Abstract
Background
We examined the role of menopause on cardiac dimensions and function and assessed the efficacy of exercise training before and after menopause.
Methods and Results
Two groups of healthy premenopausal (n=36, 49.4±0.3 years) and postmenopausal (n=37, 53.5±0.5 years) women with no history of cardiovascular disease and with a mean age difference between groups of only 4 years were studied. Cardiac dimensions and systolic and diastolic function were determined by transthoracic echocardiography with tissue Doppler imaging and 2‐dimensional speckle tracking. Measurements were performed at baseline and after a 12‐week period of high‐intensity aerobic cycle training. LV internal diastolic diameter and LV mass were similar in the 2 groups at baseline and increased by ≈2% to 8% (P=0.04–0.0007) with training in both groups. Left atrial end‐diastolic and end‐systolic volumes were similar for both groups and increased by 23% to 36% (P=0.0006–0.0001) with training. Systolic function assessed by mean global strain was similar in both groups at baseline and increased by ≈8% (P=0.0004) with training in the postmenopausal group. LV displacement increased by ≈3% (P=0.04) in the premenopausal women only. Diastolic function assessed by E/A ratio was similar at baseline and increased by ≈7% (P=0.01) in the premenopausal group and 11% (P=0.0001) in the postmenopausal group with training.
Conclusions
These results suggest that training‐induced cardiac adaptations are preserved in the early postmenopausal phase. Furthermore, the hormonal changes associated with the menopausal transition do not appear to affect cardiac dimensions and function.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02135575.
Keywords: E/A ratio, exercise training, menopause, transthoracic echocardiography, women
Subject Categories: Basic Science Research, Physiology, Women, Primary Prevention, Exercise
Clinical Perspective
What Is New?
This study shows that aerobic training improves cardiac structure and function in middle‐aged women irrespective of menopausal status.
What Are the Clinical Implications?
Implementation of aerobic training can be used as a safe prophylactic strategy to oppose deteriorations in cardiac function known to be prevalent in older postmenopausal women.
Introduction
Cardiovascular disease is the leading cause of death in women globally and constitutes a major socioeconomic burden for society.1, 2 Risk factors for cardiovascular disease include smoking, physical inactivity, age, metabolic syndrome, and loss of estrogen in women.2 Estrogen has been shown to have a protective effect on the cardiovascular system in women and the risk for cardiovascular events increases markedly after the menopausal transition when substantial hormonal changes occur, including the loss of estrogen production.3 However, the role of age versus hormonal changes for cardiovascular risk after menopause in women remains debated,4 in part because of the fact that comparisons of premenopausal and postmenopausal women are often conducted with significant age differences between the groups.5 We and others have recently provided support for the notion that the menopausal transition per se leads to rapid impairments in vascular function.6, 7 In Nyberg et al,7 leg vascular function, assessed by infusion of vasoactive compounds, was markedly impaired in recent postmenopausal women compared with age‐matched premenopausal women. Although evidence for altered cardiac structure and function exists in older postmenopausal women,8, 9 it is unclear to what extent these alterations are detectable in the first few years after the menopausal transition.
Aerobic exercise training is well known to induce improvements in maximal oxygen uptake (VO2max) in sedentary individuals.10, 11 This improvement is mainly a consequence of an increase in cardiac dimensions and function12, 13 although an enhanced blood volume may also contribute to improved ventricular filling.14 Cardiac adaptations in response to aerobic training in sedentary individuals have been documented in a number of studies,15, 16, 17 but adaptations appear to be smaller in premenopausal women than in men.18, 19, 20 Hormonal changes after menopause may further reduce the ability of women to achieve cardiovascular adaptations in response to exercise training6, 21 and it has been proposed that estrogen supplementation is required for vascular adaptations in response to physical activity in postmenopausal women.22 However, findings in the literature on adaptations to training may also vary as a result of different training regimens, where high‐intensity aerobic training may be more effective than low‐ to moderate‐intensity exercise.23, 24 A few studies have examined cardiac function and structure in postmenopausal elite athletes,25, 26 but studies comparing the effect of training on cardiac adaptations in premenopausal versus postmenopausal women are lacking. This knowledge is of clinical importance, in particular for recommendations of hormonal therapy and physical activity in the prevention of cardiovascular disease in middle‐aged women.
The method used for determination of cardiac function may be a decisive factor for the ability to detect early changes after menopause and adaptations to exercise. Most studies on cardiac adaptations to physical activity have utilized conventional echocardiographic measures, but development of advanced imaging modalities in recent years with the advent of tissue Doppler imaging (TDI) and 2‐dimensional speckle tracking have allowed for more sensitive detection of early subclinical changes in systolic and diastolic function.27, 28, 29, 30 These modalities provide sensitive assessment of subclinical changes in LV contractility and relaxation.31 Previous studies have found subclinical indications of altered systolic function by TDI in patients with normal ventricular diastolic function and ejection fraction32 supporting that these modalities provide more sensitive measures of early changes. Moreover, cardiac dysfunction determined by TDI has been shown to be an independent predictor of mortality.33
The aim of the present study was 2‐fold: (1) to compare cardiac dimensions and systolic and diastolic function in premenopausal and early postmenopausal women with a small age difference and (2) to assess the efficacy of 12 weeks of high‐intensity aerobic training on cardiac adaptations in premenopausal and recent postmenopausal women. We hypothesized that recent postmenopausal women would have similar cardiac dimensions but impaired systolic and diastolic function compared with premenopausal women and that high‐intensity aerobic exercise training would be more effective in inducing cardiac adaptations in premenopausal than postmenopausal women.
Methods
Participants
Participants were recruited from the Copenhagen area through advertisement in local newspapers. Study participants were premenopausal or postmenopausal women with an age range of 45 to 57 years (Table 1). The participants were all informed about potential risks and discomforts associated with the study and written consent was signed before the study experiments.
Table 1.
General Characteristics
| Premenopausal | Postmenopausal | Group Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Training Effect | Baseline | 12 Weeks | Training Effect | Baseline | 12 Weeks | |
| n=36 | P Value | n=37 | P Value | P Value | P Value | |||
| Anthropometrics | ||||||||
| Age, y | 49.4±2.1 | 53.3±3.0 | <0.0001 | |||||
| Height, m | 1.69±0.01 | 1.67±0.01 | 0.68 | |||||
| Weight, kg | 67.4±6.9 | 66.7±6.8 | 0.03 | 66.3±8.5 | 65.8±8.7 | 0.14 | 0.54 | 0.60 |
| Body mass index, kg/m2 | 23.9±2.4 | 23.7±2.3 | 0.02 | 23.7±2.4 | 23.5±2.6 | 0.17 | 0.62 | 0.71 |
| Cardiorespiratory fitness | ||||||||
| Resting heart rate, beats/min | 67±8 | 64±8 | 0.006 | 64±7 | 61±8 | <0.0001 | 0.15 | 0.07 |
| VO2max, mL/min | 2068±295 | 2283±291 | <0.001 | 2017±217 | 2204±230 | <0.0001 | 0.40 | 0.22 |
| Maximum heart rate, beats/min | 177±12 | 175±9 | 0.03 | 174±11 | 172±9 | 0.27 | 0.55 | 0.19 |
| Maximum RER VO2max | 1.31±0.10 | 1.27±0.08 | 0.02 | 1.30±0.08 | 1.29±0.09 | 0.40 | 0.61 | 0.46 |
| Maximum power VO2max, W | 194±26 | 222±26 | <0.001 | 189±21 | 215±21 | <0.0001 | 0.35 | 0.25 |
| Systolic BP, mm Hg | 111±15 | 113±15 | 0.40 | 113±16 | 110±13 | 0.11 | 0.56 | 0.38 |
| Diastolic BP, mm Hg | 72±11 | 73±11 | 0.92 | 73±10 | 70±8 | 0.03 | 0.68 | 0.26 |
| Cholesterol | ||||||||
| Total cholesterol, mmol/L | 4.90±0.73 | 4.76±0.63 | 0.11 | 5.64±0.72 | 5.48±0.61 | 0.03 | <0.0001 | <0.0001 |
| Training | ||||||||
| Total training sessions | 37±6 | 38±5 | 0.82 | |||||
| Time in intervals of HRmax, % | ||||||||
| 60% to 85% | 58±10 | 56±10 | 0.66 | |||||
| 86% to 100% | 41±12 | 41±13 | 0.97 | |||||
BP indicates blood pressure; HRmax, maximum heart rate; RER, respiratory exchange ratio; VO2max, maximal oxygen uptake.
Values are expressed as mean±SD.
The study was approved by the ethics committee of Copenhagen and Frederiksberg municipalities Region H (H‐1‐2012‐150) and conducted in accordance with the guidelines of the Declaration of Helsinki. All participants signed an informed consent form before participation in the study. The study was registered at ClinicalTrials.gov (NCT02135575).
The premenopausal women were all healthy with regular menstrual cycles. The postmenopausal women were all healthy and had not experienced a menstrual cycle during the previous 12 months but were less than 8 years past their final menstrual period. Menopausal status was verified by measurements of hypothalamic and reproductive hormones from a blood sample (estradiol <0.20 nmol/L and follicle‐stimulating hormone: 22–138 IU/L, indicative of postmenopausal status). If the blood samples were indicative of perimenopause hormone levels, the women were excluded. Inclusion criteria were age 45 to 57 years, body mass index <30, and <2 hours of light to moderate physical activity per week of age. Exclusion criteria were smoking, use of hormonal contraceptives, hormone replacement treatment, prescription medicine, hypertension, cardiovascular disease, renal dysfunction, insulin resistance, diabetes mellitus, and hypercholesterolemia.
None of the participants had pathological ECG findings that were incompatible with the exercise intervention on examination. To ensure that all participants were normotensive, blood pressure (BP) was measured 7 consecutive times by an automatic upper‐arm BP monitor (M7, OMRON) after at least 15 minutes of rest in the supine position. Heart rate (HR) was measured during the BP monitoring.
The data presented are part of a larger study on the effects of exercise training on women in the menopausal transition funded by the University of Copenhagen Excellence Programme for Interdisciplinary Research and the data presented in Table 1 have previously been published.7, 34
Study Design
The study was part of a larger study on the effect of menopause and physical activity on cardiovascular and metabolic health. The overall study design was a prospective intervention study with one group of premenopausal and one group of postmenopausal women. The women were selected to be close in age to minimize age as a confounder. All women underwent a 12‐week period of high‐intensity aerobic training with 3 sessions per week. For the present part of the study, the women underwent echocardiography measurements, a test for VO2max, and measurements of BP before and after the intervention period.
Exercise Training Intervention
All participants underwent the exercise training intervention. The training was performed on a cycle ergometer (Body Bike). Instructors from the research group supervised 2 training sessions per week and instructors from a local fitness center supervised 1 session. HR was monitored during all training sessions (TEAM2 Wearlink+, Polar). The training sessions were characterized by ≈50 minutes of intermittent high‐intensity intervals where the participants reached HRs over 85% of maximum HR (Table 2). For more information and details on the participants’ variation in exercise intensities during the training see Nyberg et al7 and Mandrup et al.34
Table 2.
Echocardiographic measures before and after 12 weeks of high aerobic intensity cycle training
| Echocardiographic Variables | Premenopausal | Postmenopausal | Group Differences | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n=36 | Training Effect | n=37 | Training Effect | Baseline | 12 Weeks | ||||
| Unit | Baseline | 12 Weeks | P Value | Baseline | 12 Weeks | P Value | P Value | P Value | |
| LV morphology | |||||||||
| LVIDD | cm | 4.5±0.1 | 4.6±0.1 | 0.0007 | 4.5±0.1 | 4.6±0.1 | 0.04 | 0.56 | 0.94 |
| LVIDS | cm | 2.8±0.8 | 2.7±0.8 | 0.064 | 2.9±0.8 | 2.8±0.8 | 0.49 | 0.61 | 0.15 |
| LVEDV | mL | 93.2±2.6 | 101.7±2.6 | 0.0006 | 95.4±2.5 | 97.6±2.5 | 0.36 | 0.36 | 0.25 |
| LVESV | mL | 37.5±1.7 | 42.0±1.7 | 0.054 | 40.3±1.7 | 41.8±1.7 | 0.49 | 0.50 | 0.93 |
| LV stroke volume | mL | 55.6±1.9 | 59.7±1.9 | 0.064 | 55.1±1.9 | 55.8±1.9 | 0.76 | 0.85 | 0.15 |
| LV mass index | g/m2 | 76.9±2.5 | 82.1±2.5 | 0.001 | 81.4±2.4 | 87.6±2.4 | 0.001 | 0.19 | 0.11 |
| LV mass | g | 136±5 | 145±5 | 0.001 | 142±5 | 152±5 | 0.002 | 0.35 | 0.25 |
| IVSD | cm | 0.92±0.2 | 0.92±0.02 | 0.91 | 0.94±0.02 | 0.97±0.02 | 0.08 | 0.42 | 0.053 |
| IVSS | cm | 1.32±0.04 | 1.37±0.04 | 0.23 | 1.35±0.04 | 1.37±0.04 | 0.69 | 0.54 | 0.96 |
| LVPWd | cm | 0.93±0.02 | 0.926±0.02 | 0.97 | 0.94±0.02 | 0.95±0.02 | 0.68 | 0.77 | 0.55 |
| LVPWs | cm | 1.64±0.05 | 1.80±0.05 | 0.002 | 1.74±0.05 | 1.78±0.05 | 0.37 | 0.16 | 0.81 |
| Systolic function | |||||||||
| EF, biplane | % | 59.3±1.3 | 58.8±1.3 | 0.79 | 57.9±1.3 | 57.5±1.3 | 0.85 | 0.45 | 0.50 |
| GLS | % | −19.8±0.4 | −20.2±0.4 | 0.27 | −19.3±0.4 | −20.8±0.4 | 0.0004 | 0.38 | 0.25 |
| s′TDI color, mean | cm/s | 6.1±0.2 | 6.3±0.3 | 0.41 | 5.7±0.2 | 5.8±0.2 | 0.55 | 0.10 | 0.07 |
| Pulsed TDI s′, mean | cm/s | 8.3±0.2 | 8.0±0.2 | 0.12 | 7.6±0.2 | 7.4±0.2 | 0.39 | 0.003 | 0.02 |
| LV displacement | mm | 12.4±0.2 | 12.8±0.2 | 0.04 | 11.8±0.2 | 12.1±0.2 | 0.16 | 0.09 | 0.04 |
| Diastolic function | |||||||||
| Mitral inflow velocity E/A ratio | ··· | 1.29±0.05 | 1.38±0.05 | 0.01 | 1.20±0.04 | 1.32±0.04 | 0.0001 | 0.13 | 0.40 |
| Mitral deceleration time | ms | 207±8 | 185±8 | 0.02 | 190±8 | 196±8a | 0.48 | 0.11 | 0.32 |
| E/e′ | ··· | 6.5±0.3 | 7.1±0.3 | 0.001 | 7.0±0.3 | 7.5±0.3 | 0.02 | 0.13 | 0.19 |
| a′TDI color, mean | cm/s | −6.5±0.2 | −6.1±0.2 | 0.025 | −6.4±0.2 | −6.5±0.2a | 0.49 | 0.78 | 0.11 |
| Pulsed TDI a′, mean | cm/s | 9.1±0.2 | 8.9±0.2 | 0.33 | 9.2±0.2 | 9.0±0.2 | 0.62 | 0.92 | 0.69 |
| e′TDI color, mean | cm/s | −9.0±0.3 | −9.2±0.3 | 0.22 | −7.9±0.2 | −8.0±0.2 | 0.53 | 0.002 | 0.0004 |
| Pulsed TDI e′, mean | cm/s | 11.6±0.3 | 11.3±0.3 | 0.18 | 10.1±0.3 | 10.1±0.3 | 0.87 | 0.0002 | 0.01 |
| IVRT, mean | ms | 86±2 | 87±2 | 0.54 | 96±2 | 94±2 | 0.26 | 0.002 | 0.03 |
| Mitral valve E velocity | m/s | 0.81±0.02 | 0.87±0.02 | 0.0002 | 0.77±0.02 | 0.82±0.02 | 0.002 | 0.20 | 0.08 |
| Mitral valve A velocity | m/s | 0.64±0.02 | 0.65±0.02 | 0.54 | 0.66±0.02 | 0.64±0.02 | 0.19 | 0.46 | 0.78 |
| Left atrium | |||||||||
| LAEDV | mL | 22.9±1.5 | 28.4±1.5 | 0.0006 | 23.5±1.5 | 31.6±1.5 | <0.0001 | 0.77 | 0.13 |
| LAEDV index | mL/m2 | 12.9±0.8 | 16.3±0.8 | 0.0004 | 13.3±0.8 | 18.2±0.8 | <0.0001 | 0.73 | 0.10 |
| LAESV index | mL/m2 | 29.4±1.2 | 36.1±1.2 | <0.0001 | 30.3±1.2 | 37.8±1.2 | <0.0001 | 0.59 | 0.31 |
| LAESV | mL | 51.9±2.2 | 63.3±2.2 | <0.0001 | 51.2±2.2 | 65.6±2.2 | <0.0001 | 0.67 | 0.45 |
| Right ventricle | |||||||||
| TAPSE | cm | 2.59±0.1 | 2.62±0.1 | 0.63 | 2.55±0.1 | 2.55±0.1 | 0.99 | 0.59 | 0.36 |
| Pulsed TDI s′ | cm/s | 13.6±0.3 | 13.9±0.4 | 0.34 | 13.4±0.3 | 13.3±0.3 | 0.58 | 0.79 | 0.22 |
EF indicates ejection fraction; GLS, global longitudinal strain; IVRT, isovolumic relaxation time; IVSD, left ventricular interventricular septum diastole; IVSS, left ventricular interventricular septum systole; LAEDV, left atrial diastolic volume; LAESV, left atrial systolic volume; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; LVIDD, left ventricular end‐diastolic diameter; LVIDS, left ventricular end‐systolic diameter; LVPWd, left ventricular posterior wall thickness diastole; LVPWs, left ventricular posterior wall thickness systole; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging.
Values are expressed as mean±SEM.
P<0.05: significant delta change with training between group.
Echocardiography
Transthoracic echocardiography was performed using a GE Vivid 9 ultrasound machine with a 2.5‐MHz transducer (GE Healthcare). The echocardiographic examinations were performed by 2 investigators but the examination before and after the training intervention were always performed by the same investigator for each participant. During the examination, participants rested in a supine position on their left side in a darkened room. All examinations were analyzed offline in unidentifiable random order, using the Echo Pac software version BT 13.0 (GE Healthcare). To reduce analytical variability, all echocardiographic examinations were analyzed by one of the investigators.
All participants were examined with the same protocol as previously described.35 In short, cardiac structure was evaluated from parasternal long axis 2‐dimensional recordings according to current guidelines.36 The calculated left ventricular (LV) mass was indexed according to body surface area (m2=0.20247×height [m]0.725×weight [kg]0.425, DuBois formula). LV volume and LV ejection fraction (%) were evaluated using Simpson's biplane method. Peak transmitral blood inflow velocity in early (E) and late (A) diastole and the corresponding E/A ratio was measured using pulsed‐wave Doppler in the apical 4‐chamber view. Peak systolic velocity (s′; cm/s), peak early diastolic velocity (e′; cm/s), and peak late diastolic velocity (a′; cm/s) were measured with pulsed‐wave TDI in the 6 LV segments at the level of the mitral annulus. The value of e′ represented the average of the segments of early peak diastolic velocities. Color TDI (TDIcolor) at a frame rate >120/s was evaluated at the 6 mitral annular sites and values were averaged. Measurements included peak systolic velocity (s′TDI color), peak early diastolic velocity (e′TDI color), and peak late diastolic velocity (a′TDI color). LV displacement was evaluated using tissue tracking. LV systolic function was evaluated by speckle tracking analysis where a semiautomated function tracks speckles from frame to frame identified within a specified region of interest and reported as the absolute value of global longitudinal strain.
Right ventricular function was evaluated as tricuspid annular plane systolic excursion and pulsed‐wave TDI derived peak systolic velocity (s′).
Determination of VO2max
VO2max was measured with an Oxycon Pro (Intramedic, Denmark). The protocol was an incremental exercise test on a cycling ergometer (Monark, E9). The participants started with a 10‐minute warm‐up and thereafter the test was initiated with a start load of 50 W and increased by 25 W/min until volitional fatigue. Criteria for determination of VO2max were: a plateau in VO2, even with increased workload and/or respiratory exchange ratio >1.1 and/or an HR >90% of expected value. Two of 3 criteria had to be attained before the test was approved.
The VO2max tests as well as the echocardiography measurements were conducted in the weeks before the initiation of training and between 2 and 5 days after the training.
HR Monitoring and Compliance of Training
The participants had an individual HR monitor (TEAM2 Wearlink+, Polar) to record HR during the training sessions and ensure the training quality and that intervals reached a target HR >85% of maximum HR. All training sessions were registered throughout the training period.
Statistical Analysis
Our hypotheses could be directly evaluated as differences between groups and within groups in a linear mixed model framework. Fixed‐effects factors were “group” (premenopausal, postmenopausal) and “time” (before training, after training). Between‐subject variation was modeled using random effects. Model assumptions on homogeneity of variance and normal distribution were confirmed through residual and Q–Q plots. Pairwise differences were performed using post hoc t tests (based on the mixed models); no adjustment for multiplicity was applied. Data are reported as mean±SEM. The statistical analysis was executed with R version 3.2.2 (R Core Team, 2015) through the interface RStudio (RStudio Team [2015]. RStudio: Integrated Development for R. RStudio, Inc.) and the extension packages lme4 and multcomp. A significance level of 0.05 was used. Sample size was calculated on the basis of detecting a 10% change in E/A ratio with training, with a power of 0.8 and a significance level of 0.05.
Results
Participant Characteristics
A total of 83 participants (43 premenopausal and 40 postmenopausal) were initially recruited. A CONSORT flow diagram for the participants has been included in Mandrup et al.34 Ten participants were either excluded or dropped out because of illness (1), during run‐in (4), because of insufficient training adherence (2), and because of pregnancy (1). Two premenopausal women were not assessed by echocardiography for logistic reasons. Therefore, a total of 36 premenopausal and 37 postmenopausal women were included in the study. All of the included participants fulfilled the training intervention and participated in the echocardiography examination both before and after the intervention period. The premenopausal women participated on average in 37±6 training sessions (≈93%) and the postmenopausal women in 38±5 training sessions (≈95%). Systolic arterial BP was similar in the 2 groups at baseline and after the training period. In the postmenopausal group, diastolic BP decreased (P=0.03) during the training period (by −4.3%) with no change in systolic BP. BP did not change significantly with training in the premenopausal group. It is important to point out that BP data were previously reported on these groups of women and were found to be unaltered with training.34 The reason for this discrepancy is likely that the number of included participants was lower in the present group and there was a slight difference in statistical approach.
HR at rest was similar in the 2 groups both at baseline and after the training period. There was a decrease (P=0.0001) in resting HR after the training period in the postmenopausal group (5.2%), but the change in HR at rest in the premenopausal group did not reach statistical significance. Data on low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglycerides are presented elsewhere.34
Cardiac Structure and Function
Intervariability and intravariability of measurements
A sample of 10 participants was reanalyzed for intraobserver and interobserver variability by 2 investigators. The results showed a variation between 0% and 15.2% for the different common variables for interobserver and between 0% and 6.7% for the intraobserver variation.
Cardiac dimensions
Left atrial end‐diastolic volume index and left atrial end‐systolic volume index were similar in both groups at baseline (Table 2 and Figure 1A). Training increased left atrial end‐diastolic volume index by ≈26 (P=0.0004) and 36% (P<0.0001) and left atrial end‐systolic volume index by ≈23 (P<0.0001) and 25% (P<0.0001)) in the premenopausal and postmenopausal groups, respectively. LV anatomy (determined as LV end‐diastolic volume), LV internal diastolic diameter, and LV mass index were similar in the 2 groups at baseline (Table 2 and Figure 1). Training increased LV end‐diastolic volume by ≈9% (P=0.0006) in the premenopausal group (Figure 2B); LV internal diastolic diameter by ≈3 (P=0.0007) and 2% (0.04) in the premenopausal and postmenopausal groups, respectively (Figure 2C); and LV mass index by ≈7 (P=0.001) and 8% (P=0.001) in the premenopausal and postmenopausal groups, respectively (Figure 2D).
Figure 1.
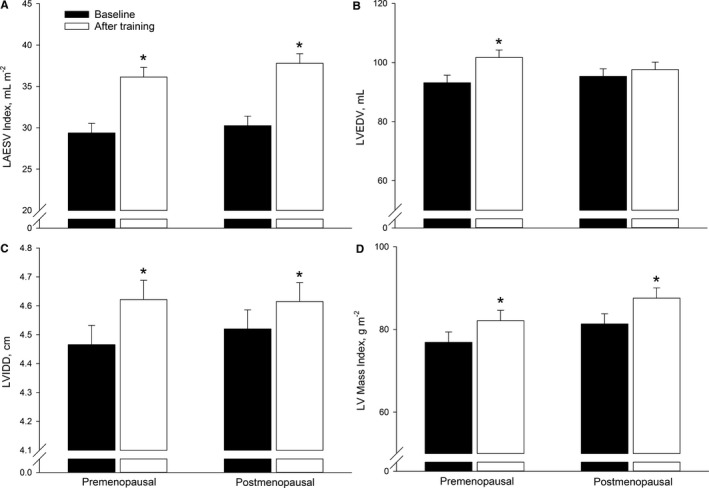
Cardiac structure evaluated as left atrial end‐systolic volume (LAESV) index (A), left ventricular (LV) end‐diastolic volume (LVEDV, B), LV internal diastolic dimension (LVIDD, C), and LV mass index (D) in premenopausal and postmenopausal women at baseline and after training. *P<0.05 significantly different from before training.
Figure 2.
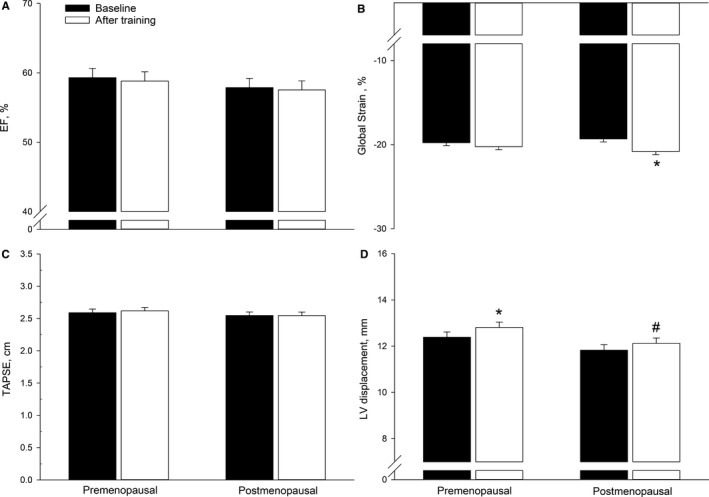
Left ventricular (LV) systolic function evaluated as LV ejection fraction (EF) by Simpson's biplane method (A), 2‐dimensional LV longitudinal global strain (global strain, B), right ventricular function by tricuspid annular plane systolic excursion (TAPSE, C), and LV longitudinal displacement by tissue tracking tissue Doppler imaging (D) in premenopausal and postmenopausal women at baseline and after training. *P<0.05 significantly different from before training, # P<0.05 significantly different from premenopausal at that time point.
Interventricular septum width at diastole and LV posterior wall thickness diastole were similar in both groups at baseline and these variables remained unaltered after the intervention (Table 2).
Systolic function
LV ejection fraction was similar in the 2 groups at baseline and was not altered by the training period in either group (Figure 2A). Global longitudinal strain was similar in both groups at baseline and increased (P=0.0004) in the postmenopausal group only (by ≈8%, Figure 2B). LV longitudinal displacement was similar in both groups at baseline (Figure 2D). In the premenopausal group, LV displacement was significantly higher (P=0.04) after the training period (by ≈3%), whereas no change was detected in the postmenopausal group. LV displacement was ≈6% lower (P=0.04) in the postmenopausal compared with the premenopausal group after the training period. Color TDI (s′TDI color) was similar in both groups at baseline and after the training intervention (Table 2).
Diastolic function
The E/A ratio was similar in both groups at baseline and was higher in ≈7% (P=0.01) and 11% (P=0.0001) in the groups after the training period (Figure 3A). Mitral valve deceleration time was similar in both groups at baseline (Table 2). None of the participants had detectable diastolic dysfunction.
Figure 3.
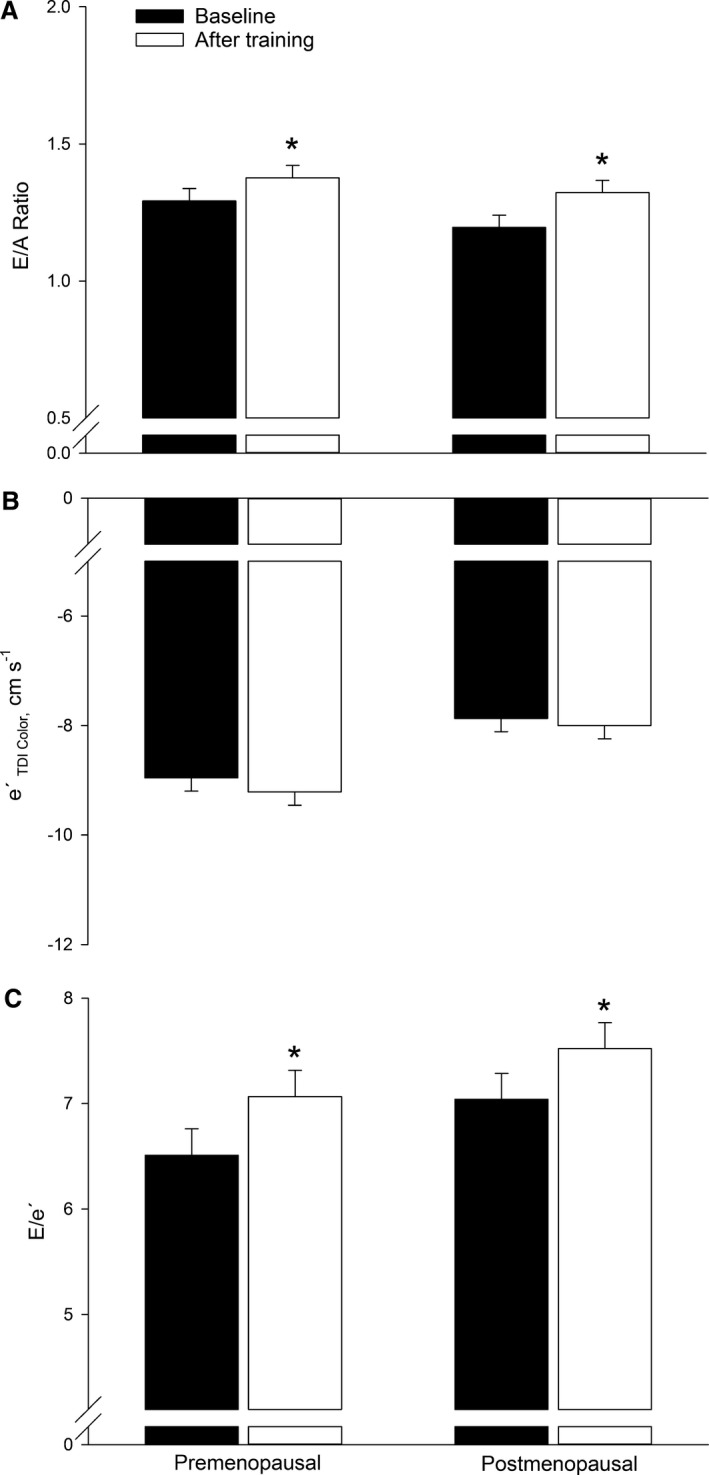
Left ventricular diastolic function evaluated as the ratio between early mitral inflow (E) and late (A) mitral inflow (E/A ratio, A), peak early diastolic velocity (e′, B), and E/e′ (C) in premenopausal and postmenopausal women at baseline and after training. *P<0.05 significantly different from before training.
Mitral valve deceleration time was significantly lower (P=0.02) in the premenopausal (≈11%) but not in the postmenopausal group after the training period. The training‐induced change in mitral valve deceleration time was different (P=0.03) between the 2 groups. The e′ was similar in the 2 groups before training and remained unaltered with training (Figure 3B and 3C). At baseline, isovolumetric relaxation time (IVRT) was ≈12% lower (P=0.002) in the premenopausal compared with the postmenopausal group (Table 2). The training intervention did not alter IVRT in either group.
Right ventricular function
Tricuspid annular plane systolic excursion (Figure 2C) and right ventricular pulsed‐wave TDI s′ was similar in both groups at baseline and remained unaltered with the training intervention.
Discussion
The present study compared cardiac structure and functions in premenopausal and postmenopausal women and assessed the effect of a high‐intensity aerobic training period in the 2 groups. Overall, the main findings were that cardiac dimensions and cardiac systolic and diastolic functions were similar in premenopausal and postmenopausal women separated by only 4 years of age. Moreover, a period of high‐intensity aerobic training induced adaptations in cardiac dimensions as well as systolic and diastolic function in the 2 groups. These findings indicate that cardiac structure and function are not altered in the early postmenopausal phase and that cardiac adaptations to high‐intensity aerobic training are preserved in recent postmenopausal women.
It is well established that estrogen has favorable effects on endothelial and cardiac function5, 37 and loss of estrogen at menopause is associated with an increased risk of cardiovascular disease.38 In the present study, only minor differences in cardiac dimensions and systolic and diastolic function were detected between the premenopausal and recent postmenopausal women at baseline. This finding was therefore surprising and contrasts our own findings of reduced peripheral vascular function in postmenopausal women, as determined in a subgroup of the same premenopausal and postmenopausal women.7 The reason for this discrepancy is unclear; however, it could suggest that impairments in vascular function are manifested before changes in cardiac function are detectable. The current study was specifically designed to achieve a small age difference (4 years) between the groups, and therefore cardiac impairments caused by hormonal changes at menopause may not yet have occurred. This possibility is supported by a study showing that approximately half of postmenopausal women at a mean age of 62 years had mild diastolic dysfunction as indicated by E/A ratios ≤1.0, whereas only very few women had E/A ratios <1.0 in the current study. Another possibility is that estrogen loss is more important for vascular function than for cardiac impairments, for which age may be a more influential factor. However, previous studies have shown that estrogen treatment improves diastolic function in postmenopausal women.39, 40 Thus, at least with time, estrogen is likely to also impact cardiac function.
TDI and 2‐dimensional speckle tracking used in the examinations of the participants allows for a more sensitive measure of early subclinical changes in systolic and diastolic function. Cardiac dysfunction determined by TDI has been shown to independently predict mortality.33 Previous studies have reported training‐induced adaptations in systolic and diastolic function as assessed by TDI in men16, 35, 41 and premenopausal women42, 43 but no studies have examined training‐induced cardiac adaptations in postmenopausal women. We compared a large number of parameters related to cardiac functionality in the contraction phase (systole) and the filling phase of the ventricles (diastole) before and after 12 weeks of high‐intensity aerobic training. Within this relatively short time of training we were able to detect changes in a majority of the parameters determined, confirming the sensitivity of TDI for determination of cardiac functionality.
The finding of similar training‐induced adaptations in the premenopausal and postmenopausal women rejects the study hypothesis of lesser adaptation in postmenopausal compared to premenopausal women. Our hypothesis was based on previous studies showing that postmenopausal women present lesser adaptations to training with regard to vascular function21, 22 compared with age‐matched men, and that improvements in vascular function only occur when combined with estrogen supplementation.22 However, one explanation for the discrepancy in findings could be differences in age, because the postmenopausal women in the study by Moreau et al22 and Pierce et al21 were older than the women in the present study. Numerous studies have shown that inactive aging has a marked influence on cardiac structure and function44 and it is plausible that the ability to respond to training accordingly is lost with age after menopause.
The magnitude of adaptations to training in the postmenopausal group, both in terms of dimensions and function, is similar to that observed after a period of football training in middle‐aged and older men16, 35 and premenopausal women,43, 45 suggesting that high‐intensity aerobic training provides an effective means of improving cardiac function in general, including in postmenopausal women. There may, however, be some difference in cardiac adaptation to training between men and women. This was evidenced in a previous study comparing the effect of 1 year of intensive endurance training on cardiac function and dimensions in young men and women. The study showed that the women had similar adaptations in cardiac function to men, whereas adaptations in cardiac dimensions were smaller and plateaued earlier in the women.20
Experimental Considerations
Tissue Doppler, atleast for some variables, is thought to be superior for assessing cardiac function compared with conventional echocardiography; however, the method is associated with some potential limitations as the results are sensitive to probe angle and noise interference and are associated with intraobserver and interobserver variability.46, 47, 48 To evaluate the reproducibility and accuracy of the measurements, we therefore assessed intraobserver and interobserver variability and found the variabilities to be within an acceptable range. The women participating in this study were likely more healthy than women in general and not representative of all female populations. The study participants were recruited from greater Copenhagen, which is considered an area with more focus on health than in more rural areas of Denmark, and it seems that Danish women tend to be habitually more physically active than reported in many other Western countries. It is likely that a healthy population such as this has relatively less room for cardiac improvements with training compared with women with metabolic disorders, cardiovascular disease and/or overweight. It would therefore be expected that the adaptations observed herein with exercise training would be similar or even greater in less healthy women. Finally, because of the exploratory nature of the present study, a significance level of 0.05 was used. Hence, it should be acknowledged that this approach implies an increased risk of false‐positive findings. Since we only tested recent postmenopausal women and late premenopausal women, we do not know whether late postmenopausal women would adapt just as well to a high‐intensity training intervention.
Conclusions
The present study demonstrates that cardiac dimensions and subclinical measures of cardiac function are similar in premenopausal and postmenopausal women with a small age difference. The finding indicates that, despite the known beneficial effects of estrogen on cardiac tissue, loss of estrogen and other hormonal changes at menopause, do not seem to alter cardiac dimensions or functionality in the near term. Moreover, a period of high‐intensity aerobic exercise training led to significant and similar adaptations in cardiac dimensions and systolic and diastolic function in premenopausal and postmenopausal women. These findings contrast with the notion that postmenopausal women adapt less well to exercise training and clearly suggest that high‐intensity aerobic training is an effective means of attaining beneficial cardiac adaptations in both premenopausal and recent postmenopausal women.
Sources of Funding
The study was funded by the University of Copenhagen Excellence Programme for Interdisciplinary Research and The Danish Ministry of Culture, Council for Sports Science.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005469 DOI: 10.1161/JAHA.117.005469.)28862950
References
- 1. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. [DOI] [PubMed] [Google Scholar]
- 2. Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ouyang P, Wenger NK, Taylor D, Rich‐Edwards JW, Steiner M, Shaw LJ, Berga SL, Miller VM, Merz NB. Strategies and methods to study female‐specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaidya D, Becker DM, Bittner V, Mathias RA, Ouyang P. Ageing, menopause, and ischaemic heart disease mortality in England, Wales, and the United States: modelling study of national mortality data. BMJ. 2011;343:d5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novella S, Dantas AP, Segarra G, Medina P, Hermenegildo C. Vascular aging in women: is estrogen the fountain of youth? Front Physiol. 2012;3:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97:4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J, Hellsten Y. Early postmenopausal phase is associated with reduced prostacyclin‐induced vasodilation that is reversed by exercise training: the Copenhagen Women Study. Hypertension. 2016;68:1011–1020. [DOI] [PubMed] [Google Scholar]
- 8. Albu A, Fodor D, Bondor C, Poanta L. Arterial stiffness, carotid atherosclerosis and left ventricular diastolic dysfunction in postmenopausal women. Eur J Intern Med. 2013;24:250–254. [DOI] [PubMed] [Google Scholar]
- 9. Hirokawa M, Daimon M, Lee SL, Nakao T, Kawata T, Kimura K, Kato TS, Mizuno Y, Watanabe M, Yatomi Y, Yamazaki T, Komuro I. Early menopause does not influence left ventricular diastolic dysfunction: a clinical observational study in healthy subjects. J Cardiol. 2016;68:548–553. [DOI] [PubMed] [Google Scholar]
- 10. Saltin B, Hartley LH, Kilbom A, Astrand I. Physical training in sedentary middle‐aged and older men. II. Oxygen uptake, heart rate, and blood lactate concentration at submaximal and maximal exercise. Scand J Clin Lab Invest. 1969;24:323–334. [DOI] [PubMed] [Google Scholar]
- 11. Pedersen PK, Jorgensen K. Maximal oxygen uptake in young women with training, inactivity, and retraining. Med Sci Sports. 1978;10:233–237. [PubMed] [Google Scholar]
- 12. Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–189. [DOI] [PubMed] [Google Scholar]
- 13. Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol. 2015;6:1–32. [DOI] [PubMed] [Google Scholar]
- 14. Bonne TC, Doucende G, Fluck D, Jacobs RA, Nordsborg NB, Robach P, Walther G, Lundby C. Phlebotomy eliminates the maximal cardiac output response to six weeks of exercise training. Am J Physiol Regul Integr Comp Physiol. 2014;306:R752–R760. [DOI] [PubMed] [Google Scholar]
- 15. Arbab‐Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams‐Huet B, Haykowsky MJ, Levine BD. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation. 2014;130:2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen LJ, Randers MB, Hansen PR, Hornstrup T, Schmidt JF, Dvorak J, Sogaard P, Krustrup P, Bangsbo J. Structural and functional cardiac adaptations to 6 months of football training in untrained hypertensive men. Scand J Med Sci Sports. 2014;24:27–35. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt JF, Hansen PR, Andersen TR, Andersen LJ, Hornstrup T, Krustrup P, Bangsbo J. Cardiovascular adaptations to 4 and 12 months of football or strength training in 65‐ to 75‐year‐old untrained men. Scand J Med Sci Sports. 2014;24:86–97. [DOI] [PubMed] [Google Scholar]
- 18. Pelliccia A, Maron BJ, Culasso F, Spataro A, Caselli G. Athlete's heart in women. Echocardiographic characterization of highly trained elite female athletes. JAMA. 1996;276:211–215. [DOI] [PubMed] [Google Scholar]
- 19. Wernstedt P, Sjostedt C, Ekman I, Du H, Thuomas KA, Areskog NH, Nylander E. Adaptation of cardiac morphology and function to endurance and strength training. A comparative study using MR imaging and echocardiography in males and females. Scand J Med Sci Sports. 2002;12:17–25. [DOI] [PubMed] [Google Scholar]
- 20. Howden EJ, Perhonen M, Peshock RM, Zhang R, Arbab‐Zadeh A, Adams‐Huet B, Levine BD. Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol. 2015;119:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex‐specific effects of habitual aerobic exercise on brachial artery flow‐mediated dilation in middle‐aged and older adults. Clin Sci (Lond). 2011;120:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98:4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersen LJ, Randers MB, Westh K, Martone D, Hansen PR, Junge A, Dvorak J, Bangsbo J, Krustrup P. Football as a treatment for hypertension in untrained 30‐55‐year‐old men: a prospective randomized study. Scand J Med Sci Sports. 2010;20:98–102. [DOI] [PubMed] [Google Scholar]
- 24. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. [DOI] [PubMed] [Google Scholar]
- 25. Hagmar M, Hirschberg AL, Lindholm C, Schenck‐Gustafsson K, Eriksson MJ. Athlete's heart in postmenopausal former elite endurance female athletes. Clin J Sport Med. 2005;15:257–262. [DOI] [PubMed] [Google Scholar]
- 26. Knebel F, Spethmann S, Schattke S, Dreger H, Schroeckh S, Schimke I, Hattasch R, Makauskiene R, Kleczka J, Sanad W, Lock J, Brechtel L, Baumann G, Borges AC. Exercise‐induced changes of left ventricular diastolic function in postmenopausal amateur marathon runners: assessment by echocardiography and cardiac biomarkers. Eur J Prev Cardiol. 2014;21:782–790. [DOI] [PubMed] [Google Scholar]
- 27. Ha JW, Lee HC, Kang ES, Ahn CM, Kim JM, Ahn JA, Lee SW, Choi EY, Rim SJ, Oh JK, Chung N. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart. 2007;93:1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ernande L, Bergerot C, Rietzschel ER, De Buyzere ML, Thibault H, Pignonblanc PG, Croisille P, Ovize M, Groisne L, Moulin P, Gillebert TC, Derumeaux G. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr. 2011;24:1268–1275. [DOI] [PubMed] [Google Scholar]
- 29. Poulsen MK, Henriksen JE, Dahl J, Johansen A, Gerke O, Vach W, Haghfelt T, Hoilund‐Carlsen PF, Beck‐Nielsen H, Moller JE. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging. 2010;3:24–31. [DOI] [PubMed] [Google Scholar]
- 30. Ernande L, Rietzschel ER, Bergerot C, De Buyzere ML, Schnell F, Groisne L, Ovize M, Croisille P, Moulin P, Gillebert TC, Derumeaux G. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle‐tracking imaging study. J Am Soc Echocardiogr. 2010;23:1266–1272. [DOI] [PubMed] [Google Scholar]
- 31. Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. [DOI] [PubMed] [Google Scholar]
- 32. Jensen MT, Sogaard P, Andersen HU, Gustafsson I, Bech J, Hansen TF, Theilade S, Almdal T, Rossing P, Jensen JS. Early myocardial impairment in type 1 diabetes patients without known heart disease assessed with tissue Doppler echocardiography: the Thousand & 1 study. Diab Vasc Dis Res. 2016;13:260–267. [DOI] [PubMed] [Google Scholar]
- 33. Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, Goetze JP, Jensen JS. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. [DOI] [PubMed] [Google Scholar]
- 34. Mandrup CM, Egelund J, Nyberg M, Lundberg Slingsby MH, Andersen CB, Logstrup S, Bangsbo J, Suetta C, Stallknecht B, Hellsten Y. Effects of high‐intensity training on cardiovascular risk factors in premenopausal and postmenopausal women. Am J Obstet Gynecol. 2017;216:384. [DOI] [PubMed] [Google Scholar]
- 35. Schmidt JF, Andersen TR, Horton J, Brix J, Tarnow L, Krustrup P, Andersen LJ, Bangsbo J, Hansen PR. Soccer training improves cardiac function in men with type 2 diabetes. Med Sci Sports Exerc. 2013;45:2223–2233. [DOI] [PubMed] [Google Scholar]
- 36. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 37. Alhurani RE, Chahal CA, Ahmed AT, Mohamed EA, Miller VM. Sex hormone therapy and progression of cardiovascular disease in menopausal women. Clin Sci (Lond). 2016;130:1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins P, Webb CM, de Villiers TJ, Stevenson JC, Panay N, Baber RJ. Cardiovascular risk assessment in women—an update. Climacteric. 2016;19:329–336. [DOI] [PubMed] [Google Scholar]
- 39. Duzenli MA, Ozdemir K, Sokmen A, Gezginc K, Soylu A, Celik C, Altunkeser BB, Tokac M. The effects of hormone replacement therapy on myocardial performance in early postmenopausal women. Climacteric. 2010;13:157–170. [DOI] [PubMed] [Google Scholar]
- 40. Duygu H, Akman L, Ozerkan F, Akercan F, Zoghi M, Nalbantgil S, Erturk U, Akilli A, Onder R, Akin M. Comparison of the effects of new and conventional hormone replacement therapies on left ventricular diastolic function in healthy postmenopausal women: a Doppler and ultrasonic backscatter study. Int J Cardiovasc Imaging. 2009;25:387–396. [DOI] [PubMed] [Google Scholar]
- 41. Schmidt JF, Andersen TR, Andersen LJ, Randers MB, Hornstrup T, Hansen PR, Bangsbo J, Krustrup P. Cardiovascular function is better in veteran football players than age‐matched untrained elderly healthy men. Scand J Med Sci Sports. 2015;25:61–69. [DOI] [PubMed] [Google Scholar]
- 42. Krustrup P, Hansen PR, Andersen LJ, Jakobsen MD, Sundstrup E, Randers MB, Christiansen L, Helge EW, Pedersen MT, Sogaard P, Junge A, Dvorak J, Aagaard P, Bangsbo J. Long‐term musculoskeletal and cardiac health effects of recreational football and running for premenopausal women. Scand J Med Sci Sports. 2010;20:58–71. [DOI] [PubMed] [Google Scholar]
- 43. Andersen LJ, Hansen PR, Sogaard P, Madsen JK, Bech J, Krustrup P. Improvement of systolic and diastolic heart function after physical training in sedentary women. Scand J Med Sci Sports. 2010;20:50–57. [DOI] [PubMed] [Google Scholar]
- 44. Parker BA, Kalasky MJ, Proctor DN. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur J Appl Physiol. 2010;110:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Randers MB, Andersen LJ, Orntoft C, Bendiksen M, Johansen L, Horton J, Hansen PR, Krustrup P. Cardiovascular health profile of elite female football players compared to untrained controls before and after short‐term football training. J Sports Sci. 2013;31:1421–1431. [DOI] [PubMed] [Google Scholar]
- 46. Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. [DOI] [PubMed] [Google Scholar]
- 47. Barmeyer A, Mullerleile K, Mortensen K, Meinertz T. Diastolic dysfunction in exercise and its role for exercise capacity. Heart Fail Rev. 2009;14:125–134. [DOI] [PubMed] [Google Scholar]
- 48. Caglar AO, Epcacan S, Uner A, Ece I, Dogan M. Evaluation of left and right ventricular functions using conventional and tissue Doppler echocardiography in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2016;29:885–891. [DOI] [PubMed] [Google Scholar]


