Abstract
Background
Sudden cardiac death (SCD) is often the first presentation of ischemic heart disease; however, there is limited information on SCD among women with and without obstructive coronary artery disease (CAD). We evaluated SCD incidence in the WISE (Women's Ischemia Syndrome Evaluation) study.
Methods and Results
Overall, 904 women with suspected ischemic heart disease with preserved ejection fraction and core laboratory coronary angiography were followed for outcomes. In case of death, a death certificate and/or a physician or family narrative of the circumstances of death was obtained. A clinical events committee rated all deaths as cardiovascular or noncardiovascular and as SCD or non‐SCD. In total, 96 women (11%) died over a median of 6 years (maximum: 8 years). Among 65 cardiovascular deaths, 42% were SCD. Mortality per 1000 person‐hours increased linearly with CAD severity (no CAD: 5.8; minimal: 15.9; obstructive: 38.6; P<0.0001). However, the proportion of SCD was similar across CAD severity: 40%, 58%, and 38% for no, minimal, and obstructive CAD subgroups, respectively (P value not significant). In addition to traditional risk factors (age, diabetes mellitus, smoking), a history of depression (P=0.018) and longer corrected QT interval (P=0.023) were independent SCD predictors in the entire cohort. Corrected QT interval was an independent predictor of SCD in women without obstructive CAD (P=0.033).
Conclusions
SCD contributes substantially to mortality in women with and without obstructive CAD. Corrected QT interval is the single independent SCD risk factor in women without obstructive CAD. In addition to management of traditional risk factors, these data indicate that further investigation should address mechanistic understanding and interventions targeting depression and corrected QT interval in women.
Keywords: coronary atherosclerosis, ischemic heart disease, sudden cardiac death, women
Subject Categories: Sudden Cardiac Death, Chronic Ischemic Heart Disease, Women
Clinical Perspective
What Is New?
Sudden cardiac death contributes substantially to mortality in women with and without obstructive coronary artery disease.
In addition to traditional risk factors (age, diabetes mellitus, smoking), a history of depression and longer corrected QT interval were independent predictors of sudden cardiac death in women.
Corrected QT interval is the single independent risk factor for sudden cardiac death in women without obstructive coronary artery disease.
What Are the Clinical Implications?
Traditional risk factors should be identified and treated in women with and without obstructive coronary artery disease.
Interventions targeting depression and corrected QT interval should be studied in women for reduction of sudden cardiac death risk.
Sudden cardiac death (SCD) is a major health threat, and although the incidence varies depending on how SCD is defined, ≈120 000 SCDs occur annually in American women.1, 2, 3, 4 The recent decade's decline in SCD incidence among women has been relatively less compared with that among men,5 and recent reports indicate that women represent ≈40% of SCDs.6 Whereas obstructive coronary artery diseases (CAD) is a predictor of SCD in women,7 SCD in women occurs more often in the absence of obstructive CAD compared with men,7, 8, 9 similar to the lower rate of obstructive CAD found on angiography for suspected ischemia in women compared with men.10, 11, 12, 13, 14
Traditional ischemic heart disease (IHD) risk factors such as hypertension, hyperlipidemia, and tobacco use are associated with but insufficient to predict SCD7; new markers and improved models to better predict SCD risk are needed in women. Current risk‐stratification methods using left ventricular ejection fraction are limited in adequately predicting which patients are at risk of SCD15, 16 because two thirds of those with SCD do not have significant left ventricular dysfunction.17
To our knowledge, incidence of SCD in women with signs and symptoms of IHD without obstructive CAD has not been reported. Accordingly, we examined the incidence of SCD in women with suspected IHD without obstructive CAD by angiography and compared them with women with obstructive CAD enrolled in the WISE (Women's Ischemia Syndrome Evaluation) study. We also explored risk variables for SCD in our well‐characterized observational cohort of women.
Methods
Study Population
WISE is a National Heart, Lung, and Blood Institute–sponsored 4‐center study of women undergoing clinically ordered coronary angiography to evaluate symptoms, signs, and pathophysiology of IHD and to improve diagnostic testing and outcomes in women. The WISE rationale, objectives, and organization have been described in detail previously.18 A total of 936 women were enrolled between 1996 and 2000. The current analysis includes a subgroup of 904 women enrolled in WISE who had sufficient baseline and angiographic information and were prospectively followed annually. Exclusion criteria included acute coronary syndrome (defined as acute myocardial infarction or unstable angina) within 30 days of study entry; coronary angioplasty or coronary bypass surgery within 6 months of study entry; significant congenital or primary valvular heart disease; New York Heart Association class IV heart failure; other significant illnesses such as severe lung, renal, or hepatic disease; life expectancy <6 months; pregnancy; and any condition likely to affect study retention (alcoholism, drug abuse, or severe psychiatric illness). The protocol was approved by institutional review committees at each site, and all participants provided written informed consent.
Baseline Evaluation and Angiographic Assessment
The baseline evaluation and coronary angiographic assessment of CAD have been described previously.11, 18, 19 Briefly, baseline evaluations included collection of demographic variables, risk factors, medical history, medication use, symptom history, functional capacity using the Duke Activity Score Index, psychosocial evaluation using standardized forms, and physical examination, in addition to an ECG and blood sampling for lipids and other blood risk markers. Body mass index was calculated as kg/m2, and homeostasis model assessment of insulin resistance was calculated as (glucose [mmol/L]×insulin [μU/L])/22.5. Lipid low‐density lipoprotein was calculated using the Friedewald formula, and glomerular filtration rate was calculated using the Cockroft–Gault formula.
All angiograms were analyzed, masked to clinical data, by the WISE angiographic core laboratory (Rhode Island Hospital), as described previously,20 and results were classified into 3 groups: (1) no CAD, defined as normal‐appearing coronary arteries and no diameter stenosis ≥20% in any epicardial artery; (2) minimal CAD, defined as 20% to 49% stenosis in ≥1 epicardial artery; or (3) obstructive CAD, defined as ≥50% stenosis in ≥1 epicardial coronary artery. No CAD and minimal CAD were classified as no obstructive CAD. A CAD severity score based on extent of stenosis and number of epicardial vessels involved and adjusted for lesion location and collaterals was determined.20, 21 A severity score of 5 was assigned to women with no detectable CAD. The interobserver variability for WISE angiographic core coronary artery diameter measurement was 0.196 mm with a 6.3% coefficient of variation.21
Follow‐up Procedures
Follow‐up was conducted by an experienced research coordinator or physician at each site using a scripted telephone interview at 6 weeks and annually thereafter. The women were followed over a median of 6 years. Each woman was queried about symptoms, medication use, cardiovascular events since last contact, hospitalizations, and diagnostic or revascularization procedures. During follow‐up, if the woman was found to be deceased, we obtained a death certificate, physician narrative, and/or family narrative of circumstances of death. The WISE events committee, masked to angiographic findings, categorized all deaths as cardiovascular versus noncardiovascular; if possible, using narratives in addition to death certificates, cardiovascular deaths were further adjudicated as SCD versus non‐SCD. SCD was defined as unwitnessed, unexplained, nonhospital death; sudden, fatal, acute myocardial infarction; or in‐hospital cardiac arrest during emergency care.
Statistical Analysis
Values are expressed as mean±SD or percentage, as indicated in Table 1. Mortality rates are given as events per 1000 person‐years with Poisson 95% confidence intervals (CIs) in Table 2. P values for mortality trends across increasing CAD severity categories were calculated using the Mantel–Haentzel statistic.
Table 1.
Baseline Characteristics (n=904)
| Characteristic | Result |
|---|---|
| Age, ya | 58±12b |
| Postmenopausal | 679 (76) |
| Non‐White race | 171 (19) |
| High school graduateb | 714 (80) |
| Diabetes mellitus | 223 (25) |
| History of dyslipidemia | 458 (55) |
| History of hypertension | 535 (59) |
| Family history of CAD | 581 (66) |
| BMI, kg/m2 | 29.6±6.6 |
| BMI ≥30 | 362 (41) |
| Ever smoking | 478 (53) |
| Current smoking | 178 (20) |
| ≥3 CAD risk factorsc | 609 (67) |
| Systolic blood pressure, mm Hg | 137±22 |
| Diastolic blood pressure, mm Hg | 76±11 |
| Total cholesterol, mg/dL | 195±45 |
| Triglycerides, mg/dL | 156±122 |
| HDL‐C, mg/dL | 54±13 |
| LDL‐C, mg/dL | 112±39 |
| Fasting glucose, mg/dL | 119±56 |
| Creatinine, mg/dL | 0.88±0.50 |
| Hemoglobin, g/dL | 12.9±1.3 |
| HOMA‐IR | 4.2±6.2 |
| Estimated GFR mL/min/1.73m2 | 95±39 |
| Ever HRT use | 455 (51) |
| Current HRT use | 343 (38) |
| Beta blockers | 353 (39) |
| Statins | 231 (26) |
| ACE inhibitors | 235 (26) |
| CAD status | |
| No CAD | 328 (36) |
| Minimal CAD | 223 (25) |
| Obstructive CAD | 353 (39) |
| Prior MI or revascularization | 257 (29) |
| Ejection fraction | 65±11 |
| Corrected QT interval, ms | 429±30 |
ACE indicates angiotensin‐converting enzyme; BMI, body mass index; CAD, coronary artery disease; GFR, glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; HRT, hormone replacement therapy; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction.
Continuous variables are summarized as mean±1 SD, and qualitative variables summarized as n (%).
Age range is 21 to 86 years.
Includes diabetes mellitus, history of dyslipidemia, history of hypertension, family history of CAD, BMI ≥30, history of smoking, prior MI, or revascularization.
Table 2.
All‐Cause Death Rates Per 1000 Person‐Years, Numbers, and Percentages of Cardiovascular Deaths and SCD by CAD Severity
| CAD Severity | Number of All‐Cause Deaths (and Rates)a | Number of Deaths (%) | ||
|---|---|---|---|---|
| Cardiovascular Deaths | SCD | SCD | ||
| Among 904 Women | Among 96 All‐Cause Deaths | Among 96 All‐Cause Deaths | Among 65 Cardiovascular Deaths | |
| No CAD (n=328) | 10 (5.8 [2.2–11.7]) | 5 (50) | 2 (20) | 2 (40) |
| Minimal CAD (n=223) | 19 (15.9 [9.1–24.7]) | 12 (63) | 7 (37) | 7 (58) |
| Obstructive CAD (n=353) | 67 (38.6 [27.7–52.2]) | 48 (73) | 18 (27) | 18 (38) |
| Missing information | 0 | 1 | 17 | 17 |
| Total | 96 (20.8 [13.0–30.9]) | 65 (68) | 27 (28) | 27 (42) |
| P value | <0.0001b | 0.12c | 0.33c | 0.99c |
CAD indicates coronary artery disease; SCD, sudden cardiac death.
Rates per 1000 person‐years (Poisson 95% confidence intervals).
P‐value by log‐rank statistic.
P‐value by Mantel–Haentzel statistic for trend across CAD subgroups.
For survival analyses, patients who did not die were censored at the date of their last contact with the study coordinator. Using competing risk models, age‐adjusted Cox proportional hazards regression was used to determine variables that predicted SCD and non‐SCD cardiovascular mortality. For these models, women who died of noncardiovascular causes were censored, and those whose deaths could not be classified as SCD were excluded from the analyses, yielding a total sample size of 886. Because of the large number of tests run, we chose a nominal P≤0.01 as statistically significant in Table 3. In addition, we calculated the Wald χ2 statistics to test whether the effects of the predictors differed significantly across event types.
Table 3.
Significant Predictors of SCD and Non‐SCD Cardiovascular Mortality in All Women (N=886)
| Predictors | Non‐SCD (21 Events) | SCD (27 Events) | P of Non‐SCD vs SCDb |
|---|---|---|---|
| HR (95% CI)a | HR (95% CI)a | ||
| Age, y | 1.11 (1.06–1.17)c | 1.05 (1.01–1.09)d | 0.032 |
| White race | 0.44 (0.17–1.14) | 0.38 (0.17–0.85)d | >0.99 |
| Obstructive CAD | 4.43 (1.43–13.70)e | 2.84 (1.23–6.52)d | 0.53 |
| CAD severity score (log) | 2.67 (1.50–4.73)c | 2.41 (1.51–3.84)c | >0.99 |
| Left ventricular ejection fraction | 0.95 (0.92–0.98)e | 0.96 (0.93–0.98)e | >0.99 |
| Number of CAD risk factorsf | 1.48 (1.11–1.96) e | 1.52 (1.18–1.96) e | >0.99 |
| Prior MI or revascularization | 5.02 (1.91–13.21) c | 1.97 (0.91–4.26) | 0.09 |
| History of Revascularization | 2.82 (1.18–6.78)d | 2.25 (1.00–5.06)d | >0.99 |
| QTc, per 10 ms | 1.08 (0.93–1.25) | 1.19 (1.07–1.32)e | 0.23 |
| Systolic blood pressure, mm Hg | 1.02 (1.005–1.04)d | 1.02 (0.999–1.03) | 0.74 |
| Diastolic blood pressure, mm Hg | 1.06 (1.02–1.10)e | 0.99 (0.96–1.03) | 0.013 |
| Ever smoking | 1.38 (0.58–3.28) | 3.08 (1.29–7.32)e | 0.14 |
| Diabetes mellitus | 2.15 (0.90–5.13) | 2.59 (1.21–5.55)d | >0.99 |
| Metabolic syndrome | 1.16 (0.46–2.93) | 3.91 (1.57–9.75)e | 0.042 |
| Duke Activity Score Index | 0.91 (0.86–0.97)e | 0.99 (0.96–1.02) | 0.011 |
| COPD | 5.00 (1.67–16.98)e | 2.64 (0.79–8.76) | 0.39 |
| Estimated GFR, mL/min/1.73 m2 | 0.97 (0.95–0.99)e | 1.003 (0.99–1.02) | 0.005 |
| History of depression | 1.46 (0.52–4.05) | 2.63 (1.21–5.72)d | 0.29 |
| Hemoglobin | 0.70 (0.52–0.95)d | 1.04 (0.76–1.42) | 0.047 |
Not tabulated: moderate predictors (defined as P>0.01 but <0.05 and not differing significantly between outcomes) of non‐SCD were history of hypertension, history of peripheral vascular disease, log IL6, and resting heart rate. Log SAA, use of antidepressants, diuretics and nitrates were moderately predictive of SCD. Variables not predicting either outcome included postmenopausal status, history of oral contraceptive or hormone therapy use, body mass index, lipids, homeostasis model assessment, and use at baseline of digitalis, statins, beta blockers, or hormone therapy. CAD indicates coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HR, hazard ratio; IL6, Interleukin 6; MI, myocardial infarction; QTc, corrected QT interval; SAA, Serum Amyloid A; SCD, sudden cardiac death.
Except for age, all P values are age‐adjusted.
P value when comparing the predictors across different outcomes. A P value <0.05 suggests a variable to be a unique predictor.
P≤0.001.
P≤0.05.
P≤0.01.
Includes diabetes mellitus, history of dyslipidemia, history of hypertension, family history of CAD, body mass index ≥40, history of smoking, and prior MI or revascularization.
The unique predictors of SCD from Table 3 meeting this criterion of significance were entered into a combination of forward and backward selection procedures to aid in determining the best model of independent predictors of SCD. This step was followed by forcing, one at a time, each variable from Table 3 into the model to determine its effect on the relationships of interest. The likelihood ratio test was used to compare the incremental goodness of fit of nested models. Fitted models were evaluated for departures from the proportional hazards assumption by using diagnostic residual analyses, including plotting Schoenfeld residuals against time, and were found to meet assumptions. All tests for the final models were 2‐sided, and P≤0.05 was considered statistically significant.
Using the ROC option of PROC LOGISTIC, we used receiver operating characteristic curve analysis to identify the optimal cut point in corrected QT interval (QTc) for predicting SCD. A cut point was chosen that maximized sensitivity and specificity. The area under the curve and 95% CIs were reported to indicate the accuracy of QTc to predict those who will and will not die of SCD. All analyses were performed with SAS software version 9.3 (SAS Institute).
Results
Pertinent baseline characteristics for the 904 women are summarized in Table 1. The group was predominantly middle‐aged and postmenopausal, and a majority were white with ≥3 IHD risk factors (67%). The women were about equally distributed across the 3 CAD severity categories. There were only a few differences between the 904 women included and the 32 who were excluded from the current analysis, mainly because of missing follow‐up information (data not tabulated). The excluded women were more likely to be obese (with 60% versus 41% having a body mass index >30, P=0.052) or current smokers (35% versus 20%, P=0.032). They had lower mean high‐density lipoprotein (47 versus 54 mg/dL, P=0.012), lower creatinine levels (0.77 versus 0.88 mg/dL, P=0.0002), lower homeostasis model assessment (2.3 versus 4.2, P<0.001), and higher estimated glomerular filtration rate (113 versus 95 mL/min/1.73m2, P=0.024). No differences were found regarding presence or absence of obstructive CAD or CAD severity level.
All‐Cause, Cardiovascular, and Noncardiovascular Mortality
A total of 96 women died over a median of 6.0 years (maximum: 8 years), equivalent to a rate of 20.8 deaths per 1000 person‐years (95% CI, 13.0–30.9). Among the deaths, 65 of 96 (68%) were due to cardiovascular causes, 30 of 96 (31%) due to noncardiovascular causes, and 1 (1%) lacked sufficient information to classify her death (Figure 1). Among the 65 cardiovascular deaths in the entire cohort, 48 of 65 (74%) could be classified in terms of SCD; 17 did not have enough information to classify their deaths as SCD versus non‐SCD, although their deaths were related to cardiovascular causes. Of the 48 classifiable cardiovascular deaths, 27 of 48 (56%) were SCD (Figure 1), for a minimal overall rate of 5.8 SCDs per 1000 person‐years (95% CI, 2.2–11.7). Sensitivity analysis including the 1 woman (who had obstructive CAD) with no information to classify her death as a probable cardiovascular death did not change the results. The median time between the final WISE contact with the patient and SCD death was 6.2 months (interquartile range: 3.0–11.9 months).
Figure 1.
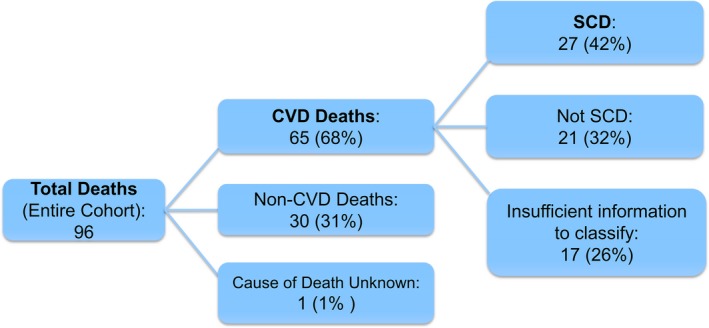
Deaths in WISE (Women's Ischemia Syndrome Evaluation). Overall, 904 women with signs and symptoms of ischemia who underwent a clinically indicated invasive coronary angiography were followed over a median of 5.9 years (maximum: 9 years), and deaths were classified. CVD indicates cardiovascular disease; SCD, sudden cardiac death.
Mortality and CAD Severity
The all‐cause and cardiovascular death rates increased linearly across CAD severity (P<0.0001) (Table 2 and Figure 2). SCD rates similarly increased across the CAD severity groups, with estimated 1000‐person‐year SCD rates of 1.2, 5.8, and 10.4 for no, minimal, and obstructive CAD, respectively (P=0.002; Figure 3); however, the proportion of SCD among deaths was similar across the CAD severity subgroups (P value not significant; Table 2). In women with obstructive CAD, a moderate but nonsignificant difference in SCD rates was found among those with versus without a history of revascularization (14.6 [range: 8.4–23.5] versus 8.4 [range: 4.1–15.8] events per 1000 person‐years, respectively; P=0.24).
Figure 2.
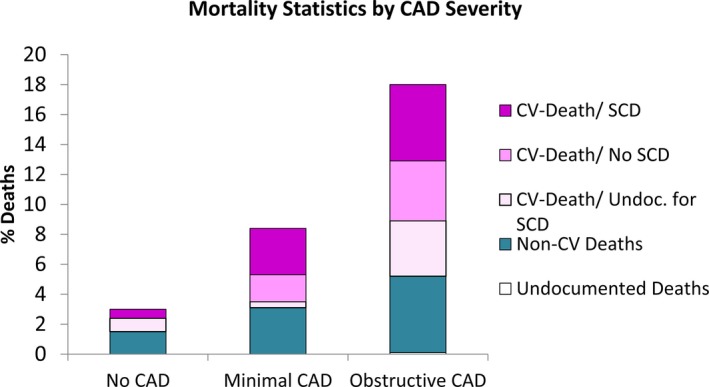
Percentages of women dying over 8 years, by cause of death and CAD severity. CAD indicates coronary artery disease; CV, cardiovascular; SCD, sudden cardiac death.
Figure 3.
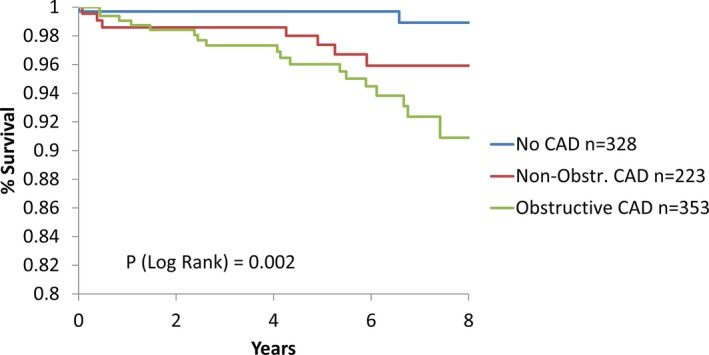
Freedom from SCD by CAD severity. Kaplan–Meier estimated mortality rates of SCD by CAD severity per woman, per 8 years: no CAD, 0.011/8 years; minimal CAD, 0.041/8 years; and obstructive CAD, 0.093/8 years. When comparing the no‐CAD group with the minimal‐CAD group, the P value is 0.023. CAD indicates coronary artery disease; SCD, sudden cardiac death.
Predictors of SCD
Baseline predictors of non‐SCD and SCD cardiovascular mortality in all women are summarized in Table 3. The traditional predictors, higher CAD severity, lower left ventricular ejection fraction, and higher number of CAD risk factors all predicted both non‐SCD and SCD cardiovascular events. Higher age (P=0.032), high diastolic blood pressure (P=0.013), low functional capacity (Duke Activity Score Index, P=0.011), and low estimated glomerular filtration rate (P=0.005) and hemoglobin (P=0.047) uniquely predicted non‐SCD cardiovascular death. The only significant unique predictor of SCD was metabolic syndrome (P=0.042); however, longer QTc, ever smoking, and history of depression, although not statistically significant, were potentially unique predictors of SCD (Table 3). Notably, the mean QTc for women was 446±27 versus 428±30 ms for those with and without SCD, respectively (P=0.003). Among women with no obstructive CAD, those without SCD had a mean QTc of 427±28 ms, whereas those with SCD had a mean QTc of 447±31 ms (P=0.034). Symptoms at baseline and those recorded at the final contact with the patient did not predict SCD; specifically, presence of symptoms at final contact (P=0.56), final symptom frequency (P=0.40), and high symptom frequency compared with prior follow‐up contacts (P=0.69) did not predict SCD (data not shown).
In multivariable modeling, age, diabetes mellitus, ever smoking, a history of depression, and QTc were significant independent predictors of SCD in the entire cohort (Table 4). No interactions were found between CAD and these or other potential predictors. Although CAD severity is an important predictor of SCD, it is not uniquely so, namely, it also predicted all‐cause and cardiovascular mortality. We sought to determine the risk variables that predicted SCD independent of CAD status. When entering CAD severity (log) into the model (Table 5), both a history of depression and QTc remained independent predictors of SCD, whereas age, diabetes mellitus, and smoking history dropped out. The final model did not change with sensitivity analyses, which assumed that the 3 women with no CAD whose causes of death could not be determined had SCD. Importantly, QTc (in intervals of 10 ms) was the single significant predictor of SCD in those with no obstructive CAD, even after forcing other potential predictors into the model (Table 6). There was no significant interaction between QTc and depression.
Table 4.
Independent Predictors of SCD (Multivariate Modeling)—Model Not Including CAD Severity in All Women
| Predictor | n=748, 27 Events | P Value (Sensitivity Analysis)a n=751, 30 Events | |
|---|---|---|---|
| HR (95% CI) | P Value | ||
| Age, y | 1.06 (1.02–1.10) | 0.003 | 0.006 |
| Diabetes mellitus | 2.69 (1.21–6.01) | 0.016 | 0.018 |
| History of depression | 2.28 (1.03–5.05) | 0.041 | 0.006 |
| Ever smoking | 3.01 (1.25–7.23) | 0.014 | 0.015 |
| QTc, per 10 ms | 1.14 (1.02–1.27) | 0.024 | 0.009 |
CAD indicates coronary artery disease; CI, confidence interval; HR, hazard ratio; SCD, sudden cardiac death.
The sensitivity analyses assume the 3 women with no obstructive CAD and missing classifiable mortality information all have SCD.
Table 5.
Independent Predictors of SCD (Multivariate Modeling)—Model Including CAD Severity in All Women
| Predictor | n=739, 27 Events | P Value (Sensitivity Analysis)a n=742, 30 Events | |
|---|---|---|---|
| HR (95% CI) | P Value | ||
| CAD severity (log) | 2.86 (1.80–4.53) | <0.0001 | <0.001 |
| History of depression | 2.66 (1.19–5.95) | 0.018 | 0.003 |
| QTc, per 10 ms | 1.13 (1.02–1.26) | 0.023 | 0.008 |
CAD indicates coronary artery disease; CI, confidence interval; HR, hazard ratio; QTc, corrected QT interval; SCD, sudden cardiac death.
The sensitivity analyses assume the 3 women with no obstructive CAD and missing classifiable mortality information all have SCD.
Table 6.
Independent Predictors of SCD (Multivariate Modeling)—Model Including CAD Severity in Women Without Obstructive CAD
| Predictor | n=477, 9 Events | P Value (Sensitivity Analysis)a n=480, 12 Events | |
|---|---|---|---|
| HR (95% CI) | P Value | ||
| QTc, per 10 ms | 1.22 (1.02–1.47) | 0.033 | 0.003 |
The sensitivity analyses assume the 3 women with no obstructive CAD and missing classifiable mortality information all have SCD.
QTc interval as a predictor of SCD
In addition to a continuous specification of QTc, we further evaluated whether a QTc >470 ms, a standard cut point for women, predicted SCD. This was not the case (P=0.28 in all women and P=0.09 in women without obstructive CAD). Using receiver operating characteristic analysis, a cut point of 437 ms offered optimal prediction of SCD (area under the curve: 0.66 [95% CI], 0.57–0.77; P=0.0009), with sensitivity of 0.63 and specificity of 0.70.
We further investigated the association between use of antidepressants at baseline and QTc. In the WISE cohort of 904 women, 217 (24%) reported recent antidepressant medication use. In all women, use of antidepressant medications was borderline associated with increased QTc (QTc in women not on antidepressants was 428±29 versus 434±32 ms in those on antidepressants; P=0.06). This association was not found in women without obstructive CAD (QTc 427±29 versus 429±29 ms, respectively; P=0.48). No association was found for antianxiety medications and QTc (P=0.17 in all women; P=0.36 in women without obstructive CAD). In the model shown in Table 5, when history of depression was replaced with antidepressant medication use, no significant relationship to SCD was noted, and the overall model was not substantively changed (data not shown).
Discussion
In this well‐characterized prospective cohort study of women with signs and symptoms of ischemia undergoing coronary angiography, we observed that SCD is a substantial contributor to all‐cause and cardiovascular death among women undergoing coronary angiography for suspected ischemia with and without obstructive CAD. Although traditional risk factors and depression predict SCD in women with and without obstructive CAD, QTc interval is the single independent risk factor in women without obstructive CAD. These results indicate that SCD is a substantial health threat for women independent of angiographic characterization. Notably, the SCD risk variables predict risk across the CAD status, for example, they are not exclusive predictors within the obstructive CAD cohort. Together these results indicate that low angiographic CAD burden in women with evidence of ischemia does not guarantee immunity from cardiovascular death or SCD. In the WISE study, we have typically found chest pain indicators to be unreliable predictors of presence or severity of obstructive heart disease or of downstream events; similarly, presence of symptoms and symptom frequency at final contact did not predict SCD in this cohort.
Among women with no obstructive CAD, longer QTc was the single predictor of SCD, even after forcing other variables into the model. Longer QTc is well known to be independently associated with SCD in men and women.6, 22 In the case‐control Oregon Sudden Unexpected Death Study of patients with CAD (40% women), QTc was longer in those with SCD compared with controls (450±45 versus 433±37 ms, P<0.0001), and abnormally prolonged QTc was associated with a 5‐fold increased risk of SCD.23 In our study, women with SCD had a mean QTc of 446±27 ms. It is important to note that healthy adult women are known to have longer QT than men; the upper limit for QTc is 450 ms for women but only 430 ms for men.22, 24 QTc of >470 ms was not predictive of SCD among women without CAD (P=0.09), probably due to loss of statistical power in this binary specification given relatively low event rates. Similar to the challenge of determining cut points for increased risk in other biological variables with continuous risk (eg, blood pressure, cholesterol levels, ejection fraction), our data seem to suggest a linear relationship between QTc and SCD in women with signs and symptoms of ischemia. Of note, in a study of those with SCD (predominantly men), 80% had significant coronary stenosis on autopsy, and traditional CAD risk factors were highly prevalent; in these men, mutations in the long QT gene were not prevalent.9
To our knowledge, our study is the first to report incidence of SCD in a consecutive cohort of women with suspected IHD with and without obstructive CAD among women with angiographic core laboratory assessment of CAD. We previously reported that among 917 women suspected of IHD in the WISE study, 161 died (103 cardiovascular deaths), and that the WISE angiographic CAD severity score is a useful risk predictor of adverse cardiovascular outcomes.20 We extend these findings and show that CAD severity also relates to overall, cardiovascular, and SCD mortality. Others25 have reported that SCD occurred in 136 of 254 (53.5%) cardiac deaths in postmenopausal women with known CAD over a mean follow‐up of 6.8 years; the independent predictors of SCD were myocardial infarction, heart failure, low estimated glomerular filtration rate, atrial fibrillation, physical inactivity, and diabetes mellitus. In comparison, we similarly found that diabetes mellitus is an independent predictor of SCD in our cohort not restricted to postmenopausal women. Women with significant heart failure, renal disease, or atrial fibrillation were not included in this WISE cohort. The population in WISE is a clinically referred cohort for coronary angiography with preserved left ventricular ejection fraction and high density of risk factors. Compared with general population surveys, this high density of risk factors sometimes limits the detection of associations because of the lack of participants without these risk factors.
Notably, we found that the novel risk factor of a history of depression independently predicted SCD in the WISE cohort. It is known that psychological factors such as depression, anger, and anxiety may trigger arrhythmias,26, 27, 28 and these are believed to be related to altered sympathetic and parasympathetic balance. Others have reported an association of depression and SCD in both men and women. In a study of 2228 cases and 4164 controls, compared with nondepressed patients, the risk of SCD was increased in depressed patients (odds ratio: 1.88, [95% CI, 1.59–2.23]).29 In the Nurses Health Study, depressive symptoms were associated with coronary heart disease, and the use of antidepressant medications was associated with SCD.30 In our study, use of psychotropic medications was not a significant predictor of SCD mortality, but our cohort excluded those with significant psychiatric illness. We previously reported that use of antidepressants and anxiolytics are associated with major adverse cardiovascular events in WISE.31 We did not find a significant interaction between QTc and depression in this cohort. We conclude that although the use of antidepressants was borderline associated with increased QTc, it does not explain the association of QTc with SCD, nor does it explain the association of history of depression with SCD.
Several hypotheses exist regarding the mechanisms of SCD in women without obstructive CAD. Endothelial dysfunction and/or coronary microvascular dysfunction are mechanisms of ischemia observed in many women who have no obstructive CAD.19, 32 Autopsy data show that young women (aged <50 years) who died from myocardial infarction have more erosions and microemboli versus the overt plaque rupture typically seen in men.33 The high percentage of SCD (about one‐third) of all‐cause deaths observed in our participants without obstructive CAD highlights that signs and symptoms of myocardial ischemia are not benign and that the absence of obstructive CAD does not infer an absence of SCD risk. Further mechanistic research of ischemic pathophysiology in women, including cardiac autonomic dysfunction, is clearly needed. Because CAD risk factors are independently associated with SCD in this group, interventional research investigating the potential benefit of treating and reducing CAD risk factors in symptomatic women without obstructive CAD should be prioritized to inform guidelines.
Limitations
This prospective observational cohort study cannot determine causality, and results may not be generalizable to the population of women at large. The raw number of deaths is an underestimate because of varying follow‐up times of the women at study completion. The Cox proportional hazards method, however, accounts for such attrition over time in calculating hazard ratios and CIs. Despite considerable effort, documentation for some deaths (1 for classifying cardiovascular mortality and 17 for classifying SCD) could not be obtained. The percentages may be underestimated because of missing information: 3 of 10 (30%) of the women with no CAD, although dying from cardiovascular causes, were missing information to classify deaths as SCD versus non‐SCD compared with only 1 of 19 (5%) in women with minimal CAD and 14 of 67 (20%) in those with obstructive CAD (P=0.19). Our use of a National Death Index search for our longer follow‐up period resulted in some deaths that could not be accurately determined to be SCD from the records; no autopsy data were collected in this study. In contrast to other studies, however, our study included periprocedural deaths in the SCD category. Such deaths may have different predictors or correlates than nonprocedural deaths. There was a small number of deaths in the no CAD group compared with the minimal and obstructive CAD groups, thus statistical power was too low to detect differences. Given the long follow‐up period, it is possible that those who initially had no obstructive CAD on angiography could have developed significant CAD. Ischemic burden was not uniformly quantified in this study, and various site‐specific stress‐testing modalities were included; electrocardiographic parameters such as t‐wave alternans and QTc dynamicity were not collected. Although all predictive data were age‐adjusted, larger predictive models were precluded by low power, given the relatively low number of deaths, particularly in women without obstructive CAD.
Conclusions
SCD is a substantial contributor to all‐cause and cardiovascular death among women undergoing coronary angiography for suspected ischemia with and without obstructive CAD. Although traditional risk factors and depression predict SCD in women with and without obstructive CAD, QTc is the single independent risk factor in a low‐risk population of women without obstructive CAD. These data underscore that SCD is an important health threat for women regardless of coronary angiographic status. In addition to attention to management of traditional risk factors in women, further investigation should address mechanistic understanding and interventions targeting depression and QTc in women.
Sources of Funding
This work was supported by contracts from the National Heart, Lung, and Blood Institute's nos. N01‐HV‐68161, N01‐HV‐68162, N01‐HV‐68163, N01‐HV‐68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, General Clinical Research Center grant MO1‐RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars‐Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and Qmed Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women's Heart Research Fellowships, Cedars‐Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars‐Sinai Medical Center, Los Angeles, the Society for Women's Health Research (SWHR), Washington, DC, The Linda Joy Pollin Women's Heart Health Program, and the Erika Glazer Women's Heart Health Project, and the Adelson Family Foundation, Cedars‐Sinai Medical Center, Los Angeles, California. Dr Pepine was also supported by National Institutes of Health grants HL33610, HL56921; UM1 HL087366; the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine; NIH NCATS—University of Florida Clinical and Translational Science UL1TR001427; and PCORnet‐OneFlorida Clinical Research Consortium Clinical Data Research Network‐1501‐26692. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Disclosures
Dr Johnson, Dr Sharaf, Dr Kenkre, Dr Reis, Dr Rogers, Dr Kelsey, Ms Thompson, Dr Shaw, Dr Eteiba and Dr Sopko have nothing to disclose. Dr Mehta reports research funding from Gilead and General Electric. Dr Pepine reports grants from NIH/NHLBI, during the conduct of the study; grants from Amarin, Amgen, AstraZeneca, Baxter, Boehringer Ingleheim, Catadasis, Cytori, Daiichi Sankyo, Esperion, Genentech, ISIS Pharmaceuticals, Neostem, Sanofi/Aventis, and Unified Therapeutics, outside the submitted work. Dr Bittner reports research funding from the following: Steering Committee or National Coordinator (contracted through UAB): Sanofi/Regeneron, Dalcor, AstraZeneca, Eli Lilly; Site PI for multicenter clinical trial: Bayer, Astra Zeneca; Co‐Investigator in School of Public Health Contract: Amgen Consulting/Advisory Board: Amgen, Lilly. Dr Bairey Merz reports consulting revenue paid to CSMC from Gilead, Medscape, Research Triangle Institute, research money from Gilead, Erika Glazer, payments for lectures from Beaumont Hospital, European Horizon, Florida Hospital, INOVA, Korean Cardiology Society, Practice Point Communications, Pri‐Med, University of Chicago, VBWG, University of Colorado, University of Utah, WomenHeart, Harold Buchwald Heart‐Health, Tufts.
(J Am Heart Assoc. 2017;6:e005501 DOI: 10.1161/JAHA.117.005501.)28862961
References
- 1. Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al‐Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. [DOI] [PubMed] [Google Scholar]
- 5. Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. [DOI] [PubMed] [Google Scholar]
- 6. Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate‐based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. [DOI] [PubMed] [Google Scholar]
- 7. Simmons A, Pimentel R, Lakkireddy D. Sudden cardiac death in women. Rev Cardiovasc Med. 2012;13:e37–e42. [DOI] [PubMed] [Google Scholar]
- 8. Chugh SS, Uy‐Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: the Ore‐SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol. 2009;54:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. [DOI] [PubMed] [Google Scholar]
- 11. Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ; Investigators W . Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 12. Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena‐Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pepine CJ, Ferdinand KC, Shaw LJ, Light‐McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN; Committee ACiW . Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM Jr, Chen PS, Chugh SS, Costantini O, Exner DV, Kadish AH, Lee B, Lloyd‐Jones D, Moss AJ, Myerburg RJ, Olgin JE, Passman R, Stevenson WG, Tomaselli GF, Zareba W, Zipes DP, Zoloth L. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516–526. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki T, Nazarian S, Jerosch‐Herold M, Chugh SS. Imaging for assessment of sudden death risk: current role and future prospects. Europace. 2016;18:1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population‐based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two‐year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. [DOI] [PubMed] [Google Scholar]
- 18. Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. [DOI] [PubMed] [Google Scholar]
- 19. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, Pepine CJ, Bairey Merz CN. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, Miele NJ, Williams DO, Merz CN; Group WS . Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol. 2001;87:937–941; A933. [DOI] [PubMed] [Google Scholar]
- 22. Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. [DOI] [PubMed] [Google Scholar]
- 23. Chugh SS, Reinier K, Singh T, Uy‐Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization. Circulation. 1989;80:1301–1308. [DOI] [PubMed] [Google Scholar]
- 25. Deo R, Vittinghoff E, Lin F, Tseng ZH, Hulley SB, Shlipak MG. Risk factor and prediction modeling for sudden cardiac death in women with coronary artery disease. Arch Intern Med. 2011;171:1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peacock J, Whang W. Psychological distress and arrhythmia: risk prediction and potential modifiers. Prog Cardiovasc Dis. 2013;55:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carney RM, Freedland KE, Sheps DS. Depression is a risk factor for mortality in coronary heart disease. Psychosom Med. 2004;66:799–801. [DOI] [PubMed] [Google Scholar]
- 28. Watkins LL, Blumenthal JA, Davidson JR, Babyak MA, McCants CB Jr, Sketch MH Jr. Phobic anxiety, depression, and risk of ventricular arrhythmias in patients with coronary heart disease. Psychosom Med. 2006;68:651–656. [DOI] [PubMed] [Google Scholar]
- 29. Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, Simon G, Siscovick DS. Clinical depression and risk of out‐of‐hospital cardiac arrest. Arch Intern Med. 2006;166:195–200. [DOI] [PubMed] [Google Scholar]
- 30. Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, Garan H, Albert CM. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol. 2009;53:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krantz DS, Whittaker KS, Francis JL, Rutledge T, Johnson BD, Barrow G, McClure C, Sheps DS, York K, Cornell C, Bittner V, Vaccarino V, Eteiba W, Parashar S, Vido DA, Merz CN. Psychotropic medication use and risk of adverse cardiovascular events in women with suspected coronary artery disease: outcomes from the Women's Ischemia Syndrome Evaluation (WISE) study. Heart. 2009;95:1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA, National Heart L, Blood I. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:722–725. [DOI] [PubMed] [Google Scholar]
- 33. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–728. [DOI] [PubMed] [Google Scholar]


