Abstract
Background
Atrial fibrillation (AF) and heart failure with reduced ejection fraction frequently coexist. The AATAC (Ablation versus Amiodarone for Treatment of persistent Atrial fibrillation in patients with Congestive heart failure and an implantable device) trial suggests that catheter ablation may benefit these patients. However, applicability to contemporary ambulatory cardiology practice is unknown.
Methods and Results
Using the outpatient National Cardiovascular Data Registry® Practice Innovation and Clinical Excellence Registry, we identified participants meeting AATAC enrollment criteria between 2013 and 2014. Treatment with medications and procedures was assessed at registry inclusion. From 164 166 patients with AF and heart failure, 8483 (7%) patients potentially met AATAC inclusion criteria. Eligible subjects, compared to AATAC trial participants, were older (mean age, 71.2±11.4 years) and had greater comorbidity (coronary artery disease 79.2%, hypertension 82.4%, and diabetes mellitus 31.8%). AF was predominantly paroxysmal (65.5%), rather than persistent/permanent (16.7%) or new onset (17.8%), whereas all patients in the AATAC trial had persistent AF. Commonly used atrioventricular‐nodal blocking agents were carvedilol (71.2%), digoxin (31.9%), and metoprolol (27.1%). Rhythm control with anti‐arrhythmic drugs was reported in 29.0% of AATAC eligible patients (predominantly amiodarone [24.6%]) and 9.3% had undergone catheter ablation. Patients who underwent ablation were more likely to be younger and have less comorbidities than those who did not.
Conclusions
Among the contemporary ambulatory AF/heart failure with reduced ejection fraction population, treatment is predominantly rate control with few catheter ablations. Application of AATAC findings has the potential to markedly increase the use of catheter ablation in this population, although significant differences in clinical profiles might influence ablation outcomes in practice.
Keywords: ablation, antiarrhythmic drug, atrial fibrillation heart failure
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Heart Failure, Arrhythmias
Clinical Perspective
What Is New?
The recent AATAC trial suggests a benefit for catheter ablation of atrial fibrillation in those with AF and heart failure (HF).
Using the ambulatory NCDR® PINNACLE registry, we identified 8483 patients with atrial fibrillation and HF meeting AATAC enrollment criteria and showed that rate control was the predominant approach whereas antiarrhythmic medications were used in 29% and catheter ablation was used in 9.3%.
What Are the Clinical Implications?
Applying the AATAC trial to practice could significantly increase the use of catheter ablation in this population, although differences in clinical profiles may limit the benefits in ablation outcomes observed in real‐world practice.
Introduction
Atrial fibrillation (AF) and heart failure (HF) are frequently coincident. Patients with both conditions have worse outcomes than patients with either alone.1, 2, 3 Rhythm control may be appealing for selected patients with AF and HF, reversing rapid and irregular heart rates and restoring atrial contraction may result in increased cardiac performance, which, in turn, could improve symptoms and quality of life. However, the side effects, toxicities, and incomplete effectiveness of antiarrhythmic drugs (AADs) may offset their potential benefits.4 Indeed, studies of AADs as a rhythm control strategy—namely amiodarone—have not identified benefits of maintaining sinus rhythm,5, 6 a finding that appears to extend to patients with HF irrespective of ejection fraction (EF).7, 8 Thus, alternative approaches to rhythm control are being explored.
Catheter ablation has emerged as a promising approach for rhythm control of AF patients with concomitant HF. Whereas this therapy has become a first‐line strategy for patients without HF,9 the relative effectiveness of catheter ablation as compared with AADs in patients with HF with reduced ejection fraction (HFrEF) has only recently begun to be studied. The AATAC (Ablation versus Amiodarone for Treatment of persistent Atrial fibrillation in patients with Congestive heart failure and an implanted device) trial compared catheter ablation to AADs in patients with persistent AF, a dual‐chamber implantable cardioverter‐defibrillator (ICD) or cardiac resynchronization therapy with defibrillator (CRT‐D), Class II or III HF, and an EF ≤40%. The trial demonstrated that catheter ablation was superior to amiodarone not only in freedom from AF at 2 years of follow‐up, but also in hospitalization and mortality.10
The relevance of AATAC to real‐world practice is unclear. It is unknown how closely the AATAC population resembles clinical practice and to what proportion rate and rhythm therapies consist of current treatment practice. Contemporary clinical registries can assist with the translation of this trial to current practice. To assist in this effort, the ACC has recently launched the R2P (Research to Practice) initiative (formerly titled Rapid Registry Response), which facilitates rapid analysis of registry data to understand how clinical trials, such as AATAC, inform clinical practice.11 To understand the impact of AATAC, we used data from the National Cardiovascular Data Registry (NCDR®) Practice Innovation and Clinical Excellence (PINNACLE) registry. We identified how many patients with a history of AF and HF in PINNACLE would have qualified for AATAC based upon the trial eligibility criteria, examined recent patterns of AADs and procedural use in this population, and determined the potential impact of the AATAC findings.
Methods
Cohort
The NCDR® PINNACLE Registry was launched in 2008 by the American College of Cardiology to improve the quality of ambulatory cardiovascular care in the United States.12 Both academic and private practices participate. Patient data, including symptoms, comorbidities, vital signs, and medications, are extracted from the electronic medical record. Given extraction of de‐identified data from an electronic medical record under a quality improvement model, approval from an institutional review board and informed consent were waived. Data quality assessments and analyses were performed at Saint Luke's Mid America Heart Institute (Kansas City, Missouri), an analytic center for the registry.
Cohort creation was guided by the AATAC trial inclusion and exclusion criteria. Between January 2013 and December 2014, we identified patients with an index encounter including both the diagnoses of AF and HF. The initial cohort of patients was then narrowed by excluding sites with >80% subjects missing any measure of left ventricular ejection fraction (LVEF). Among eligible sites, individual subjects were excluded if they had no measure of LVEF or no ICD with or without CRT‐D. Individuals with liver disease, New York Heart Association functional class IV, or a left ventricular assist device were also excluded. Additional AATAC criteria were applied excluding individuals with LVEF >40%, a reversible cause of AF, cardiac surgery (within 3 months of inclusion in registry), prohibitive social factors (≥15 drinks of alcohol per week), no ICD or CRT‐D, or who were not treated with both a beta‐blocker and angiotensin‐converting enzyme inhibitor in their medical regimen.
Rate Control
Individuals treated with a rate control strategy were identified as receiving a beta‐blocker (atenolol, propranolol, metoprolol, carvedilol, or bisoprolol), a nondihydropyridine calcium‐channel blocker (diltiazem or verapamil), or digoxin and the absence of an AAD, direct current cardioversion (DCCV), or AF catheter ablation at the study inclusion.
Rhythm Control
Individuals receiving rhythm control were defined by a medication history of an AAD (flecainide, propafenone, amiodarone, dronedarone, sotalol, dofetilide, or quinidine) or a procedural history of a DCCV or AF ablation at study inclusion.
Statistical Analysis
Frequencies of clinical characteristics and therapies are reported as proportions. Summary statistics examining baseline differences in demographics and comorbidities by catheter ablation (yes versus no) and by cohort (PINNACLE AATAC subgroup versus AATAC trial) were evaluated at registry inclusion. A chi‐square analysis was performed to assess for differences in categorical variables, and the Student t test was applied for continuous variables. To assess site‐level variation, ablation rates were examined over the study period to examine the use of AF catheter ablation by site. SAS software (version 9.4; SAS Institute Inc, Cary, NC) was used to generate these frequencies and comparisons.
Results
Baseline Cohort Characteristics
We identified 164 166 patients with an index encounter with diagnoses of both AF and HF in 127 ambulatory practices. From this initial cohort, patients were excluded by location in sites with >80% missing measures of LVEF (n=14 760), by the absence of both an LVEF measure and history of ICD or CRT‐D implantation (n=34 977), and by the presence of liver disease (n=115), New York Heart Association functional class IV (n=285), or a left ventricular assist device (n=7), leaving 114 022 patients. After excluding patients with LVEF >40% (n=77 236), there were 36 786 patients with AF and HFrEF. With further application of the AATAC trial exclusion criteria, individuals were also omitted if they lacked an ICD or CRT‐D (n=16 503), were not prescribed a beta‐blocker or angiotensin‐converting enzyme inhibitor (n=10 657), or if they had a reversible cause of AF (n=4), prohibitive social factors (n=144), or cardiac surgery within 3 months of registry inclusion (n=995; Figure 1; Table 1), leaving a final PINNACLE AATAC cohort of 8483 subjects (7%).
Figure 1.
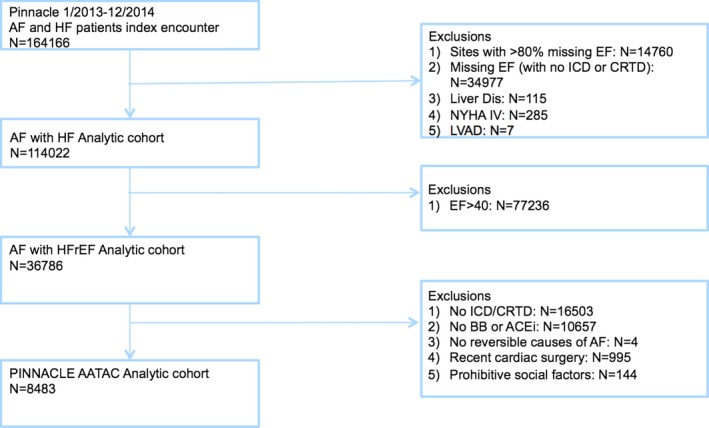
NCDR ® PINNACLE cohort creation. ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; BB, beta‐blocker; CRTD, cardiac resynchronization therapy with defibrillator; EF, ejection fraction; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter‐defibrillator; LVAD, left ventricular assist device; NYHA, New York Heart Association Class; PINNACLE, Practice Innovation and Clinical Excellence.
Table 1.
Clinical Characteristics by Cohort
| AF+HF N=114 022 (100%) | AF+HFrEF N=36 786 (22%) | PINNACLE AATAC Eligible Cohort N=8483 (7%) | |
|---|---|---|---|
| Demographics | |||
| Age, y | 74.7±11.2 | 73.2±11.5 | 71.2±11.4 |
| Male | 67 330 (59.1%) | 26 780 (72.9%) | 6659 (78.5%) |
| Race | |||
| White | 66 761 (92.7%) | 20 604 (90.7%) | 4740 (90.4%) |
| Black | 3951 (5.5%) | 1720 (7.6%) | 417 (8.0%) |
| Other | 1318 (1.8%) | 388 (1.7%) | 86 (1.6%) |
| Comorbidities | |||
| BMI, kg/m2 | 30.4±9.9 | 29.8±9.7 | 30.1±9.7 |
| Hypertension | 92 307 (88.4%) | 27 718 (83.3%) | 6299 (82.4%) |
| Diabetes mellitus | 34 649 (30.8%) | 11 573 (32.0%) | 2660 (31.8%) |
| Hyperlipidemia | 76 380 (73.2%) | 23 783 (72.2%) | 5580 (74.7%) |
| Heart failure | 114 022 (100.0%) | 36 786 (100.0%) | 8483 (100.0%) |
| Coronary artery disease | 69 652 (66.9%) | 25 338 (75.3%) | 6088 (79.2%) |
| Unstable angina | 3479 (3.1%) | 1229 (3.4%) | 311 (3.7%) |
| Stable angina | 14 250 (13.3%) | 4493 (13.0%) | 1050 (13.2%) |
| Chronic liver disease | 200 (0.2%) | 0 (0.0%) | 0 (0.0%) |
| Stroke/TIA | 7175 (9.0%) | 2246 (8.7%) | 523 (8.6%) |
| Peripheral arterial disease | 14 895 (14.5%) | 4959 (15.1%) | 1140 (15.1%) |
| Ischemic vascular disease | 66 229 (61.0%) | 24 428 (69.9%) | 6264 (76.2%) |
| Cardiovascular events | |||
| Myocardial infarction | 26 262 (25.5%) | 10 529 (32.1%) | 2575 (34.3%) |
| Systemic embolism | 587 (0.7%) | 278 (1.0%) | 65 (1.0%) |
| Intracranial hemorrhage | 3611 (3.2%) | 1209 (3.3%) | 289 (3.4%) |
| Other major hemorrhage | 2796 (2.5%) | 925 (2.5%) | 176 (2.1%) |
| Vascular complication | 3606 (3.2%) | 1223 (3.3%) | 323 (3.8%) |
| Echocardiographic parameters | |||
| LVEF | 50.1±15.0 | 30.1±8.1 | 28.4±8.0 |
| Atrial fibrillation characteristics | |||
| Duration | |||
| First detected | 3277 (11.9%) | 1279 (15.4%) | 345 (17.8%) |
| Paroxysmal | 19 631 (71.5%) | 5570 (67.2%) | 1268 (65.5%) |
| Persistent/permanent | 4547 (16.6%) | 1443 (17.4%) | 323 (16.7% |
| Etiology | |||
| Nonvalvular | 4243 (95.3%) | 1335 (96.1%) | 339 (98.8%) |
| Valvular | 211 (4.7%) | 54 (3.9%) | 4 (1.2%) |
| Transient/reversible | 198 (0.2%) | 38 (0.1%) | 0 (0%) |
| INR value | 2.2±1.0 | 2.2±1.0 | 2.2±1.0 |
| Devices | |||
| ICD | 25 074 (22.1%) | 18 438 (50.5%) | 8483 (100%) |
| CRT‐D | 23 172 (20.5%) | 15 604 (42.8%) | 6626 (78.6%) |
| LVAD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Medications | |||
| Rate control | |||
| Beta‐blockers | 72 170 (63.3%) | 24 159 (65.7%) | 8483 (100.0%) |
| Calcium‐channel blockers | 25 751 (22.6%) | 4521 (12.3%) | 1037 (12.2%) |
| Digoxin | 19 405 (17.0%) | 7549 (20.5%) | 2710 (31.9%) |
| Rhythm control | |||
| Amiodarone | 17 859 (15.7%) | 8010 (21.8%) | 2090 (24.6%) |
| Dofetilide | 591 (0.5%) | 263 (0.7%) | 96 (1.1%) |
| Dronedarone | 2074 (1.8%) | 395 (1.1%) | 78 (0.9%) |
| Flecainide | 566 (0.5%) | 44 (0.1%) | 9 (0.1%) |
| Propafenone | 300 (0.3%) | 41 (0.1%) | 8 (0.1%) |
| Quinidine | 27 (0.0%) | 15 (0.0%) | 4 (0.0%) |
| Sotalol | 1261 (1.1%) | 431 (1.2%) | 178 (2.1%) |
| Anticoagulation | |||
| ADP antagonist | 13 811 (12.1%) | 5209 (14.2%) | 1237 (14.6%) |
| Aspirin | 65 592 (57.5%) | 22 112 (60.1%) | 5391 (63.6%) |
| DOAC | 20 800 (18.2%) | 6338 (17.2%) | 1316 (15.5%) |
| Warfarin | 52 406 (46.0%) | 18 008 (49.0%) | 4476 (52.8%) |
| Procedures | |||
| DCCV | 14 434 (12.7%) | 4776 (13.0%) | 1113 (13.1%) |
| Electrophysiology study | 6394 (5.6%) | 2641 (7.2%) | 835 (9.8%) |
| Catheter ablation | 6072 (5.3%) | 2492 (6.8%) | 785 (9.3%) |
| PCI (BMS) | 2271 (2.1%) | 928 (2.6%) | 228 (2.8%) |
| PCI (DES) | 3900 (3.9%) | 1308 (4.0%) | 327 (4.3%) |
| CABG | 20 929 (18.6%) | 8673 (23.9%) | 1719 (20.5%) |
| Cardiac valve surgery | 4619 (4.3%) | 1413 (4.1%) | 221 (2.8%) |
| Heart transplant | 1749 (1.5%) | 583 (1.6%) | 157 (1.9%) |
AF indicates atrial fibrillation; BMI, body mass index; BMS, bare metal stent; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy with defibrillation; DCCV, direct‐current cardioversion; DES, drug‐eluting stent; DOAC, direct oral anticoagulant; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable‐cardioverter defibrillator; INR, international normalized ration; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PINNACLE, Practice Innovation and Clinical Excellence; TIA, transient ischemic attack.
The PINNACLE AATAC eligible subgroup was slightly younger and had a larger proportion of male patients than the overall AF and HF cohort. Similar to the overall cohort, the PINNACLE AATAC group was comparable in racial composition and several comorbidities, including obesity, diabetes mellitus, hyperlipidemia, coronary artery disease, and stroke/transient ischemic attack. AF characteristics were similar across groups in type (first‐detected, paroxysmal, or persistent/permanent) and etiology (valvular, nonvalvular, or secondary causes).
When compared with the AATAC trial population,10 the PINNACLE AATAC cohort was older (71±11 versus 61±11, y), had more comorbidities (hypertension [82% versus 47%], diabetes mellitus [32% versus 23%], and coronary artery disease [79% versus 64%]), and was on greater medical therapy, including angiotensin‐converting enzyme inhibitor (100% versus 90%) and beta‐blocker (100% versus 78%).10 Male predominance and EF were comparable among all groups.
AF Therapies: PINNACLE AATAC Subgroup
Among the PINNACLE AATAC subgroup, rate control was used in 100% of the cohort because of the requirement for beta‐blocker prescription for inclusion in the cohort. The most commonly used atrioventricular‐nodal blocking agents were carvedilol (71.2%), digoxin (31.9%), and metoprolol (27.1%; Table 2).
Table 2.
AF Rate Control Medications
| AF+HF (N=114 022) | AF+HFrEF (N=36 786) | PINNACLE AATAC Eligible Cohort (N=8483) | |
|---|---|---|---|
| Beta‐blockers | 72 170 (63.3%) | 24 159 (65.7%) | 8483 (100.0%) |
| Atenolol | 6497 (5.7%) | 1023 (2.8%) | 350 (4.1%) |
| Bisoprolol | 966 (0.8%) | 242 (0.7%) | 64 (0.8%) |
| Carvedilol | 28 971 (25.4%) | 14 959 (40.7%) | 6036 (71.2%) |
| Metoprolol | 30 117 (26.4%) | 7567 (20.6%) | 2302 (27.1%) |
| Nebivolol | 1663 (1.5%) | 342 (0.9%) | 90 (1.1%) |
| Calcium‐channel blockers | 25 751 (22.6%) | 4521 (12.3%) | 1037 (12.2%) |
| Diltiazem | 2827 (2.5%) | 463 (1.3%) | 77 (0.9%) |
| Verapamil | 181 (0.2%) | 29 (0.1%) | 8 (0.1%) |
| Digoxin | 19 405 (17.0%) | 7549 (20.5%) | 2710 (31.9%) |
AF indicates atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; PINNACLE, Practice Innovation and Clinical Excellence.
Of rhythm control strategies (Table 3), amiodarone was the predominant AAD (24.6% of the PINNACLE AATAC subgroup) over other Class III drugs (sotalol [2.1%], dronedarone [0.9%], and dofetilide [1.1%]). AAD use increased as an adjunctive therapy to DCCV or AF ablation (Tables 4 and 5). Procedures were utilized less often than AAD with DCCV (13.1%) used more frequently than catheter ablation (9.3%).
Table 3.
AF Rhythm Control Medications
| AF+HF (N=114 022) | AF+HFrEF (N=36 786) | PINNACLE AATAC Eligible Cohort (N=8483) | |
|---|---|---|---|
| Amiodarone | 17 859 (15.7%) | 8010 (21.8%) | 2090 (24.6%) |
| Dofetilide | 591 (0.5%) | 263 (0.7%) | 96 (1.1%) |
| Dronedarone | 2074 (1.8%) | 395 (1.1%) | 78 (0.9%) |
| Flecainide | 566 (0.5%) | 44 (0.1%) | 9 (0.1%) |
| Propafenone | 300 (0.3%) | 41 (0.1%) | 8 (0.1%) |
| Quinidine | 27 (0.0%) | 15 (0.0%) | 4 (0.0%) |
| Sotalol | 1261 (1.1%) | 431 (1.2%) | 178 (2.1%) |
AF indicates atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction.
Table 4.
Antiarrhythmic Medications Used With Cardioversion
| AF+HF (N=14 434) | AF+HFrEF (N=4776) | PINNACLE AATAC Eligible Cohort (N=1113) | |
|---|---|---|---|
| Amiodarone | 4073 (28.2%) | 1769 (37.0%) | 410 (36.8%) |
| Dofetilide | 181 (1.3%) | 79 (1.7%) | 26 (2.3%) |
| Dronedarone | 542 (3.8%) | 112 (2.3%) | 15 (1.3%) |
| Flecainide | 99 (0.7%) | 11 (0.2%) | 1 (0.1%) |
| Propafenone | 52 (0.4%) | 8 (0.2%) | 2 (0.2%) |
| Quinidine | 3 (0.0%) | 1 (0.0%) | 0 (0.0%) |
| Sotalol | 241 (1.7%) | 88 (1.8%) | 26 (2.3%) |
AF indicates atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; PINNACLE, Practice Innovation and Clinical Excellence.
Table 5.
Antiarrhythmic Medications Used With AF Ablation
| AF+HF (N=6072) | AF+HFrEF (N=2492) | PINNACLE AATAC Eligible Cohort (N=785) | |
|---|---|---|---|
| Amiodarone | 1005 (16.6%) | 566 (22.7%) | 195 (24.8%) |
| Dofetilide | 79 (1.3%) | 39 (1.6%) | 12 (1.5%) |
| Dronedarone | 136 (2.2%) | 42 (1.7%) | 10 (1.3%) |
| Flecainide | 24 (0.4%) | 1 (0.0%) | 0 (0.0%) |
| Propafenone | 22 (0.4%) | 4 (0.2%) | 1 (0.1%) |
| Quinidine | 1 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sotalol | 84 (1.4%) | 43 (1.7%) | 19 (2.4%) |
AF indicates atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; PINNACLE, Practice Innovation and Clinical Excellence.
PINNACLE AATAC Ablation Cohort
When compared with PINNACLE AATAC–eligible patients who did not undergo AF ablation (N=7698), patients who underwent ablation (N=785) were slightly younger and had less comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, and myocardial infarctions (Table 6). Other demographics, including sex and racial composition as well as vascular disease and EF, were comparable between groups. Although PINNACLE AATAC–eligible patients who underwent ablation were more likely to undergo other rhythm control procedures, such as DCCV, AAD prescription was similar with or without catheter ablation.
Table 6.
Clinical Characteristics by AF Ablation Status in PINNACLE AATAC Cohort
| PINNACLE AATAC Cohort N=8483 (100%) | PINNACLE AATAC Cohort With AF Ablation N=785 (9%) | PINNACLE AATAC Cohort Without AF Ablation N=7698 (91%) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 71.2±11.4 | 69.5±11.5 | 71.4±11.4 | <0.001 |
| Male | 6659 (78.5%) | 620 (79.0%) | 6039 (78.5%) | 0.74 |
| Race | ||||
| White | 4740 (90.4%) | 416 (90.0%) | 4324 (90.4%) | 0.47 |
| Black | 417 (8.0%) | 41 (8.9%) | 376 (7.9%) | |
| Other | 86 (1.6%) | 5 (1.1%) | 81 (1.7%) | |
| Comorbidities | ||||
| BMI, kg/m2 | 30.1±9.7 | 30.7±9.5 | 30.1±9.8 | 0.18 |
| Hypertension | 6299 (82.4%) | 488 (78.8%) | 5811 (82.7%) | 0.01 |
| Diabetes mellitus | 2660 (31.8%) | 209 (27.2%) | 2451 (32.3%) | 0.003 |
| Hyperlipidemia | 5580 (74.7%) | 416 (70.3%) | 5164 (75.1%) | 0.008 |
| Heart failure | 8483 (100.0%) | 785 (100.0%) | 7698 (100.0%) | |
| Coronary artery disease | 6088 (79.2%) | 555 (76.8%) | 5533 (79.5%) | 0.09 |
| Unstable angina | 311 (3.7%) | 41 (5.2%) | 270 (3.5%) | 0.02 |
| Stable angina | 1050 (13.2%) | 88 (12.3%) | 962 (13.3%) | 0.47 |
| Chronic liver disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Stroke/TIA | 523 (8.6%) | 38 (7.4%) | 485 (8.7%) | 0.30 |
| Peripheral arterial disease | 1140 (15.1%) | 89 (14.4%) | 1051 (15.1%) | 0.63 |
| Ischemic vascular disease | 6264 (76.2%) | 577 (75.1%) | 5687 (76.3%) | 0.47 |
| Cardiovascular events | ||||
| Myocardial infarction | 2575 (34.3%) | 193 (28.8%) | 2382 (34.8%) | 0.001 |
| Systemic embolism | 65 (1.0%) | 3 (0.5%) | 62 (1.0%) | 0.24 |
| Intracranial hemorrhage | 289 (3.4%) | 26 (3.3%) | 263 (3.4%) | 0.85 |
| Other major hemorrhage | 176 (2.1%) | 18 (2.3%) | 158 (2.1%) | 0.65 |
| Vascular complication | 323 (3.8%) | 49 (6.3%) | 274 (3.6%) | <0.001 |
| Echocardiographic parameters | ||||
| LVEF | 28.4±8.0 | 28.8±8.2 | 28.3±8.0 | 0.19 |
| Atrial fibrillation characteristics | ||||
| Duration | 0.02 | |||
| First detected | 345 (17.8%) | 12 (9.2%) | 333 (18.4%) | |
| Paroxysmal | 1268 (65.5%) | 97 (74.6%) | 1171 (64.8%) | |
| Permanent | 323 (16.7% | 21 (16.2%) | 302 (16.7%) | |
| Etiology | 1.00 | |||
| Non‐valvular | 339 (98.8%) | 2 (100.0%) | 337 (98.8%) | |
| Valvular | 4 (1.2%) | 0 (0.0% | 4 (1.2%) | |
| Transient/reversible | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| INR value | 2.2±1.0 | 2.1±1.0 | 2.2±1.0 | 0.49 |
| AF recurrence | 12 (0.1%) | 1 (0.1%) | 11 (0.1%) | 1.00 |
| Devices | ||||
| ICD | 8483 (100%) | 722 (92.0%) | 7198 (93.5%) | 0.10 |
| CRT‐D | 6626 (78.6%) | 746 (95.2%) | 5880 (76.9%) | <0.001 |
| LVAD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Medications | ||||
| Rate control | ||||
| Beta‐blockers | 8483 (100.0%) | 785 (100.0%) | 7698 (100.0%) | |
| Calcium‐channel blockers | 1037 (12.2%) | 84 (10.7%) | 953 (12.4%) | 0.17 |
| Digoxin | 2710 (31.9%) | 220 (28.0%) | 2490 (32.3%) | 0.01 |
| Rhythm control | ||||
| Amiodarone | 2090 (24.6%) | 195 (24.8%) | 1895 (24.6%) | 0.89 |
| Dofetilide | 96 (1.1%) | 12 (1.5%) | 84 (1.1%) | 0.27 |
| Dronedarone | 78 (0.9%) | 10 (1.3%) | 68 (0.9%) | 0.27 |
| Flecainide | 9 (0.1%) | 0 (0.0%) | 9 (0.1%) | 1.00 |
| Propafenone | 8 (0.1%) | 1 (0.1%) | 7 (0.1%) | 0.54 |
| Quinidine | 4 (0.0%) | 0 (0.0%) | 4 (0.1%) | 1.00 |
| Sotalol | 178 (2.1%) | 19 (2.4%) | 159 (2.1%) | 0.51 |
| Anticoagulation | ||||
| ADP antagonist | 1237 (14.6%) | 88 (11.2%) | 1149 (14.9%) | 0.004 |
| Aspirin | 5391 (63.6%) | 487 (62.0%) | 4904 (63.7%) | 0.36 |
| DOAC | 1316 (15.5%) | 145 (18.5%) | 1171 (15.2%) | 0.02 |
| Warfarin | 4476 (52.8%) | 400 (51.0%) | 4076 (52.9%) | 0.29 |
| Procedures | ||||
| DCCV | 1113 (13.1%) | 273 (34.9%) | 840 (10.9%) | <0.001 |
| Electrophysiology study | 835 (9.8%) | 541 (68.9%) | 294 (3.8%) | <0.001 |
| PCI (BMS) | 228 (2.8%) | 6 (0.8%) | 222 (3.0%) | <0.001 |
| PCI (DES) | 327 (4.3%) | 14 (1.9%) | 313 (4.5%) | 0.001 |
| CABG | 1719 (20.5%) | 146 (18.6%) | 1573 (20.6%) | 0.18 |
| Cardiac valve surgery | 221 (2.8%) | 32 (4.2%) | 189 (2.6%) | 0.01 |
| Heart transplant | 157 (1.9%) | 19 (2.4%) | 138 (1.8%) | 0.21 |
AF indicates atrial fibrillation; BMI, body mass index; BMS, bare metal stent; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy with defibrillation; DCCV, direct‐current cardioversion; DES, drug‐eluting stent; DOAC, direct oral anticoagulant; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable‐cardioverter defibrillator; INR, international normalized ration; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PINNACLE, Practice Innovation and Clinical Excellence; TIA, transient ischemic attack.
Of the sites performing AF ablation (64 of 127 sites), there was significant variation in ablation volume by site (Figure 2). During the study period, the median ablation rate was 0.1 ablations/year (interquartile range, 0.05, 0.15) with a corresponding median patient volume of 77 encounters (interquartile range, 65, 99) at sites performing AF ablation. Nearly half of participating sites did not perform AF ablation in this population whereas 3 sites had ablation rates exceeding 25%.
Figure 2.
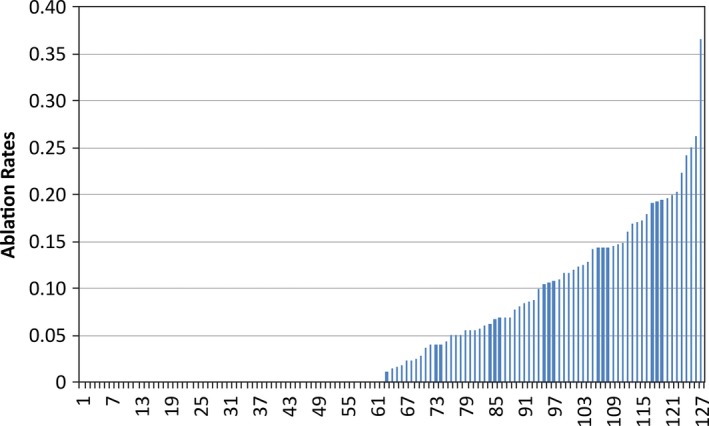
Site‐level variation in atrial fibrillation ablation rates. Site‐level variation in atrial fibrillation ablation volume across sites by assessing ablation rates (ablations per year) during the study period across participating sites. The median ablation rate was 0.1 ablations/year (interquartile range, 0.05, 0.15).
AF Therapies: Overall HF Cohort
In the overall AF with HF cohort, rate control was the predominant strategy over rhythm control (Tables 2 and 3), with beta‐blockers the preferred medication over calcium‐channel blockers or digoxin. Of the beta‐blockers, carvedilol (25.4%) and metoprolol (26.4%) were the most frequently used agents.
A rhythm control approach was used in just under 20% of the overall cohort, with amiodarone use accounting for over three quarters of this strategy (Table 3). Use of other AADs was less than 2%. Amiodarone again remained the predominant medication when AADs were used adjunctively with DCCV and AF ablation (Tables 4 and 5). Procedures were also less common, with DCCV (12.7%) used around twice as frequently as catheter ablation (5.3%).
Discussion
In this contemporary cohort of more than 100 000 patients in the United States with AF and HF, 7% appeared to be eligible for catheter ablation based on the AATAC trial criteria. Within this AATAC‐eligible subgroup, as also observed across the larger AF‐HF group, rate control therapies predominated. Of the patients meeting AATAC trial eligibility criteria, AAD—mostly amiodarone—was the predominant rhythm control therapy, used over DCCV and catheter ablation. Application of catheter ablation for AF showed significant variation across sites where ablation was offered.
Previous studies have described practice patterns of rate and rhythm control for the treatment of AF alone.13, 14 Similar to our findings, rate control therapies were consistently utilized more frequently than rhythm control strategies. Furthermore, DCCV and AADs were employed more often than catheter ablation, which, in 1 contemporary cohort of AF patients, accounted for 5.2% of all AF therapies and 11% of rhythm control treatments.13 The only study to describe AF management in an HF cohort was a retrospective, single‐center study by Al‐Khatib et al.15 They queried the Duke cardiovascular disease inpatient database to describe therapies and outcomes of patients with AF and HFrEF identified between 1995 and 2002. In this cohort, rate and rhythm control prescription were equivalent. However, these findings may reflect the practice patterns of a single center and the comorbidities and poor prognosis of a cohort defined by an index admission. Additionally, AF procedural data were not available. Notably, with the rapid changes in the techniques and frequency of use of catheter ablation for AF, older data have less applicability to current practice.
The predominance of rate control over rhythm control in our results is not surprising, given the equivalent results of landmark randomized, controlled trials that failed to show improved outcomes with rhythm control.5, 6, 16, 17, 18 These results were mirrored in subgroup analyses of those with HFrEF as well.19 However, the limitations of these studies prevent their generalization to all patients. Sinus rhythm was maintained in only 39% to 64% of patients assigned to rhythm control,20 crossover between treatment arms was frequent, and under‐representation of patient subgroups, including the young, elderly, and those with structural heart disease also hampered applicability to contemporary populations. Concerns about extension to the HFrEF population were mitigated by the advent of the AF‐CHF (Atrial Fibrillation and Congestive Heart Failure) trial.7 In the absence of symptoms, AF‐CHF concluded that there was no advantage of a rhythm control approach with AADs over a rate control strategy to treat AF in HFrEF. The predominance of rate control in our results likely reflects, in part, the influence of AF‐CHF, which has formed the paradigm for AF treatment in HFrEF.
Given the failure rate of AADs as well as their side effects, catheter ablation has emerged as a potential alternative to pharmacological rhythm control. Clinical trials have shown reduced recurrence of atrial tachyarrhythmias with an ablation strategy over AADs. The clinical effectiveness of AF ablation for patients with HFrEF has also shown comparable results with freedom from recurrent arrhythmias ranging from 50% to 88%.21 Until recently, many clinicians felt that such results did not generalize to the HF population. However, the AATAC trial provides evidence that catheter ablation in appropriately selected HFrEF patients can provide significant benefit. Despite selecting for persistent AF, AATAC trial patients were relatively healthy, had an AF duration of just over 8 months, had relatively small atria, and had less comorbidities than observed in our ambulatory cohort. Within this context, ablation was associated with improved clinical outcomes compared to amiodarone, with an over 2‐fold difference in freedom from recurrence in follow‐up as well as decreased hospitalizations and mortality and improvements in quality of life, exercise capacity, and EF. These associations, which mirror those noted in comparable populations in other studies, likely reflect a number of factors, including selection of a relatively healthy cohort of patients for catheter ablation (Table 6).
In the period immediately preceding the recent release of the AATAC findings, our results indicate that catheter ablation of AF has been used infrequently in the United States in patients with HFrEF, and when a rhythm control strategy is desired, AADs have been the approach of choice. These observations mirror clinical guidelines where catheter ablation is often reserved as a second‐line therapy with the failure of AADs (Class I) for symptomatic AF rather than as first‐line therapy in select patients (Class IIa or IIb).9 It is also encouraging to see that AADs, such as sotalol or dronedarone, that would not be indicated in this HF population were used infrequently.
Although the cohort described here reflects recent practice, the AATAC findings became available after these data were collected. Additionally, catheter ablation remains a relatively new therapy and involves a complex procedure requiring a skilled practitioner. How quickly and to what extent the AATAC findings are taken up into clinical practice remains to be determined. Additionally, as previously mentioned, heterogeneity in patient selection, ablation technique, and procedural experience may lead to differences in patient outcomes. Furthermore, our results also highlight site‐level variation that reflects differences in clinical practice. As a result, referral for AF ablation may also reflect not only patient specific differences, but also regional and system‐wide differences that may favor rate control over rhythm control.
A number of limitations of this PINNACLE analysis should be noted. First, we were unable to comment on outcomes associated with each treatment type given the differential follow‐up in the registry. Future studies should focus on comparing real‐world outcomes in treatment strategies in the AF and HF population, recognizing major limitations imposed by treatment selection bias in observational data. We were also unable to assess symptom burden as it relates to measures such as quality of life and 6‐minute walk. As a result, we could not comment on the primary indication for ablation in our population—mitigation of symptomatic AF. Given the lack of these measures, the inability to comment on outcome, and the lack of a rate control arm in AATAC, we did not have the ability—nor was it our objective—to determine the appropriate treatment approach for these patients. However, the improvements in hospitalization and mortality observed in the AATAC trial make its results compelling and call for replication in this population. Furthermore, specifics regarding preprocedure cardiac structure and function beyond EF are not available. As a result, we cannot comment on the candidacy of these patients for AF ablation based on parameters such as left atrial size. Third, because patients were enrolled in the registry on their index encounter, previous medication use and procedural data are lacking. Fourth, catheter ablation includes catheter ablation of “atrial arrhythmias.” As a result, catheter ablation as defined in PINNACLE includes not only procedures for atrial fibrillation, but may also include atrial flutter and other supraventricular tachycardias. We believe this nonspecificity is diminished given selection for patients with encounters coding for AF. Finally, given the short follow‐up time, we could not effectively describe crossover between groups and, consequently, we cannot comment on failure of each arm in current practice.
Conclusion
Rate control is primarily used to treat AF and HFrEF in a contemporary, outpatient US population, and escalation to rhythm control therapies most often utilizes AADs or DCCV. Catheter ablation has been rarely used in this population. Future studies focusing on patient symptoms, quality of life, and real‐world outcomes will improve our understanding of catheter ablation in this population.
Sources of Funding
PINNACLE is funded by the American College of Cardiology. Dr Allen is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award No. K23HL105896.
Disclosures
Dr Masoudi receives support from the ACC for his role as Chief Science Officer of the NCDR®. Dr Allen reports receiving research grants from the National Institutes of Health, the American Heart Association, and the Patient‐Centered Outcomes Research Institute; and serving as a consultant for Janssen, Novartis, St. Jude, and ZS Pharma.
(J Am Heart Assoc. 2017;6:e005273 DOI: 10.1161/JAHA.116.005273.)28862932
References
- 1. Israel CW, Lawo T, Lemke B, Gronefeld G, Hohnloser SH. Atrial pacing in the prevention of paroxysmal atrial fibrillation: first results of a new combined algorithm. Pacing Clin Electrophysiol. 2000;23:1888–1890. [DOI] [PubMed] [Google Scholar]
- 2. Hohnloser SH. Prevention of recurrent life‐threatening arrhythmias: will lipid‐lowering therapy make a difference? J Am Coll Cardiol. 2000;36:773–775. [DOI] [PubMed] [Google Scholar]
- 3. Gronefeld GC, Mauss O, Li YG, Klingenheben T, Hohnloser SH. Association between atrial fibrillation and appropriate implantable cardioverter defibrillator therapy: results from a prospective study. J Cardiovasc Electrophysiol. 2000;11:1208–1214. [DOI] [PubMed] [Google Scholar]
- 4. Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG; Investigators A . Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow‐up investigation of rhythm management (AFFIRM) study. Circulation. 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 5. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD; Atrial Fibrillation Follow‐up Investigation of Rhythm Management I . A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 6. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study G . A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. [DOI] [PubMed] [Google Scholar]
- 7. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL; Atrial F, Congestive Heart Failure I . Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 8. Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC, Cleland JG. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE‐II Study). Heart. 2009;95:924–930. [DOI] [PubMed] [Google Scholar]
- 9. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; Members AATF . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 10. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 11. Maddox TM, Masoudi FA, Oetgen WJ, Rumsfeld JS. The capacity of evidence to inform practice: the rapid registry response (RRR) initiative. J Am Coll Cardiol. 2015;65:2252–2253. [DOI] [PubMed] [Google Scholar]
- 12. Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, Spertus JA. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (practice innovation and clinical excellence) program. J Am Coll Cardiol. 2010;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J, Sylwestrzak G, Barron J, Rosenberg A, White J, Whitney J, Redberg R, Malenka D. Evaluation of practice patterns in the treatment of atrial fibrillation among the commercially insured. Curr Med Res Opin. 2014;30:1707–1713. [DOI] [PubMed] [Google Scholar]
- 14. Allen LaPointe NM, Lokhnygina Y, Rimmler J, Sanders GD, Peterson ED, Al‐Khatib SM. Use of rate and rhythm control drugs in patients younger than 65 years with atrial fibrillation. J Atr Fibrillation. 2014;7:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al‐Khatib SM, Shaw LK, Lee KL, O'Connor C, Califf RM. Is rhythm control superior to rate control in patients with atrial fibrillation and congestive heart failure? Am J Cardiol. 2004;94:797–800. [DOI] [PubMed] [Google Scholar]
- 16. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. [DOI] [PubMed] [Google Scholar]
- 17. Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U; Investigators S . Randomized trial of rate‐control versus rhythm‐control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. [DOI] [PubMed] [Google Scholar]
- 18. Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska‐Kaplon B, Kolodziej P, Achremczyk P; Investigators of the Polish How to Treat Chronic Atrial Fibrillation S . Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the polish how to treat chronic atrial fibrillation (HOT CAFE) study. Chest. 2004;126:476–486. [DOI] [PubMed] [Google Scholar]
- 19. Freudenberger RS, Wilson AC, Kostis JB; Investigators A, Committees . Comparison of rate versus rhythm control for atrial fibrillation in patients with left ventricular dysfunction (from the AFFIRM Study). Am J Cardiol. 2007;100:247–252. [DOI] [PubMed] [Google Scholar]
- 20. Zimetbaum P. Is rate control or rhythm control preferable in patients with atrial fibrillation? An argument for maintenance of sinus rhythm in patients with atrial fibrillation. Circulation. 2005;111:3150–3156; discussion 3156–3157. [DOI] [PubMed] [Google Scholar]
- 21. Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol. 2014;64:710–721. [DOI] [PubMed] [Google Scholar]


