Abstract
Background
Data on the clinical utility of coronary computed tomography angiography–derived fractional flow reserve (FFRCT) are sparse. In patients with intermediate (40–70%) coronary stenosis determined by coronary computed tomography angiography, we investigated the association of replacing standard myocardial perfusion imaging with FFRCT testing with downstream utilization of invasive coronary angiography (ICA) and the diagnostic yield of ICA (rate of no obstructive disease, and rate of revascularization).
Methods and Results
This was a single‐center observational study of symptomatic patients with suspected coronary artery disease referred to coronary computed tomography angiography between 2013 and 2015. Patients were divided into 3 historical groups based on the adjunctive functional testing approach: myocardial perfusion imaging (n=1332) or FFRCT “implementation” (n=800) or “clinical use” (n=1391). Propensity score matching was used to estimate the average period effect on outcomes. Patients in the FFRCT clinical use group versus the myocardial perfusion imaging group were older and had higher pretest probability of obstructive disease. After adjusting for baseline risk characteristics, there was a reduction in downstream ICA utilization (absolute risk difference: −4.2; 95% CI, −6.9 to −1.6; P=0.002). In patients referred to ICA, findings of no obstructive coronary artery disease decreased (−12.8%; 95% CI, −22.2 to −3.4; P=0.008) and rate of coronary revascularization increased (14.1%; 95% CI, 3.3–24.9; P=0.01), as did availability of functional information for guidance of revascularization (27.8%; 95% CI, 11.3–44.4; P<0.001) after clinical adoption of FFRCT.
Conclusions
Replacing adjunctive myocardial perfusion imaging with FFRCT testing for functional assessment of intermediate stenosis determined by coronary computed tomography angiography in stable coronary artery disease was associated with less ICA utilization, and a higher ICA diagnostic yield. The findings in this observational study needs confirmation in prospective, randomized trials.
Keywords: computed tomography angiography, coronary artery disease, imaging, positron emission tomography
Subject Categories: Computerized Tomography (CT), Diagnostic Testing, Imaging, Nuclear Cardiology and PET, Angiography
Clinical Perspectives
What Is New?
This single‐center, real‐world‐practice, observational study used a strategy of replacing adjunctive rubidium‐82 positron emission tomography with coronary computed tomography angiography–derived fractional flow reserve testing for functional assessment of intermediate stenosis, determined by coronary computed tomography angiography, in patients with stable coronary artery disease. This approach was associated with a reduction in the number of patients who had to return for a second noninvasive test, less downstream invasive coronary angiography utilization, a reduction in the rate of finding no obstructive coronary artery disease at invasive coronary angiography, an increase in availability of lesion‐specific functional information for guidance of coronary interventions, and a higher rate of revascularization with no sacrifice of short‐term adverse clinical outcomes.
What Are the Clinical Implications?
Large‐scale multicenter prospective studies are needed to delineate the relative diagnostic yield, safety, and costs of adjunctive coronary computed tomography angiography–derived fractional flow reserve versus myocardial perfusion imaging testing in stable patients undergoing coronary computed tomography angiography.
Introduction
Coronary computed tomography angiography (CTA) is increasingly used as an alternative to frontline functional testing in patients with suspected stable coronary artery disease (CAD). Coronary CTA, however, tends to overestimate stenosis severity, and the correlation to downstream myocardial ischemia is poor.1 Consequently, guidelines recommend that ischemia testing should be performed before referral to invasive coronary angiography (ICA) when coronary CTA shows significant CAD.2
Recent advances in individual image‐based modeling and computational fluid dynamics allow for estimation of coronary blood flow and pressure from coronary CTA images.3 Based on these techniques, noninvasive fractional flow reserve (FFR) can be computed.3, 4, 5, 6 In studies including patients with known or suspected stable CAD and blinded comparison to FFR, coronary CTA‐derived FFR (FFRCT) has shown high diagnostic performance.4, 5, 6 Recently, it was demonstrated in stable patients referred to ICA that initial FFRCT assessment reduced the downstream use of ICA procedures, the rate of ICA showing no obstructive CAD, and overall costs.7 Although results are promising, the real‐world clinical utility of FFRCT is not known. The purpose of this observational study was to assess replacement of an adjunctive diagnostic strategy of conventional myocardial perfusion imaging (MPI) with FFRCT in stable patients with intermediate‐range coronary stenosis determined by coronary CTA on (1) the rate of downstream ICA utilization and (2) the diagnostic yield of ICA, defined as proportion of ICA showing no obstructive CAD, and the rate of revascularization.
Methods
We conducted a single‐center observational all‐comer study of symptomatic patients with suspected CAD undergoing coronary CTA at Aarhus University Hospital in Denmark between May 1, 2013, and December 31, 2015. At our institution, coronary CTA testing has been the preferred initial diagnostic modality since 2009 for patients with new‐onset chest pain without known CAD and low‐intermediate pretest probability of CAD. Noninvasive ischemia testing (using exercise electrocardiography) was performed before the time of coronary CTA acquisition only in patients referred from private cardiolgist practices. In patients with intermediate‐range (30–70%) coronary stenosis, adjunctive functional testing is recommended before decision making about referral to ICA. Until 2014, adjunctive functional testing at this institution was performed by conventional MPI. Based on local pilot experiences together with published reports on the diagnostic performance of FFRCT testing4, 5, 6 and potential improvements in clinical workflows, we decided in 2014 to replace MPI with FFRCT for functional assessment of CAD determined by coronary CTA. At our institution, frontline MPI of patients with new‐onset chest pain without known CAD is recommended only for those with contraindications to coronary CTA.8 Patients were divided into 3 historical groups based on the adjunctive functional testing approach: (1) rest–stress rubidium‐82 positron emission tomography (Rb‐PET) or sestamibi SPECT (single‐photon emission CT; May 2013 to April 2014, “MPI era”), (2) FFRCT as an alternative to MPI assessment (May 2014 to December 2014, during which our institution and physicians became familiar with the use of the FFRCT technology), and (3) FFRCT testing fully incorporated into clinical practice (January 2015 to December 2015). Local recommendations on diagnostic workflows in the MPI era and during both periods of FFRCT use (FFRCT era) are described in Table 1. Demographic and clinical characteristics were obtained from patient files and registries.9 The study was approved by the Danish Data Protection Agency (1‐16‐02‐229‐16) with a waiver of individual informed consent from the regional ethics committee.
Table 1.
Local Recommendations Regarding Downstream Diagnostic Work‐up in Patients Following Coronary CTA, MPI, and FFRCT Testing Between May 1, 2013, and December 31, 2015
| Test Outcome | Diagnostic Recommendationsa | |
|---|---|---|
| Frontline coronary CTA | ||
| Diagnostic conclusive | High riskb | ICA |
| Intermediate riskc | Functional testing | |
| Low riskd | OMTd | |
| Diagnostic inconclusive | ··· | OMTe, MPI or ICA |
| Functional testing | ||
| MPI (May 2013 to April 2014) | Positive or equivocal | ICA |
| Negative | OMTe | |
| FFRCT (May 2014 to December 2015) | Positivef | ICA |
| Negativef | OMTe | |
| Inconclusive | MPI or ICA | |
CTA indicates computed tomography angiography; FFRCT indicates coronary computed tomography angiography–derived fractional flow reserve; ICA, invasive coronary angiography; MPI, myocardial perfusion imaging; OMT, optimal medical treatment without additional testing.
Other factors than test results (eg, symptom severity, patient preference) may have influenced decisions on downstream patient management.
Patients with left main, 3‐vessel disease and/or high‐grade proximal left anterior descending artery stenosis.
Patients with ≥1 intermediate coronary stenosis (lumen reduction 30–70%).
Patients without coronary disease or with maximum coronary stenosis <30%.
If disease was present, statin, aspirin, and antianginal medication were generally recommended.
We recommended ICA to be performed in patients with FFRCT ≤0.80 between May 2014 and April 2015, after which the FFRCT threshold for referral to ICA was adjusted to 0.75.8
Coronary CTA
Coronary CTA was performed using a dual‐source scanner, as described previously.8 In brief, image acquisition was performed in accordance with best practice guidelines.10 Contraindications to coronary CTA were known CAD, scenarios in which a nondiagnostic test result could appear with high probability (eg, patients with arrhythmia or obesity, high Agatston score), severe renal insufficiency, pregnancy, or allergy to contrast. The decision to perform coronary CTA in patients with a high Agatston score was based on symptom severity and the probability of good image quality, taking into account issues related to, for example, patient cooperation, weight, and heart rate, at the discretion of the treating physician. Oral and/or intravenous β‐blockers or oral ivabradine were administered if necessary, targeting a heart rate <60 beats per minute, and patients received sublingual spray nitroglycerin 0.8 mg <5 minutes before the scan. An initial nonenhanced scan was performed using high‐pitch spiral acquisition, from which the Agatston score was assessed. Coronary CTA was performed using prospective electrocardiographic triggering. Images were reconstructed using filtered back projection. Scans were assessed using axial images and multiplanar reconstructions by experienced cardiologists with a mean of 6 years of experience interpreting coronary CTA. Vessel segments ≥2 mm were evaluated for lumen narrowing. For guidance of downstream patient management, the CT‐reading cardiologists grouped patients into 1 of 3 risk categories8 (Table 1).
Myocardial Perfusion Imaging
Rb‐PET is our preferred MPI test. Rb‐PET scans (rest and stress) using bolus injections of 1110 MBq rubidium‐82 were performed on a GE Discovery 690 PET/CT system (GE Healthcare). International standard adenosine infusion (0.140 mg/kg per minute) and reconstruction protocols (static and dynamic images) were applied.11 Rb‐PET myocardial perfusion images were analyzed qualitatively using differences in relative counts between rest and stress.12 Any significant visual regional tracer redistribution between the rest and stress studies was interpreted as an inducible perfusion defect. Finally, absolute flow values calculated based on data from the dynamic acquisition data were reported in cases of balanced 3‐vessel disease or diffuse disease. In these cases, global myocardial blood flow <2 mL/g per minute was reported to be abnormally low. A positive Rb‐PET result was present in the event of visual regional tracer redistribution between the rest and stress studies or myocardial blood flow <2 mL/g per minute. In the event of contraindications to adenosine, exercise sestamibi SPECT was performed.13 Local recommendations regarding the clinical consequence of test results are shown in Table 1.
Coronary CTA‐Derived FFR
Standard coronary CTA data sets were transmitted for analysis.6 The scientific basis of FFRCT computation has been described previously.3 FFRCT values in the major epicardial arteries (left main, left anterior descending, left circumflex, and right coronary; including side branches) ≥2 mm in diameter were registered. Between May 2014 and April 2015, we recommended that ICA should be performed in patients with FFRCT ≤0.80; subsequently, we lowered the threshold to 0.75 for referral to ICA.8 This decision was made because we observed substantial discordance in our clinical practice between measured FFR and FFRCT in patients with lowest FFRct values between 0.76 and 0.80.8 In the latter period, in patients with FFRCT between 0.75 and 0.80, a strategy of optimal medical treatment was recommended together with ambulatory follow‐up after 3 months, with referral to ICA only in the event of ongoing chest pain.8 Local recommendations for downstream patient management based on FFRCT results are shown in Table 1.
ICA and FFR
ICA and FFR were performed according to standard practice by experienced invasive cardiologists. All information relevant for patient management, including the MPI and FFRCT results, were available for decision making, and measurement of FFR and coronary revascularization were performed at the discretion of the treating physicians. Patients without obstructive CAD (no coronary lesions ≥50% lumen reduction) were registered. For this study, the degree of angiographic vessel stenosis was determined by experienced interventionalists blinded to other patient information and the results of preceding noninvasive tests (including MPI and FFRCT). FFR measurements were performed using the Verrata (Volcano Therapeutics) or Aeris (St. Jude Medical) pressure wire.
Patient Follow‐up
Rates of follow‐up in the 3 time periods of ICA, FFR, and coronary revascularization (percutaneous coronary intervention or coronary bypass grafting) within 3 months from the time of coronary CTA were obtained from patient files and registries.9 For patients with FFRCT ≤0.80 for whom a strategy of optimal medical treatment with subsequent ambulatory follow‐up was initiated (Table 1), those diagnostic procedures performed within 3 months after the ambulatory visit were registered. All‐cause death occurring within 6 months from date of the CT investigation was ascertained from the Danish Civil Registration system, which maintains complete data on mortality.14
Radiation Exposure
Radiation exposure, including all diagnostic tests, are reported in millisieverts using the formula mSv=(dose length product)×0.014 for coronary CTA, and with conversion factors of 0.18 mSv/(Gy·cm2) for ICA15 and 0.00126 MBq for MPI.16
Statistical Analysis
Categorical data are presented as numbers and proportions, and continuous data are presented as mean±SD, as appropriate. The Fisher exact test was used to compare categorical data, the t test was used for comparison of means, and the bootstrap procedure was used to compare means for nonnormally distributed data. Propensity score matching was used to adjust for differences in baseline patient characteristics between time periods.17 Propensity score matching was chosen rather than regression analysis because of the limited number of events. The average period effect on outcomes was quantified as a risk difference for the second and third periods (test periods) compared with the first period (reference period). The propensity score was computed using a logistic regression model including age as a second‐order polynomial (to account for a possible nonlinear effect of age), sex, symptoms (nonanginal chest pain, atypical angina, or typical angina), and Agatston score group (0, 1–100, 101–400, >400). In subanalyses of patients with ICA and/or coronary revascularization, propensity scoring was based only on age and symptoms to account for the smaller sample size. The average period effect was computed by taking the average of the difference between the observed and matched outcomes for each participant. Overlap in distribution of baseline risk factors between time periods was large enough to allow a propensity score matching strategy to adjust for these differences. We performed an informal power calculation by considering the comparison of 2 proportions of size (10% and 15%) in samples of 1300 patients, and found that such a comparison would have a power of 97% in an unadjusted analysis. All 95% confidence intervals (CIs) and P values were computed taking into account both the uncertainty of the estimated propensity score and matching with replacement. The distributions of risk factors were compared between time periods before and after matching. A subanalysis comparing outcomes in the MPI and FFRCT eras was performed. Statistical analyses were performed using Stata version 14.1 (StataCorp).
Results
The study cohort comprised 3523 consecutive patients. Baseline characteristics of study patients are shown in Table 2. Coronary CTA acquisition characteristics, information on radiation exposure, and Agatston scores are shown in Table 3. Radiation dose associated with coronary CTA increased from 2.9 mSv in the MPI era to 3.4 mSv in the FFRCT era (P<0.001).
Table 2.
Patient Characteristics
| Characteristic | Period 1 (n=1332) | Period 2 (n=800) | Period 3 (n=1391) | Periods 1 vs 2 (P Value) | Periods 1 vs 3 (P Value) |
|---|---|---|---|---|---|
| Mean (SD) age, y | 56 (11) | 57 (11) | 58 (11) | 0.05 | 0.001 |
| Male sex | 629 (47) | 371 (46) | 655 (47) | 0.7 | 1.0 |
| Diabetes mellitus | 105 (8) | 57 (7) | 118 (9) | 0.6 | 0.6 |
| Hypertension | 482 (36) | 267 (33) | 497 (36) | 0.15 | 0.8 |
| Hyperlipidemia | 411 (31) | 240 (30) | 425 (31) | 0.7 | 1.0 |
| Current smoker | 303 (23) | 176 (22) | 303 (22) | 0.7 | 0.6 |
| Family history of CAD | 669 (50) | 382 (48) | 635 (48) | 0.24 | 0.05 |
| Symptoms | |||||
| Typical angina | 146 (11) | 99 (12) | 226 (16) | <0.001 | <0.001 |
| Atypical angina | 835 (63) | 606 (76) | 908 (66) | ||
| Nonanginal chest pain | 347 (26) | 93 (12) | 249 (18) | ||
| Mean (SD) updated Diamond‐Forrester risk score, % | 32 (19) | 35 (18) | 35 (20) | <0.001 | <0.001 |
| Intermediate (20–80%) pretest risk | 871 (65) | 585 (73) | 979 (70) | <0.001 | <0.001 |
| Noninvasive ischemia testing performed before coronary CTAa | 146 (11) | 96 (12) | 149 (11) | 0.5 | 1.00 |
| Mean (SD) body mass index, kg/m2 | 26 (4) | 26 (5) | 26 (4) | 0.8 | 0.8 |
| Mean (SD) serum creatinine, μmol/L | 75 (22) | 75 (16) | 76 (28) | 0.9 | 0.4 |
Values are mean (SD), or number (proportion). CAD indicates coronary artery disease; CTA, computed tomography angiography.
Pre‐coronary CTA noninvasive ischemia testing (using exercise electrocardiography) was performed in patients referred from private cardiologist practices. Time period 1, May 2013 to April 2014; period 2, May 2014 to December 2014; period 3, January 2015 to December 2015.
Table 3.
Coronary CTA Acquisition Characteristics, Radiation Exposure, and Agatston Scores
| Characteristic | Period 1 (n=1332) | Period 2 (n=800) | Period 3 (n=1391) | Periods 1 vs 2 (P Value) | Periods 1 vs 3 (P Value) |
|---|---|---|---|---|---|
| Mean (SD) heart rate, bpm | 59 (10) | 58 (10) | 59 (10) | 0.03 | 0.81 |
| Sinus rhythm | 1259 (95) | 761 (95) | 1340 (96) | 0.62 | 0.03 |
| Prescan administration of nitrates | 1272 (95) | 770 (96) | 1348 (97) | 0.40 | 0.05 |
| Prescan administration of beta‐blockers | 1146 (86) | 675 (84) | 1154 (83) | 0.29 | 0.03 |
| Prospective acquisition | 1276 (96) | 776 (97) | 1363 (98) | 0.13 | <0.001 |
| Retrospective acquisition | 16 (1) | 8 (1) | 14 (1) | 0.67 | 0.63 |
| High‐pitch spiral acquisition | 40 (3) | 16 (2) | 14 (1) | 0.19 | <0.001 |
| Mean (SD) diagnostic radiation exposure, mSv | |||||
| Cumulative radiation exposurea | 4.0 (2.2) | 3.8 (2.0) | 4.1 (2.1) | 0.09 | 0.62 |
| Coronary CTAb | 2.9 (1.4) | 3.0 (1.5) | 3.4 (1.7) | 0.10 | <0.001 |
| Mean (SD) Agatston score | 98 (316) | 111 (337) | 143 (496) | 0.43 | 0.001 |
| Agatston >400 | 80 (6) | 57 (7) | 113 (8) | 0.23 | 0.02 |
Values are mean (SD) or number (proportion). CTA indicates computed tomography angiography; MPI, myocardial perfusion imaging.
Including diagnostic coronary CTA, index and downstream MPI, and invasive coronary angiography. For MPI, both rest and stress tests were included in the radiation estimate. For coronary CTA, both the scout, noncontrast and contrast scans were included in the estimate.
Coronary CTA investigations only.
Figure 1 displays the diagnostic flow of patients in the 3 time periods. The coronary CTA result was inconclusive in 202 patients (6%). Noninvasive functional testing was performed in 711 patients (20%) with intermediate‐range coronary lesions. Of 347 MPI investigations, 95% were Rb‐PET and 5% were SPECT. The MPI result was positive in 45 patients (13%), negative in 292 (84%), and in equivocal in 10 (3%). Over the entire study period, the mean time between referral to MPI and availability of the test result was 33 days (range: 8–59 days). Of 364 FFRCT assessments, FFRCT was >0.80 in all vessels in 215 patients (59%) and ≤0.80 and ≤0.75 in at least 1 vessel in 133 (37%) and 77 (21%) patients, respectively. For 16 patients (4.3%), FFRCT could not be computed because of motion, misalignment, low contrast, and/or calcium blooming (n=12), missing myocardium or lack of CT diastole phase (n=4). The FFRCT result was available for 96% of the patients <24 hours and in 100% <48 hours after the coronary CTA examination.
Figure 1.
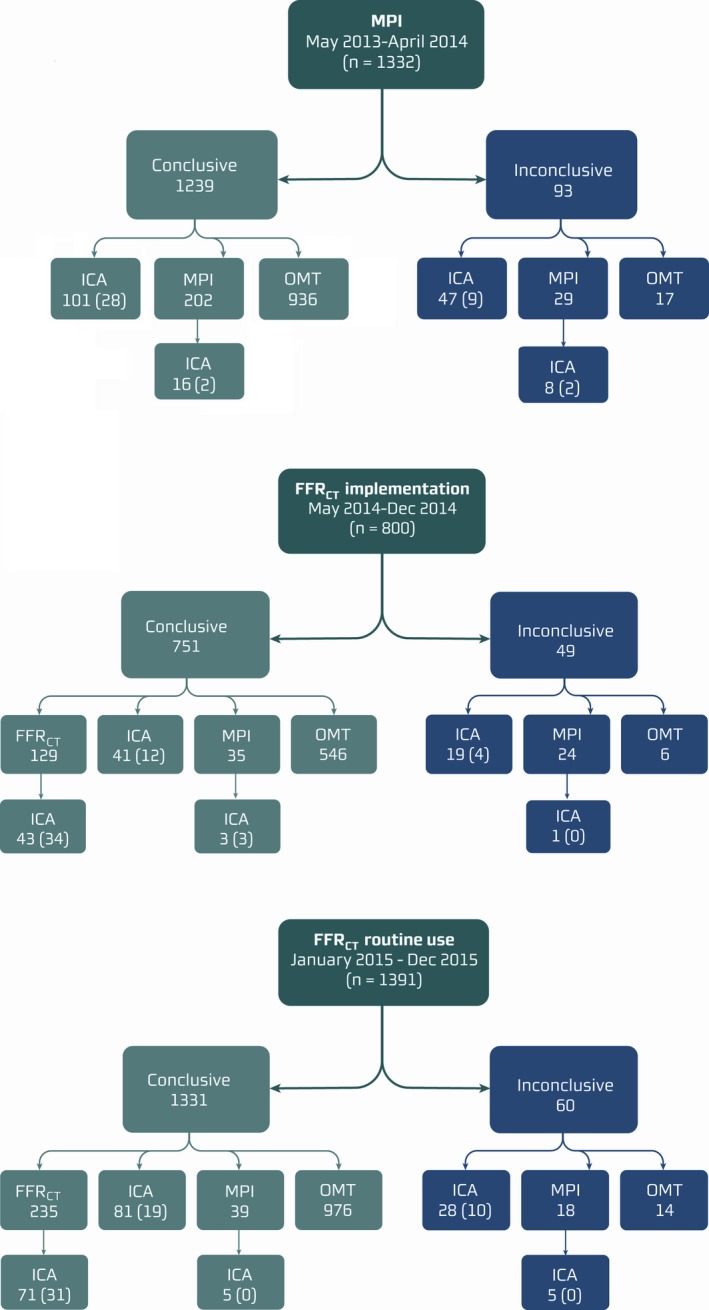
Flow of study patients. The functional significance of intermediate‐range lesions (30–70%) determined by coronary CTA was assessed by MPI (period 1) or FFRCT (periods 2 and 3). Numbers refer to the number of patients in each group. Numbers in parentheses refer to number of patients having fractional flow reserve (95%) or instantaneous wave‐free ratio (5%) performed. CTA indicates computed tomography angiography; FFRCT indicates coronary computed tomography angiography–derived fractional flow reserve; ICA, invasive coronary angiography; MPI, myocardial perfusion imaging; OMT, optimal medical treatment without additional testing.
Temporal Changes in Diagnostic Workflows, Findings of No Obstructive CAD, Revascularizations, and Clinical Outcomes
The proportion of inconclusive coronary CTA investigations decreased from 7.0% to 6.1% and 4.3% over the 3 time periods (P<0.001). The proportion of patients with high‐risk‐anatomy findings determined by coronary CTA referred directly to ICA without functional testing were 8.2%, 5.5%, and 6.1% in periods 1, 2, and 3, respectively (P<0.001). Temporal changes in downstream utilization of MPI, FFRCT, and FFR are shown in Figure 2. The proportion of patients who had to come back for a second noninvasive test after coronary CTA declined after FFRCT adoption, from 17.3% (231/1332) in period 1 to 4.1% (57/1391) in period 3 (P<0.001). Rates of ICA utilization, findings of no obstructive CAD, rates of revascularization, and proportions of patients undergoing revascularization with available functional information are shown in Table 4. Unadjusted risk differences in rates of ICA, findings of no obstructive disease, availability of functional information, and revascularization between the MPI and FFRCT eras are shown in Figure 3. The rate of patients for whom functional information (MPI, FFRCT, and/or FFR) was available for guiding revascularization was higher in period 3 (70%) than in period 1 (35%; Figure S1). In 18 patients with FFRCT ranging between 0.75 and 0.80, optimal medical treatment was initiated with 3 months of ambulatory follow‐up (Table 1). Of these patients, 22% were referred to ICA after the planned ambulatory visit. Eight patients (0.23%) died during 6‐month follow‐up: 3 (0.23%) in period 1, 2 (0.25%) in period 2, and 3 (0.22%) in period 3. In these patients, the coronary CTA result was normal (n=4), showed CAD requiring no additional testing (n=2), or showed high‐risk CAD leading to direct ICA referral (n=2).
Figure 2.
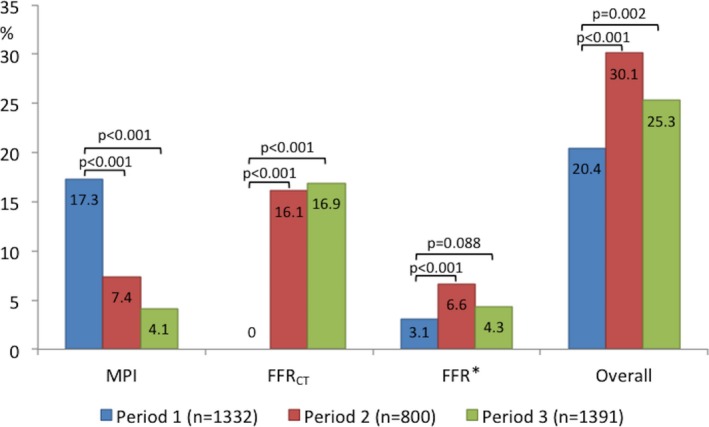
Temporal changes in downstream utilization of functional tests. *Instantaneous wave‐free ratio was performed in 5% of the patients. FFR indicates fractional flow reserve; FFRCT, coronary computed tomography angiography–derived fractional flow reserve; MPI, myocardial perfusion imaging.
Table 4.
Rates of Downstream ICA, Finding of No Obstructive CAD, Coronary Revascularization, and Procedural Functional Guidance
| Group | Period 1 | Period 2 | Period 3 |
|---|---|---|---|
| All patients, n | 1332 | 800 | 1391 |
| ICA | 172 (12.9) | 107 (13.4) | 190 (13.7) |
| No obstructive CAD | 52 (3.9) | 32 (4.0) | 32 (2.3) |
| Coronary revascularization | 72 (5.4) | 47 (5.9) | 102 (7.3) |
| ≥1 functional testa | 268 (20.1) | 204 (25.5) | 321 (23.1) |
| ICA, n | 172 | 107 | 190 |
| No obstructive CAD | 52 (30.2) | 32 (29.9) | 32 (16.8) |
| Coronary revascularization | 72 (41.9) | 47 (43.9) | 102 (53.7) |
| ≥1 functional testa | 61 (35.5) | 63 (58.9) | 110 (57.9) |
| Coronary revascularization, n | 72 | 47 | 102 |
| ≥1 functional testa | 23 (31.9) | 26 (55.3) | 61 (59.8) |
Values are numbers (proportions). CAD indicates coronary artery disease; ICA, invasive coronary angiography.
Myocardial perfusion imaging, coronary computed tomography angiography–derived fractional flow reserve, and/or fractional flow reserve. Time period 1, May 2013 to April 2014; period 2, May 2014 to December 2014; period 3, January 2015 to December 2015.
Figure 3.
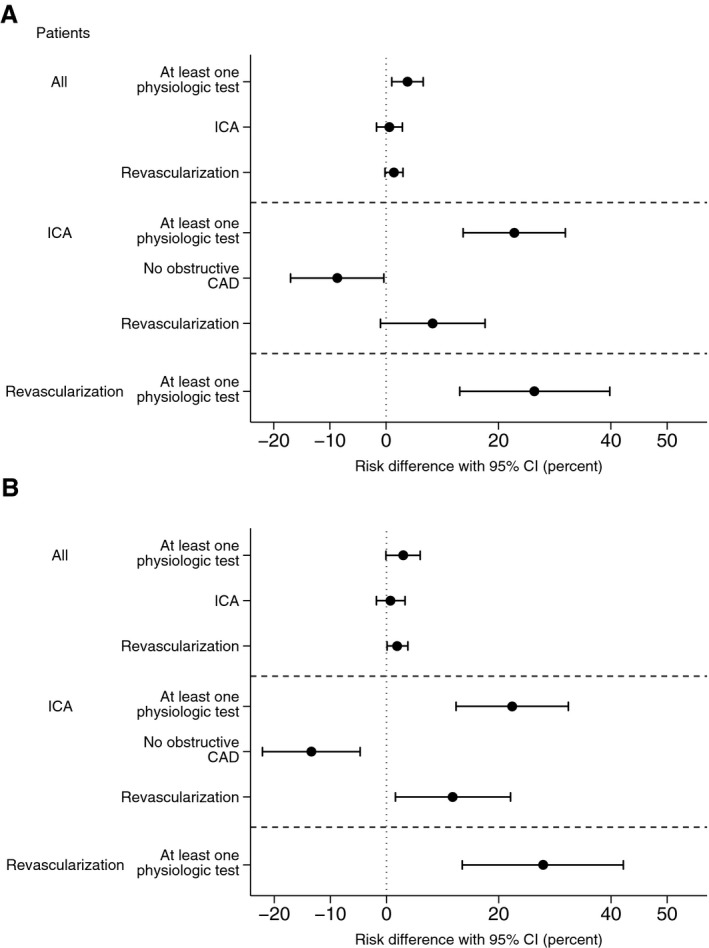
Unadjusted risk differences in rates of ICA, availability of functional information, findings of no obstructive disease, and revascularization in all patients, patients undergoing ICA, and patients undergoing revascularization. A, Time period 1 (May 2013 to April 2014) vs periods 2 and 3 (May 2014 to December 2015). B, Time periods 1 vs 3 (January 2015 to December 2015). CAD indicates coronary artery disease; CI, confidence interval; ICA, invasive coronary angiography.
Adjusted Analyses
Following propensity score match analysis, comparisons of periods 1 and 3 showed decreases in the use of ICA (absolute risk difference: −4.2; 95% CI, −6.9 to −1.6; P=0.002) and in the rate of finding no obstructive CAD (absolute risk difference: −12.8%; 95% CI, −22.2 to −3.4; P=0.008; Figure 4). Moreover, in patients who underwent ICA, the rate of revascularization increased (14.1%; 95% CI, 3.3–24.9; P=0.01), as did availability of information regarding lesion‐specific ischemia for guiding therapeutic decisions (27.8%; 95% CI, 11.3–44.4; P<0.001) after clinical adoption of FFRCT.
Figure 4.
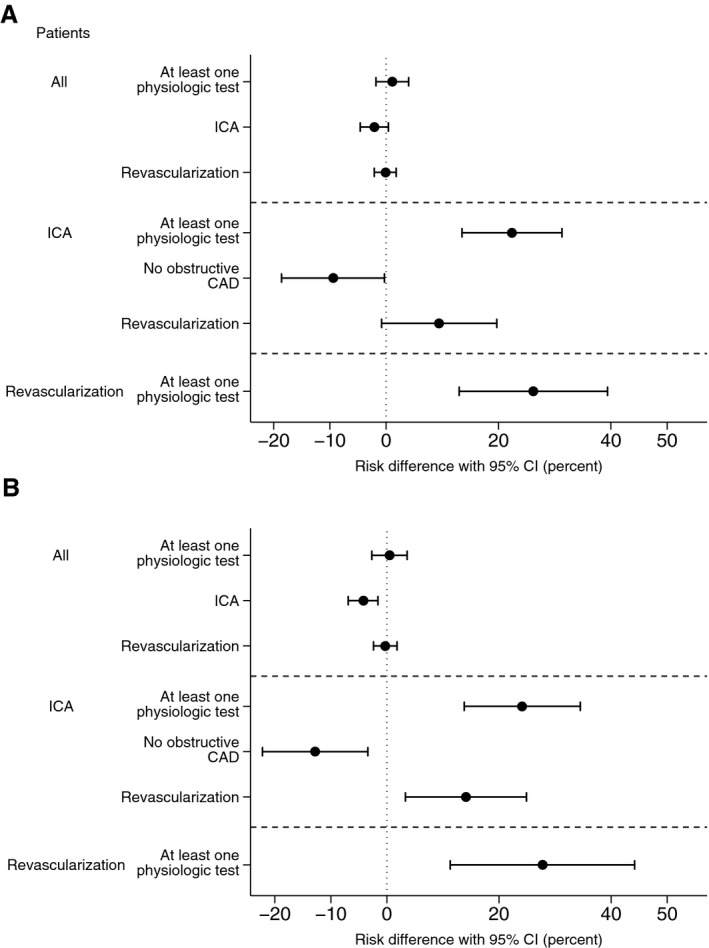
Adjusted risk differences in rates of ICA, availability of functional information, findings of no obstructive disease, and revascularization in all patients, patients undergoing ICA, and patients undergoing revascularization. A, Time period 1 (May 2013 to April 2014) vs periods 2 and 3 (May 2014 to December 2015); (B) Time periods 1 vs 3 (January 2015 to December 2015). CAD indicates coronary artery disease; CI, confidence interval; ICA, invasive coronary angiography.
Discussion
In this single‐center observational study of symptomatic patients with suspected CAD undergoing frontline coronary CTA testing, a change in functional testing strategy from standard MPI to FFRCT in patients with intermediate‐range coronary lesions did not affect overall downstream ICA utilization. However, following adjustment for differences in baseline risk variables, adoption of adjunctive FFRCT testing was associated with a reduction in downstream use of ICA. Moreover, after adoption of FFRCT, we observed a 75% reduction in patients who had to return for a second noninvasive test. Finally, the introduction of FFRCT was associated with a reduction in the rate of finding no obstructive CAD at ICA, a higher rate of revascularization, and an increase in availability of lesion‐specific functional information for guidance of coronary interventions.
Compared with MPI, the strength of coronary CTA is the ability to accurately exclude, detect, and localize CAD. In the recent PROMISE and SCOT‐HEART trials, frontline coronary CTA compared with noninvasive functional assessment in patients with stable CAD enhanced diagnostic certainty and favorably influenced the diagnostic workflow and therapeutic plans.18, 19 Coronary CTA, however, overestimates the severity of lesions and cannot reliably determine their functional significance.1 This gap in noninvasive diagnostic testing may be addressed by combining anatomic and physiologic data.20
In accord with the present findings, the prognosis in contemporary patients undergoing noninvasive testing for suspected CAD is favorable, with an annual risk of nonfatal myocardial infarction or all‐cause death of ≈1%.7, 18, 19 Consequently, demonstrating a benefit for clinical outcome with a new diagnostic test for stable CAD is complex. To the best of our knowledge, no previous study has assessed the potential impact of frontline coronary CTA testing in combination with different functional test strategies on downstream diagnostic workflows and the quality of information for guiding care. In the recent PLATFORM study of patients with suspected stable CAD and planned ICA, FFRCT testing was associated with a safe reduction in downstream ICA utilization, a reduced rate of ICA showing no obstructive CAD, and an increase in the availability of functional information to guide therapeutic decision making.7 The present study adds to these findings by demonstrating comparable results in a real‐world clinical setting of stable patients with chest pain in whom coronary CTA testing was performed according to guidelines2 and in whom the overall ICA referral rate was low (≈13%).
Because MPI or FFRCT testing strategies in this report were used almost exclusively as gatekeepers for ICA in patients with intermediate‐range stenosis, the frequency of inappropriate referrals to ICA may be considered as an indirect metric of false‐positive rates. In this study, the rate of finding no obstructive CAD at ICA declined by almost 50% following clinical adoption of FFRCT. These findings are in accordance with the PLATFORM study, which showed an 83% reduction in findings of no obstructive CAD at ICA when using FFRCT testing after coronary CTA rather than direct referral to ICA.7 Of note, the proportion of patients with no obstructive CAD at ICA in this study (17–30%) was lower than previously reported (50–60%).21 This finding most likely reflects the rigorous anatomical–functional assessment in the majority of patients before referral to ICA at this institution. The higher diagnostic yield in the FFRCT era versus the MPI era in this study is supported by observations that, even after adjustment for differences in baseline characteristics between time periods, there was a 14% absolute increase in the revascularization rate together with a 28% increase in availability of lesion‐specific functional information at the time of revascularization.
Guidelines recommend functional information for decision making regarding revascularization in patients with intermediate‐range coronary lesions.2 In contemporary clinical practice, however, less than two‐thirds of patients undergo noninvasive ischemia testing before ICA.21, 22 FFR is a robust tool for the adjudication of the hemodynamic significance of a stenosis; however, FFR interrogation carries risk of complications, it cannot be performed in all vessels (eg, occluded vessels, severe tortuosity, and/or calcification), and it is costly. Consequently, the adoption of FFR in clinical practice is limited.23, 24 In this study, from the MPI era to the FFRCT era, we observed doubling in the rate of patients with functional information available for decision making on downstream diagnostic and therapeutic management. Surprisingly, during the FFRCT implementation phase, there was a notable increase in the use of FFR. This finding most likely reflects a generally higher recognition of the need for functional information to guide patient care over time, together with an initial FFRCT diagnostic uncertainty among the treating physicians, prompting “confirmatory” FFR interrogation in many vessels.8 The latter is supported by the observation that following FFRCT adoption, FFR measurements decreased in parallel with an increased reliance on FFRCT for revascularization guidance.
We observed that the proportion of patients being referred directly to ICA without functional testing declined during the study period. This finding may reflect an increased focus on coronary CTA image quality during the clinical adoption of FFRCT.8 Moreover, it may be speculated that in challenging cases with high‐risk anatomy, FFRCT testing encourages higher observer confidence in test results compared with standard practice. This is supported by the high and superior diagnostic performance of FFRCT compared with coronary CTA interpretation in patients with high levels of coronary calcification25 and by our observation of increasing Agatston scores during the study period.
There has been much focus on the radiation dose associated with coronary CTA testing.14 In this study reflecting contemporary practice, mean radiation associated with coronary CTA testing was relatively low. Despite the reduction in the rate of MPI testing during the study period, we could not demonstrate a decline in the overall mean radiation dose (≈4 mSv) following the introduction of FFRCT testing. This reflects a modest increase in radiation dose associated with coronary CTA testing over time (2.9–3.4 mSv). The latter finding presumably relates to higher focus on image quality in parallel with the introduction of FFRCT. Accordingly, we observed a reduction in the use of low‐radiation, high‐pitch spiral acquisition scans over time. Moreover, an increase in prospective CTA acquisition padding duration over time may have occurred (data not available).
In the present study, only patients with new‐onset chest pain were included. It should be acknowledged that the diagnostic performance of FFRCT in patients with known CAD (eg, prior coronary intervention, previous myocardial infarction) is not known. At present, the use of FFRCT in patients with suspected or known acute coronary syndromes cannot be recommended.26 Furthermore, the diagnostic performance of FFRCT in patients with documented microvascular disease has not been investigated. It cannot be excluded that discrepancies between FFRCT and invasive FFR may be due to microvascular disease. In these circumstances, MPI may provide additional prognostic information. Currently, FFRCT requires offsite computer processing with a 24‐hour response time. Reduced order models for FFRCT computation may allow for onsite FFRCT assessment in the future.27 However, further investigation in prospective multicenter trials are needed to determine the diagnostic performance of these techniques.28 Of note, not all patients are suitable for coronary CTA testing (eg, because of contrast allergy, renal impairment, obesity, arrhythmia, and/or high coronary calcification). Because FFRCT is derived from coronary CTA imaging data, CT artifacts may inherently impair the diagnostic utility of FFRCT. Accordingly, in the present study, coronary CTA was deemed inconclusive in 5% of cases during the FFRCT testing era. In contrast to coronary CTA (and FFRCT), MPI is a well‐validated modality for assessment of symptomatic patients with known CAD (eg, previous coronary intervention or myocardial infarction). Accordingly, the recently updated UK NICE (National Institute for Health and Care Excellence) guidelines on the management of stable patients with chest pain recommend frontline coronary CTA testing in patients without known CAD and MPI in patients with known CAD.29 Of note, US guidelines do not recommend frontline coronary CTA testing in patients with chest pain.
Study Limitations
This was a single‐center, nonrandomized, observational study. Although we adjusted for factors that were expected to influence outcomes, residual confounding may remain. This study, however, includes a large all‐comer consecutive symptomatic cohort with limited exclusion criteria, relevant for frontline coronary CTA testing, and thus is representative of patients encountered in clinical practice. General changes in time trends in relation to referrals for ICA, decision making on coronary revascularization, and availability of functional information may have influenced outcomes. However, data were collected over a relatively narrow time span, and there were no significant changes in medical guidelines on the management of stable CAD throughout the study period.2 Moreover, there was no change in CT technology or CT interpretation experience (data not shown) during the study course. In Denmark, we have universal tax‐supported health care that guarantees unfettered access to the hospital, to general practitioners, and to prescribed medications, thus, no healthcare service is “reimbursement driven.” Therefore, major changes in management of patients unrelated to the adjunctive functional testing strategy in this study seem unlikely. In this study, MPI was performed using Rb‐PET, which is less widely applied in clinical practice than SPECT30; therefore, the findings in this study may not be generalizable to many sites. Rb‐PET, however, is associated with less radiation, more expedited examination time, and higher diagnostic performance compared with SPECT.30 It is possible that lack of standardized core laboratory analysis of MPI data in this study may have biased the study outcome in favor of FFRCT. Indeed, central processing of FFRCT allows for uniform CT image quality control, processing of image data, accumulation of data and machine learning with continuous refinements in technology and physiologic modeling, and improvement in diagnostic performance6; however, the diagnostic strategies in this study represent real‐world practice. The change during period 3 in the FFRCT threshold for referral to ICA from 0.80 to 0.75 involved a limited number of patients and thus had no significant impact on study outcomes (data not shown); however, safety and impact on downstream testing and treatment following introduction of the adjusted FFRCT interpretation strategy needs delineation in future studies. This study is further limited by the lack of information on downstream angina or changes in medication. Information on coronary stenosis severity would have provided valuable information. Moreover, the follow‐up period was short, leaving questions related to costs and long‐term adverse clinical outcomes unanswered.
Conclusions
Replacing adjunctive MPI with FFRCT testing for functional assessment of intermediate stenosis determined by coronary CTA in patients with stable CAD was associated with less ICA utilization and higher ICA diagnostic yield. Further testing in randomized settings with longer follow‐up is needed to assess the relative diagnostic yield, safety, and costs of adjunctive MPI versus FFRCT testing in stable patients undergoing coronary CTA.
Sources of Funding
This work was supported by the Faculty of Health Sciences, Aarhus University Hospital; and the Danish Heart Foundation.
Disclosures
Leipsic has received speaker's honoraria from GE and is a consultant for Edwards Lifesciences and HeartFlow. Christiansen has received unrestricted research grants from St Jude Medical. Jensen has received speaker's honorarium from Bracco Imaging. Nørgaard has received unrestricted research grants from Edwards Lifesciences, Siemens, and HeartFlow. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Supporting information
Figure S1. Temporal changes in rates of patients for whom functional information was available for guidance of revascularization.
(J Am Heart Assoc. 2017;6:e005587 DOI: 10.1161/JAHA.117.005587.)28862968
References
- 1. Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, Boersma E, Regar E, van Geuns RJ, de Jaegere PJ, Serruys PW, Krestin GP, de Feyter PJ. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52:636–643. [DOI] [PubMed] [Google Scholar]
- 2. Task Force Members , Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers , Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61:2233–2241. [DOI] [PubMed] [Google Scholar]
- 4. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia‐causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER‐FLOW (Diagnosis of Ischemia‐Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–1997. [DOI] [PubMed] [Google Scholar]
- 5. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, Erglis A, Lin FY, Dunning AM, Apruzze P, Budoff MJ, Cole JH, Jaffer FA, Leon MB, Malpeso J, Mancini GB, Park SJ, Scgwartz RS, Shaw LJ, Mauri L. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S; NXT Trial Study Group . Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial. J Am Coll Cardiol. 2014;63:1145–1155. [DOI] [PubMed] [Google Scholar]
- 7. Douglas PS, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, Hlatky MA; PLATFORM Investigators . 1‐year outcomes of FFRCT‐guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol. 2016;68:435–445. [DOI] [PubMed] [Google Scholar]
- 8. Nørgaard BL, Hjort J, Gaur S, Hansson N, Bøtker HE, Leipsic J, Mathiassen ON, Grove EL, Pedersen K, Christiansen EH, Kaltoft A, Gormsen LC, Mæng M, Terkelsen CJ, Kristensen SD, Krusell LR, Jensen JM. Clinical Use of Coronary CTA‐Derived FFR for Decision‐Making in Stable CAD. JACC Cardiovasc Imaging. 2017;10:541–550. pii: S1936‐878X(16)30040‐7. DOI: 10.1016/j.jcmg.2015.11.025. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen LH, Nørgaard BL, Tilsted HH, Sand NP, Jensen JM, Bøttcher M, Diederichsen AC, Lambrechtsen J, kristensen LD, Mickley H, Munkholm H, Gøtzsche O, Knudsen LL, Bøtker HE, Pedersen L, Schmidt M. The western Denmark cardiac computed tomography registry: a review and validation study. Clin Epidemiol. 2014;7:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbara S, Arbab‐Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, Weigold WG. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. [DOI] [PubMed] [Google Scholar]
- 11. Nakazato R, Berman DS, Dey D, Le Meunier L, Hayes SW, Fermin JS, Cheng VY, Thomson LE, Friedman JD, Germano G, Slomka PJ. Automated quantitative 82Rb 3D PET/CT myocardial perfusion imaging: normal limits and correlation with invasive coronary angiography. J Nucl Cardiol. 2012;19:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nesterov SV, Deshayes E, Sciagrà R, Settimo L, Declerck JM, Pan XB, Yoshinaga K, Katoh C, Slomka PJ, Germano G, Han C, Aalto V, Alessio AM, Ficaro EP, Lee BC, Nekolla SG, Gwet KL, deKamp RA, Klein R, Dickson J, Case JA, Bateman T, Prior JO, Knuuti JM. Quantification of myocardial blood flow in absolute terms using (82)Rb PET imaging: results of RUBY‐10 study. JACC Cardiovasc Imaging. 2014;7:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winther S, Svensson M, Jørgensen HS, Bouchelouche K, Gormsen LC, Pedersen BB, Holm NR, Bøtker HE, Ivarsen P, Bøttcher M. Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates. JACC Cardiovasc Imaging. 2015;8:553–562. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Maeng M, Jakobsen CJ, Madsen M, Thuesen L, Nielsen PH, Bøtker HE, Sørensen HT. Existing data sources for clinical epidemiology. The Western Denmark Heart Registry. Clin Epidemiol. 2010;2:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eistein AJ, Moser KW, Thompson RC, Cerqueria MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. [DOI] [PubMed] [Google Scholar]
- 16. Senthamizhchelvan S, Bravo PE, Esaias C, Lodge MA, Merril J, Hobbs RF, Sqouros G, Bengel FM. Human biodistribution and radiation dosimetry of 82Rb. J Nucl Med. 2010;51:1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abadie A, Imbens GW. Large sample properties of matching estimators for average treatment effects. Econometrica. 2006;74:235–267. [Google Scholar]
- 18. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al‐Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL; PROMISE Investigators . Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. SCOT‐HEART Investigators . CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT‐HEART): an open‐label, parallel‐group, multicenter trial. Lancet. 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 20. Nørgaard BL, Jensen JM, Leipsic J. Fractional flow reserve derived from coronary CT angiography in stable coronary disease: a new standard in non‐invasive testing? Eur Radiol. 2015;25:2282–2290. [DOI] [PubMed] [Google Scholar]
- 21. Patel MR, Dai D, Hernandez AF, Douglas PS, Messenger J, Garratt KN, Maddox TM, Peterson ED, Roe MT. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary practice. Am Heart J. 2014;167:846–852. [DOI] [PubMed] [Google Scholar]
- 22. Vavalle JP, Shen L, Broderick S, Shaw LK, Douglas PS. Effect of the presence and type of angina on cardiovascular events in patients without known coronary artery disease referred for elective coronary angiography. JAMA Cardiol. 2016;1:232–234. [DOI] [PubMed] [Google Scholar]
- 23. Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60:2337–2339. [DOI] [PubMed] [Google Scholar]
- 24. Park SJ, Ahn JM, Park GM, Cho YR, Lee JY, Kim WJ, Han S, Kang SJ, Park DW, Lee SW, Kim YH, Lee CW, Mintz GS, Park SW. Trends in the outcomes of percutaneous coronary intervention with the routine incorporation of fractional flow reserve in real practice. Eur Heart J. 2013;34:3353–3361. [DOI] [PubMed] [Google Scholar]
- 25. Nørgaard BL, Gaur S, Leipsic J, Ito H, Miyoshi T, Park SJ, Zvaigzne L, Tzemos N, Jensen JM, Hansson N, Ko B, Bezerra H, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S. Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease; a substudy of the NXT trial. JACC Cardiovasc Imaging. 2015;8:1045–1055. [DOI] [PubMed] [Google Scholar]
- 26. Gaur S, Taylor CA, Jensen JM, Bøtker HE, Christiansen EH, Kaltoft AK, Holm NR, Leipsic J, Zarins CK, Achenbach S, Khem S, Wilk A, Bezerra HG, Lassen JF, Nørgaard BL. FFR derived from coronary CT angiography in nonculprit lesions of patients with recent STEMI. JACC Cardiovasc Imaging. 2017;10:424–433. [DOI] [PubMed] [Google Scholar]
- 27. Coenen A, Lubbers MM, Kurata A, Kono A, Dedic A, Chelu RG, Dijkshoorn ML, Gijsen FJ, Ouhlous M, van Geuns RJ, Nieman K. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on‐site clinician‐operated computational fluid dynamics algorithm. Radiology. 2015;274:674–683. [DOI] [PubMed] [Google Scholar]
- 28. Nørgaard BL, Leipsic J. From Newton to the coronaries: computational fluid dynamics has entered the clinical scene. JACC Cardiovasc Imaging. 2016;9:700–702. [DOI] [PubMed] [Google Scholar]
- 29.Available at: https://www.nice.org.uk/guidance/cg95/chapter/recommendations.
- 30. Ghobti AA, Kjær A, Hasbak P. Comparison of PET rubidium‐82 with conventional SPECT myocardial perfusion imaging. Review. Clin Physiol Funct Imaging. 2014;34:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Temporal changes in rates of patients for whom functional information was available for guidance of revascularization.


