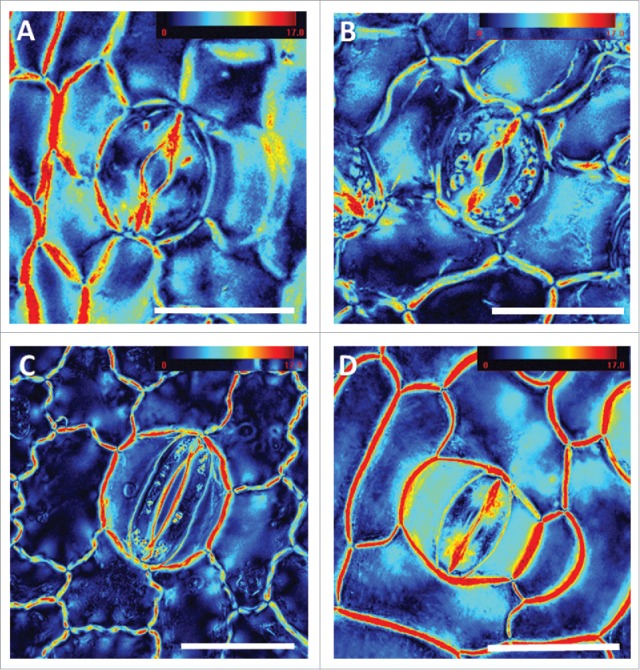
Stomatal crystallinity patterns of floating aquatic plant species. Liquid crystal polarized light microscopy (LC-PolScope) images of the adaxial leaf epidermis from the early-diverging angiosperms Nuphar lutea (A), Nymphaea alba (B), and the monocotyledons Limnobium laevigatum (C) and Alisma plantago-aquatica (D). The retardance color scale bar codes the retardance range. Scale: 50 µm.
Interestingly, the ventral wall (cell wall at the pore margins) of all 4 plant species examined displayed high crystallinity. The ventral wall has to remain flexible to allow the guard cells to elongate and shorten during the repeated cycles of stomata opening and closing which occur as part of plant adaptation to changing environment. High crystallinity in this area is assumed to be associated with increased cell wall stiffness and therefore might interfere with stomatal movement. These observations suggest that the altered pattern of cell wall crystallinity in floating plants is probably associated with the loss of stomatal function, though more research is needed to come to definite conclusions. However, the observation that in the partially functional stomata of Alisma the crystallinity in the polar-regions is not significantly reduced strengthens this hypothesis.
Aquatic floating plants have specific adaptations to their unique habitat. Their permanently open stomata display similar alterations in cellulose crystallinity pattern, presumably as a consequence of similar environmental pressure(s) yielding another fascinating example of convergent evolution. It would be very interesting to know whether other cell wall constituents (such as pectins, lignins, phenols, etc.) are modified in this unique context. Better understanding of the biomechanical impacts of key changes in cell wall composition in response to environmental adaptation could extend our knowledge of how different plant species may respond to climate- and anthropologically-induced environmental changes including drought and salinity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Chen Z-H, Chen G, Dai F, Wang Y, Hills A, Ruan Y-L, Zhang G, Franks PJ, Nevo E, Blatt MR. Molecular evolution of grass stomata. Trends Plant Sci 2017; 22:124-39; PMID:27776931; https://doi.org/ 10.1016/j.tplants.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Qu X, Peterson KM, Torii KU. Stomatal development in time: The past and the future. Curr Opin Genet Dev 2017; 45:1-9; PMID:28219014; https://doi.org/ 10.1016/j.gde.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Brodribb TJ, McAdam SAM. Evolution of the stomatal regulation of plant water content. Plant Physiol 2017; 174:639-49:pp.00078.2017. Available from: http://www.plantphysiol.org/lookup/doi/10.1104/pp.17.00078; PMID:28404725; https://doi.org/ 10.1104/pp.17.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mcadam SAM, Brodribb TJ. Stomatal innovation and the rise of seed plants. Ecol Lett 2012; 15:1-8; PMID:22017636; https://doi.org/ 10.1111/j.1461-0248.2011.01700.x [DOI] [PubMed] [Google Scholar]
- 5.Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM. Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 2011; 21:1030-5; PMID:21658945; https://doi.org/ 10.1016/j.cub.2011.04.044 [DOI] [PubMed] [Google Scholar]
- 6.Cai S, Chen G, Wang Y, Huang Y, Marchant B, Wang Y, Yang Q, Dai F, Hills A, Franks PJ, et al.. Evolutionary conservation of ABA signaling for stomatal closure in ferns. Plant Physiol 2017; 174:732-47:pp.01848.2016. Available from: http://www.plantphysiol.org/lookup/doi/10.1104/pp.16.01848; PMID:28232585; https://doi.org/ 10.1104/pp.16.01848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field KJ, Duckett JG, Cameron DD, Pressel S. Stomatal density and aperture in non-vascular land plants are non-responsive to above-ambient atmospheric CO<inf>2</inf>concentrations. Ann Bot 2015; 115:915-22; PMID:25858324; https://doi.org/ 10.1093/aob/mcv021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franks PJ, Britton-Harper ZJ. No evidence of general CO2 insensitivity in ferns: One stomatal control mechanism for all land plants? New Phytol 2016; 211:819-27; https://doi.org/ 10.1111/nph.14020 [DOI] [PubMed] [Google Scholar]
- 9.Amsbury S, Hunt L, Elhaddad N, Baillie A, Lundgren M, Verhertbruggen Y, Scheller HV, Knox JP, Fleming AJ, Gray JE. Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr Biol 2016; 26:2899-906; PMID:27720618; https://doi.org/ 10.1016/j.cub.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones L, Milne JL, Ashford D, McCann MC, McQueen-Mason SJ. A conserved functional role of pectic polymers in stomatal guard cells from a range of plant species. Planta 2005; 221:255-64; PMID:15578215; https://doi.org/ 10.1007/s00425-004-1432-1 [DOI] [PubMed] [Google Scholar]
- 11.Rui Y, Anderson CT. Functional analysis of cellulose and xyloglucan in the walls of stomatal guard cells of Arabidopsis thaliana. Plant Physiol 2016; 170:1398-419:pp.01066.2015. Available from: http://www.plantphysiol.org/content/early/2016/01/04/pp.15.01066.abstract?maxtoshow=&hits = 1&RESULTFORMAT=&titleabstract = plant+plants+arabidopsis+tobacco+potato&fulltext = stomata+stomate+%22 guard+cell%22+stomates+stoma&searchid = 1&usestrictdates = yes&resource; PMID:26729799; https://doi.org/ 10.1104/pp.15.01066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shtein I, Shelef Y, Marom Z, Popper Z, Zelinger E, Schwartz A, Bar-On B, Harpaz-Saad S. Stomatal cell wall composition and cellulose crystallinity: Distinctive structural patterns are associated with different phylogenetic groups. Ann Bot 2017; 119:1021-33; PMID:28158449; https://doi.org/ 10.1093/aob/mcw275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esau K. Anatomy of seed plants. New York: John Wiley and Sons; 1965. [Google Scholar]
- 14.Ziegler H. The evolution of stomata In: Zeiger E, Farquhar G, Cowan I, editors. Stomatal function. Stanford: Stanford University Press; 1987. page 29- 57. [Google Scholar]
- 15.Kaul RB. Anatomical observation of floating leaves. Aquat Bot 1976; 2:215-34; https://doi.org/ 10.1016/0304-3770(76)90022-X [DOI] [Google Scholar]
- 16.Jung J, Lee SC, Choi HK. Anatomical patterns of aerenchyma in aquatic and wetland plants. J Plant Biol 2008; 51:428-39; https://doi.org/ 10.1007/BF03036065 [DOI] [Google Scholar]
- 17.Tsuchiya T. Leaf life span of floating-leaved plants. Vegetatio 1991; 97:149-60; https://doi.org/ 10.1007/BF00035388 [DOI] [Google Scholar]
- 18.Olsen JL, Rouzé P, Verhelst B, Lin Y, Bayer T, Collen J, Dattolo E, De Paoli E, Dittami S, Maumus F, et al.. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 2016; 530:331-5; PMID:26814964; https://doi.org/ 10.1038/nature16548 [DOI] [PubMed] [Google Scholar]
- 19.de la Sota ER, Cassa de Pazos LA. On the stomata of Salvinia herzogii (Salviniaceae, Pteridophyta). Plant Syst Evol 1990; 172:119-25; https://doi.org/ 10.1007/BF00937802 [DOI] [Google Scholar]
- 20.Rudall PJ, Knowles EVW. Ultrastructure of stomatal development in early-divergent angiosperms reveals contrasting patterning and pre-patterning. Ann Bot 2013; 112:1031-43; PMID:23969762; https://doi.org/ 10.1093/aob/mct169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catian G, Scremin-Dias E. Compared leaf anatomy of Nymphaea (Nymphaeaceae) species from Brazilian flood plain. Braz J Biol 2013; 73:809-17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24789398; PMID:24789398; https://doi.org/ 10.1590/S1519-69842013000400018 [DOI] [PubMed] [Google Scholar]
- 22.Popper ZA, Michel G, Herve C, Domozych DS, Willats WG, Tuohy MG, Kloareg B, Stengel DB. Evolution and diversity of plant cell walls: From algae to flowering plants. Annu Rev Plant Biol 2011; 62:567-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21351878; PMID:21351878; https://doi.org/ 10.1146/annurev-arplant-042110-103809 [DOI] [PubMed] [Google Scholar]
- 23.Darwin F. On a self-recording method applied to the movements of stomata. Bot Gaz 1908; 37:81-105; https://doi.org/ 10.1086/328451 [DOI] [Google Scholar]