Abstract
Background
Elderly women are at high risk of coronary heart disease (CHD) and heart failure. High‐sensitivity assays allow detection of cardiac troponin I (hsTnI) well below diagnostic cutoffs for acute coronary syndrome. We investigated the association between these levels with future cardiac events in community‐based ambulant white women aged over 70 years initially recruited for a 5‐year randomized, controlled trial of calcium supplements.
Methods and Results
This was a prospective study of 1081 elderly women without clinical CHD at baseline (1998) or hsTnI above the diagnostic cutoffs for acute coronary syndrome with 14.5‐year follow‐up hospitalization and mortality (events). Two hundred forty‐three (22%) women had CHD events, 163 (15%) myocardial infarction or CHD death (hard CHD), and 109 (10%) heart failure. In 99.6% of available serum samples, hsTnI was above the level of detection (median, 4.5 ng/L; interquartile range, 3.6–5.8). After adjusting for Framingham risk factors, each SD natural log‐transformed hsTnI increase was associated with an increased hazard for CHD (hazard ratio, 1.34; 95% CI, 1.18–1.53; P<0.001) hard CHD (hazard ratio, 1.51; 95% CI, 1.29–1.76; P<0.001), and heart failure (hazard ratio, 1.65; 95% CI, 1.36–1.99; P<0.001). Step‐wise increases in relative hazards were observed with increasing quartiles of hsTnI (P for trend, <0.001), whereas the addition of hsTnI to conventional risk factors modestly improved discrimination indices: Harrell's c‐statistic, net reclassification, and integrated discrimination (P<0.05).
Conclusions
Cardiac troponin I is independently associated with future cardiac events in elderly women without apparent clinical manifestations. The addition of cardiac troponin I to conventional risk factors may modestly improve risk prediction in this setting.
Keywords: elderly, heart disease, heart failure, troponin, women
Subject Categories: Women, Risk Factors, Primary Prevention, Epidemiology, Aging
Clinical Perspective
What Is New?
In a cohort of elderly women, increasing levels of circulating cardiac troponin I well below the diagnostic level for acute coronary syndrome were strongly associated with 14.5‐year coronary heart disease and heart failure hospitalizations and deaths before and after adjustment for established cardiovascular risk factors.
These findings extend to an older age group the growing body of evidence that circulating cardiac troponin I levels below the diagnostic level for acute coronary syndrome are associated with the risk of future cardiac events.
What Are the Clinical Implications?
Elderly women in the highest quartile of circulating cardiac troponin I (5.8–15.5 ng/L) had 2.4‐ to 3.7‐fold higher relative hazards of cardiac events than those in the lowest quartile (<3.6 ng/L) independent of established cardiovascular risk factors.
Incorporating cardiac troponin I into risk prediction models in this often overlooked high‐risk population may modestly improve cardiac event risk prediction.
Introduction
Coronary heart disease (CHD) is the leading cause of cardiovascular deaths in the United States.1 The prediction of cardiovascular disease risk in asymptomatic individuals remains a major challenge to clinicians. The commonly used risk calculators are derived from conventional cardiovascular disease risk factors, such as age, sex, serum lipids, or body mass index (BMI), blood pressure, diabetes mellitus, and the use antihypertensive medications,2, 3 but either do not include, or do not perform well in, the elderly.4 Early identification and treatment of at risk elderly individuals may potentially prevent or delay the development of future clinical adverse cardiovascular disease events, and as such the study of novel biomarkers is an active area of clinical research.
Atherosclerosis begins with increased permeability of the endothelium due to biochemical or inflammatory stimuli that leads to atheromatous plaques on the endothelial surface.5, 6 As a result of reduced blood flow causing partial or complete vascular occlusion, ischemic damage to the myocardium occurs with transient release of cardiac troponins from myocytes into the circulation. Cardiac troponin measurement is an integral part of the modern diagnostic and prognostic workup for patients suspected of coronary artery disease and acute coronary syndrome.7
Over recent years, highly sensitive assays for cardiac troponins have revealed that the majority of older individuals have detectable levels of circulating cardiac troponin I and T at levels below those used as the clinical threshold for the diagnosis of myocardial ischemia.8, 9, 10, 11, 12 Furthermore, in apparently healthy older individuals, there is evidence of increased risk of vascular events even in those with higher circulating cardiac troponin concentrations that are still well below the diagnostic level for acute coronary syndrome.13
In general, men develop cardiovascular disease earlier than women, leading to the misconception that women are at a lower risk of cardiovascular disease. Over the past decade, there has been a focus by clinicians, professional bodies, policy makers, and insurers to raise awareness and recognize the importance of heart disease in women as well as improving risk prediction and prevention in women. Despite elderly women being at high risk of cardiac events, there remains a considerable lack of awareness of the risk, leading to undertesting and treatment as well as poorer long‐term prognosis in this group.14
In middle‐aged women, high‐sensitivity cardiac troponin I (hsTnI) has been shown to be more strongly associated with myocardial infarction and heart failure (HF) outcomes than in men.15 To expand understanding of the clinical utility of this marker to an older female population at higher risk of CHD, we examined the association between baseline circulating cardiac hsTnI and the risk of long‐term cardiac hospitalizations and deaths. Furthermore, we assessed the clinical utility of measuring hsTnI in addition to the currently accepted Framingham risk factors.
Methods
Ethics Statement
The Human Ethics Committee of the University of Western Australia approved the study, and written informed consents were obtained from all participants. Human ethics approval for the use of linked data for the project was provided by the Human Research Ethics Committee of the Western Australian Department of Health, project number #2009/24. Because the high‐sensitivity troponin assay was undertaken 16 years after the samples were collected, participants were not informed whether high levels were detected.
Study Population
The initial study participants were recruited in 1998 to a 5‐year prospective, randomized, controlled trial of oral calcium supplements to prevent osteoporotic fractures, the CAIFOS (Calcium Intake Fracture Outcome study), as described previously.16 Because this trial commenced and completed before the advent of the clinical trials registry, the trial was retrospectively registered in the Australian New Zealand Clinical Trials Registry ACTRN12615000750583 (URL https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368778&isReview=true). At the conclusion of the CAIFOS randomized, controlled trial, women were subsequently invited to participate in 2 further 5‐year follow‐up studies to identify determinants of healthy aging (longitudinal study of aging women). All participants were ambulant with an expected survival beyond 5 years and were not receiving any medication (including hormone replacement therapy) known to affect bone metabolism. Baseline disease burden and medications were comparable between these participants and the general population of similar age, although these women were more likely to be from higher socioeconomic groups.16 Of the 1500 women at baseline, 1235 had available samples for hsTnI measurement in 2013. The 265 women without samples available for assessment had similar baseline conventional cardiovascular risk factors (P>0.05) compared with those included in the study, but were, on average, 0.4 years older (P<0.05). Of those remaining, 112 individuals had a history of CHD hospitalizations from 1980 to 1998 and were excluded from further analyses. According to the manufacturer, the cutoff for the 99th percentile among healthy women for this hsTnI assay is 15.6 ng/L. There were a further 42 women whose hsTnI values were above this value (International Federation for Clinical Chemistry website: http://www.ifcc.org/media/276664/IFCC%20Troponin%20Tables%20ug_L_DRAFT%20Update%20NOVEMBER%202014.pdf). After excluding women with a history of CHD and those above the 99th percentile there were 1081 women available for this study. Of these 1042 of 1081 (96%) had complete data for the multivariable‐adjusted analyses.
Baseline Cardiovascular Risk Assessment
Baseline medical history, including the presence of diabetes mellitus, hypertension, and medications, including antihypertensive medications, was obtained from all participants. Participants' medical histories and medications were verified by their general practitioners, where possible. Smoking status was coded as nonsmoker or ex‐smoker/current smoker if they had consumed more than 1 cigarette per day for more than 3 months at any time in their life. Weight was obtained using digital scales with participants wearing light clothes and no shoes, height was measured using a stadiometer, and body mass index (BMI) was calculated in kg/m2. Blood pressure was measured in 1044 of 1081 (97%) of participants on the right arm with a mercury column manometer using an adult cuff after the participants had been seated in an upright position and had rested for 5 minutes.
Framingham Risk Score Scores
Framingham risk scores using BMI were calculated based on the publication by D'Agostino et al17 and were then confirmed using the online calculator prepared by R.B. D'Agostino and M.J. Pencina (https://www.framinghamheartstudy.org/riskfunctions/cardiovascular-disease/10-year-risk.php#;).
Biochemistry
hsTnI was measured in 2013 in baseline serum from 1235 samples stored at −80°C using the Abbott ARCHITECT i2000SR STAT hsTnI assay. The assay had a 10% coefficient of variation with a level of detection of 1.9 ng/L; however, observed values below this limit (n=5; range, 1.4–1.9 ng/L) were included in the results (assay range, 0–50 000 ng/L).
Cardiac Hospitalizations and Deaths (Events)
The first episode of cardiac hospitalizations or death was identified using the linked data provided by the Western Australian Data Linkage System. The Western Australian Data Linkage System combines 7 core databases, including inpatient hospital morbidity, inpatient, and records, and includes working links with special‐purpose health service and research databases dating back to as early as 1966.18 The quality of the Western Australian Data Linkage System linkage has been rigorously assessed by comparison with clerical investigation with estimates of invalid links (false positives) and missed links (false negatives) occurring in 0.11% of records.19 Data for all hospitalizations from baseline (1998) until 14.5 years (2013) after their baseline visit were provided by the Hospital Morbidity Data Collection whereas deaths were provided by the Mortality Register, both of which are regularly audited.19 Therefore, if the elderly women remained in Western Australia we had complete follow‐up for all hospitalizations and deaths over 14.5 years. Principal discharge diagnosis data from the Hospital Morbidity Data Collection were used to ascertain hospitalizations as the validity of these codes is higher than the additional discharge diagnoses fields20 whereas cause of death data were retrieved from the coded death certificate or Parts 1 and 2 of the death certificate where coded cause of death data were not yet available. The validity of the diagnoses of myocardial infarction from Hospital Morbidity Data Collection, CHD death from the death registry and heart failure from the Hospital Morbidity Data Collection have been previously tested.20, 21
Cardiac hospitalizations were defined using primary diagnosis codes from the International Classification of Diseases, Injuries and Causes of Death Clinical Modification22 and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification.23 These codes included: coronary heart disease (International Classification of Diseases, Injuries and Causes of Death Clinical Modification codes 410–414 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification codes I20–I25), hard coronary heart disease (myocardial infarction [International Classification of Diseases, Injuries and Causes of Death Clinical Modification codes 410 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification codes I21] and/or CHD death [as above]) and heart failure (International Classification of Diseases, Injuries and Causes of Death Clinical Modification code 428 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification code I50).
Statistical Analysis
Baseline data are presented as either mean±SD, median, and interquartile range, or number and (%), where appropriate, with chi‐squared tests or ANOVA to test the difference between quartiles of hsTnI, where appropriate. Distributions of hsTnI values were skewed and, as such, were transformed using the natural logarithm. hsTnI was included in separate models in 2 ways: as a continuous variable on the natural log scale and as a categorical variable in quartiles. The primary outcome was a composite outcome including cardiac hospitalization or death up to ≤14.5 years. Unadjusted and all of the variables in the Framingham risk score–adjusted linear regression were undertaken to test the association of cardiovascular risk factors with hsTnI. For the primary Cox regression analyses, we treated noncardiac deaths as censored (nonevent). This approach means that the hazard ratios (HRs) can be interpreted as the risk of cardiac event for any time during follow‐up assuming that a woman stays alive for that long. Unadjusted and Framingham risk factor (age, BMI or total cholesterol and high‐density lipoprotein cholesterol, systolic blood pressure, prescription of antihypertensive medications, current smoking, and diabetes mellitus) and treatment‐adjusted (calcium or placebo) Cox regression analyses were undertaken to test the association of hsTnI with cardiac hospitalizations and deaths. To assess the influence of the duration of the follow‐up on estimated of the association between hsTnI and cardiac outcomes, we performed further analyses restricting the follow‐up to 10 years. No violations of the Cox proportional hazards assumptions were detected. The models discriminative performance were calculated using Harrell's c‐statistic,24 net reclassification, or integrated discrimination improvement25 with conventional (Framingham) risk factors with treatment code (calcium or placebo) and with conventional risk factors with treatment code (calcium or placebo) plus hsTnI. The integrated discrimination improvement and net reclassification were calculated for logistic regression models and, as such, censored observations (noncardiac deaths) would be considered as nonevents. All continuous variables were naturally logarithmically transformed before they were entered into the models to improve discrimination and to minimize the influence of extreme observations. For net reclassification participants over the 14.5 years of follow‐up, the net reclassification categories were (<15%, 15–19%, and ≥20%) for CHD events (<10%, 10–14%, and ≥15%), hard CHD events, and (<5%, 5–9%, and ≥10%) for HF events. All analyses were undertaken using IBM SPSS Statistics (Version 22; 2012; IBM Corp, Armonk, NY), Stata (version 13; StataCorp LP, College Station, TX), or SAS software (Version 9.4; SAS Institute Inc, Chicago, IL). P values of less than 0.05 in 2‐tailed testing were considered statistically significant.
Results
Baseline Characteristics
An overview of the study is presented in Figure 1 and the distribution of the hsTnI results in Figure 2, whereas baseline characteristics of participants are presented in Table 1. The median cohort hsTnI value was 4.5 ng/L (range, 1.4–15.1).
Figure 1.
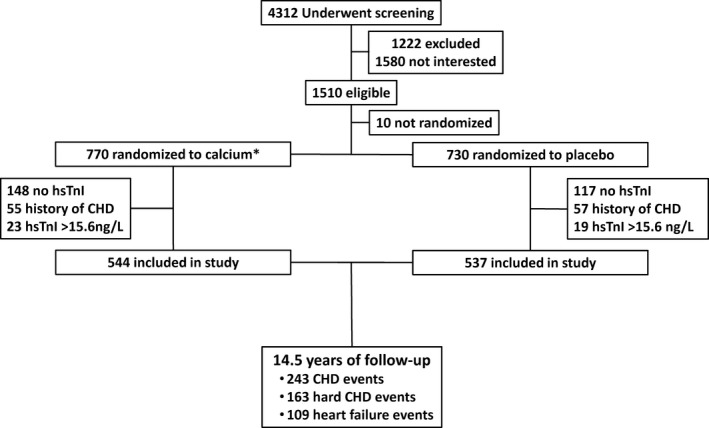
Flow chart of the study participants. CHD indicates coronary heart disease, hsTnI, high sensitivity cardiac troponin I. *39 participants received calcium supplements with 1000 IU vitamin D2.
Figure 2.
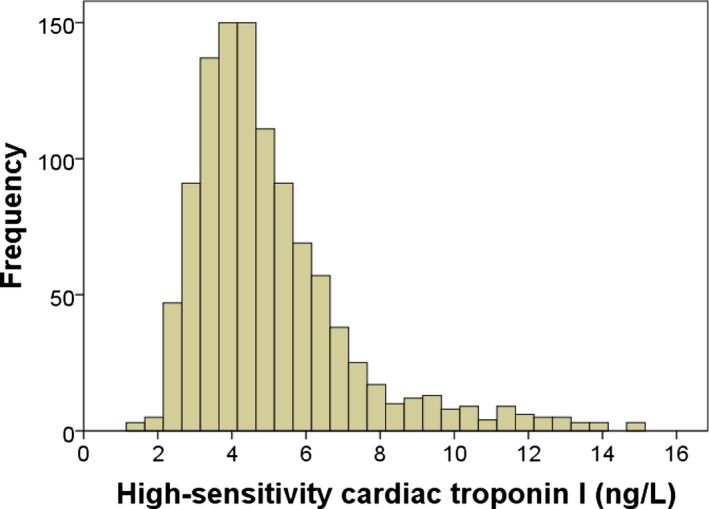
Distribution of high‐sensitivity cardiac troponin I in elderly women.
Table 1.
Cardiovascular Risk Factors Stratified by Quartiles of hsTnI
| Quartile 1 (<3.6 ng/L) | Quartile 2 (3.6–4.4 ng/L) | Quartile 3 (4.5–5.7 ng/L) | Quartile 4 (≥5.8 ng/L) | P Value | |
|---|---|---|---|---|---|
| Number | 249 | 273 | 278 | 281 | |
| Age, y | 74.5±2.5 | 74.9±2.7 | 75.5±2.8 | 75.8±2.8 | <0.001 |
| Body mass index, kg/m2 | 25.9±4.0 | 26.6±5.0 | 27.2±4.4 | 28.2±4.9 | <0.001 |
| Smoker, yes (%) | 4 (1.6) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0.020 |
| Ever smoked, yes (%) | 90 (36.3) | 100 (36.6) | 94 (33.8) | 113 (40.6) | 0.413 |
| Diabetes mellitus, yes (%) | 9 (3.6) | 14 (5.1) | 12 (4.3) | 24 (8.5) | 0.024 |
| Antihypertensive, yes (%) | 67 (26.9) | 97 (35.5) | 121 (43.5) | 164 (58.4) | <0.001 |
| Low‐dose aspirin, yes (%) | 27 (10.8) | 42 (15.4) | 46 (16.5) | 71 (25.3) | <0.001 |
| Statins, yes (%) | 43 (17.3) | 36 (13.2) | 51 (18.3) | 44 (15.7) | 0.385 |
| Systolic blood pressure, mm Hga | 133±15 | 135±18 | 140±19 | 142±20 | <0.001 |
| Diastolic blood pressure, mm Hga | 70±10 | 72±11 | 73±11 | 75±12 | <0.001 |
| Total cholesterol, mg/dLb | 222±39 | 228±38 | 231±46 | 229±47 | 0.220 |
| HDLC, mg/dLb | 60±14 | 57±15 | 55±14 | 54±14 | <0.001 |
Data expressed as mean±SD or number and (%). P value between groups by ANOVA or chi‐squared test, where appropriate. HDLC indicates high‐density lipoprotein cholesterol; hsTnI, high‐sensitivity cardiac troponin I; mm Hg, millimeters mercury.
Measured in 1044 women.
Measured in 774 women.
Relationship Between Current Cardiovascular Risk Factors and Circulating hsTnI
Using multivariable‐adjusted (all factors in the Framingham risk scores) linear regression analysis, all of the cardiovascular risk factors were significantly associated with baseline hsTnI (Table 2) except diabetes mellitus (P=0.064). Taken together, these factors explained ≈12% of the variation in hsTnI at baseline (r 2=0.12; P<0.001). Similar results were observed when replacing BMI with total cholesterol and high‐density lipoprotein cholesterol (n=774; r 2=0.13; P<0.001). A step‐wise increase in elevated cardiovascular risk as assessed by high 10‐year Framingham risk scores (≥20%) by quartiles of hsTnI was observed (Figure 3).
Table 2.
Clinical Predictors of hsTnI at Baseline
| Unstandardized β Coefficient (95% CI) | P Value | |
|---|---|---|
| Whole cohort (n=1081) | ||
| Age, per 10 y | 0.23 (0.15, 0.31) | <0.001 |
| Body mass index, per 10 kg/m2 | 0.10 (0.05, 0.15) | <0.001 |
| Systolic blood pressure, per 10 mm Hg | 0.02 (0.00, 0.03) | 0.020 |
| Antihypertensive medications, yes | 0.15 (0.19, 0.19) | <0.001 |
| Current smoker, yes | −0.41 (−0.72, −0.09) | 0.012 |
| Diabetes mellitus, yes | 0.09 (−0.01, 0.19) | 0.064 |
| Those with lipids assessed (n=774) | ||
| Age, per 10 y | 0.20 (0.10, 0.30) | <0.001 |
| Total cholesterol, per 10 mg/dL | 0.01 (0.00, 0.01) | 0.014 |
| High‐density lipoprotein cholesterol, per 10 mg/dL | −0.05 (−0.06, −0.03) | <0.001 |
| Systolic blood pressure, per 10 mm Hg | 0.01 (−0.01, 0.02) | 0.212 |
| Antihypertensive medications, yes | 0.153 (0.10, 0.21) | <0.001 |
| Current smoker, yes | −0.55 (−0.96, −0.14) | 0.009 |
| Diabetes mellitus, yes | 0.14 (0.01, 0.27) | 0.035 |
Adjusted r 2 in whole cohort=0.12, P<0.001 and subset with lipids r 2=0.13, P<0.001. Multivariable model adjusted for age, body mass index (or total cholesterol and high‐density lipoprotein cholesterol), current smoker, diabetes mellitus, systolic blood pressure, and prescription of antihypertensive medications and treatment code (calcium or placebo). hsTnI indicates high‐sensitivity cardiac troponin I.
Figure 3.
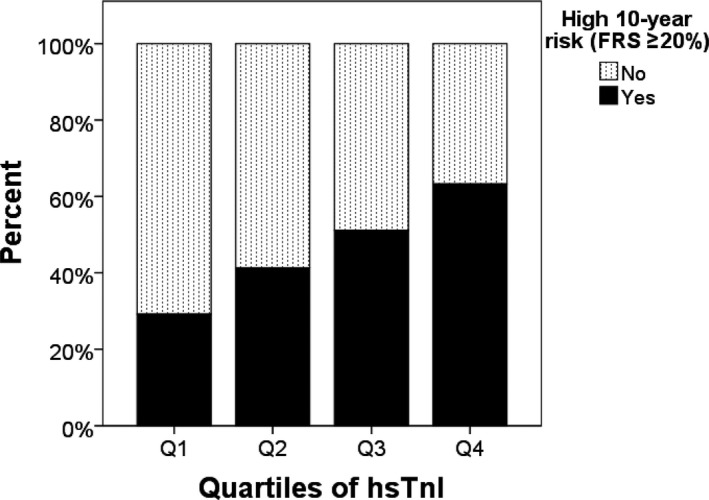
High Framingham risk score by quartiles of high‐sensitivity cardiac troponin I. FRS indicates Framingham risk score, hsTnI, high‐sensitivity cardiac troponin I.
hsTnI and Cardiac Events
Over 14.5 years, there were 243 of 1081 (22.5%) women with CHD hospitalizations or deaths, 163 of 1081 (15.1%) with hard CHD (myocardial infarction and/or CHD death), and 109 of 1081 (10.1%) with HF. Per SD increase in the natural log transformed circulating hsTnI (0.38), there was a 48% increase in the unadjusted hazard for CHD (HR, 1.48; 95% CI, 1.31–1.66; P<0.001), a 65% increase in the hazard for hard CHD (HR, 1.65; 95% CI, 1.43–1.90; P<0.001), and an 80% increase in the hazard for HF (HR, 1.80; 95% CI, 1.52–2.13; P<0.001) that remained significant after adjustment for Framingham risk with using BMI (Table 3). Similar findings were observed when adjusting for the Framingham risk factors with lipids (Table 4). When separating women by quartiles of hsTnI, step‐wise increases in the relative hazards of having cardiac events was observed (Figure 4). Compared with those in the lowest quartile of hsTnI, those in the second, third, and fourth quartile had an increased hazard for CHD in unadjusted analysis (Q2: HR, 1.85; 95% CI, 1.18–2.90; P=0.007; Q3: HR, 2.52; 95% CI, 1.63–3.88; P<0.001; Q4: HR, 3.36; 95% CI, 2.21–5.11; P<0.001) that remained similar after adjustment for Framingham risk factors (Table 3). When events were separated into hospitalizations and deaths individually, similar results were observed to events for hospitalizations whereas only those in the highest quartile had an increased hazard for CHD death (Tables 3 and 4). Similar results were observed in participants when replacing BMI with total cholesterol and high‐density lipoprotein cholesterol (n=774; Table 4).
Table 3.
Cardiac Events (14.5‐Year) by an Increase in an SD and Quartiles of hsTnI Adjusted for Framingham Risk Factors With BMI
| No. of Events (%) | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|
| Coronary heart disease events | |||
| Per SD of LN‐hsTnI | 243/1081 | 1.34 (1.18–1.53) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 29/249 (11.6) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 55/273 (20.1) | 1.55 (0.97–2.48) | 0.065 |
| Q3 hsTnI (4.5–5.7 ng/L) | 70/278 (25.2) | 2.07 (1.32–3.24) | 0.002 |
| Q4 hsTnI (≥5.8 ng/L) | 89/281 (31.7) | 2.45 (1.57–3.82) | <0.001 |
| Hard coronary heart disease eventsa | |||
| Per SD of LN‐hsTnI | 163/1081 | 1.51 (1.29–1.76) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 18/249 (7.2) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 37/273 (13.6) | 1.85 (1.04–3.32) | 0.038 |
| Q3 hsTnI (4.5–5.7 ng/L) | 39/278 (14.0) | 1.80 (1.00–3.23) | 0.048 |
| Q4 hsTnI (≥5.8 ng/L) | 69/281 (24.6) | 3.15 (1.81–5.49) | <0.001 |
| Heart failure events | |||
| Per SD of LN‐hsTnI | 109/1081 | 1.65 (1.36–1.99) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 15/249 (6.0) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 14/273 (5.1) | 0.85 (0.39–1.87) | 0.684 |
| Q3 hsTnI (4.5–5.7 ng/L) | 22/278 (7.9) | 1.35 (0.66–2.74) | 0.414 |
| Q4 hsTnI (≥5.8 ng/L) | 58/281 (20.6) | 3.74 (1.99–7.05) | <0.001 |
Cox regression models were adjusted for Framingham risk factors with BMI plus treatment code. BMI indicates body mass index; HR, hazard ratio; hsTnI, high‐sensitivity cardiac troponin I.
Hard coronary heart disease is defined as myocardial infarction hospitalization or coronary heart disease death.
Table 4.
Cardiac Events (14.5‐Year) by SD and Quartiles of hsTnI Adjusted for Framingham Risk Factors With Lipids
| No. of Events (%) | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|
| Coronary heart disease events | |||
| Per SD of LN‐hsTnI | 164/774 | 1.34 (1.14–1.56) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 18/180 (10.0) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 34/199 (17.1) | 1.50 (0.82–2.72) | 0.186 |
| Q3 hsTnI (4.5–5.7 ng/L) | 48/193 (24.9) | 2.33 (1.32–4.13) | 0.004 |
| Q4 hsTnI (≥5.8 ng/L) | 64/202 (31.7) | 2.84 (1.62–4.99) | <0.001 |
| Hard CHD eventsa | |||
| Per SD of LN‐hsTnI | 108/774 | 1.48 (1.23–1.80) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 10/180 (5.6) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 24/199 (12.1) | 2.37 (1.10–5.13) | 0.028 |
| Q3 hsTnI (4.5–5.7 ng/L) | 27/193 (14.0) | 2.47 (1.14–5.35) | 0.022 |
| Q4 hsTnI (≥5.8 ng/L) | 47/202 (23.3) | 4.10 (1.95–8.62) | <0.001 |
| Heart failure events | |||
| Per SD of LN‐hsTnI | 78/774 | 1.55 (1.24–1.93) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 12/180 (6.7) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 11/199 (5.5) | 0.78 (0.32–1.88) | 0.577 |
| Q3 hsTnI (4.5–5.7 ng/L) | 14/193 (7.3) | 1.20 (0.52–2.74) | 0.667 |
| Q4 hsTnI (≥5.8 ng/L) | 41/202 (20.3) | 3.39 (1.66–6.91) | 0.001 |
Cox regression models were adjusted for Framingham risk factors with lipids plus treatment code. CHD indicates coronary heart disease; HR, hazard ratio; hsTnI, high‐sensitivity cardiac troponin I.
Hard CHD is defined as myocardial infarction hospitalization or coronary heart disease death.
Figure 4.
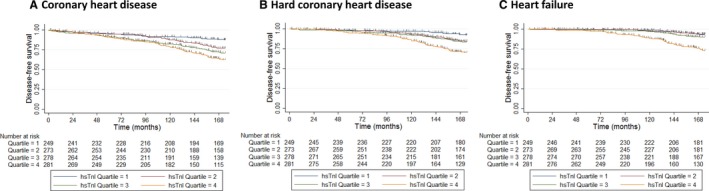
Kaplan–Meier disease‐free survival estimates for coronary heart disease (A), hard coronary heart disease (myocardial infarction or coronary heart disease death—B) and heart failure (C). Vertical gray lines indicate censoring for noncardiac death. All P<0.001 by log‐rank test. hsTnI indicates high‐sensitivity cardiac troponin I.
Improvement to Risk Prediction Over 14.5 Years of Follow‐up
In use of conventional risk factors, all variables used in the Framingham risk equation plus treatment code had moderate discrimination quantified by Harrell's c‐statistic for CHD (0.622), hard CHD (0.640), and heart failure (0.637) whereas the natural logarithm of hsTnI (LN‐hsTnI) alone had similar moderate discrimination quantified by Harrell's c‐statistic for CHD (0.616), hard CHD (0.647), and heart failure (0.683). The addition of LN‐hsTnI improved the measures of discrimination, including Harrell's c‐statistic (CHD, +0.023; hard CHD, +0.041; heart failure, +0.062; all P<0.05), net reclassification (Table 5; CHD, +0.108; hard CHD, +0.112; HF, +0.206; all P<0.05), and integrated discrimination (CHD, +0.013; hard CHD, +0.018; HF, +0.022; all P<0.05). Similar improvements to Harrell's c‐statistic were observed when adding hsTnI to conventional risk factors with lipids rather than BMI (data not shown).
Table 5.
Reclassification of Individual's Risk of 14.5 Year (A) CHD, (B) hard CHD, and (C) HF
| (A) CHD | Framingham Risk Factorsa+LN‐hsTnI | |||||
|---|---|---|---|---|---|---|
| Framinghama Risk Factors | <15% | 15% to 19% | ≥20% | Reclassified Higher Risk | Reclassified Lower Risk | Reclassification Improvement |
| Participants with CHD | ||||||
| <15% | 19 | 10 | 2 | 30 (13.0%) | 20 (8.7%) | 10 (4.3%) |
| 15% to 19% | 14 | 16 | 18 | |||
| ≥20% | 0 | 6 | 145 | |||
| Participants without CHD | ||||||
| <15% | 177 | 24 | 12 | 77 (9.5%) | 129 (16.0%) | 52 (6.4%) |
| 15% to 19% | 70 | 97 | 41 | |||
| ≥20% | 12 | 47 | 327 | |||
| (B) Hard CHD | Framingham Risk Factorsa+LN‐hsTnI | |||||
|---|---|---|---|---|---|---|
| Framingham Risk Factorsa | <10% | 10% to 14% | ≥15% | Reclassified Higher Risk | Reclassified Lower Risk | Reclassification Improvement |
| Participants with hard CHD | ||||||
| <10% | 18 | 8 | 4 | |||
| 10% to 14% | 9 | 15 | 10 | 22 (14.1%) | 19 (12.2%) | 3 (1.9%) |
| ≥15% | 1 | 9 | 82 | |||
| Participants without hard CHD | ||||||
| <10% | 205 | 33 | 12 | |||
| 10% to 14% | 109 | 125 | 63 | 108 (12.3%) | 190 (21.6%) | 82 (9.3%) |
| ≥15% | 12 | 69 | 253 | |||
| (C) HF | Framingham Risk Factorsa+LN‐hsTnI | |||||
|---|---|---|---|---|---|---|
| Framingham Risk Factorsa | <5% | 5% to 9% | ≥10% | Reclassified Higher Risk | Reclassified Lower Risk | Reclassification Improvement |
| Participants with HF | ||||||
| <5% | 2 | 2 | 1 | |||
| 5% to 9% | 8 | 17 | 16 | 19 (19.0%) | 18 (18.0%) | 1 (1.0%) |
| ≥10% | 1 | 9 | 44 | |||
| Participants without HF | ||||||
| <5% | 65 | 13 | 3 | |||
| 5% to 9% | 160 | 285 | 68 | 84 (9.0%) | 268 (28.6%) | 184 (19.6%) |
| ≥10% | 4 | 104 | 235 | |||
(A) Coronary heart disease (CHD): Overall net reclassification improvement (NRI)=0.108, P=0.002. Those with CHD 4.3%, P=0.157 and those without CHD 6.4%, P<0.001. (B) Hard CHD: Overall net reclassification improvement (NRI)=0.112, P=0.014. Those with hard CHD 1.9%, P=0.639 and those without hard CHD 9.3%, P<0.001. (C) Heart failure (HF): Overall net reclassification improvement (NRI)=0.206, P=0.001. Those with HF 1.0%, P=0.869 and those without HF 19.6%, P<0.001.
Includes Framingham risk factors and treatment code.
Further Analyses
Models were then retested with 10 years of follow‐up, only with similar associations observed between hsTnI for CHD and hard CHD and a stronger association with HF (Table 6). To explore the extent of reverse causality bias, we excluded cardiac events that occurred within the first 2 years, which did not change the overall results (data not shown).
Table 6.
Ten‐Year Cardiac Events by SD and Quartiles of hsTnI
| No. of Events (%) | 10‐Years Follow‐up | ||
|---|---|---|---|
| Adjusted HR (95% CI) | P Value | ||
| Coronary heart disease events | |||
| Per SD of LN‐hsTnI | 153/1081 | 1.31 (1.11–1.54) | 0.001 |
| Q1 hsTnI (<3.6 ng/L) | 20/229 (8.0) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 32/273 (11.7) | 1.26 (0.70–2.27) | 0.438 |
| Q3 hsTnI (4.5–5.7 ng/L) | 46/278 (16.5) | 1.86 (1.07–3.22) | 0.027 |
| Q4 hsTnI (≥5.8 ng/L) | 55/281 (19.6) | 2.03 (1.17–3.51) | 0.012 |
| Hard CHD eventsa | |||
| Per SD of LN‐hsTnI | 81/1081 | 1.64 (1.32–2.04) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 8/249 (3.2) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 17/273 (6.2) | 2.00 (0.82–4.88) | 0.128 |
| Q3 hsTnI (4.5–5.7 ng/L) | 19/278 (6.8) | 2.13 (0.89–5.13) | 0.091 |
| Q4 hsTnI (≥5.8 ng/L) | 37/281 (13.2) | 3.68 (1.59–8.55) | 0.002 |
| Heart failure events | |||
| Per SD of LN‐hsTnI | 44/1081 | 1.81 (1.36–2.40) | <0.001 |
| Q1 hsTnI (<3.6 ng/L) | 3/249 (1.2) | 1 (reference) | |
| Q2 hsTnI (3.6–4.4 ng/L) | 5/273 (1.8) | 1.46 (0.35–6.13) | 0.606 |
| Q3 hsTnI (4.5–5.7 ng/L) | 8/278 (2.9) | 2.26 (0.59–8.65) | 0.234 |
| Q4 hsTnI (≥5.8 ng/L) | 28/281 (10.0) | 6.93 (2.02–23.76) | 0.002 |
Cox regression models were adjusted for Framingham risk factors with body mass index and treatment code. CHD indicates coronary heart disease; HR, hazard ratio; hsTnI, high‐sensitivity cardiac troponin I.
Hard CHD is defined as myocardial infarction hospitalization or coronary heart disease death.
Discussion
We identified robust relationships between circulating cardiac troponin I measured by a high‐sensitivity assay, cardiovascular risk factors, and 14.5‐year cardiac hospitalizations and deaths in elderly women. This graded relationship was observed with hsTnI levels well below the 99th percentile observed in acute coronary syndromes and persisted after adjustment for Framingham risk factors and modestly improved measures of risk prediction, such as Harrell's c‐statistic, as well as newer measures of discrimination, such as the net reclassification and integrated discrimination improvements. These findings add further support to the growing body of evidence in younger individuals that cardiac troponin I are specific markers of myocyte necrosis and are related to the risk of future cardiovascular events.10, 11, 12, 26, 27, 28
In this cohort of elderly women with a mean age of 75 years, 99.6% of women had levels of cardiac troponin I above the level of detection. Although this is a higher proportion of individuals with detectable hsTnI than 2 previous cohorts studies with younger mean cohort ages (50 and 48 years, respectively),11, 12 it is very similar to a previous report in elderly men and women from Uppsala, Sweden, aged 70 or over where 96.4% had detectable hsTnI levels and the median levels increased by 45% over 5 years.10 Indeed, Neumann et al12 found that using a super sensitive assay identified a further 12% of individuals with evidence of myocardial necrosis below the threshold of the high‐sensitivity assay. Taken together, studies suggest that, with increasing age, there are progressive pathological ischemic insults to the myocardium leading to increased proportions of individuals with detectable levels of cardiac troponins.
There have been a number of studies that have found that despite men having higher hsTnI than women, hsTnI is more strongly related to cardiovascular outcomes in women.11, 15, 28 In this study, we found women's baseline global cardiovascular risk profiles were strongly related to circulating hsTnI, and per SD increase in natural log transformed hsTnI, there was a 34% to 64% increase in the hazard for cardiac events after adjustment for Framingham risk factors. These findings are very similar to the HUNT (Nord‐Trøndelag Health Study) study of middle‐aged women where hsTnI was more strongly associated with myocardial infarction and HF in women than men with an adjusted HR of 1.50 for myocardial infarction and an HR of 1.69 for HF.15 Similarly, in the study by Zeller et al,11 in middle‐aged men and women per SD increase in hsTnI were associated with an adjusted HR for coronary death of 1.51 in women and 1.35 in men. Taken together, these findings suggest that hsTnI may be a better biomarker of cardiovascular risk in women than in men.
In this cohort, we observed a positive relationship between hsTnI and long‐term cardiac events. When stratified into quartiles, there was a graded association with step‐wise increases in the hazard with increasing hsTnI with individuals in the highest quartile having HRs of between 2.4‐ and 3.7‐fold higher than those in the lowest quartile independent of Framingham risk factors. Interestingly, we observed a stronger associations between hsTnI and HF than CHD outcomes, which is consistent with the published literature in middle‐aged men and women15 and studies of high sensitivity cardiac troponin T (hsTnT).29 Elevated levels of TnI or TnT are commonly observed in patients with both acute and chronic HF,30 whereas a meta‐analysis found levels of both cardiac troponins predict prognosis in patients with chronic stable HF.31 A recent study found hsTnI predicted HF events independent of cardiac structural abnormalities assessed by echocardiography,32 suggesting that cardiac troponins capture additional prognostic information for HF risk by an as yet unidentified mechanism.
Model performance using conventional risk factors alone was moderate (0.6–0.8), and the addition of hsTnI to conventional cardiovascular risk models only led to modest improvements to discrimination, presumably attributed to the strong relationship between the current risk factors with circulating hsTnI levels. These improvements observed in elderly women to Harrell's c‐statistic for CHD and HF were greater than the significant improvement observed for measures of discrimination in younger individuals with the addition of hsTnT for coronary events33 and hsTnI in younger women for cardiovascular events.11 In addition, we observed significant improvements to newer measures of discrimination, such as the net reclassification and integrated discrimination improvements. The addition of hsTnI moved women with events up in risk correctly and also moved women who did not have events down correctly. For HF and hard CHD, the greatest net reclassification improvements were moving women who did not suffer an event correctly down in risk, suggesting that measuring hsTnI may be useful for screening out individuals with low risk of these events. However, it should be noted that their use for risk prediction remains controversial.34 However, given that all these methods were significantly improved with the addition of hsTnI, this study adds to the growing body of evidence that low levels of cardiac troponin are likely to be associated with poorer long‐term outcomes. This suggests that testing cardiac troponins, even at low levels, may be considered a promising candidate for improving cardiac disease risk prediction in elderly women.
Although our findings are consistent with previous studies, the reported improvements to Harrell's c‐statistic for both CHD (+0.023–0.041) and HF (+0.06) were larger than those observed in middle‐aged cohorts11 or for hsTnT and HF.29 There are a number of potential explanations for these findings; first, we focused on cardiac events as the putative pathological mechanism and excluded women with a history of hospitalizations for these events over a long look‐back period of 18 years. Second, most women were older than 74 years, which is the upper limit of the Framingham risk scores and these risk scores do not discriminate cardiovascular risk well in this population. Third, it is likely that a large proportion of these women had asymptomatic advanced coronary artery disease at baseline with events captured over a very long follow‐up, potentially leading to differences attributed to spectrum bias or “spectrum effect.”35 Additionally, we excluding women above the 99th percentile who may have had elevated cardiac troponin I attributed to causes not related to coronary artery disease, such as chronic kidney disease, that are often observed in the elderly.13 As such, these findings may not be generalizable to men, younger women, and people of other ethnicities.
There are, however, a number of important limitations of this study that must be considered; these include the observational nature of the study, meaning that causality cannot be established; the lack of repeated measures of hsTnI and missing assessment of hsTnI in 18% of women in the original cohort of 1500 and lipids in 28% of the elderly women with hsTnI, meaning that the possibility of introducing bias cannot be excluded. Furthermore, because we only measured hsTnI and not hsTnT, we cannot compare findings reported in this study to studies measuring hsTnT. Finally, because the data linkage only records hospitalizations and deaths that occur within Western Australia, a small proportion of women may have migrated out of the state; however, this is unlikely to have substantially affected the findings given the age of the women. The strengths of this study include the assessment of a high‐sensitivity assay to measure circulating cardiac troponin I in a large cohort of elderly women. Additionally, the complete and accurate data collection on long‐term clinical outcomes over a 14.5‐year period independent of self‐report, removing the possibility of recall or retention bias, is a strength of this study. Finally, the cohort collected extensive and accurate information regarding medication history and known cardiovascular risk factors.
In conclusion, our findings suggest that cardiac troponin I is associated with conventional risk factors in elderly women, with the majority of these women having detectable levels using this high‐sensitivity assay. These levels predicted future CHD and HF hospitalizations and deaths. The incorporation of cardiac troponin I into current risk prediction models may modestly improve risk prediction in elderly women.
Author Contributions
Dr Lewis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Lewis, Lim W.H., Wong, and Prince. Acquisition of data: Lewis, Lim W.H., Lim E.M., Abbs, Thompson, Wong, Zhu, and Prince. Analysis and interpretation of data: Lewis, Lim, Wong, and Prince. Drafting of the manuscript: Lewis, Lim W.H., Wong, and Prince. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Lewis, Lim, Wong, and Prince. Obtained funding: Lim W.H., Lewis, Lim E.M., Zhu, and Prince. Administrative, technical, and material support: Lewis, Lim W.H. and Prince. Study supervision: Lewis, Lim W.H., and Prince.
Sources of Funding
The study was supported by Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant and by project grants 254627, 303169, and 572604 from the National Health and Medical Research Council of Australia. Dr Lewis's salary is supported by a National Health and Medical Research Council of Australia Career Development Fellowship (ID: 1107474). None of the funding agencies had any role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Disclosures
None.
Acknowledgments
The authors wish to thank the staff at the Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for their work on providing the data for this study.
(J Am Heart Assoc. 2017;6:e004174 DOI: 10.1161/JAHA.116.004174.)28757482
References
- 1. Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 2. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 3. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 4. Koller MT, Steyerberg EW, Wolbers M, Stijnen T, Bucher HC, Hunink MG, Witteman JC. Validity of the Framingham point scores in the elderly: results from the Rotterdam study. Am Heart J. 2007;154:87–93. [DOI] [PubMed] [Google Scholar]
- 5. Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. [DOI] [PubMed] [Google Scholar]
- 6. Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. [DOI] [PubMed] [Google Scholar]
- 7. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR; White HD and Writing Group on behalf of the Joint ESCAAHAWHFTFftUDoMI . Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. [DOI] [PubMed] [Google Scholar]
- 8. Apple FS, Simpson PA, Murakami MM. Defining the serum 99th percentile in a normal reference population measured by a high‐sensitivity cardiac troponin I assay. Clin Biochem. 2010;43:1034–1036. [DOI] [PubMed] [Google Scholar]
- 9. Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–1581. [DOI] [PubMed] [Google Scholar]
- 10. Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin I levels measured with a high‐sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Coll Cardiol. 2013;61:1906–1913. [DOI] [PubMed] [Google Scholar]
- 11. Zeller T, Tunstall‐Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, Woodward M, Struthers A, Hughes M, Kee F, Salomaa V, Kuulasmaa K, Blankenberg S; Investigators M . High population prevalence of cardiac troponin I measured by a high‐sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–281. [DOI] [PubMed] [Google Scholar]
- 12. Neumann JT, Havulinna AS, Zeller T, Appelbaum S, Kunnas T, Nikkari S, Jousilahti P, Blankenberg S, Sydow K, Salomaa V. Comparison of three troponins as predictors of future cardiovascular events—prospective results from the FINRISK and BiomaCaRE studies. PLoS One. 2014;9:e90063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannitsis E, Katus HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol. 2013;10:623–634. [DOI] [PubMed] [Google Scholar]
- 14. Mosca L, Barrett‐Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyngbakken MN, Rosjo H, Holmen OL, Nygard S, Dalen H, Hveem K, Omland T. Gender, high‐sensitivity troponin I, and the risk of cardiovascular events (from the Nord‐Trondelag Health Study). Am J Cardiol. 2016;118:816–821. [DOI] [PubMed] [Google Scholar]
- 16. Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5‐year, double‐blind, placebo‐controlled trial in elderly women. Arch Intern Med. 2006;166:869–875. [DOI] [PubMed] [Google Scholar]
- 17. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 18. Holman CD, Bass AJ, Rosman DL, Smith MB, Semmens JB, Glasson EJ, Brook EL, Trutwein B, Rouse IL, Watson CR, de Klerk NH, Stanley FJ. A decade of data linkage in Western Australia: strategic design, applications and benefits of the WA data linkage system. Aust Health Rev. 2008;32:766–777. [DOI] [PubMed] [Google Scholar]
- 19. Holman CD, Bass AJ, Rouse IL, Hobbs MS. Population‐based linkage of health records in Western Australia: development of a health services research linked database. Aust N Z J Public Health. 1999;23:453–459. [DOI] [PubMed] [Google Scholar]
- 20. Jamrozik K, Dobson A, Hobbs M, McElduff P, Ring I, D'Este K, Crome M. Monitoring the Incidence of Cardiovascular Disease in Australia. Canberra, ACT: AIHW;2001. CVD Series 17. [Google Scholar]
- 21. Teng TH, Finn J, Hung J, Geelhoed E, Hobbs M. A validation study: how effective is the Hospital Morbidity Data as a surveillance tool for heart failure in Western Australia? Aust N Z J Public Health. 2008;32:405–407. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death: Based on the Recommendations of the Ninth Revision Conference, 1975, and Adopted by the Twenty‐Ninth World Health Assembly. 1975 revision. ed. Geneva: World Health Organization; 1977. [Google Scholar]
- 23. World Health Organization . ICD‐10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. 2nd ed Geneva: World Health Organization; 2004. [Google Scholar]
- 24. Pencina MJ, D'Agostino RB Sr, Song L. Quantifying discrimination of Framingham risk functions with different survival C statistics. Stat Med. 2012;31:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 26. Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Everett BM, Zeller T, Glynn RJ, Ridker PM, Blankenberg S. High‐sensitivity cardiac troponin I and B‐type natriuretic peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation. 2015;131:1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Omland T, de Lemos JA, Holmen OL, Dalen H, Benth JS, Nygard S, Hveem K, Rosjo H. Impact of sex on the prognostic value of high‐sensitivity cardiac troponin I in the general population: the HUNT study. Clin Chem. 2015;61:646–656. [DOI] [PubMed] [Google Scholar]
- 29. deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Omland T, Rosjo H, Giannitsis E, Agewall S. Troponins in heart failure. Clin Chim Acta. 2015;443:78–84. [DOI] [PubMed] [Google Scholar]
- 31. Nagarajan V, Hernandez AV, Tang WH. Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart. 2012;98:1778–1786. [DOI] [PubMed] [Google Scholar]
- 32. McKie PM, AbouEzzeddine OF, Scott CG, Mehta R, Rodeheffer RJ, Redfield MM, Burnett JC Jr, Jaffe AS. High‐sensitivity troponin I and amino‐terminal pro–B‐type natriuretic peptide predict heart failure and mortality in the general population. Clin Chem. 2014;60:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pencina MJ, D'Agostino RB, Demler OV, Janssens AC, Greenland P. Pencina et al. respond to “The incremental value of new markers” and “Clinically relevant measures? A note of caution”. Am J Epidemiol. 2012;176:492–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299:926–930. [DOI] [PubMed] [Google Scholar]


