Abstract
Background
It is unknown whether the astronaut occupation or exposure to microgravity influences the risk of long‐term cardiovascular disease (CVD). This study explored the effects of being a career National Aeronautics and Space Administration (NASA) astronaut on the risk for clinical CVD end points.
Methods and Results
During the Longitudinal Study of Astronaut Health, data were collected on 310 NASA astronauts and 981 nonastronaut NASA employees. The nonastronauts were matched to the astronauts on age, sex, and body mass index, to evaluate acute and chronic morbidity and mortality. The primary outcomes were composites of clinical CVD end points (myocardial infarction, congestive heart failure, stroke, and coronary artery bypass surgery) or coronary artery disease (CAD) end points (myocardial infarction and coronary artery bypass surgery). Of the astronauts, 5.2% had a clinical CVD end point and 2.9% had a CAD end point compared with the nonastronaut comparisons with 4.7% and 3.1% having CVD and CAD end points, respectively. In the multivariate models adjusted for traditional risk factors, astronauts had a similar risk of CVD compared with nonastronauts (adjusted hazard ratio, 1.08; 95% CI, 0.60–1.93; P=0.80). Risk of a CAD end point was similar between groups (hazard ratio, 0.97; CI, 0.45–2.08; P=0.93). In astronauts with early spaceflight experience, the risk of CVD (hazard ratio, 0.80; CI, 0.25–2.56; P=0.71) and CAD (hazard ratio, 1.23; CI: 0.27–5.61; P=0.79) compared with astronauts with no experience were not different.
Conclusions
These findings suggest that being an astronaut is not associated with increased long‐term risk of CVD development.
Keywords: cardiovascular disease, heart failure, myocardial infarction, spaceflight, stroke
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
The results of this study provide insight into cardiovascular disease (CVD) risk in a large cohort of National Aeronautics and Space Administration (NASA) astronauts.
Career NASA astronauts as a whole had the same risk of developing a clinical CVD end point or a specific coronary artery disease end point as did a group of NASA civil servant employees, and this risk did not change after adjusting for known CVD covariates.
Among the NASA astronauts who had spaceflight experience by the fifth decade of life, the risk of CVD was not different from that of astronauts without a history of spaceflight by the fifth decade of life.
What Are the Clinical Implications?
These findings have important implications for the lifelong assessment of astronaut health and suggest that individuals in the astronaut corps are not at an increased risk of developing CVD and that spaceflight experience may not contribute to the risk of CVD events.
The broadly defined spaceflight experiences do not appear to put NASA astronauts at greater risk of critical CVD end points across the life span.
Whether specific types of missions like long‐duration missions or those with greater exposure to cosmic radiation beyond low‐earth orbit elicit long‐term CVD risk merits further study.
Introduction
The maintenance of short‐ and long‐term cardiovascular health of the National Aeronautics and Space Administration (NASA) astronaut corps is a primary concern given the established physiological adaptations associated with spaceflight and the aging of the retired astronaut population. Attributed to reduced gravity, astronauts manifest acute physiological adaptations that may be detrimental to long‐term cardiovascular health. Maladaptation in cardiac structure and function are consistently reported following short‐duration spaceflight or head‐down tilt bed rest, a ground‐based analog for microgravity exposure, that return to preflight levels in the following days to weeks.1, 2, 3, 4, 5, 6, 7 However, despite the physiological adaptations associated with short‐duration spaceflight, there is contradictory evidence to date, linking career astronauts and spaceflight experience to an increased lifetime risk for cardiovascular disease (CVD).8, 9 Hamilton et al estimated the all‐cause cardiac risk in US astronauts by calculating individual Framingham Risk Scores. These researchers suggest that male and female astronauts have an ≈3% to 5% risk of developing CVD between the age of 40 and 50 years.10 Although the analysis by Hamilton et al provides valuable insight, it provides only a risk estimate and not an assessment of whether astronauts are at an elevated actual risk relative to other occupations. In a subsequent study recently published, Delp et al evaluated deaths attributed to CVD in US NASA astronauts through the year 2015.11 These researchers raise the possibility that all astronauts with spaceflight experience had a similar proportional mortality rate attributed to CVD as the general US population. They also suggest that in the small number of Apollo astronauts, death attributed to CVD was significantly greater than nonflight astronauts. However, neither Hamilton et al nor Delp et al included important cardiovascular risk factors (eg, age, smoking history, or statin prescription) in their analysis, nor did either explore nonfatal CVD events. As such, this recent work has highlighted the need to perform a more in‐depth analysis of whether spaceflight is associated with an increased risk of CVD in astronauts.12 Therefore, the primary objective of the present study was to determine the effects of being a NASA astronaut and spaceflight experience on the risk for CVD end points. We hypothesized that after controlling for known CVD risk factors, the risk of developing CVD would be similar between US NASA astronauts and nonastronaut comparisons. Similarly, there would be no difference between astronauts with and without a history of spaceflight experience.
Methods
Study Design and Study Population
The current study is a secondary analysis of cardiovascular outcome data from the LSAH (Longitudinal Study of Astronaut Health). The LSAH was a cohort study previously described in detail.13 The purpose of the LSAH was to evaluate acute and chronic morbidity and mortality of NASA career astronauts. For each astronaut selection class, comparison participants were enrolled at a 3:1 ratio of comparisons to astronauts. The comparison cohort was selected from the pool of NASA civil servants who worked at Johnson Space Center (JSC) and had a physical examination at the JSC Clinic within 3 years of each astronaut selection class. The comparison participants were recruited if age and body mass index (BMI) were within 2 SDs of the means for the incoming selection class of astronauts by sex. Recruitment was conducted in 2 phases, a historical phase and a prospective phase. The study began in 1992 when the comparison participants were retrospectively matched to each astronaut selection class from 1959 to 1990. From 1992 to 2000, comparison participants were matched prospectively as each new astronaut class was selected. All study participants were followed until May 2010 when the study ended. Because the astronauts and comparison participants were matched only for sex, age, and BMI, but not cardiovascular risk factor burden, we adjusted for known risk factors in our multivariate model.
Longitudinal Study Physical Examination
As part of the LSAH study, each comparison and astronaut was provided a comprehensive physical examination by a JSC physician in the Occupational Health Clinic or the Flight Medicine Clinic. The JSC physician reviewed and documented interval medical history since the previous exam, including any hospitalizations or illnesses (cardiovascular and noncardiovascular). Furthermore, the JSC physician collected medical data on any events that occurred between exams and was able to do a thorough examination (including lab work, ECG, etc) to corroborate the events. For this study, participants in both groups were monitored from their first medical exam at the time of enrollment into the LSAH until death, voluntary withdrawal, or the end of the study in May 2010, whichever occurred first. Participants who retired or otherwise left NASA before the end of the study were encouraged to return to JSC for clinical examinations according to the study schedule.
Retrospective Data Collection of Cardiovascular End Points and Risk Factors
For the purposes of the current study, data were collected retrospectively for all comparisons and astronauts. Both cardiovascular event data and risk factor data were mined from all physical examination data in the previously collected JSC medical records by qualified LSAH epidemiologists. All data were reviewed by a second epidemiologist for quality assurance. Clinical CVD events were defined as cardiovascular death and nonfatal cardiovascular disease identified using International Classification of Disease, Ninth Revision (ICD‐9) codes obtained from medical records. The defined clinical events consisted of myocardial infarction (ICD‐9 codes 410–412, death certificate, or self‐report), congestive heart failure (ICD‐9 codes 425 or 428, death certificate, or self‐report), stroke (ICD‐9 codes 434–436, death certificate, or self‐report), and coronary artery bypass graft (ICD‐9 codes V45.81, Current Procedural Terminology 92980, 92982, 93451–93461, or self‐report). Mortality data pertaining to cardiovascular death were derived from death certificates when no other source was available. Because of historical changes to ICD‐9 and Current Procedural Terminology codes and because personal history fields are not coded, text searches of keywords were also conducted. All records were reviewed for completeness and duplicate entries were removed. Coronary artery disease (CAD) events consisted of myocardial infarction and coronary artery bypass graft as described above. The total number of person‐years (PY) was calculated for each astronaut and comparison based on time from the date of the first medical exam to the date of the cardiovascular event or end of the study.
Cardiovascular risk factor data were obtained at the first medical exam after enrollment in the study and at each study‐related physical exam thereafter. Relevant covariates obtained from the medical records included resting systolic and diastolic blood pressure, total cholesterol, BMI, fasting blood glucose, smoking status and history (current, former, or ever), CVD medication history (use of antihypertensive or use of antilipemic drugs), and age. Variables that could potentially identify an astronaut were binned into groups. Participant age was reported in decades (ie, 20–29. 30–39, 40–49, 50–59, 60–69, or ≥70).
Statistical Analysis
Cardiovascular risk factor data from astronauts and nonastronaut employees were compared using the chi‐square test for categorical variables and Wilcoxon rank‐sum test for continuous variables. The unadjusted incidence rate was calculated by dividing the number of participants who had a CVD or CAD event by the total number of PY of follow‐up. Kaplan–Meier plots were used to show the difference in time to event by study group and statistically compared with the log‐rank test. Cox proportional hazard analysis was used to compare the risk of all CVD events and CAD events between astronauts and comparisons in 3 step‐wise models: a univariate model (model 1); a multivariate model adjusted for age and sex (model 2); and a multivariate model adjusted for age, sex, and baseline measurements of BMI, systolic blood pressure, fasting blood glucose, total cholesterol, smoking status, and CVD medication history (model 3). The risk for each separate type of CVD event (myocardial infarction, stroke, etc) could not be performed because of the small number of events per independent variable in the multivariate model.14
An analysis of only male astronaut data was also conducted to determine whether spaceflight experience was related to CVD event risk. The male astronauts were subdivided into 2 mutually exclusive groups on the basis of history of spaceflight experience at the time of their first available medical exam in the fifth decade of life (age 40–49 years). The first exam in the fifth decade was chosen because it was preceding the first recorded cardiovascular event for any astronaut. This analysis was limited to men because spaceflight data for women could not be obtained from the LSAH because of privacy concerns. The unadjusted incidence rate was calculated for each group. Kaplan–Meier plots were used to show the difference in time to event by study group and statistically compared with the log‐rank test. Cox proportional hazard analysis was used to compare the risk of clinical CVD events and CAD events between the 2 groups using a multivariate model adjusted for age, sex, and baseline measurements of BMI, systolic blood pressure, fasting blood glucose, total cholesterol, smoking status, and CVD medication history.
Human Subjects and Data Privacy
Institutional review boards from both NASA JSC and Kansas State University (Manhattan, KS) approved the study protocol by study exemption and informed consent was waived because analysis of the data and variables provided would not directly identify a particular individual. Because of this exemption, the data that were provided to the investigators by NASA were stripped of all potentially identifying characteristics, such as date of birth, date of medical exam, exact number of missions flown, exact days in space, and exact age at diagnosis. Age and spaceflight mission characteristics were provided by NASA to the investigators binned in groups.
Results
Astronauts Versus Comparisons
The baseline characteristics of the astronaut and nonastronaut comparison groups are displayed in Table 1. At the first exam, the age of the astronauts ranged from the 20th to 40th decade of life, with 14% of the astronauts female. In the comparison group, the age ranged from the 20th to 60th decade of life with 14% female. At baseline, astronauts had statistically significantly lower resting blood pressure, total cholesterol, and higher fasting blood glucose compared with nonastronaut comparisons; however, these differences do not appear clinically meaningful.
Table 1.
Baseline Characteristics of Astronaut and Nonastronaut Comparisons
| Variable | Astronaut (n=310) | Nonastronaut Comparisons (n=981) | P Value |
|---|---|---|---|
| Age, decadea | |||
| 20 to 29 y, n (%) | 22 (7) | 169 (17) | ··· |
| 30 to 39 y, n (%) | 261 (84) | 703 (72) | ··· |
| 40 to 49 y, n (%) | 27 (9) | 104 (11) | ··· |
| 50 to 59 y, n (%) | 0 | 4 (<1) | ··· |
| 60 to 69 y, n (%) | 0 | 1 (<1) | ··· |
| >70 y, n (%) | 0 | 0 | ··· |
| Female, n (%) | 43 (14) | 141 (14) | 0.83 |
| BMI, kg/m2 | 23.6 (22.1–25.1) | 23.6 (22.4–25.0) | 0.76 |
| SBP, mm Hg | 114 (108–120) | 116 (110–124) | <0.001 |
| DBP, mm Hg | 70 (66–76) | 72 (70–80) | <0.001 |
| Total cholesterol, mg/dL | 181 (160–207) | 188 (165–217) | 0.002 |
| Fasting serum glucose, mg/dL | 90 (86–95) | 88 (83–94) | 0.004 |
| Smoking (ever), n (%) | 35 (11) | 141 (14) | 0.18 |
| CVD medication history (%) | |||
| Antihypertensive drug | 0 (0) | 20 (2) | 0.01 |
| Antilipemic drug | 2 (0.6) | 5 (0.5% | 0.77 |
| Spaceflight history, n (%) | 289 (93) | n/a | ··· |
| >2 missions flown, n (%) | 112 (36) | n/a | ··· |
| >30 d in space, n (%) | 90 (29) | n/a | ··· |
Values are number of subjects (percentages), or medians (interquartile ranges). BMI indicates body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; n/a, not applicable; SBP, systolic blood pressure.
Indicates variables that were binned.
Table 2 shows the frequency of CVD end points and the crude incidence rates (per 1000 PY) in astronauts and nonastronaut comparisons. A total of 16 astronauts had a clinical CVD end point over a mean follow‐up of 22.1±11.7 years, and 46 nonastronaut comparisons developed a clinical CVD end point over a mean follow‐up of 21.9±12.8 years. The incidence rate in astronauts for all CVD end points were 2.34 per 1000 PY compared with 2.15 per 1000 PY in the comparison group. There was no significant difference in the time to a CVD end point between astronauts and comparisons (log‐rank=0.26; P=0.61; Figure 1A). Table 3 displays the results of the fully adjusted Cox proportional hazards model. There were no differences in risk of having a clinical CVD end point between astronauts and nonastronaut comparisons (hazard ratio, 1.08; 95% CI, 0.60–1.93; P=0.80).
Table 2.
Frequency and Crude Incidence of CVD Events
| Astronauts | Nonastronaut Comparisons | |||
|---|---|---|---|---|
| Frequency | Incidence Rate (Per 1000 PY) | Frequency | Incidence Rate (Per 1000 PY) | |
| All CVD events | 16 | 2.34 | 46 | 2.15 |
| MI | 7 | 1.02 | 21 | 0.98 |
| CHF | 5 | 0.73 | 4 | 0.19 |
| Stroke | 5 | 0.73 | 17 | 0.80 |
| CABG | 5 | 0.73 | 22 | 1.64 |
| Multiple | 5 | 0.73 | 17 | 0.79 |
| CAD events | 9 | 1.32 | 30 | 1.40 |
CABG indicates coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cardiovascular disease; MI, myocardial infarction; Multiple, individuals with multiple CVD events; PY, person‐years.
Figure 1.
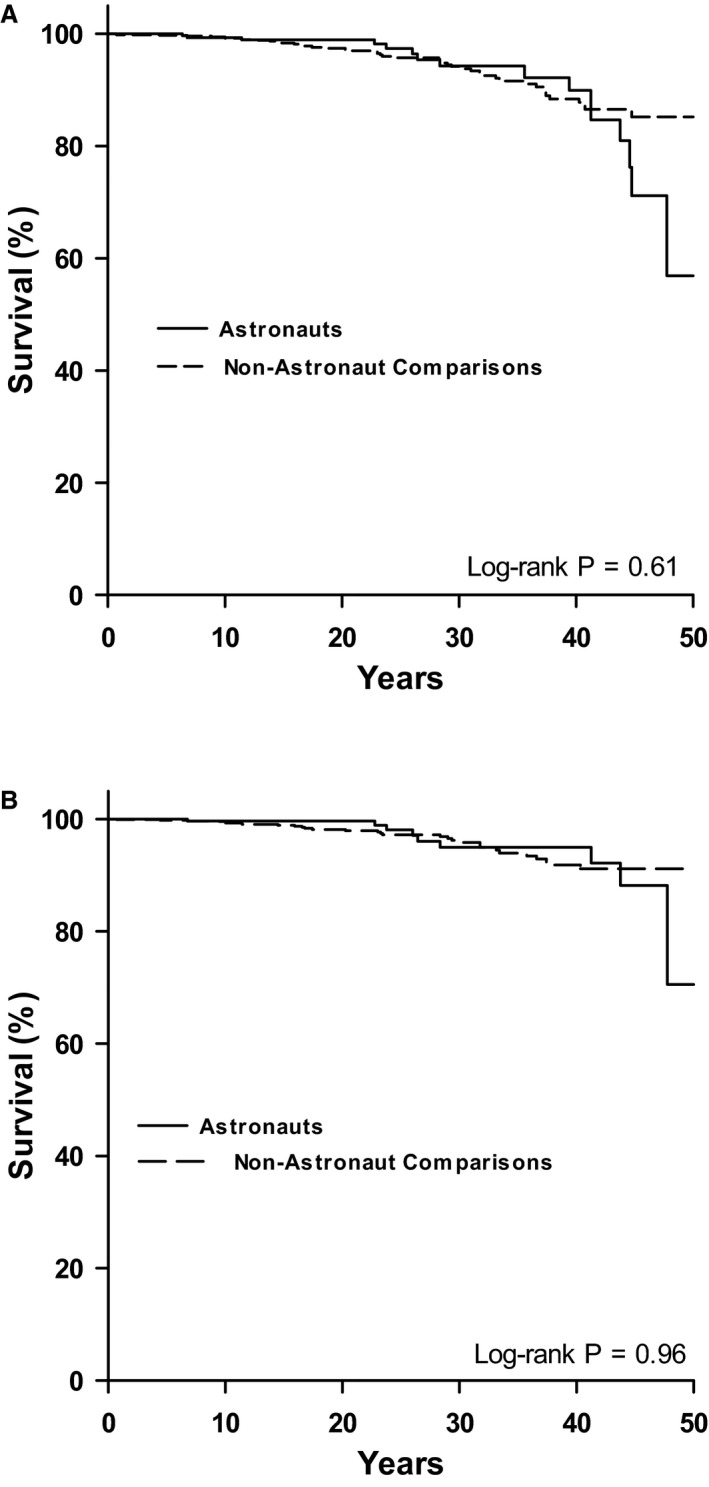
Kaplan–Meier curves of CVD end points (A) and CAD end points (B) in astronauts and nonastronaut comparisons. CAD indicates coronary artery disease; CVD, cardiovascular disease.
Table 3.
Cox Proportional Hazard Models for CVD and CAD End Points in Astronauts vs Nonastronaut Comparisons
| Covariates | All CVD Events | CAD Events | ||
|---|---|---|---|---|
| HR | 95% | HR | 95% | |
| Model 1 | 1.16 | 0.66 to 2.05 | 0.98 | 0.47 to 2.07 |
| Model 2 | 1.10 | 0.62 to 1.95 | 0.97 | 0.46 to 2.04 |
| Model 3 | 1.08 | 0.60 to 1.93 | 0.97 | 0.45 to 2.08 |
Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: adjusted for baseline age, sex, body mass index, smoking status, CVD medication history, fasting glucose, total cholesterol, systolic blood pressure. CAD indicates coronary artery disease; CVD, cardiovascular disease; HR, hazard ratio.
A CAD end point occurred in 9 astronauts and 30 nonastronaut comparisons (Table 2). For astronauts, this represented an incidence rate of 1.32 per 1000 PY compared with 1.40 per 1000 PY in nonastronaut comparisons. As with the clinical CVD end point, there was no significant difference in the time to a CAD end point between astronauts and comparisons (log‐rank=0.002; P=0.96; Figure 1B). In the fully adjusted Cox proportional hazards model (Table 3), there were no differences in risk of having a CAD end point between astronauts and nonastronaut comparisons (hazard ratio, 0.97; CI, 0.45–2.08; P=0.93).
Astronaut Spaceflight Experience Analysis
Of the 267 male astronauts, 135 had a history of spaceflight experience and 132 had no spaceflight experience at the time of their first medical exam in the fifth decade of life. Table 4 shows the frequency of CVD end points and the crude incidence rates (per 1000 PY). A total of 9 astronauts with a history of spaceflight had a clinical CVD end point over a mean follow‐up of 25.5±11.3 years and 7 without spaceflight experience developed a clinical CVD end point over a mean follow‐up of 19.8±12.1 years. There was no significant difference in the time to a CVD (Figure 2A; log‐rank=0.96; P=0.33) or CAD (Figure 2B; log‐rank=0.005; P=0.95) end point between the astronaut groups. In a fully adjusted Cox proportional hazards model, there was no difference in CVD risk between astronauts with no spaceflight experience and those without (hazard ratio, 0.80; CI, 0.25–2.56; P=0.71). Similarly, in a fully adjusted Cox proportional hazards model, there was no difference in CAD risk between astronauts with spaceflight experience those without (hazard ratio, 1.23; CI, 0.27–5.61; P=0.79).
Table 4.
Frequency and Crude Incidence of CVD End Points in Male Astronauts With History of Spaceflight at Fifth Decade of Life
| Astronauts With Spaceflight Experiencea (n=135) | Astronauts With No Spaceflight Experiencea (n=132) | |||
|---|---|---|---|---|
| Frequency | Incidence Rate (Per 1000 PY) | Frequency | Incidence Rate (Per 1000 PY) | |
| All CVD events | 9 | 2.61 | 7 | 2.68 |
| MI | 4 | 1.16 | 3 | 1.15 |
| CHF | 2 | 0.87 | 2 | 0.76 |
| Stroke | 3 | 0.58 | 3 | 1.15 |
| CABG | 3 | 0.87 | 2 | 0.76 |
| Multiple | 3 | 2 | ||
| CAD events | 6 | 1.74 | 3 | 1.15 |
CABG indicates coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cardiovascular disease; MI, myocardial infarction; Multiple, individuals with multiple CVD events; PY, person‐years.
Spaceflight experience at the time of first exam in the fifth decade of life.
Figure 2.
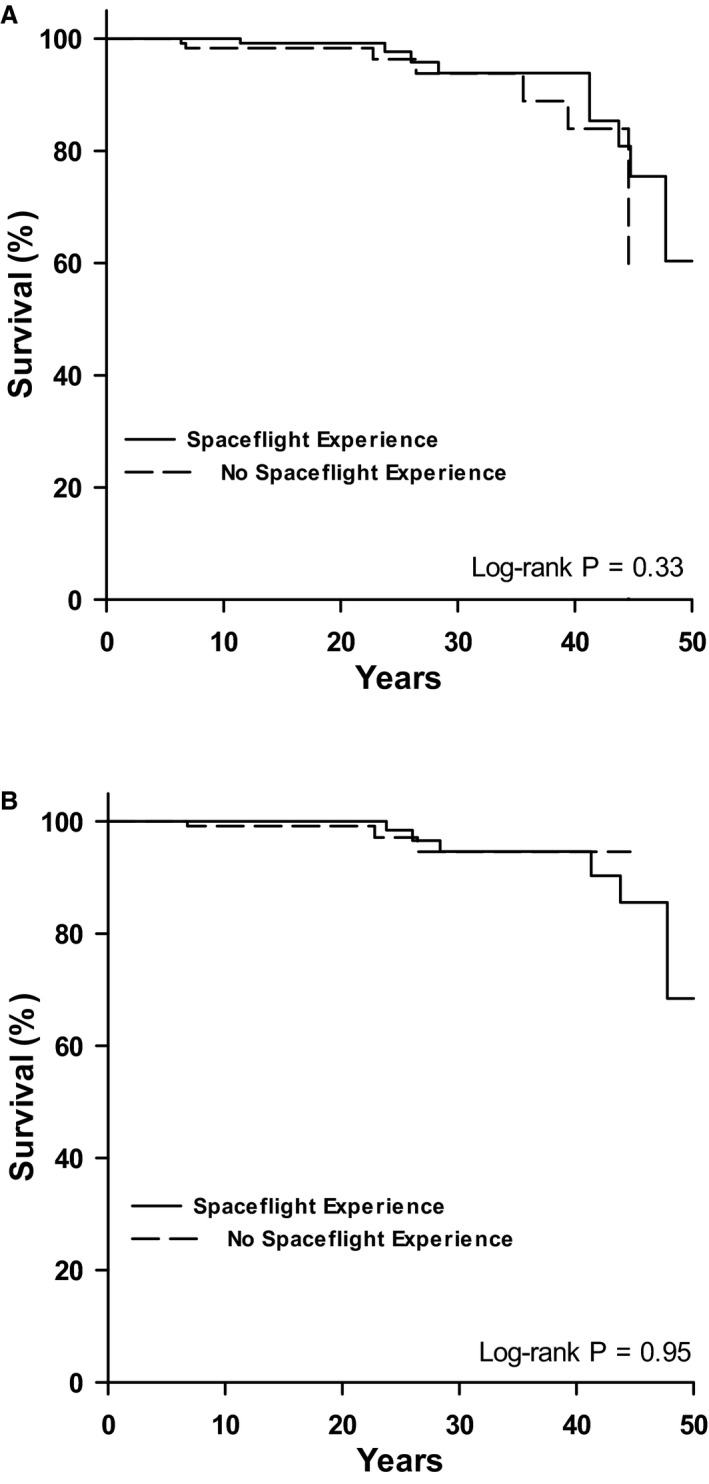
Kaplan–Meier curves of CVD end points (A) and CAD end points (B) in astronauts with and without a history of spaceflight experience at the fifth decade of life (age 40–49 years). CAD indicates coronary artery disease; CVD, cardiovascular disease.
Discussion
In a large cohort of astronauts, we evaluated the effects of being a career NASA astronaut on clinical CVD and CAD end points. In this study, the career astronauts as a whole had the same risk of developing a CVD end point or a specific CAD end point as did a group of NASA civil servant employees, and this risk did not change after adjusting for known CVD covariates. Second, among the astronauts who had spaceflight experience by the fifth decade of life, the risk of CVD was not different from that of astronauts without a history of spaceflight by the fifth decade of life. These findings have important implications for the lifelong assessment of astronaut health and suggest that individuals in the astronaut corps are not at an increased risk of developing CVD and that spaceflight experience may not contribute to the risk of CVD events.
Compared with that of the general US population, the incidence rate for CVD in the present study is low. According to a 2015 American Heart Association report, men and women between the ages of 45 and 54 have an incidence rate, respectively, of 10.1 and 4.2 per 1000 PY for coronary heart disease, heart failure, stroke, or intermittent claudication.15 Similarly, the incidence rates for heart failure (6.5 per 1000 PY), myocardial infarction (4.6 per 1000 PY), and stroke (3.1 per 1000 PY) from the ARIC (Atherosclerosis Risk in Communities Study) in the general US population between the ages of 45 and 84 years, is higher than the incidence rate for either the NASA astronauts or the nonastronaut comparisons.16 These findings are very similar to those from the “1000 Aviators” study conducted by MacIntyre et al, who report that the cardiovascular mortality rate in US Navy pilots was less than half of that predicted for unselected American men of similar age and ethnicity.17 These findings suggest that military pilots, despite the rigors of military work, have very favorable long‐term cardiovascular health compared with the general population, which supports our findings in a similar group of individuals.18 Therefore, the low incidence rate of CVD in astronauts reported in the present investigation, relative to the incidence rate in nonastronaut employees and to published reports in the general US population, suggests that astronauts are not at increased risk for CVD. In total, these findings support a 2009 review highlighting that the probability of an acute CVD event having a negative impact on a long‐duration space mission is low compared with the known mission risks associated with reduced orthostatic tolerance, aerobic exercise capacity, and muscular strength.8
To our knowledge, this is the first study to use multivariate survival analysis to investigate the risk of CVD in astronauts and matched nonastronauts. However, it is not the first to evaluate long‐term cardiovascular health in astronauts. Peterson et al evaluated cause‐specific mortality in NASA astronauts between the years 1959 and 1991.19 They determined that ≈10% of all deaths during this time were attributed to diseases of the circulatory system. Additionally, they report an incidence rate of 0.75 CVD deaths per 1000 PY. The focus of the present study was on diagnosis of CVD end points, not CVD death, which would account for the higher incidence rate reported here. Additionally, given that age is a primary risk factor for the development of CVD, the longer follow‐up time in the present study could also account for these differences. More recently, Delp et al evaluated CVD cause of death among NASA astronauts.11 Whereas the prevalence of CVD mortality for astronauts who had spaceflight experience was similar to that for the general US population, the Apollo astronauts seemed to have a significantly greater risk of CVD death than of death from other causes, relative to nonflight and low‐earth orbit astronauts. These data raised the possibility that the spaceflight experience during deep‐space missions (ie, beyond low‐earth orbit and the protection of the Van Allen belts) is associated with a greater risk of CVD. However, it is important to note that known CVD covariates (eg, fasting blood glucose, total cholesterol, smoking status, blood pressure, statin prescription, sex, and BMI) were not controlled for between groups of astronauts with different flight experiences, as was done in the current study.
Within our astronaut cohort, diversity in spaceflight experience existed. This is an important consideration given that previous findings have highlighted the effects of microgravity experience on important physiological parameters of cardiac function. Ade et al have recently suggested that maximal aerobic exercise capacity, which is an integrative measure of cardiovascular function, decreases in a dose‐dependent manner with prolonged spaceflight, as a result of changes in cardiovascular function.20, 21 Similarly, Dorfman et al demonstrated a strong relationship between the number of days in microgravity and the degree of cardiac atrophy.2 However, allthough these reports provide insight into the acute physiological changes following a single space mission, they do not address the potential cardiovascular consequences associated with repeated missions.
In the present investigation, approximately half of the NASA astronauts in the study had a history of spaceflight experience by the fifth decade of life (age 40–49 years). The first exam in the fifth decade was chosen as the first exam for the study, because it represented, for all astronauts, a time point before the first recorded cardiovascular event. Given that onset of a CVD end point could determine an individual's ability to be assigned to a spaceflight mission, use of lifetime history of spaceflight would have confounded the results. Using midlife spaceflight experience, we determined that the risk of a CVD end point was not significantly different between groups. These findings suggest that our broadly defined history of midlife spaceflight experience is not associated with greater CVD risk. Although this is a key finding, it should be interpreted with some caution given the diversity of spaceflight experiences (eg, duration, number of missions, and flight plan). It is also important to note that of the 132 astronauts who did not have spaceflight experience at the fifth decade of life, 112 did obtain spaceflight experience later in life. These findings suggest that exposure to microgravity and associated radiation in low‐earth orbit may not play a role in the development of CVD in astronauts. However, this interpretation can only be generalized to the characteristics of the missions flown by NASA astronauts through 2010 when the study ended. With the exception of 24 Apollo astronauts, all NASA astronauts’ spaceflight missions have been confined to low‐earth orbit. A mission to Mars may result in exposure to higher levels of galactic cosmic radiation for up to 3 years and will include increased radiation experience given that exposure rate increases when a spacecraft leaves low‐earth orbit.22 Given the link between radiation exposure and risk of circulatory disease, additional work is required to determine the long‐term CVD risk in astronauts performing long‐duration, deep‐space missions.23, 24
Study Limitations
In an epidemiological study, the ability to detect clinically meaningful differences between groups is dependent on the prevalence of the event and the number of subjects in the study sample. Relative to other epidemiological studies,16 the number of subjects is small, but cannot be readily increased because of the unique population of NASA astronauts studied. We combined all defined CVD end points together as a single primary end point in an attempt to improve our ability to detect differences between groups. As such, we were limited in our ability to evaluate individual disease types or evaluate the risk in subgroups. The analysis was also constrained by the use of age groups instead of individual ages. This was done to maintain anonymity and may have impacted the risk analysis. Additionally, the exact age at which a disease event occurred could not be provided, only the age decade. Selection of the nonastronaut comparison group may have also introduced error into the risk assessment. Although our comparison cohort was well matched at selection for sex, age, and BMI, it was not matched for CVD risk factors at baseline. As described in Table 1, the astronaut cohort had statistically significantly lower resting blood pressure, lower total cholesterol, and higher fasting blood glucose levels, but these do not appear to be clinically significant. To minimize this limitation, these CVD risk factors were included in our multivariate analyses and provided a more‐robust comparison between our group of astronauts and nonastronaut comparisons. There may have also been bias in the ascertainment of the cardiovascular events. The nonastronaut comparisons received physical examinations approximately half as frequently as the astronauts.13 Additionally, the attending physician was not blinded to the subject's employment, which may have resulted in astronauts being screened for disease to a greater extent than nonastronauts. However, regardless of study group, each participant received thorough examinations that allowed the flight surgeon to adjudicate any self‐reported events at the time of the exam, which can provide information similar to medical record abstraction.25 Furthermore, only significant CVD events, which are less open to interpretation and recollection errors (more likely to recall severe diagnoses) were used to evaluate CVD risk. Last, survivorship bias may exist given that Kaplan–Meier survival analysis and Cox proportional hazards regression are limited in their ability to adjust for competing risk of death.
Conclusions
The findings of the present study suggest that astronauts may not be at an increased long‐term risk of CVD development. Additionally, the broadly defined spaceflight experiences do not appear to put NASA astronauts at greater risk of critical CVD end points across the life span. Whether specific types of missions, like long‐duration missions or those with greater exposure to cosmic radiation beyond low‐earth orbit, elicit long‐term CVD risk merits further study, especially given the future of space exploration.
Sources of Funding
This study was supported by the National Aeronautics and Space Administration (NASA) research grant NNJ12ZSA002N awarded to Ade and Barstow.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005564 DOI: 10.1161/JAHA.117.005564.)28784652
References
- 1. Convertino VA. Exercise and adaptation to microgravity environments. Compr Physiol 2011, Supplement 14: Handbook of Physiology, Environmental Physiology: 815–843. 1996. doi: 10.1002/cphy.cp040236 [Google Scholar]
- 2. Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR. Cardiac atrophy in women following bed rest. J Appl Physiol. 2007;103:8–16. [DOI] [PubMed] [Google Scholar]
- 3. Dorfman TA, Rosen BD, Perhonen MA, Tillery T, McColl R, Peshock RM, Levine BD. Diastolic suction is impaired by bed rest: MRI tagging studies of diastolic untwisting. J Appl Physiol. 2008;104:1037–1044. [DOI] [PubMed] [Google Scholar]
- 4. Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol. 2001;91:645–653. [DOI] [PubMed] [Google Scholar]
- 5. Bungo MW, Goldwater DJ, Popp RL, Sandler H. Echocardiographic evaluation of space‐shuttle crewmembers. J Appl Physiol. 1987;62:278–283. [DOI] [PubMed] [Google Scholar]
- 6. Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed‐rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. [DOI] [PubMed] [Google Scholar]
- 7. Spaak J, Montmerle S, Sundblad P, Linnarsson D. Long‐term bed rest‐induced reductions in stroke volume during rest and exercise: cardiac dysfunction vs. volume depletion. J Appl Physiol. 2005;98:648–654. [DOI] [PubMed] [Google Scholar]
- 8. Convertino VA. Status of cardiovascular issues related to space flight: implications for future research directions. Respir Physiol Neurobiol. 2009;169(suppl 1):S34–S37. [DOI] [PubMed] [Google Scholar]
- 9. Convertino VA, Cooke WH. Evaluation of cardiovascular risks of spaceflight does not support the NASA bioastronautics critical path roadmap. Aviat Space Environ Med. 2005;76:869–876. [PubMed] [Google Scholar]
- 10. Hamilton DR, Murray JD, Kapoor D, Kirkpatrick AW. Cardiac health for astronauts: current selection standards and their limitations. Aviat Space Environ Med. 2005;76:615–626. [PubMed] [Google Scholar]
- 11. Delp MD, Charvat JM, Limoli CL, Globus RK, Ghosh P. Apollo lunar astronauts show higher cardiovascular disease mortality: possible deep space radiation effects on the vascular endothelium. Sci Rep. 2016;6:29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. NASA HRP . NASA research announcement—research and technology development to support crew health and performance in space exploration missions—nnj12zsa002n. 2012:1–106.
- 13. Institute of Medicine (U.S.). Committee on the Longitudinal Study of Astronaut Health , Longnecker DE, Manning FJ, Worth MH; United States. National Aeronautics and Space Administration, National Academy of Sciences (U.S.) . Review of NASA'S Longitudinal Study of Astronaut Health. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 14. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. [DOI] [PubMed] [Google Scholar]
- 15. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu SM, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, Comm AHAS, Subcomm SS. Executive summary: heart disease and stroke statistics—2015 update a report from the American Heart Association. Circulation. 2015;131:434–441. [DOI] [PubMed] [Google Scholar]
- 16. National Heart, Lung, and Blood Institute . Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Disease. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 17. MacIntyre NR, Mitchell RE, Oberman A, Harlan WR, Graybiel A, Johnson E. Longevity in military pilots: 37‐year followup of the Navy's “1000 aviators”. Aviat Space Environ Med. 1978;49:1120–1122. [PubMed] [Google Scholar]
- 18. York E, Mitchell RE, Graybiel A. Cardiovascular epidemiology, exercise, and health: 40‐year followup of the U.S. Navy's “1000 aviators”. Aviat Space Environ Med. 1986;57:597–599. [PubMed] [Google Scholar]
- 19. Peterson LE, Pepper LJ, Hamm PB, Gilbert SL. Longitudinal study of astronaut health: mortality in the years 1959‐1991. Radiat Res. 1993;133:257–264. [PubMed] [Google Scholar]
- 20. Ade CJ, Broxterman RM, Barstow TJ. VO(2max) and microgravity exposure: convective versus diffusive O(2) transport. Med Sci Sports Exerc. 2015;47:1351–1361. [DOI] [PubMed] [Google Scholar]
- 21. Ade CJ, Broxterman RM, Moore AD, Barstow TJ. Decreases in maximal oxygen uptake following long‐duration spaceflight: role of convective and diffusive O2 transport mechanisms. J Appl Physiol (1985). 2017;122:968–975. [DOI] [PubMed] [Google Scholar]
- 22. Cucinotta FA, Kim MH, Chappell LJ, Huff JL. How safe is safe enough? Radiation risk for a human mission to Mars. PLoS One. 2013;8:e74988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darby SC, Ewertz M, McGale P, Bennet AM, Blom‐Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 24. Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, Harrison JD, Hildebrandt G, Ivanov V, Kashcheev VV, Klymenko SV, Kreuzer M, Laurent O, Ozasa K, Schneider T, Tapio S, Taylor AM, Tzoulaki I, Vandoolaeghe WL, Wakeford R, Zablotska LB, Zhang W, Lipshultz SE. Systematic review and meta‐analysis of circulatory disease from exposure to low‐level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye F, Moon DH, Carpenter WR, Reeve BB, Usinger DS, Green RL, Spearman K, Sheets NC, Pearlstein KA, Lucero AR, Waddle MR, Godley PA, Chen RC. Comparison of patient report and medical records of comorbidities: results from a population‐based cohort of patients with prostate cancer. JAMA Oncol. 2017. DOI: 10.1001/jamaoncol.2016.6744. Available at http://jamanetwork.com/journals/jamaoncology/article-abstract/2603224. Accessed July 31, 2017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


