Abstract
Background
The authors analyzed the effects of perforin‐dependent infiltration on long‐term mortality in patients with inflammatory cardiomyopathy (CMi). We previously demonstrated that left ventricular function deteriorates and progresses to substantial cardiac dysfunction in patients with perforin‐positive cardiac cell infiltration.
Methods and Results
Between 2003 and 2013, 2389 consecutive patients with clinically suspected CMi who underwent endomyocardial biopsies were enrolled. Endomyocardial biopsies were performed at first admission after exclusion of ischemic or valvular heart disease, and CMi was confirmed in 1717 patients. Follow‐up was up to 10.1 years (median 0.47 years; interquartile range, 0.03–2.56 years) and information on vital status was obtained from official resident data files. Multivariable statistical analysis was conducted for all patients with CMi regarding significant predictors of all‐cause mortality or need for heart transplantation. Multiple Cox regression analysis revealed perforin above the calculated cutoff point of 2.9 cells/mm² as a strong predictor of impaired survival with a hazard ratio of 1.881 (95% confidence interval, 1.177–3.008; P=0.008), independent of left ventricular function and other myocardial inflammation markers (CD3, macrophage‐1 antigen, leukocyte function–associated antigen‐1, human leukocyte antigen‐1, and intercellular cell adhesion molecule‐1). Unexpectedly, male sex emerged as another strong adverse predictor of survival in CMi (hazard ratio, 1.863; confidence interval, 1.096–3.168 [P=0.022]). Whereas left ventricular ejection fraction course is adversely affected by myocardial perforin, multivariate analysis indicates that left ventricular ejection fraction explains only part of the observed overall mortality.
Conclusions
High perforin‐positive cardiac cell infiltration and male sex are independent adverse predictors of long‐term mortality in CMi. Furthermore, exact quantification of immunohistochemically detected infiltrates is necessary to assess the prognosis.
Keywords: inflammatory cardiomyopathy, myocardial inflammation, perforin, survival
Subject Categories: Inflammatory Heart Disease, Inflammation, Heart Failure, Biomarkers
Clinical Perspective
What Is New?
The current study indicates for the first time that high perforin‐positive cardiac cell infiltration in endomyocardial biopsies of patients with CMi is a significant independent risk factor for mortality or need of heart transplantation.
What Are the Clinical Implications?
These new data suggest that perforin‐positive infiltrates should be routinely measured by immunohistochemistry in endomyocardial biopsies to improve the assessment of prognosis in these patients.
Prospective immunomodulating clinical trials are needed to clarify whether perforin is also a predictor of responsiveness to therapy.
Introduction
Inflammatory cardiomyopathy (CMi) represents a major cause of heart failure with potential for transition to the clinical picture of dilated cardiomyopathy.1, 2, 3 The pathogenesis of CMi encompasses immune responses as well as autoimmune reactions involving autoantigen‐specific T cells. Myocardial inflammation is reflected by infiltration of lymphocytes, macrophages, and cell adhesion molecules.4, 5 Several studies have shown that cytotoxic cells expressing cytotoxic effector molecules are also increased in this cardiac inflammatory process.6, 7, 8, 9, 10, 11 One key mediator of cytotoxicity is perforin, a molecule released by T cells and natural killer cells. Its expression on T‐cell maturation is strongly regulated after activation, and effector T cells have been shown to express the highest levels of intracellular perforin. Once conjucated to target cells, the cytotoxic secretory granules traffic to the immunological synapse and release the deadly protein perforin. We previously demonstrated in a cohort of 495 patients with endomyocardial biopsy (EMB)–proven myocardial inflammation that left ventricular function deteriorates in patients with detection of perforin‐positive cardiac cell infiltration and progresses towards substantial cardiac dysfunction.6 In the present study, in a series of 2389 patients with suspected CMi, we investigated the influence of high perforin‐positive cardiac cell infiltration and other clinical and immunological parameters on long‐term mortality or need for heart transplantation.
Patients and Methods
Patients
Between January 2003 and November 2013, we screened all patients admitted to our clinic for further evaluation of suspected CMi by the complete spectrum of clinical and EMB‐based diagnostics. These patients complained about symptoms of heart failure with fatigue, reduced physical capacity or dyspnea on exertion, and cardiac dysfunction. Coronary artery disease and other possible causes of myocardial dysfunction (valvular heart disease) had been excluded by angiography and echocardiography before EMB in all patients.
Patients presenting with signs of acute myocarditis with recent onset of symptoms (eg, mimicking acute myocardial infarction with elevated serum markers of troponin T and creatine kinase/creatine kinase‐MB) were excluded, as well as those with proof of intramyocardial genomes of enterovirus, adenovirus, human herpesvirus 6, Epstein‐Barr virus, or erythrovirus (B19V) (primers used to test the presence of viral genome in Table S1). Other exclusion criteria were antiviral or immunosuppressive therapy in the past, clinical or biochemical evidence for concomitant chronic inflammatory disease (eg, rheumatological disorders), inability to understand the consent form, or participation or consent to participate in another study. EMBs from the right ventricular septum were obtained. Left ventricular ejection fraction (LVEF) was determined by echocardiography.
The follow‐up period was up to 10.1 years (median 0.47 years; interquartile range, 0.03–2.56 years). Information on vital status was obtained from direct contact with the patient or official resident data files. The demographic and clinical characteristics of these 2389 patients (1655 men, 734 women) are summarized in Table 1.
Table 1.
Clinical and Hemodynamic Data of Study Groups
| Patients | Group IA CMi (Perforin >2.9 cell/mm²) | Group IB CMi (Perforin ≤2.9 cell/mm²) | Group IC CMi (Without Perforin) | Group II Noninflammatory cardiomyopathy |
|---|---|---|---|---|
| No. | 305 | 890 | 522 | 672 |
| Age, y | 51.7±15.0 | 50.2±14.6 | 48.8±14.7 | 50.2±14.5 |
| Men/women | 184/121 | 604/286 | 361/161 | 506/166 |
| Preceding infection, No. (%) | 113 (37.0) | 360 (40.4) | 181 (34.6) | 206 (30.6)a |
| LVEF, % | 52.0 (32.5–68.5) | 46.0 (29.0–64.0) | 48.0 (30.0–65.0) | 46.0 (30.0–65.0) |
| LVEDD, mm | 53.0 (47.0–61.0) | 56.0 (49.0–63.0) | 55.0 (49.0–63.0) | 56.0 (49.0–65.0) |
| Dyspnea, No. (%) | 249 (81.6) | 719 (80.7) | 380 (72.7) | 385 (52.2)a |
| NYHA class I, No. (%) | ··· | ··· | ··· | ··· |
| NYHA class II, No. (%) | 166 (54.4) | 480 (53.9) | 280 (53.6) | 270 (40.1) |
| NYHA class III, No. (%) | 83 (27.2) | 239 (26.8) | 100 (19.1) | 115 (17.1)a |
| NYHA class IV, No. (%) | ··· | ··· | ··· | ··· |
| Fatigue/reduced capacity, No. (%) | 217 (71.1) | 705 (79.2) | 412 (78.9) | 490 (72.9) |
| ICD/pacemaker, No. (%) | 36 (11.8) | 98 (11.0) | 71 (13.6) | 89 (13.2) |
| Cardiovascular risk | ||||
| Smoking, No. (%) | 110 (36.0) | 297 (33.3) | 180 (34.4) | 252 (37.5) |
| Diabetes mellitus, No. (%) | 31 (10.1) | 98 (11.0) | 50 (9.5) | 60 (8.9) |
| Arterial hypertension, No. (%) | 22 (7.2) | 59 (6.6) | 46 (8.8) | 57 (8.4) |
Data are presented as mean±SD, median and range (75–95%), or number (percentage) of patients. ICD indicates implantable cardioverter‐defibrillator; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Significantly different inflammatory cardiomyopathy (CMi) (perforin >2.9 cell/mm²) vs non‐CMi.
Ethical Approval
The study was approved by the local ethics committees of the participating clinical centers and the committees of the respective federal states. Informed written consent was obtained from each study patient and the protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Myocardial Inflammation
Up to 8 EMBs were obtained from the right ventricular septum. Histology was developed by hematoxilin eosin staining in light microscopy. For immunohistological evaluation, specimens were embedded in Tissue Tec (SLEE Medical) and immediately snap‐frozen in methylbutane, which had been cooled in liquid nitrogen, and then stored at −80°C until processing. Embedded specimens were cut serially into cryosections of 5‐mm thickness and placed on 10% poly‐L‐lysine‐precoated slides. Immunohistochemistry was used for the characterization of inflammatory infiltrates. Myocardial inflammation was diagnosed by >14.0 lymphocytes/mm², including >7.0 CD3+ lymphocytes/mm2 according to the European Society of Cardiology guidelines.1 Furthermore, we analyzed macrophages (threshold >35.0 CD11b+/Mac‐1+ macrophages/mm2). Antibodies used included: CD3+ lymphocytes (Dako; dilution 1:25), CD11a+/LFA‐1+ lymphocytes (ImmunoTools; dilution 1:250), CD11b+/Mac‐1+ macrophages (ImmunoTools; dilution 1:500), HLA‐1 (Dako; dilution 1:2000), and ICAM‐1 (ImmunoTools; dilution 1:800). Perforin‐positive cardiac cell infiltration was defined by immunohistochemistry (clone δG9, BD Bioscience; dilution 1:150). As a secondary antibody we used enhancing EnVision peroxidase‐conjugated anti‐mouse antibody (DakoCytomation). Immunohistological staining was visualized using 3‐amino‐9‐ethylcarbazole (Merck) as chromogenic substrate. Finally, slides were counterstained in hematoxylin and mounted with Kaiser's gelatinR (Merck). The staining and peroxidase reactions in all samples were performed identically and in parallel for all samples. Specimens with perforin cellular infiltrates were classified as perforin positive.6 Immunoreactivity was quantified by digital image analysis. The images for the quantification of infiltrates were grabbed at ×200 magnification. The calculated objects were related to the unit Heart Area (mm2).
We divided the overall cohort in group I with immunohistological evidence of myocardial inflammation and designated it “confirmed CMi”. Group I with confirmed CMi was subdivided (group IA and group IB) at a cutoff value of 2.9 cells/mm2 for perforin‐positive cardiac cell infiltration, and group IC without perforin, according to our previous study.6 There, we presented 95% confidence intervals (CIs) for sensitivity and specificity and calculated the optimal cutoff point (2.95 with 94.2% sensitivity and 80.4% specificity) according to the maximal Youden index with a high risk for LVEF deterioration. Group II included those without evidence of myocardial inflammation and were designated non‐CMi (Table 2).
Table 2.
Histological and Immunohistological Data of Study Groups
| Patient | Group IA CMi (Perforin >2.9 cell/mm²) | Group IB CMi (Perforin ≤2.9 cell/mm²) | Group IC CMi (Without Perforin) | Group II Noninflammatory cardiomyopathy |
|---|---|---|---|---|
| Cardiomyocyte diameter, mm | 20.0 (18.0–22.0) | 19.0 (17.0–22.0) | 20.0 (16.0–22.0) | 20.0 (17.0–23.0) |
| CD3+, cells/mm2 | 8.7 (5.3–13.4) | 7.5 (3.9–11.0) | 5.4 (2.5–9.4) | 2.1 (1.0–3.6)a |
| Mac‐1+, cells/mm2 | 44.6 (30.7–61.8) | 36.8 (27.2–48.0) | 32.4 (21.8–44.2) | 15.7 (10.6–21.0)a |
| LFA‐1+, cells/mm2 | 21.5 (13.9–29.3) | 15.8 (10.7–25.3) | 14.8 (9.0–23.0) | 5.8 (3.4–8.0)a |
| Perforin+, cells/mm² | 4.5 (3.4–7.0) | 1.1 (0.7–1.8) | 0 | 0 a |
| HLA‐1/AF, % | 8.4 (6.5–10.2) | 7.5 (5.9–9.2) | 6.9 (5.4–8.8) | 5.1 (4.0–6.6)a |
| ICAM‐1/AF, % | 2.9 (1.9–3.9) | 2.3 (1.6–3.2) | 2.0 (1.4–2.8) | 1.1 (0.9–1.9)a |
| VCAM‐1/AF, % | 0.09 (0.04–0.14) | 0.05 80.02–0.11) | 0.04 (0.02–0.08) | 0.03 (0.01–0.06)a |
Data are presented as mean±SD, median and range (75–95%), or number (percentage) of patients. AF indicates area fraction; Perforin+, perforin above the optimal cutoff value (2.9 cells/mm²) as previously defined6; HLA‐1, human leukocyte antigen‐1; ICAM‐1, intercellular cell adhesion molecule‐1; LFA‐1, lymphocyte function–associated antigen‐1; Mac‐1, macrophage‐1 antigen; VCAM‐1, vascular cell adhesion molecule‐1.
Significantly different inflammatory cardiomyopathy (CMi; perforin >2.9 cell/mm²) vs non‐CMi.
Statistical Analyses
Data are shown as means and SDs. The nonparametric Mann–Whitney U test was used for group comparisons. Variables predicting survival were identified via Kaplan–Meier analysis and log‐rank test in a first step. Adjustment of potential confounders such as baseline LVEF and parameters significantly differing in the univariate analyses was performed using multiple Cox regression analysis with backward and forward selection. Thus, with perforin as the independent variable, baseline LVEF, left ventricular end‐diastolic diameter, CD3, CD45, sex, and age as potentially confounding covariates were included into the Cox regression model before backward and forward selection. In order to statistically confirm an observed mutually reinforcing effect of (male) sex and (high) perforin in the CMi group, a cox regression analysis with a perforin‐sex interaction term, adjusted for the known additional predictors, age and LVEF, was performed. A probability value of <0.05 was considered statistically significant. No Bonferroni correction was been performed. All statistical analyses were performed with SPSS (version 22.0; IBM Corp), STATA.13 (Stata Corporation), and Prism7 (PRISM).
Results
Clinical and immunological data of the study patients (n=2389, mean age 50.5±14.7 years) are summarized in Table 1. Table 1 encompasses 2 patient groups. Patients in group I had immunohistological evidence of myocardial inflammation and were designated “confirmed CMi” (n=1717). Patients in group II included those without evidence of myocardial inflammation and were designated “non‐CMi” (n=672). There were no significant differences between these 2 groups with regard to baseline LVEF (47.4±21.0% versus 48.1±20.3%, P=0.5), age (50.1±14.7 years versus 50.2±14.5 years, P=0.6), or sex. Group I with confirmed CMi was subdivided (group IA and group IB) at a cutoff value of 2.9 cells/mm2 for perforin‐positive cardiac cell infiltration according to our previous study,6 where this value was statistically calculated as the optimal cutoff point regarding prediction of a high risk for LVEF deterioration. A third group (group IC) had evidence of myocardial inflammation but no perforin (Table 2). There was no significant difference between the 3 groups regarding clinical or hemodynamic data.
Multivariate statistical analysis (Table 3) of all examined clinical and immunological parameters was conducted for the entire CMi cohort of 1717 patients to identify predictors of all‐cause mortality or need for heart transplantation. Multiple Cox regression analysis revealed (besides LVEF and age) that perforin was a strong predictor of impaired survival, with a hazard ratio of 1.881 (95% CI, 1.177–3.008; P=0.008) (Table 3). Figure 1A shows 4 survival curves: first patients with CMi with perforin above 2.9 cells/mm2, second CMi patients with perforin below 2.9 cells/mm2, third patients with CMi without perforin, and fourth patients with non‐CMi.
Table 3.
Multivariable Analysis of Factors Influencing Survival Rate
| Group | Parameter | Hazard Ratio | 95% CI for Hazard Ratio | P Value |
|---|---|---|---|---|
| I | Perforin >2.9 | 1.881 | 1.177–3.008 | 0.008 |
| Sex | 1.863 | 1.096–3.168 | 0.022 | |
| Age | 1.031 | 1.014–1.049 | 0.000 | |
| LVEF | 0.989 | 0.979–1.000 | 0.042 | |
| II | LVEF | 0.977 | 0.956–0.999 | 0.043 |
CI indicates confidence interval; LVEF, left ventricular ejection fraction.
Figure 1.
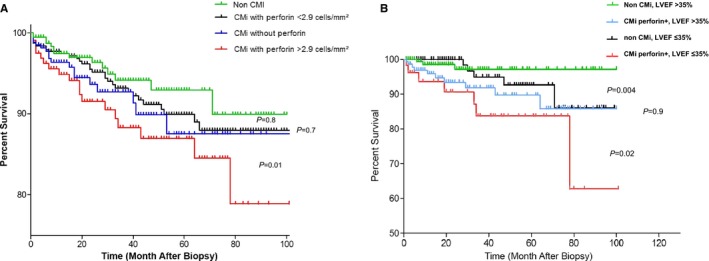
A, Kaplan–Meier analysis of patients with noninflammatory cardiomyopathy and inflammatory cardiomyopathy (CMi) for parameters with significant influence on survival by multivariable statistical analysis. Survival rate according to perforin analysis in endomyocardial biopsies of patients with CMi (group IA, IB, and IC) compared with patients with non‐CMi (group II). Perforin at the optimal cutoff point of 2.9 cells/mm² was associated with increased mortality or need for heart transplantation. Non‐CMi vs CMi without perforin, P=0.8 (not significant [ns]); CMi with perforin <2.9 cells/mm2 vs CMi without perforin, P=0.7 (ns); CMi with perforin <2.9 cells/mm2 vs CMi with perforin >2.0 cells/mm2, P=0.01. B, Survival rate among patients with non‐CMi and patients with CMi and high perforin (group IA) according to baseline left ventricular ejection fraction (LVEF) >35% vs LVEF ≤35%. Perforin and LVEF are significant predictors in this patient group as indicated by multivariable analysis (Table 3). Non‐CMi, LVEF >35% vs CMi perforin+, LVEF >35%, P=0.004; non‐CMi, LVEF ≤35% vs CMI perforin+, LVEF ≤35%, P=0.02.
To investigate whether either the existence of intramyocardial inflammation with high perforin or the severity of baseline cardiac dysfunction were predictive of survival in univariate analyses, Figure 1B shows that during the follow‐up period, patients with CMi with increased perforin but low cardiac dysfunction (LVEF >35%) had a significantly increased risk of cardiovascular death in contrast to those without intramyocardial inflammation. Of note, patients with CMi with increased perforin and severe cardiac dysfunction had the worst prognosis (non‐CMi, LVEF >35% versus CMi perforin positive, LVEF >35% [P=0.004]; non‐CMI, LVEF ≤35% versus CMi perforin positive, LVEF ≤35% [P=0.02]).
In addition, perforin and LVEF are significant predictors in patients with CMi as indicated by multivariable analysis (Table 3).
Unexpectedly, male sex emerged as another strong predictor of adverse survival in group I patients with confirmed CMi, with a hazard ratio of 1.863 (CI, 1.096–3.168; P=0.022) (Table 3, Figure 2). Age‐ and LVEF‐adjusted Cox regression analysis revealed a significant mutually reinforcing interaction between inflammation and sex. Whereas LVEF course was adversely affected by myocardial perforin,6 multivariable analysis shows that LVEF explains only part of the observed overall mortality.
Figure 2.
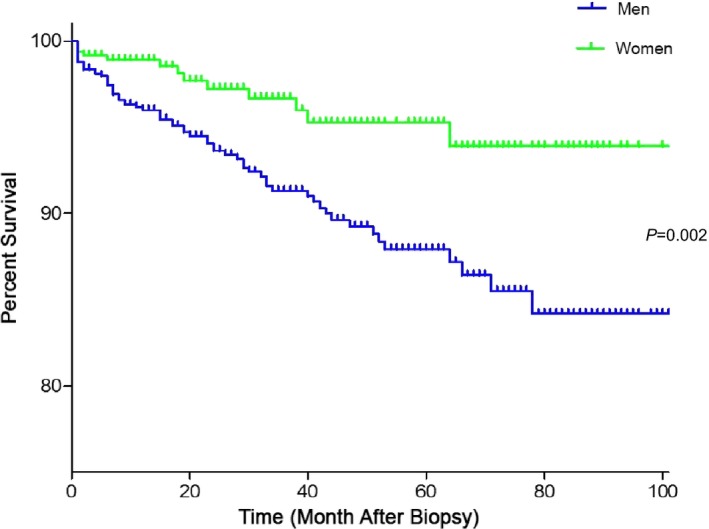
Survival rate among patients with inflammatory cardiomyopathy (CMi) according to sex. Male sex emerged as another strong predictor of adverse survival in patients in group I with confirmed CMi.
Immunohistochemical staining verifies that perforin‐positive cells attacked their targets, gradually beginning colocalized cardiomyocyte destruction. Representative aspects of immunohistologically detected infiltrates are shown in Figure 3.
Figure 3.
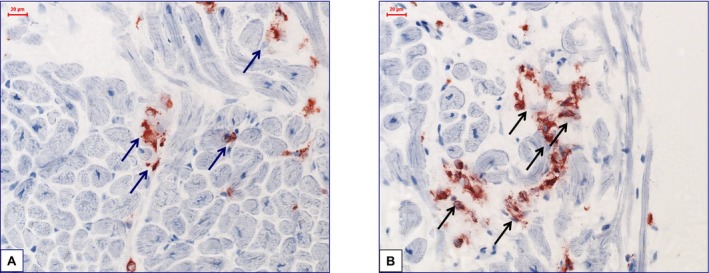
Representative images of immunohistological staining from frozen samples. A and B, Increased perforin‐positive cardiac cell infiltration with focal infiltration pattern and partially beginning of cardiomyocyte destruction in a patient with perforin‐positive inflammatory cardiomyopathy (×400).
Discussion
In a previous study6 we found in a large cohort of 495 patients with EMB‐proven myocardial inflammation that left ventricular function rapidly deteriorates in patients with perforin‐positive cardiac cell infiltration above a calculated critical cutoff value of 2.9 cells/mm2 despite continued use of heart failure medication. In contrast, perforin infiltration <2.9 cells/mm2 or lack of perforin‐positive cardiac infiltrates in first EMBs was associated with spontaneous LVEF improvement. However, the possible influence of perforin‐positive cardiac cell infiltration on survival in patients with CMi has not yet been studied.
The current study shows that high perforin‐positive cardiac cell infiltration (>2.9 cells/mm2 perforin‐positive status) is not only a risk factor for progression of cardiac dysfunction, but also a significant independent risk factor for mortality or need of heart transplantation. Whereas LVEF course is known to be adversely affected by myocardial perforin,6 multivariable analysis in the current large CMi patient cohort revealed that LVEF explains only part of the observed overall mortality rate. The current study clearly demonstrates a significant influence of a peculiar cardiac inflammatory status (high perforin‐positive cell infiltration) on survival in human myocardial disease. To our knowledge, this has been shown only in animal models of cardiomyopathies.7 Unexpectedly, male sex emerged as another independent strong adverse predictor of survival in CMi (hazard ratio, 1.863; CI, 1.096–3.168 [P=0.022]).
Beyond this, the current data are consistent with the hypothesis that “local” cardiac perforin‐positive status reflects a peculiar status of a patient's immune system in general. “General” perforin‐positive status would then not only predispose the patient to accelerated perforin‐positive cell migration into the heart, but also to a generally aggravated perforin‐associated response to diverse injuries. In fact, several important recent studies have shown aggravated perforin‐associated immunological responses in genetically perforin‐deficient or otherwise perforin‐depleted animal models and in patient cohorts with myocardia,6, 7, 8, 9, 10, 11, 12, 13 vascular,14, 15, 16 and other diseases.17, 18 Across several important cardiovascular pathologies, perforin‐mediated mechanisms play important roles during pathogenesis. Thus, a perforin‐mediated mechanism controls cardiac inflammation in Chagas disease,12 perforin is an important marker of cardiac transplant rejection,9, 13 and CD4‐positive natural killer T cells potently augment atherosclerosis by perforin‐ and granzyme B–dependent cytotoxicity.14, 15, 16
Additional knowledge regarding the immune cell populations that contribute to the complement of perforin‐positive cells detected and quantitated in our patients’ EMBs could provide novel insight into CMi etiology at the cellular level. In a post hoc subgroup analysis of 230 patients, there was no significant correlation between the numbers of CD8‐positive cells and perforin‐positive cells (P=0.3, r=0.07) (Figure S1), suggesting that natural killer cells are a key component of inflammatory activation in patients with CMi. The prognostic role of standard immunohistochemically markers (CD3‐positive cells, macrophages, and human leukocyte antigen) was demonstrated in regard to cardiovascular death and need for heart transplantation, but perforin staining was not employed in these studies.19, 20 Regarding the possible origins for the observed spectrum of patients with CMi who had high to undetectable cardiac perforin, genetic polymorphisms may account for differences in perforin‐associated immune processes.21, 22, 23
Thus, there are sex‐associated differences of perforin polymorphisms in the susceptibility to multiple sclerosis,22 but there are no data thus far regarding a role in cardiovascular diseases. In addition to such genetically determined differences in an individual's immune constitution, there are well‐known grave sex differences regarding inflammatory processes in general.22, 23, 24, 25, 26 In mice, there are gross differences between male and female mice with identical genetic backgrounds regarding the extent of myocardial inflammation.27 From these animal data, it might be deduced that the adverse survival effect of men with CMi (hazard ratio, 1.863; CI, 1.096–3.168 [P=0.022]) also reflects an unfavorable immune response in men. Statistical analysis did not, however, detect significant interaction between male sex and perforin‐positivity in humans, but rather important sex differences in the general immunological responses leading to the observed outcome differences in patients with CMi.
Conclusions
Cardiac perforin‐positive status is associated with progressive cardiac dysfunction and impaired survival in patients with CMi and should be routinely measured to improve prognosis assessment in these patients. Our study has high and immediate clinical impact. First, perforin‐positive status should prompt the clinician to conduct clinical surveillance at narrow intervals. Beyond previous data regarding cardiac dysfunction progression, the new survival data reported here support inclusion of cardiac perforin‐positive status into the routine evaluation of EMBs. Second, the indication for immunosuppressive therapy is thus far commonly based only on a standard set of myocardial inflammation markers.28, 29, 30, 31, 32 Recent studies have demonstrated that immunosuppressive treatment of patients with virus‐negative CMi results in improved LVEF during long‐term follow‐up and significant reduction of cytotoxic cells.33 Controlled prospective immunomodulating clinical trials are needed to clarify whether perforin is also a predictor of responsiveness to therapy, beyond being a marker of disease course and survival. From a scientific perspective, the origins of differential cardiac perforin infiltration need to be further investigated.
Sources of Funding
This study was supported by Deutsche Forschungsgemeinschaft through Collaborative Research Center CRC/TR‐19.
Disclosures
None.
Supporting information
Table S1. Primer Used to Test the Presence of Viral Genome
Figure S1. Regression analysis of perforin‐positive cells vs CD8‐positive T cells in a subgroup of 230 patients.
Acknowledgments
We thank K. Winter, S. Ochmann, and C. Seifert (IKDT) for their excellent expert technical assistance.
(J Am Heart Assoc. 2017;6:e005352 DOI: 10.1161/JAHA.116.005352.)28862949
References
- 1. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 2. Schultheiss HP, Kuhl U, Cooper LT. The management of myocarditis. Eur Heart J. 2011;32:2616–2625. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 4. Cooper LT Jr. Myocarditis. N Engl J Med. 2009;360:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu P, Mason J. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. [DOI] [PubMed] [Google Scholar]
- 6. Escher F, Kühl U, Lassner D, Stroux A, Westermann D, Skurk C, Tschöpe C, Poller W, Schultheiss HP. Presence of perforin in endomyocardial biopsies of patients with inflammatory cardiomyopathy predicts poor outcome. Eur J Heart Fail. 2014;16:1066–1072. [DOI] [PubMed] [Google Scholar]
- 7. Fairweather D, Cooper LT Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young LH, Joag SV, Zheng LM, Lee CP, Lee YS, Young JD. Perforin‐mediated myocardial damage in acute myocarditis. Lancet. 1990;336:1019–1021. [DOI] [PubMed] [Google Scholar]
- 9. Legros‐Maïda S, Soulié A, Benvenuti C, Wargnier A, Vallée N, Berthou C, Guillet J, Sasportes M, Sigaux N. Granzyme B and perforin can be used as predictive markers of acute rejection in heart transplantation. Eur J Immunol. 1994;24:229–233. [DOI] [PubMed] [Google Scholar]
- 10. Chun‐yan G, Bo H, Hong C, Hong‐lei J, Xiu‐zhen H. Anti‐perforin neutralizing antibody reduces myocardial injury in viral myocarditis. Cardiol Young. 2009;19:601–607. [DOI] [PubMed] [Google Scholar]
- 11. Noutsias M, Pauschinger M, Schultheiss HP, Kühl U. Cytotoxic perforin+ and TIA‐1+ infiltrates are associated with cell adhesion molecule expression in dilated cardiomyopathy. Eur J Heart Fail. 2003;5:469–479. [DOI] [PubMed] [Google Scholar]
- 12. Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15:388–400. [DOI] [PubMed] [Google Scholar]
- 13. Keslar K, Rodriguez ER, Tan CD, Starling RC, Heeger PS. Complement gene expression in human cardiac allograft biopsies as a correlate of histologic grade of injury. Transplantation. 2008;86:1319–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, To K, Kanellakis P, Hosseini H, Deswaerte V, Tipping P, Smyth MJ, Toh BH, Bobik A, Kyaw T. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin‐ and granzyme B‐dependent cytotoxicity. Circ Res. 2015;116:245–254. [DOI] [PubMed] [Google Scholar]
- 15. Selathurai A, Deswaerte V, Kanellakis P, Tipping P, Toh BH, Bobik A, Kyaw T. Natural killer (NK) cells augment atherosclerosis by cytotoxic‐dependent mechanisms. Cardiovasc Res. 2014;102:128–137. [DOI] [PubMed] [Google Scholar]
- 16. Hiebert PR, Boivin WA, Zhao H, McManus BM, Granville DJ. Perforin and granzyme B have separate and distinct roles during atherosclerotic plaque development in apolipoprotein E knockout mice. PLoS One. 2013;8:e78939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plantone D, Marti A, Frisullo G, Iorio R, Damato V, Nociti V, Patanella AK, Bianco A, Mirabella M, Batocchi AP. Circulating CD56dim NK cells expressing perforin are increased in progressive multiple sclerosis. J Neuroimmunol. 2013;265:124–127. [DOI] [PubMed] [Google Scholar]
- 18. Samaka RM, Gaber MA, Metwe NA. Perforin expression in plaque psoriasis: an immunohistochemical study. Ultrastruct Pathol. 2015;39:110–120. [DOI] [PubMed] [Google Scholar]
- 19. Mahon NG, Madden BP, Caforio AL, Elliott PM, Haven AJ, Keogh BE, Davies MJ, McKenna WJ. Immunohistologic evidence of myocardial disease in apparently healthy relatives of patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;39:455–462. [DOI] [PubMed] [Google Scholar]
- 20. Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. [DOI] [PubMed] [Google Scholar]
- 21. Camiña‐Tato M, Morcillo‐Suárez C, Bustamante MF, Ortega I, Navarro A, Muntasell A, López‐Botet M, Sánchez A, Carmona P, Julià E, Tortola MT, Audí L, Oksenberg JR, Martin R, Montalban X, Comabella M. Gender‐associated differences of perforin polymorphisms in the susceptibility to multiple sclerosis. J Immunol. 2010;185:5392–5404. [DOI] [PubMed] [Google Scholar]
- 22. Tejera‐Alhambra M, Alonso B, Teijeiro R, Ramos‐Medina R, Aristimuño C, Valor L, de Andrés C, Sánchez‐Ramón S. Perforin expression by CD4+ regulatory T cells increases at multiple sclerosis relapse: sex differences. Int J Mol Sci. 2012;13:6698–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin‐deficient mice. Nature. 1994;369:31–37. [DOI] [PubMed] [Google Scholar]
- 24. Lefèvre N, Corazza F, Duchateau J, Desir J, Casimir G. Sex differences in inflammatory cytokines and CD99 expression following in vitro lipopolysaccharide stimulation. Shock. 2012;38:37–42. [DOI] [PubMed] [Google Scholar]
- 25. Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. [DOI] [PubMed] [Google Scholar]
- 26. Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol. 2006;290:H2043–H2050. [DOI] [PubMed] [Google Scholar]
- 27. Huber S, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis‐diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. [DOI] [PubMed] [Google Scholar]
- 29. Schultheiss HP, Piper C, Sowade O, Waagstein F, Kapp JF, Wegscheider K, Groetzbach G, Pauschinger M, Escher F, Arbustini E, Siedentop H, Kuehl U. Betaferon in chronic viral cardiomyopathy (BICC) trial: effects of interferon‐β treatment in patients with chronic viral cardiomyopathy. Clin Res Cardiol. 2016;105:763–773. [DOI] [PubMed] [Google Scholar]
- 30. Kania G, Blyszczuk P, Müller‐Edenborn B, Eriksson U. Novel therapeutic options in inflammatory cardiomyopathy. Swiss Med Wkly. 2013;143:w13841. [DOI] [PubMed] [Google Scholar]
- 31. Ruschitzka F, Abraham WT. Heart failure: in search of improved therapies for acute heart failure. Nat Rev Cardiol. 2011;8:9–10. [DOI] [PubMed] [Google Scholar]
- 32. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus‐negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30:1995–2002. [DOI] [PubMed] [Google Scholar]
- 33. Escher F, Kuehl U, Lassner D, Poller W, Westermann D, Pieske B, Tschoepe C, Schultheiss HP. Long‐term outcome of patients with virus‐negative inflammatory cardiomyopathy after immunosuppressive therapy. Clin Res Cardiol. 2016;105:1011–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer Used to Test the Presence of Viral Genome
Figure S1. Regression analysis of perforin‐positive cells vs CD8‐positive T cells in a subgroup of 230 patients.


