ABSTRACT
The Ca2+ and Ca2+/calmodulin-dependent protein kinase (CCaMK) is an important effector protein of Ca2+/calmodulin-mediated signaling, and in legumes, it is a critical regulator of plant-rhizobia and mycorrhizal symbioses. CCaMK contains a kinase domain, a calmodulin-binding/autoinhibitory domain and a visinin-like domain. Previous studies revealed the presence of 2 phosphorylation sites, S343 and S344, in the calmodulin-binding domain. Mutations at these sites affected the kinase activity and downstream rhizobium and mycorrhizal symbioses, which highlighted the importance of these residues in regulating protein activity. This addendum further clarifies the regulation of CCaMK by identifying an intramolecular interaction between residue(s) in the kinase domain and phosphorylation sites S343 and S344. This interaction turns off the substrate phosphorylation capacity of CCaMK.
KEYWORDS: Autophosphorylation, CCaMK, calmodulin, intramolecular interaction
Abbreviations
- CCaMK,
Calcium/calmodulin-dependent protein kinase
- CaM,
calmodulin
- Ca2+,
calcium
Introduction
The Ca2+/calmodulin (CaM)-dependent protein kinase (CCaMK) is a unique protein in plants;1 in legumes, it regulates the formation of rhizobium and mycorrhizal symbioses by decoding the unique Ca2+ signature that is triggered in the plant by these symbionts.2,3,4 CCaMK has a novel protein structure5 that includes a catalytic domain in its N-terminus and 2 independent regulatory domains: the CaM-binding/autoinhibitory domain and the visinin-like domain, which contains 3 EF hands in the C-terminus.6,7 CCaMK is regulated by the binding of Ca2+ to the regulatory domains. It can bind either as free Ca2+ (in the visinin-like domain) or as Ca2+-loaded CaM (in the CaM-binding/autoinhibitory domain).8,9 In addition to these 2 regulatory domains, CCaMK contains autophosphorylation sites, which also have been described as regulatory sites for the activation and function of this protein kinase.10,11
Recent publications have revealed the presence of 2 phosphorylation sites (S343 and S344) located in the CaM-binding/autoinhibitory domain of the CCaMK.12,13 Biochemical studies on both phosphorylation sites have demonstrated that when S343 and/or S344 were phosphorylated, the CCaMK could not interact with CaM and its kinase activity was abolished. The phosphorylation of these sites also negatively regulated the development of nodules or arbuscules in the root system of Medicago truncatula.14,15
This addendum presents evidence of an intramolecular interaction within CCaMK that is regulated through the phosphorylation of Ser-343 and Ser-344. Our biochemical results strongly suggest that this interaction occurs between the kinase domain and the CaM-binding/autoinhibitory domain of CCaMK. Furthermore, we identified the amino acid T227 in the kinase domain as a key residue involved in this intramolecular interaction. However, altering this residue is not sufficient to recover the CaM binding in the phosphorylated forms of S343 and S344, indicating that there are other residue(s) involved in that interaction between the kinase domain and the CaM-binding/autoinhibitory domain of CCaMK.
Kinase domain of CCaMK interacts with the phosphomimic mutant S343D and S344D
In previous studies, CCaMK phosphomimic mutants S343D and S344D lost their CaM-binding capacity.12,13,16 Two possibilities could explain this result: 1) the mutated CaM-binding domain no longer interacts with CaM; or 2) these phosphomimic mutations resulted in a change in the CCaMK structure which blocks the access of CaM-binding domain to CaM. We synthesized the peptides containing the S343D and S344D mutations, and tested the peptides for their interaction with CaM by using a CaM electrophoresis mobility shift assay. Our results showed that both S343D or S344D peptides can bind to CaM at similar levels as that of the wild-type peptide (Fig. 1).
Figure 1.
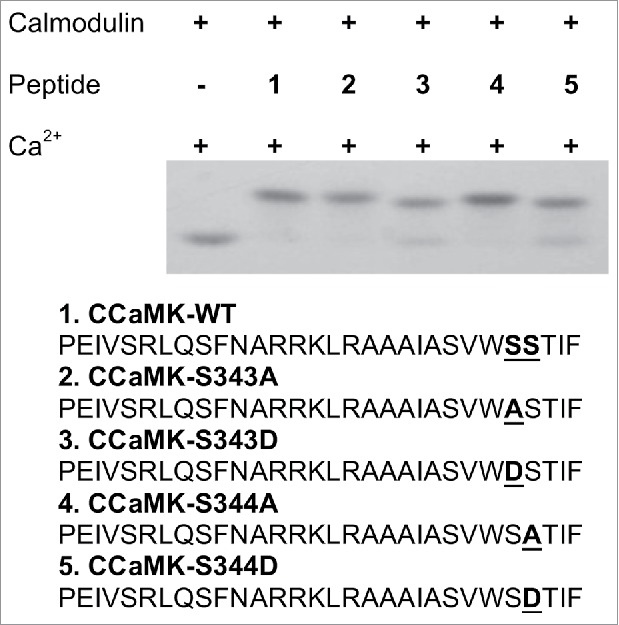
S343D and S344D mutations in the CaMBD of CCaMK did not alter the interactions between calmodulin (CaM) and the peptides. Mobility shift assay using oligo-peptides covering the CaMBD (30 amino acids in length): equal mole of peptide and CaM (1ug/per reaction) was incubated in reaction buffer (25mM Tris-HCl, 150mM NaCl and 1mM CaCl2, pH7.5) for 15 minutes at room temperature and run in non-denaturing polyacrylamide gel in the presence of 1mM CaCl2. The gel was then stained with Coomassie solution until bands were observed. Small peptides including a phosphomimic mutation at Ser-343 and Ser-344 interacted with CaM.
Although the mutant peptides bind to CaM when full-length protein undergoes phosphorylation of S343 and S344, the phosphorylated full-length protein cannot bind to CaM. This result indicates that there are intramolecular interactions involving these phosphorylation sites. This interaction may lead to structural changes that block the CaM-binding domain (CaMBD) from binding to CaM. To identify the site(s) of the intermolecular interaction with the CaMBD, we generated a series of progressive N-terminus deletions in the phosphomimic mutants, S343D and S344D, and tested the deletion mutants for their interactions with CaM. The CaM-binding assays showed that any deletion at or before amino acid 198 of CCaMK prevents it from binding to CaM. However, deletion mutant Δ250–523 can bind to CaM, indicating some residue(s) between the 198th to the 250th position is critical for blocking the interaction between CaM and the CCaMK mutant S343D or S344D (Fig. 2A).
Figure 2.
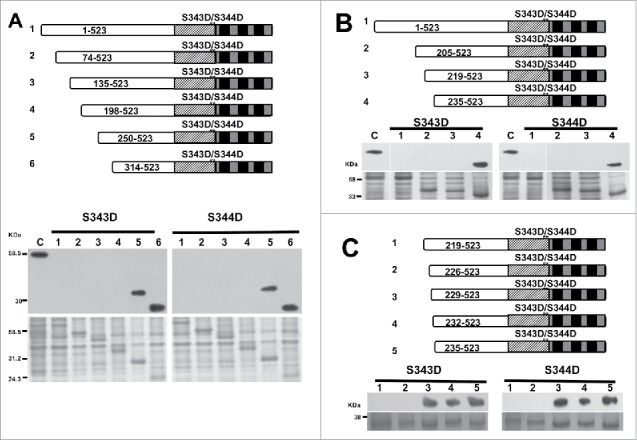
Calmodulin-binding assay of progressive deletion of S344D and S343D mutants. The CaM-binding capacity of full-length and deletion mutants of S343D and S344D was tested by transferring equal molar of target protein onto PVDF membrane, and incubated with horseradish peroxidase (HRP)-conjugated CaM in the presence of Ca2+ (1mM). The structures of full-length and deletion mutants of S343/S344D are shown in the diagram. The top panel shows the CaM-binding to 5 progressive deletions of each phosphomimic mutant (S344D and S343D). Bottom panel shows Coomassie staining (loading control). Full-length CCaMK was used as a positive control. (A) In the presence of Ca2+, the CaM-binding was recovered on S344D (250-523) and S343D (250-523). (B) Results showed that the CaM-binding was recovered in deletion mutant beginning with aa235. (C) Results showed that the CaM-binding was recovered in deletion mutants beginning with aa229.
Identification of the amino acid T227 as one of the residues interacting with phosphorylation sites S343 and S344
To further narrow down the residue(s) critical for the intramolecular interaction involving the S343D or S344D mutation, another set of progressive deletions were generated that covered the 52 amino acids between aa198 to aa250. A new series of deletions (Δ205-523, Δ219-523 and Δ235-523) were generated and then tested for CaM-binding. After position 235, the CaM-binding activity was recovered, suggesting that there is a 16-amino acid region (between position 219–235) that is critical for the intramolecular interaction in the S343D and S344D mutants (Fig. 2B). To further analyze the possible residues of this intramolecular interaction, a new series of deletions of residues were created and tested for their `CaM-binding capacity. The new constructs were deleted on the following positions: Δ226-523, Δ229-523 and Δ232-523 of the kinase domain. This experiment revealed that CaM interacted with the deletion mutant Δ229-523, reducing the number of possible interaction sites to 3 amino acids (Fig. 2C). To determine which one of the 3 amino acids is the interacting residue, a new series of single deletion residues among these 3 amino acids were generated. The CaM-binding assay clearly suggested that the amino acid Thr-227 is critical for the interaction because CaM was able to interact with the phosphomimic mutants after deleting this residue (Fig. 3A). Interestingly, our data showed that this amino acid is critical for the interaction for both phosphorylation sites, Ser-343 and Ser-344.
Figure 3.
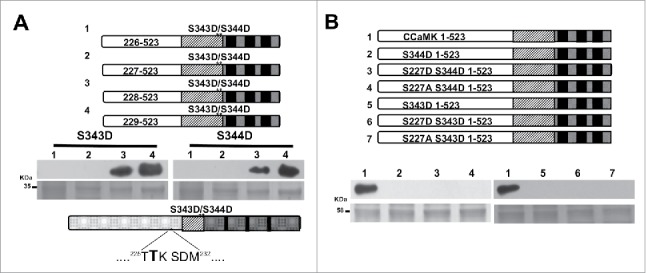
T227 is a critical residue for the intramolecular interaction which blocks the CaM-binding to S343D/S344D. (A) Single deletion amino acids between region 226 and 229 were analyzed for their CaM-binding capacity. Top gels show the recovery of CaM-binding capacity after the T227 was deleted in the phosphomimic mutants S344D and S343D. Lower gels show Coomassie staining as loading control. Amino acid sequence showing that position 227 is critical for interaction with S344D and S343D (highlighted). (B) Site directed mutants on position T227 (lanes 3, 4 and 6, 7) show that mutations T227D or T227A cannot restore the CaM-binding capacity in the background of S343D/S344D (Top gels). Lower gels show loading control of target proteins.
Since the Thr at 227 could be modified by phosphorylation, we hypothesized that any mutation at T227 could restore the CaM-binding of S343D or S344D mutants. We made T227D or T227A mutants in the CCaMK S343D or S344D mutant background and tested their CaM-binding capacity. Our results revealed that neither T227D nor T227A in S344D and S343D background could restore CaM binding. This result indicated that even though Thr-227 is a residue involved in this intramolecular interaction, there must be at least another amino acid that plays a critical role in blocking the CaM-binding of phosphorylated CCaMK at the S343 or S344 position (Fig. 3B). These results confirm the existence of an intramolecular interaction involving more than 3 residues within CCaMK.
Summary
Phosphorylation plays a critical role in inducing changes in the structural conformation of proteins.17,18,19 Protein phosphorylation can regulate metabolic pathways, symbiotic and other processes.20,21,22 In this addendum, we demonstrate that CCaMK structure can be modified through autophosphorylation. The autophosphorylation of residues S343 and S344 results in an intramolecular interaction that blocks the access of CCaMK's CaMBD to CaM. Through a study of serial CCaMK deletions, we identified a critical residue, Thr-227, which is involved in the intramolecular interaction discussed above. However, we were not able to rule out the effect of this interaction on the activity of CCaMK and further regulation of any symbiotic or other responses. Further studies to find the other critical residue(s) should provide a better understanding of the importance of this intramolecular interaction.
Funding
Research of the authors are supported by the US National Science Foundation grant (1021344), and the National Natural Science Foundation of China grant (U1130304). Support from the Washington State University Agricultural Research Center is appreciated. The authors also thank Lorie Mochel for her help in editing.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Watillon B, Kettmann R, Boxus P, Burny A. A calcium/calmodulin-binding serine/threonine protein kinase homologous to the mammalian type II calcium/calmodulin-dependent protein kinase is expressed in plant cells. Plant Physiol 1993; 101:1381-4; PMID:8310066; https://doi.org/ 10.1104/pp.101.4.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GE, Long SR. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development : Gene identification by transcript-based cloning. Proc Natl Acad U S A 2004; 101(13):4701-5; PMID:15070781; https://doi.org/ 10.1073/pnas.0400595101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Parniske M. Activation of calcium- and calmodulin-dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr Opin Plant Biol 2012; 15(4):444-53; PMID:22727503; https://doi.org/ 10.1016/j.pbi.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 4.Gobbato E. Recent developments in arbuscular mycorrhizal signaling. Curr Opin Plant Biol 2015; 26:1-7; PMID:26043435; https://doi.org/ 10.1016/j.pbi.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Patil S, Takezawa D, Poovaiah BW. Chimeric plant calcium/calmodulin-dependent protein kinase gene with a neural visinin-like calcium binding domain in plants. Proc Natl Acad Sci USA 1995; 92(5):4897-901; PMID:7761420; https://doi.org/ 10.1073/pnas.92.11.4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sathyanarayanan PV, Cremo CR, Poovaiah BW. Plant chimeric Ca2+/calmodulin-dependent protein kinase. Role of the neural visinin-like domain in regulating autophosphorylation and calmodulin affinity. J Biol Chem 2000; 275(39):30417-22. PMID:10840028; https://doi.org/ 10.1074/jbc.M000771200 [DOI] [PubMed] [Google Scholar]
- 7.Takezawa D, Ramachandiran S, Paranjape V, Poovaiah BW. Dual regulation of a chimeric plant serine/threonine kinase by calcium and calcium/calmodulin. J Biol Chem 1996; 271(14):8126-32; PMID:8626500; https://doi.org/ 10.1074/jbc.271.14.8126 [DOI] [PubMed] [Google Scholar]
- 8.Harmon AC. Calcium-regulated protein kinases of plants. Gravit Sp Biol Bull 2003; 16(6):83-90; PMID:12959135; http://gravitationalandspacebiology.org/index.php/journal/article/viewFile/298/297. [PubMed] [Google Scholar]
- 9.Miller JB, Pratap A, Miyahara A, Zhou L, Bornemann S, Morris RJ, Oldroyd GED. Calcium/Calmodulin-dependent protein kinase is negatively and positively regulated by calcium, providing a mechanism for decoding calcium responses during symbiosis signaling. Plant Cell 2013; 25(12):5053-66; PMID:24368786; https://doi.org/ 10.1105/tpc.113.116921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sathyanarayanan PV, Poovaiah BW. Autophosphorylation-dependent inactivation of plant chimeric calcium/calmodulin-dependent protein kinase. Eur J Biochem 2002; 269(10):2457-63; PMID:12027883; https://doi.org/ 10.1046/j.1432-1033.2002.02904.x [DOI] [PubMed] [Google Scholar]
- 11.Gleason C, Chaudhuri S, Yang T, Muñoz A, Poovaiah BW, Oldroyd GED. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 2006; 441(7097):1149-52; PMID:16810256; https://doi.org/ 10.1038/nature04812 [DOI] [PubMed] [Google Scholar]
- 12.Routray P, Miller JB, Du L, Oldroyd G, Poovaiah BW. Phosphorylation of S344 in the calmodulin-binding domain negatively affects CCaMK function during bacterial and fungal symbioses. Plant J 2013; 76(2):287-96; PMID:23869591; https://doi.org/ 10.1111/tpj.12288 [DOI] [PubMed] [Google Scholar]
- 13.Liao J, Singh S, Hossain MS, Undersen ST, Ross L, Bonetta D, Zhou Y, Sato S, Tabata S, Stougaard J, et al.. Negative regulation of CCaMK is essential for symbiotic infection. Plant J 2012; 72(4):572-84; PMID:22775286; https://doi.org/ 10.1111/j.1365-313X.2012.05098.x [DOI] [PubMed] [Google Scholar]
- 14.Limpens E, Bisseling T. CYCLOPS: A new vision on rhizobium-induced nodule organogenesis. Cell Host Microbe 2014; 15(2):127-9; PMID:24528858; https://doi.org/ 10.1016/j.chom.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 15.Downie JA. Legume nodulation. Curr Biol 2014; 24(5):184-90; PMID:24602880; https://doi.org/ 10.1016/j.cub.2014.01.028 [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Naismith JH. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol 2008; 8(1):91; PMID:19055817; https://doi.org/ 10.1186/1472-6750-8-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colbrant R, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of Calcium/calmodulin-dependent protein kinase II. J Biol Chem 1990; 265(7):11213-9; PMID:2162839 [PubMed] [Google Scholar]
- 18.Teichmann SA, Murzin AG, Chothia C. Determination of protein function, evolution and interactions by structural genomics. Curr Opin Struct Biol 2001; 11(3):354-63; PMID:11406387; https://doi.org/ 10.1016/S0959-440X(00)00215-3 [DOI] [PubMed] [Google Scholar]
- 19.Loog M, Toomik R, Sak K, Muszynska G, Järv J, Ek P. Peptide phosphorylation by calcium-dependent protein kinase from maize seedlings Eur J Biochem. 2000; 267:337-343; PMID:10632703; https://doi.org/ 10.1046/j.1432-1327.2000.01002.x [DOI] [PubMed] [Google Scholar]
- 20.Johnson LN. The effect of phosphorylation on the structure and function of proteins. Annu Rev Biophys Biolmol Struct 1993; 22:199-232; PMID:8347989; https://doi.org/ 10.1146/annurev.bb.22.060193.001215 [DOI] [PubMed] [Google Scholar]
- 21.Griffith LC. Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J Neurosci 2004; 24(39):8394-8; PMID:15456810; https://doi.org/ 10.1523/JNEUROSCI.3604-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pottiez G, Ciborowski P. Elucidating protein inter- and intramolecular interacting domains using chemical cross-linking and matrix-assisted laser desorption ionization-time of flight/time of flight mass spectrometry. Anal Biochem 2012; 421(2):712-8; PMID:22226790; https://doi.org/ 10.1016/j.ab.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]


