The appearance of a fruit fly—the wings, legs, even the number and size of the bristles—is remarkably constant. This archetypal appearance is surprising given the complexity of the underlying developmental programs that mold the organism. How do organisms maintain such constancy? Classic developmental studies by Conrad Waddington took a cue from the effects of environmental stresses. Waddington proposed that canalization (Table 1) is a feature of developmental pathways that ensures robust specification of form (Waddington 1942). He argued that since multiple determinants work together to define form, most single determinants would not cause phenotypic variation. However, he observed that stresses like heat shock could perturb development and induce a wide range of dramatic phenotypic changes, suggesting that environmental stress could reveal cryptic variation (Table 1). Surprisingly, Waddington showed that persistent environmental stress and selection for phenocopies (Table 1), could recover variants that were heritable even in the absence of the stress, a process he termed genetic assimilation (Table 1)(Waddington 1953). The basis of genetic assimilation has remained mysterious.
Table 1. Glossary of Terms.
| Term | Definition |
|---|---|
| Canalization | The developmental program of organisms is robust so that small environmental changes will be buffered to produce a single definite phenotype. |
| Cryptic variation | Heritable variation that is buffered during development in an unstressed state, producing a wild-type phenotype. In a stressed state, the phenotypic variant can be revealed. |
| Genetic assimilation | Organisms exposed to stress for several generations develop a phenotypic modification that persists even in the absence of the stress that first elicited the phenotype. |
| Phenocopy | Changes in developmental program by the environment producing a phenotype that is typically not heritable. |
| Pseudoassimilation | Organisms exposed to stress produce de novo somatic changes that, over several generations, lead to de novo genetic mutations in the germline. |
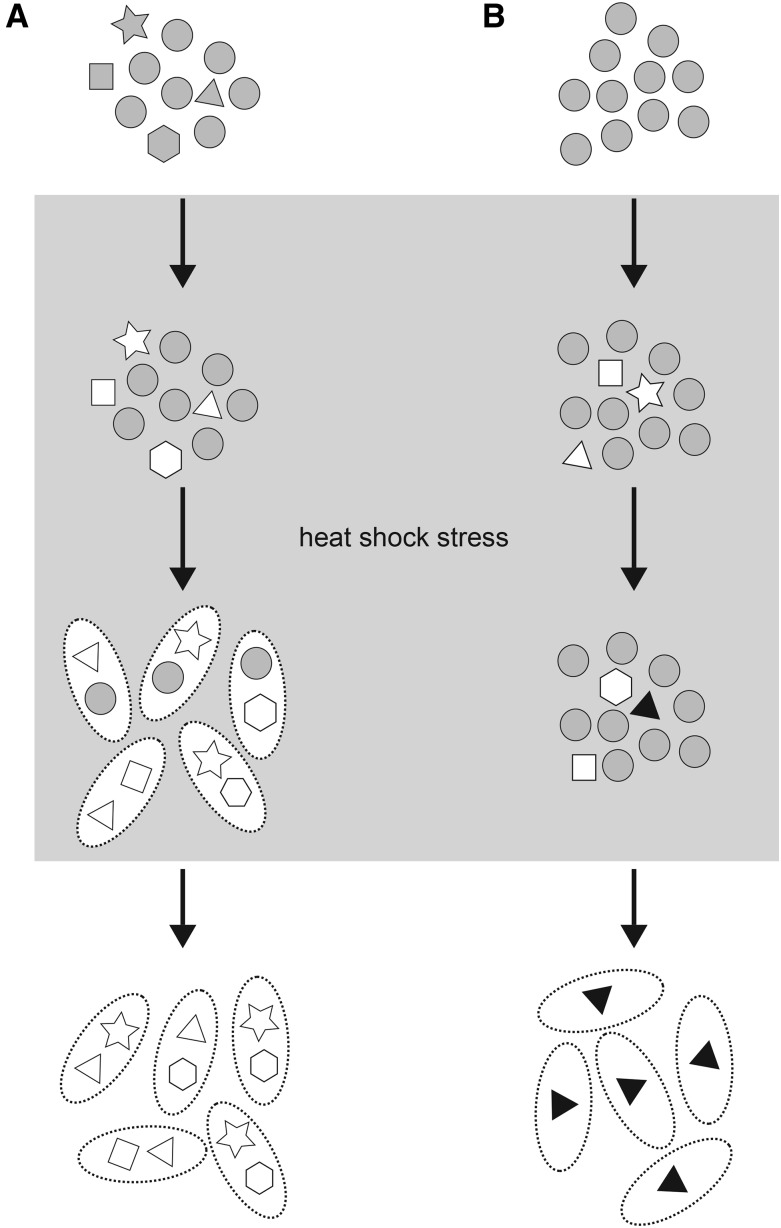
Pioneering work by Rutherford and Lindquist (1998) elaborated a molecular mechanism for genetic assimilation. Lindquist and colleagues found that the protein chaperone Hsp90 plays a role in canalization. Without Hsp90, stress-induced phenotypic aberrations are more frequent. They suggested that the basis of Waddington’s cryptic variation was polymorphic genetic variants at many genes which work together to control developmental pathways. They argued that the Hsp90 chaperone reduced the effects of cryptic genetic variants by regulating protein structure and function in nonstressed cells. The idea that the Hsp90-chaperone activities mask the effects of segregating genetic variation has been borne out by studies in fungi, plants, and animals (Rutherford and Lindquist 1998; Queitsch et al. 2002; Jarosz and Lindquist 2010; Rohner et al. 2013). Rutherford and Lindquist further argued that heat-shock stress or inhibiting Hsp90 would reveal effects of cryptic variants, and that repeated selection would enrich for multiple variants in the same genome (Figure 1A). Many cryptic variants would overcome the normal canalizing activity of Hsp90, thereby making the phenotype stress independent (Rutherford and Lindquist 1998). Under this proposal, genetic assimilation occurs via the selection and accumulation of preexisting cryptic genetic variants in a population.
Figure 1.
Two models explain Waddington’s genetic assimilation. (A) Heat-shock stress exposes cryptic variation. A phenotypically wild-type population (gray shapes) harbors preexisting cryptic variants (triangle, star, square, and hexagon) which present as wild type (gray shapes) under normal conditions but as mutant (white shapes) under heat-shock stress. Persistent heat-shock stress (gray box) leads to accumulation of cryptic variants in the same genome (represented by ovals with dashed lines). The accumulation of sufficient numbers of cryptic variants, in combinations, can manifest as phenotypically mutant even in absence of heat-shock stress. (B) Heat-shock stress induces de novo variation. A genotypically and phenotypically wild-type population (gray circles) exposed to heat-shock stress causes novel epigenetic changes. These epigenetic changes result in altered phenotype, but these phenocopies (white) are not heritable. Continued heat shock over multiple generations results in de novo mutations that occur in the germline (black triangle). Such de novo germline variants are inherited in future generations and can manifest the mutant phenotype even when heat-shock stress is removed.
In the August issue of GENETICS, Fanti et al. (2017) provide evidence for another mechanism for genetic assimilation: stress-induced de novo mutations. They propose that phenocopies result from stress-induced mutations in somatic cells, whereas de novo mutations in germline cells are selected for in genetic assimilation experiments (Figure 1B).
To come to this model, Fanti et al. (2017) began by emulating methods used in Waddington’s genetic assimilation studies (Waddington 1953). They treated two laboratory Drosophila melanogaster populations to heat shock during pupation. This heat-shock treatment led to changes in eye color, bristle morphology, and other developmental aberrations. Fanti et al. (2017) then chose individuals with these phenotypes and crossed them to one another. The resulting progeny were subjected to heat shock and selection every generation. These populations underwent a steady increase in the frequency of the phenotype, eventually leading to fixation of the phenotype. Four heritable phenotypes were isolated during the study, showing altered eye color, melanotic tumors, and two types of bristle defects. Fanti et al. (2017) then isolated the genetic loci responsible for these four phenotypes. The eye color phenotype was identified as a large deletion of the sepia gene, the tumorous phenotype resulted from a transposon insertion in the cactus gene, and the bristle phenotypes were attributed to a transposon insertion in the singed gene and a frameshift mutation in the forked gene. Critically, none of these changes were found in the parental populations, demonstrating that they are de novo mutations. The authors refer to the recovery of these mutants as pseudoassimilation (Table 1), to distinguish it from Waddington’s original proposal of genetic assimilation.
If de novo mutations are the basis for pseudoassimilation, one might expect that even one round of heat shock would be sufficient to elicit such change. But this is not the case. The assimilation experiments performed by Fanti et al. (2017) still require repeated stresses across multiple generations to isolate heritable variants, just like the Waddington and the Rutherford–Lindquist experiments. How can this be?
Fanti et al. (2017) propose that heat shock causes epigenetic changes that result in phenocopies. Indeed, stress-induced phosphorylation of Drosophila activation transcription factor-2 can induce chromatin changes (Seong et al. 2011). Such epigenetic perturbations could cause phenotypic changes, but may not be stably inherited. How do phenocopies become heritable over time? Fanti et al. (2017) propose that regions with stress-induced epigenetic change are more susceptible to genome instability, including transposon insertions. There is an increased rate of transposition events during heat-shock stress because Piwi, a transposon silencer and an Hsp90 substrate, can be crippled by stress (Gangaraju et al. 2011). As a result, heat shock leads to transposon mobilization in the germline and an increased number of mutant progeny (Specchia et al. 2010; Gangaraju et al. 2011).
Under this model, repeated rounds of heat shock alter the chromatin state, produce phenocopies, and make DNA hypermutable. Transposon insertions at hypermutable regions produce de novo mutations, thus phenotypes would manifest even in the absence of heat shock. An alternate possibility is that repeated stress and selection of phenocopies might select for hypermutable flies, increasing the chance of germline mutations over multiple generations. Fanti et al. (2017) conclude that by affecting both epigenetic and genetic changes, stress-response mechanisms have multiple general roles in perpetuating consistent organismal forms.
Acknowledgments
We thank Lisa Kursel and Keith Maggert for comments. We are supported by National Institutes of Health training grant T32 CA-009657 (B.K.), the Julie Tall Achievement Rewards for College Scientists (ARCS) endowment from the Seattle Chapter of the ARCS Foundation (B.K.), National Institutes of Health grants R01 GM-098349, R01 GM-108699 (K.A.), and R01 GM-074108 (H.S.M.). H.S.M. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Communicating editor: P. K. Geyer
Literature Cited
- Fanti L., Piacentini L., Cappucini U., Casale A. M., Pimpinelli S., 2017. Canalization by selection of de novo-induced mutations. Genetics 206: 1995–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangaraju V. K., Yin H., Weiner M. M., Wang J., Huang X. A., et al. , 2011. Drosophila Piwi functions in Hsp90-mediated suppression of phenotypic variation. Nat. Genet. 43: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz D. F., Lindquist S., 2010. Hsp90 and environmental stress transform the adaptive value of natural genetic variation. Science 330: 1820–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C., Sangster T. A., Lindquist S., 2002. Hsp90 as a capacitor of phenotypic variation. Nature 417: 618–624. [DOI] [PubMed] [Google Scholar]
- Rohner N., Jarosz D. F., Kowalko J. E., Yoshizawa M., Jeffery W. R., et al. , 2013. Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342: 1372–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S. L., Lindquist S., 1998. Hsp90 as a capacitor for morphological evolution. Nature 396: 336–342. [DOI] [PubMed] [Google Scholar]
- Seong K.-H., Li D., Shimizu H., Nakamura R., Ishii S., 2011. Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell 145: 1049–1061. [DOI] [PubMed] [Google Scholar]
- Specchia V., Piacentini L., Tritto P., Fanti L., D’Alessandro R., et al. , 2010. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature 463: 662–665. [DOI] [PubMed] [Google Scholar]
- Waddington C. H., 1942. Canalization of development and the inheritance of acquired characters. Nature 150: 563–564. [DOI] [PubMed] [Google Scholar]
- Waddington C. H., 1953. Genetic assimilation of an acquired character. Evolution 7: 118–126. [Google Scholar]