Abstract
Background
In‐hospital discontinuation of statins has been linked to poorer early stroke outcomes, but the consequences of postdischarge discontinuation or dose reduction of statin treatment are unknown. The objective of this study was to explore the effects of statin discontinuation or statin dose reduction on recurrent stroke risk.
Methods and Results
We conducted a nationwide cohort study using the data from the Taiwan National Health Insurance Research Database. Our source population comprised all patients who were prescribed a statin within 90 days of discharge after an ischemic stroke between 2001 and 2012. Patients were categorized into 3 groups: statin‐discontinued, statin‐reduced, and statin‐maintained. Cox proportional hazard models were used to estimate the hazard ratios and 95%CIs of recurrent stroke during 1‐year follow‐up in the groups who discontinued statins or reduced statin dose compared with the group who maintained statins as the reference. Among the 45 151 ischemic stroke patients meeting criteria, during the day‐90 to day‐180 period, 7.0% were on reduced statin therapy, and 18.5% were not on any statin therapy. Compared with maintained‐statin intensity therapy, discontinuation of statins was associated with an increased hazard of recurrent stroke (adjusted hazard ratio 1.42, 95%CI 1.28‐1.57), whereas reduced‐statin dose was not associated with an additional risk (adjusted hazard ratio 0.94, 95%CI 0.78‐1.12). Propensity‐matching analysis obtained similar results.
Conclusions
Discontinuation of statin therapy between 3 and 6 months after an index ischemic stroke was associated with a higher risk of recurrent stroke within 1 year after statin discontinuation.
Keywords: secondary prevention, statin, stroke, ischemic stroke, stroke prevention
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Secondary Prevention
Clinical Perspective
What Is New?
Discontinuation of statin therapy between 3 and 6 months after an index ischemic stroke is associated with a higher risk of recurrent stroke within 1 year after statin discontinuation.
What Are the Clinical Implications?
Discontinuation of statin treatment in patients with ischemic stroke of large‐ or small‐vessel atherosclerotic origin taking a statin who have reached a target low‐density lipoprotein cholesterol goal should be strongly discouraged.
Introduction
It is now well established that treatment with statins reduces the frequency of first and recurrent ischemic stroke.1, 2 Expert consensus guidelines recommend statin treatment for patients who have experienced an ischemic stroke or transient ischemic attack due to large‐ or small‐artery atherosclerosis.3 Moreover, prestroke statin use, in‐hospital statin initiation, nondiscontinuation of premorbid statins in the hospital have all been linked to better early stroke outcomes.4 However, in routine clinical practice, many high‐risk patients are not maintained on statin therapy beyond the initial period.5 The effects of discontinuing or reducing statin treatment beyond the initial period (first 3 months) after an index ischemic stroke have not been well studied. A single‐center study of modest sample size found that patients discontinuing their use of statins during the first year after the acute cerebrovascular event had higher mortality,6 but that study did not look at the outcome of recurrent stroke, and larger multicenter data are warranted.
Given the limited data on the impact of changes to statin prescription just beyond the initial period following an ischemic stroke, the objective of this study was to explore the effects of statin discontinuation or statin dose reduction on recurrent stroke risk in a large, nationwide, multicenter “real world” patient population.
Methods
Study Design and Data Set
We conducted a retrospective cohort study by retrieving all hospitalized patients (≥20 years) with a primary diagnosis of ischemic stroke from January 1, 2001 to December 31, 2012 using Taiwan National Health Insurance Research Database (NHIRD). The Taiwan National Health Insurance program was launched in 1995. It covers 99% of the population and reimburses for outpatient and inpatient services as well as prescription drugs. All contracted institutions must file claims according to standard formats, which later transform into NHIRD. This study has been approved by the institutional review board of Chang Gung Memorial Hospital, Chiayi, Taiwan.
Study Population
We identified all hospitalized patients who were admitted with a primary diagnosis of ischemic stroke (International Classification of Diseases, Ninth Revision [ICD‐9] codes 433.X1, 434.X1, 436) among subjects (≥20 years) encountered between 2001 and 2012. This was a nationwide study that included all available and eligible patients. The requirement of informed consent from subjects included in this study was waived. The accuracy of the diagnosis of stroke has been validated by a prior study showing that among confirmed cases of acute ischemic stroke in a hospital medical record, 94.5% were assigned “ischemic stroke” as the principal diagnosis in the NHIRD.7
We defined the first ischemic stroke during the study period as the index stroke. Stroke severity was evaluated by The Stroke Severity Index (airway suctioning, bacterial sensitivity test, general ward stay, intensive care unit stay, nasogastric intubation, osmotherapy, and urinary catheterization), developed specifically to evaluate the severity of strokes in Taiwan NHIRD.8 Information regarding patients’ medications during the first 180 days after discharge from the index stroke was retrieved from the pharmacy prescription database.
During the study period, the Taiwan National Health Bureau would only reimburse initial statin prescriptions for stroke patients if the total cholesterol was ≥200 mg/dL or the low‐density lipoprotein (LDL)‐cholesterol was ≥130 mg/dL. Furthermore, it was recommended that statins be discontinued or reduced in intensity once goal cholesterol levels were reached (total cholesterol <160 mg/dL or LDL‐cholesterol <100 mg/dL). To explore the effect of statin discontinuation or statin dose reduction in the chronic poststroke period, we assessed patients receiving high‐intensity or moderate‐intensity statin therapy within 90 days after discharge for the index stroke. The 2013 ACC/AHA guideline recommended initiation of a high‐intensity statin in patients with clinical atherosclerotic cardiovascular diseases (eg, stroke) aged ≤75 years and moderate‐intensity statin in patients aged >75 years.3 Therefore, we did not enroll patients on a low‐intensity statin at the start of the study. Statin intensity was defined based on 2013 ACC/AHA guidelines on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults.3 For a daily dose lowering LDL‐cholesterol by ≥50%, atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg was categorized as high‐intensity statin therapy. For a daily dose lowering LDL‐cholesterol by 30% to <50%, atorvastatin 10 to 20 mg, rosuvastatin 5 to 10 mg, simvastatin 20 mg to 40 mg, pravastatin 40 to 80 mg, lovastatin 40 mg, fluvastatin 80 mg, or pitavastatin 2 to 4 mg was categorized as moderate‐intensity statin therapy. For a daily dose lowering LDL‐cholesterol by <30%, simvastatin 10 mg, pravastatin 10 to 20 mg, lovastatin 20 mg, fluvastatin 20 to 40 mg, or pitavastatin 1 mg was categorized as low‐intensity statin therapy. Patients receiving the combination of low‐intensity statin with ezetimibe were regarded as receiving moderate‐intensity statin therapy. Patients receiving the combination of moderate‐intensity statin with ezetimibe were regarded as receiving high‐intensity statin therapy. We then categorized these patients into 3 groups. The statin‐discontinuation group was defined as patients not receiving any statin between day 91 and day 180 after discharge. The statin‐reduced group was defined as patients receiving low‐intensity statin therapy or changing their medication from high‐intensity statin to moderate‐intensity statin between day 91 and day 180 after discharge. The statin‐maintained group was defined as patients receiving high‐intensity or moderate‐intensity statin therapy between day 91 and day 180 after discharge. Because use of an oral anticoagulant, rather than statin therapy, is the major factor affecting risk of recurrent stroke for ischemic stroke patients with atrial fibrillation, we excluded patients with atrial fibrillation from this study. Because antiplatelet therapy is a standard therapy for secondary stroke prevention in ischemic stroke patients without atrial fibrillation, patients who did not receive antiplatelet therapy within 90 days or between day 91 and day 180 after discharge were excluded. We also excluded patients with end‐stage renal disease and having recurrent stroke within 180 days of discharge from index stroke. The study population was followed from day 181 after index stroke discharge to the date of recurrent stroke (code 430‐432, 433.X1, 434.X1, or 436), death, or 1 year from day 181 (ie, 545 days after discharge), whichever came first. Recurrent stroke events were identified only if patients received brain computed tomography and/or magnetic resonance imaging during hospitalization for recurrent stroke or within 7 days before hospitalization at an outpatient or emergency department. The requirement for imaging served to exclude stroke patients who were hospitalized solely for rehabilitation during the chronic stage.
We assessed the comorbidities considered to be potential confounding factors for computing the propensity score. These comorbidities were selected based on prior clinical knowledge about established risk factors for stroke recurrence, and the univariable analyses indicated an association with stroke recurrence in this particular study. In addition to age, sex, and stroke severity index, the comorbidities included in propensity score analysis were hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, stroke history, chronic kidney disease, heart failure, chronic obstructive pulmonary disease, peripheral vascular disease, and sleep apnea. Comorbidities were identified using ICD‐9 codes based on coding of concomitant diagnoses during the index stroke hospitalization and on coding for all outpatient visits before the index stroke. Comorbidities from ICD‐9 codes in Taiwan NHIRD have been validated in a prior study,7 and the comorbidities that were considered in multivariable models are listed in Table 1. Potential factors or complications, such as myalgia, rhabdomyolysis, and memory/cognitive impairment, between day 91 and day 180 that may lead to statin discontinuation or reducing statin intensity were retrieved. Information on antihypertensive therapy in each group between day 91 and day 180 was also retrieved.
Table 1.
A List of the Comorbidities That Were Considered in Multivariable Models
| Comorbidity | ICD‐9 Code |
|---|---|
| Hypertension | 401 to 405 |
| Diabetes mellitus | 250 |
| Hyperlipidemia | 272 |
| Ischemic heart disease | 410 to 414 |
| Stroke history before index stroke | 430 to 434, 436 to 438 |
| Chronic kidney disease | 585, 403 |
| Heart failure | 428 |
| Chronic obstructive pulmonary disease | 491, 492, 496 |
| Peripheral vascular disease | 443.9 |
| Sleep apnea | 327.23, 780.57 |
Main Outcome Measures
The primary outcome of this study was the first occurrence of recurrent stroke (ischemic or hemorrhagic). The secondary end points were all‐cause mortality, intracerebral hemorrhage (code 431), ischemic stroke, myocardial infarction (code 410), all major events (composite of ischemic and hemorrhagic stroke, myocardial infarction, and all‐cause mortality), and any hospitalization in the 1‐year follow‐up period (ie, 6 to 18 months after discharge from index stroke). All‐cause mortality analyzed included those that occurred during a rehospitalization. Data regarding deaths occurring without a hospitalization were not captured.
Statistical Analysis
The baseline characteristics of 3 groups (statin‐discontinued versus statin‐reduced versus statin‐maintained) were compared using a Student t test for continuous variables and a chi‐squared test for categorical variables. To mitigate differences of baseline characteristics among groups, we conducted propensity score matching with 2 contrasts separately: statin discontinuation versus maintenance, and dose reduction versus maintenance. For each of the 2 contrasts, we performed logistic regression models separately to estimate a propensity score and created matched pairs. In the 2 logistic regression models, we included the same variables including age, sex, hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, stroke history, chronic kidney disease, heart failure, chronic obstructive pulmonary disease, peripheral vascular disease, sleep apnea, and the stroke severity index.
We then employed the Cox proportional hazard model to estimate the unadjusted and adjusted hazard ratios (HRs) and 95%CIs of discontinued and reduced statin therapy groups, with the statin‐maintained group as the reference group. The model was adjusted for baseline age, sex, hypertension, diabetes mellitus, ischemic heart disease, stroke history, chronic kidney disease, heart failure, chronic obstructive pulmonary disease, peripheral vascular disease, sleep apnea, and stroke severity index. We also employed the Cox proportional hazard model to estimate HRs and 95%CIs based on propensity score matching between statin‐discontinued versus statin‐maintained and statin‐reduced versus statin‐maintained groups, respectively. We have also performed an analysis in which the index date was the date of dose reduction for the statin‐reduced group and the date of discontinuation for the statin‐discontinued group.
We performed subgroup analyses stratified by risk factors of stroke to assess whether these variables modified the association between statin treatments and risk of stroke recurrence. The subgroup analyses were prespecified, including age, sex, hypertension, ischemic heart disease, and stroke severity, which were well‐known risk factors and considered of clinical importance. We used a severity index score to classify patients into 3 groups of stroke severity: mild, estimated National Institutes of Health Stroke Scale 0 to 5; moderate, estimated Stroke Scale 6 to 12; severe, estimated Stroke Scale ≥13. We tested the significance of the interaction terms in the regression models using the Wald test for interactions.
The data were analyzed with SAS statistical software, V.9.4 (SAS Institute Inc, Cary, NC). A 2‐sided P<0.05 was considered to be statistically significant.
Results
Among the 45 151 ischemic stroke patients who received moderate or high‐intensity statin therapy for 90 days after discharge, by the day‐90 to day‐180 period, 33 623 patients (74.5%) remained on moderate or high‐intensity statin therapy as the initial statin therapy, 3175 patents (7.0%) were on reduced statin therapy, and 8353 patients (18.5%) were not on any statin therapy (Figure 1). Table 2 shows the baseline characteristics of the patients by day‐90 to day‐180 time period and their statin utilization status (statin‐maintained versus statin‐reduced versus statin‐discontinued). Age, ischemic heart disease, stroke history before index stroke, heart failure, and stroke severity index varied among the 3 groups. Potential complications that could lead to statin discontinuation or reduction were not different among the 3 groups. Propensity score matching generated patients with similar baseline characteristics in statin‐maintained versus statin‐reduced therapy, and statin‐maintained versus statin‐discontinued therapy, respectively, as shown in Table 3.
Figure 1.
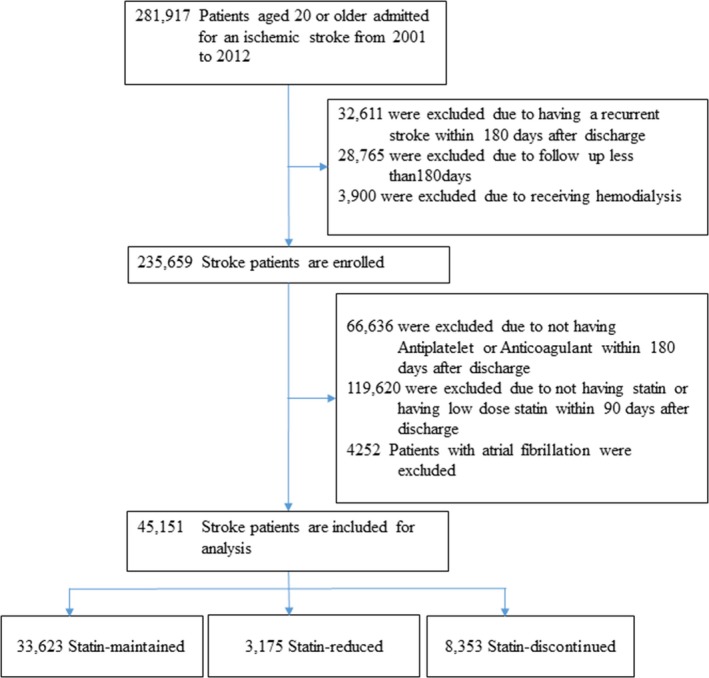
A flowchart of the study process.
Table 2.
Characteristics at Baseline (N=45 151)
| Demographic Characteristic | Statin‐Maintained (N=33 623) | Statin‐Reduced (N=3175) | Statin‐Discontinued (N=8353) | P Value |
|---|---|---|---|---|
| Men, n (%) | 19 084 (56.8) | 1849 (58.2) | 4688 (56.1) | 0.12 |
| Age, y, mean±SD | 65.1±11.7 | 64.9±11.6 | 65.6±11.8 | 0.001 |
| <65 | 15 771 (46.9) | 1515 (47.7) | 3721 (44.6) | 0.0005 |
| 65 to 74 | 10 016 (29.8) | 937 (29.5) | 2535 (30.4) | |
| ≥75 | 7836 (23.3) | 723 (22.8) | 2097 (25.1) | |
| Comorbidity, n (%) | ||||
| Hypertension | 28 287 (84.1) | 2650 (83.5) | 7066 (84.6) | 0.31 |
| Diabetes mellitus | 16 815 (50.0) | 1589 (50.1) | 4145 (49.6) | 0.81 |
| Ischemic heart disease | 10 075 (30.0) | 913 (28.8) | 2649 (31.7) | 0.001 |
| Stroke history before index stroke | 5655 (16.8) | 456 (14.4) | 1478 (17.7) | 0.0001 |
| Chronic kidney disease | 1359 (4.0) | 127 (4.0) | 360 (4.3) | 0.52 |
| Heart failure | 2671 (7.9) | 217 (6.8) | 723 (8.7) | 0.004 |
| Chronic obstructive pulmonary disease | 4996 (14.9) | 446 (14.1) | 1315 (15.7) | 0.04 |
| Peripheral vascular disease | 734 (2.2) | 58 (1.8) | 205 (2.5) | 0.10 |
| Sleep apnea | 143 (0.4) | 14 (0.4) | 33 (0.4) | 0.91 |
| Antihypertensive therapya | ||||
| On days 91 to 180 | 22 640 (67.3) | 2146 (67.6) | 5705 (68.3) | 0.24 |
| Stroke severity index | 0.0008 | |||
| Mild | 26 718 (79.5) | 2545 (80.2) | 6523 (78.1) | |
| Moderate | 4503 (13.4) | 450 (14.2) | 1201 (14.4) | |
| Severe | 2402 (7.1) | 180 (5.7) | 629 (7.5) | |
| Complications on days 91 to 180, n (%) | ||||
| Elevated transaminases | 7 (0.02) | 2 (0.06) | 1 (0.01) | 0.25b |
| Abnormal serum enzyme | 2 (0.01) | 0 (0.00) | 1 (0.01) | 0.75b |
| Rhabdomyolysis | 0 | 0 | 0 | ··· |
| Myalgia and myositis | 1828 (5.4) | 167 (5.3) | 471 (5.6) | 0.67 |
| Cognitive impairment | 65 (0.2) | 1 (0.03) | 15 (0.2) | 0.12 |
Antihypertensive therapy: angiotensin‐converting enzyme inhibitor, angiotensin II receptor blockers, calcium channel blockers, and diuretics.
More than 25% of the cells have expected counts less than 5. Chi‐squared may not be a valid test.
Table 3.
Characteristics at Baseline Using Propensity Score Matching
| Demographic Characteristic | Statin‐Maintained (N=8353) | Statin‐Discontinued (N=8353) | P Value | Statin‐Maintained (N=3175) | Statin‐Reduced (N=3175) | P Value |
|---|---|---|---|---|---|---|
| Men, n (%) | 4632 (55.5) | 4688 (56.1) | 0.38 | 1846 (58.1) | 1849 (58.2) | 0.94 |
| Age, y, mean±SD | 65.7±11.5 | 65.6±11.8 | 0.39 | 64.8±11.4 | 64.9±11.6 | 0.87 |
| <65 | 3672 (44.0) | 3721 (44.6) | 0.60 | 1524 (48.0) | 1515 (47.7) | 0.90 |
| 65 to 74 | 2593 (31.0) | 2535 (30.4) | 943 (29.7) | 937 (29.5) | ||
| ≥75 | 2088 (25.0) | 2097 (25.1) | 708 (22.3) | 723 (22.8) | ||
| Comorbidity, n (%) | ||||||
| Hypertension | 7130 (85.4) | 7066 (84.6) | 0.17 | 2675 (84.3) | 2650 (83.5) | 0.39 |
| Diabetes mellitus | 4149 (49.7) | 4145 (49.6) | 0.95 | 1592 (50.1) | 1589 (50.1) | 0.94 |
| Ischemic heart disease | 2606 (31.2) | 2649 (31.7) | 0.47 | 907 (28.6) | 913 (28.8) | 0.87 |
| Stroke history before index stroke | 1443 (17.3) | 1478 (17.7) | 0.48 | 440 (13.9) | 456 (14.4) | 0.56 |
| Chronic kidney disease | 305 (3.7) | 360 (4.3) | 0.03 | 98 (3.1) | 127 (4.0) | 0.05 |
| Heart failure | 641 (7.7) | 723 (8.7) | 0.02 | 205 (6.5) | 217 (6.8) | 0.55 |
| Chronic obstructive pulmonary disease | 1245 (14.9) | 1315 (15.7) | 0.13 | 415 (13.1) | 446 (14.1) | 0.26 |
| Peripheral vascular disease | 195 (2.3) | 205 (2.5) | 0.61 | 42 (1.3) | 58 (1.8) | 0.11 |
| Sleep apnea | 26 (0.3) | 33 (0.4) | 0.36 | 10 (0.3) | 14 (0.4) | 0.41 |
| Antihypertensive therapya | ||||||
| On days 91 to 180 | 5722 (68.5) | 5705 (68.3) | 0.78 | 2138 (67.4) | 2146 (67.6) | 0.83 |
| Stroke severity index | 0.02 | 0.41 | ||||
| Mild | 6640 (79.5) | 6523 (78.1) | 2547 (80.2) | 2545 (80.2) | ||
| Moderate | 1171 (14.0) | 1201 (14.4) | 427 (13.5) | 450 (14.2) | ||
| Severe | 542 (6.5) | 629 (7.5) | 201 (6.3) | 180 (5.7) | ||
| Complications on days 91 to 180, n (%) | ||||||
| Elevated transaminases | 2 (0.02) | 1 (0.01) | 0.56b | 2 (0.1) | 2 (0.1) | 1.00b |
| Abnormal serum enzyme | 1 (0.01) | 1 (0.01) | 1.00b | 0 | 0 | ··· |
| Rhabdomyolysis | 0 | 0 | ··· | 0 | 0 | ··· |
| Myalgia and myositis | 475 (5.7) | 471 (5.6) | 0.89 | 170 (5.4) | 167 (5.3) | 0.86 |
| Memory/cognitive impairment | 20 (0.2) | 15 (0.2) | 0.40 | 7 (0.2) | 1 (0.03) | 0.03 |
Antihypertensive therapy: angiotensin‐converting enzyme inhibitor, angiotensin II receptor blockers, calcium channel blockers, and diuretics.
More than 25% of the cells have expected counts less than 5.
There were 2120 recurrent strokes during the 1‐year follow‐up period based on the analysis from whole cohort. Table 4 shows the impact of statin‐reduced therapy and statin discontinuation on recurrent vascular events and other clinical end points. In multivariable analyses, compared with statin‐maintained therapy, discontinuation of statins was associated with an increased hazard of recurrent ischemic or hemorrhagic stroke (6.2% versus 4.4%, adjusted HR 1.42, 95%CI 1.28‐1.57, P<0.0001), whereas statin‐reduced therapy was not associated with an additional risk (4.1% versus 4.4%, adjusted HR 0.94, 95%CI 0.78‐1.12, P=0.47). Discontinuation of statins was also linked to higher risks of ischemic stroke (5.6% versus 3.9%, adjusted HR 1.45, 95%CI 1.30‐1.61, P<0.0001), all‐cause mortality (1.4% versus 1.0%, adjusted HR 1.37, 95%CI 1.11‐1.70, P=0.003), all major events (7.8% versus 5.6%, adjusted HR 1.38, 95%CI 1.26‐1.51, P<0.0001), and any hospitalization (31.7% versus 27.1%, adjusted HR 1.19, 95%CI 1.14‐1.24, P<0.0001) but had neutral effects on intracerebral hemorrhage (0.6% versus 0.5%, adjusted HR 1.19, 95%CI 0.86‐1.64, P=0.30) and myocardial infarction (0.5% versus 0.5%, adjusted HR 0.96, 95%CI 0.68‐1.35, P=0.80). Statin‐reduced therapy was not associated with increased risks of ischemic stroke, intracerebral hemorrhage, all‐cause mortality, myocardial infarction, or all major events. Results from propensity score matching comparing statin‐maintained versus statin‐discontinued, and statin‐maintained versus statin‐reduced, respectively, were similar to the results from an original cohort (Table 5). We performed an analysis in which the index date was the date of dose reduction for the statin‐reduced group and the date of discontinuation for the statin‐discontinued group and obtained similar results (Table 6).
Table 4.
Multivariable‐Adjusted Hazard Ratio of 1‐Year Recurrent Cardiovascular Events
| Number of Events (%) | Crude HR (95%CI) | Adjusted HR (95%CI) | P Value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Recurrent stroke | ||||
| Statin‐maintained (N=33 623) | 1474 (4.4) | 1.00 (reference) | ||
| Statin‐reduced (N=3175) | 129 (4.1) | 0.92 (0.77‐1.11) | 0.94 (0.78‐1.12) | 0.47 |
| Statin‐discontinued (N=8353) | 517 (6.2) | 1.43 (1.29‐1.58) | 1.42 (1.28‐1.57) | <0.0001 |
| Secondary outcomes | ||||
| Ischemic stroke | ||||
| Statin‐maintained | 1302 (3.9) | 1.00 (reference) | ||
| Statin‐reduced | 118 (3.7) | 0.96 (0.79‐1.16) | 0.97 (0.80‐1.17) | 0.74 |
| Statin‐discontinued | 466 (5.6) | 1.46 (1.31‐1.62) | 1.45 (1.30‐1.61) | <0.0001 |
| Intracerebral hemorrhage | ||||
| Statin‐maintained | 164 (0.5) | 1.00 (reference) | ||
| Statin‐reduced | 10 (0.3) | 0.64 (0.34‐1.22) | 0.65 (0.34‐1.23) | 0.19 |
| Statin‐discontinued | 48 (0.6) | 1.19 (0.86‐1.64) | 1.19 (0.86‐1.64) | 0.30 |
| Myocardial infarction | ||||
| Statin‐maintained | 169 (0.5) | 1.00 (reference) | ||
| Statin‐reduced | 16 (0.5) | 1.00 (0.60‐1.67) | 1.01 (0.60‐1.68) | 0.98 |
| Statin‐discontinued | 41 (0.5) | 0.98 (0.70‐1.38) | 0.96 (0.68‐1.35) | 0.80 |
| All‐cause mortality | ||||
| Statin‐maintained | 332 (1.0) | 1.00 (reference) | ||
| Statin‐reduced | 29 (0.9) | 0.95 (0.65‐1.39) | 0.97 (0.66‐1.42) | 0.88 |
| Statin‐discontinued | 116 (1.4) | 1.45 (1.17‐1.79) | 1.37 (1.11‐1.70) | 0.003 |
| All major eventsa | ||||
| Statin‐maintained | 1889 (5.6) | 1.00 (reference) | ||
| Statin‐reduced | 166 (5.2) | 0.93 (0.79‐1.09) | 0.94 (0.80‐1.10) | 0.46 |
| Statin‐discontinued | 649 (7.8) | 1.40 (1.28‐1.53) | 1.38 (1.26‐1.51) | <0.0001 |
| Stenting or endarterectomy | ||||
| Statin‐maintained | 91 (0.3) | 1.00 (reference) | ||
| Statin‐reduced | 1 (0.03) | 0.12 (0.02‐0.83) | 0.12 (0.02‐0.82) | 0.03 |
| Statin‐discontinued | 24 (0.3) | 1.06 (0.68‐1.67) | 1.05 (0.67‐1.65) | 0.83 |
| Any hospitalization | ||||
| Statin‐maintained | 9121 (27.1) | 1.00 (reference) | ||
| Statin‐reduced | 796 (25.1) | 0.91 (0.85‐0.98) | 0.94 (0.87‐1.01) | 0.07 |
| Statin‐discontinued | 2650 (31.7) | 1.21 (1.16‐1.26) | 1.19 (1.14‐1.24) | <0.0001 |
Model was adjusted for age, sex, hypertension, diabetes mellitus, ischemic heart disease, stroke history, chronic kidney disease, heart failure, chronic obstructive pulmonary disease, peripheral vascular disease, sleep apnea, and stroke severity index. HR indicates hazard ratio.
All major events: composite of stroke, acute myocardial infarction, and death.
Table 5.
Cox Proportional Hazard Models Stratifying on the Matched Pairs for 1‐Year Recurrent Cardiovascular Events
| Number of Events (%) | HR (95%CI) | P Value | |
|---|---|---|---|
| Statin‐maintained vs statin‐discontinued, N=16 706 | |||
| Recurrent stroke | |||
| Statin‐maintained | 374 (4.5) | 1.00 (reference) | |
| Statin‐discontinued | 517 (6.2) | 1.40 (1.22‐1.60) | <0.0001 |
| Ischemic stroke | |||
| Statin‐maintained | 330 (4.0) | 1.00 (reference) | |
| Statin‐discontinued | 466 (5.6) | 1.43 (1.24‐1.65) | <0.0001 |
| Intracerebral hemorrhage | |||
| Statin‐maintained | 42 (0.5) | 1.00 (reference) | |
| Statin‐discontinued | 48 (0.6) | 1.16 (0.77‐1.75) | 0.49 |
| Myocardial infarction | |||
| Statin‐maintained | 34 (0.4) | 1.00 (reference) | |
| Statin‐discontinued | 41 (0.5) | 1.21 (0.77‐1.90) | 0.42 |
| All‐cause mortality | |||
| Statin‐maintained | 84 (1.0) | 1.00 (reference) | |
| Statin‐discontinued | 116 (1.4) | 1.39 (1.05‐1.83) | 0.02 |
| All major eventsa | |||
| Statin‐maintained | 473 (5.7) | 1.00 (reference) | |
| Statin‐discontinued | 649 (7.8) | 1.39 (1.23‐1.56) | <0.0001 |
| Stenting and endarterectomy | |||
| Statin‐maintained | 24 (0.3) | 1.00 (reference) | |
| Statin‐discontinued | 24 (0.3) | 1.00 (0.57‐1.77) | 0.99 |
| Any hospitalization | |||
| Statin‐maintained | 2334 (27.9) | 1.00 (reference) | |
| Statin‐discontinued | 2650 (31.7) | 1.17 (1.11‐1.24) | <0.0001 |
| Statin‐maintained vs statin‐reduced, N=6350 | |||
| Recurrent stroke | |||
| Statin‐maintained | 147 (4.6) | 1.00 (reference) | |
| Statin‐reduced | 129 (4.1) | 0.88 (0.69‐1.11) | 0.27 |
| Ischemic stroke | |||
| Statin‐maintained | 129 (4.1) | 1.00 (reference) | |
| Statin‐reduced | 118 (3.7) | 0.91 (0.71‐1.17) | 0.48 |
| Intracerebral hemorrhage | |||
| Statin‐maintained | 18 (0.6) | 1.00 (reference) | |
| Statin‐reduced | 10 (0.3) | 0.56 (0.26‐1.20) | 0.14 |
| Myocardial infarction | |||
| Statin‐maintained | 15 (0.5) | 1.00 (reference) | |
| Statin‐reduced | 16 (0.5) | 1.07 (0.53‐2.16) | 0.85 |
| All‐cause mortality | |||
| Statin‐maintained | 30 (0.9) | 1.00 (reference) | |
| Statin‐reduced | 29 (0.9) | 0.97 (0.58‐1.62) | 0.91 |
| All major eventsa | |||
| Statin‐maintained | 189 (6.0) | 1.00 (reference) | |
| Statin‐reduced | 166 (5.2) | 0.88 (0.71‐1.08) | 0.22 |
| Stenting or endarterectomy | |||
| Statin‐maintained | 15 (0.5) | 1.00 (reference) | |
| Statin‐discontinued | 1 (0.03) | 0.07 (0.01‐0.50) | 0.009 |
| Any hospitalization | |||
| Statin‐maintained | 830 (26.1) | 1.00 (reference) | |
| Statin‐discontinued | 796 (25.1) | 0.96 (0.87‐1.05) | 0.36 |
Propensity score matching was computed from age, sex, stroke severity index, hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, stroke history, heart failure, chronic kidney disease, chronic obstructive pulmonary disease, peripheral vascular disease, and sleep apnea. HR indicates hazard ratio.
All major events: composite of stroke, acute myocardial infarction, and death.
Table 6.
Cox Proportional Hazard Models Stratifying on the Matched Pairs for 1‐Year Recurrent Cardiovascular Events (Followed From Index Date for Dose Change)
| Number of Events (%) | HR (95%CI) | P Value | |
|---|---|---|---|
| Statin‐maintained vs statin‐discontinued, N=16 706 | |||
| Recurrent stroke | |||
| Statin‐maintained | 284 (3.4) | 1.00 (reference) | |
| Statin‐discontinued | 387 (4.6) | 1.38 (1.18‐1.60) | <0.0001 |
| Ischemic stroke | |||
| Statin‐maintained | 255 (3.1) | 1.00 (reference) | |
| Statin‐discontinued | 349 (4.2) | 1.38 (1.18‐1.62) | <0.0001 |
| Intracerebral hemorrhage | |||
| Statin‐maintained | 27 (0.3) | 1.00 (reference) | |
| Statin‐discontinued | 36 (0.4) | 1.35 (0.82‐2.22) | 0.24 |
| Myocardial infarction | |||
| Statin‐maintained | 22 (0.3) | 1.00 (reference) | |
| Statin‐discontinued | 27 (0.3) | 1.23 (0.70‐2.16) | 0.47 |
| All‐cause mortality | |||
| Statin‐maintained | 56 (0.7) | 1.00 (reference) | |
| Statin‐discontinued | 76 (0.9) | 1.39 (0.98‐1.96) | 0.07 |
| All major eventsa | |||
| Statin‐maintained | 349 (4.2) | 1.00 (reference) | |
| Statin‐discontinued | 476 (5.7) | 1.38 (1.20‐1.58) | <0.0001 |
| Stenting and endarterectomy | |||
| Statin‐maintained | 20 (0.2) | 1.00 (reference) | |
| Statin‐discontinued | 19 (0.2) | 0.95 (0.51‐1.78) | 0.88 |
| Any hospitalization | |||
| Statin‐maintained | 1099 (13.2) | 1.00 (reference) | |
| Statin‐discontinued | 1256 (15.0) | 1.16 (1.07‐1.26) | 0.0003 |
| Statin‐maintained vs statin‐reduced, N=6350 | |||
| Recurrent stroke | |||
| Statin‐maintained | 128 (4.0) | 1.00 (reference) | |
| Statin‐reduced | 107 (3.4) | 0.84 (0.65‐1.08) | 0.17 |
| Ischemic stroke | |||
| Statin‐maintained | 114 (3.6) | 1.00 (reference) | |
| Statin‐reduced | 99 (3.1) | 0.87 (0.66‐1.14) | 0.30 |
| Intracerebral hemorrhage | |||
| Statin‐maintained | 14 (0.4) | 1.00 (reference) | |
| Statin‐reduced | 8 (0.3) | 0.57 (0.24‐1.36) | 0.21 |
| Myocardial infarction | |||
| Statin‐maintained | 12 (0.4) | 1.00 (reference) | |
| Statin‐reduced | 13 (0.4) | 1.08 (0.50‐2.38) | 0.84 |
| All‐cause mortality | |||
| Statin‐maintained | 25 (0.8) | 1.00 (reference) | |
| Statin‐reduced | 22 (0.7) | 0.88 (0.50‐1.56) | 0.67 |
| All major eventsa | |||
| Statin‐maintained | 162 (5.1) | 1.00 (reference) | |
| Statin‐reduced | 138 (4.4) | 0.85 (0.68‐1.07) | 0.16 |
| Stenting and endarterectomy | |||
| Statin‐maintained | 14 (0.4) | 1.00 (reference) | |
| Statin‐reduced | 1 (0.03) | 0.07 (0.01‐0.54) | 0.01 |
| Any hospitalization | |||
| Statin‐maintained | 349 (11.0) | 1.00 (reference) | |
| Statin‐reduced | 322 (10.1) | 0.92 (0.79‐1.07) | 0.30 |
Propensity score matching was computed from age, sex, stroke severity index, hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, stroke history, heart failure, chronic kidney disease, chronic obstructive pulmonary disease, peripheral vascular disease, and sleep apnea. HR indicates hazard ratio.
All major events: composite of stroke, acute myocardial infarction, and death.
In subgroup analyses based on a propensity score–matching data set, there was no interaction between most baseline characteristics by the day‐90 to day‐180 time period statin utilization status except that a detrimental effect of statin discontinuation was noted mostly in patients with minor stroke severity (Figure 2).
Figure 2.
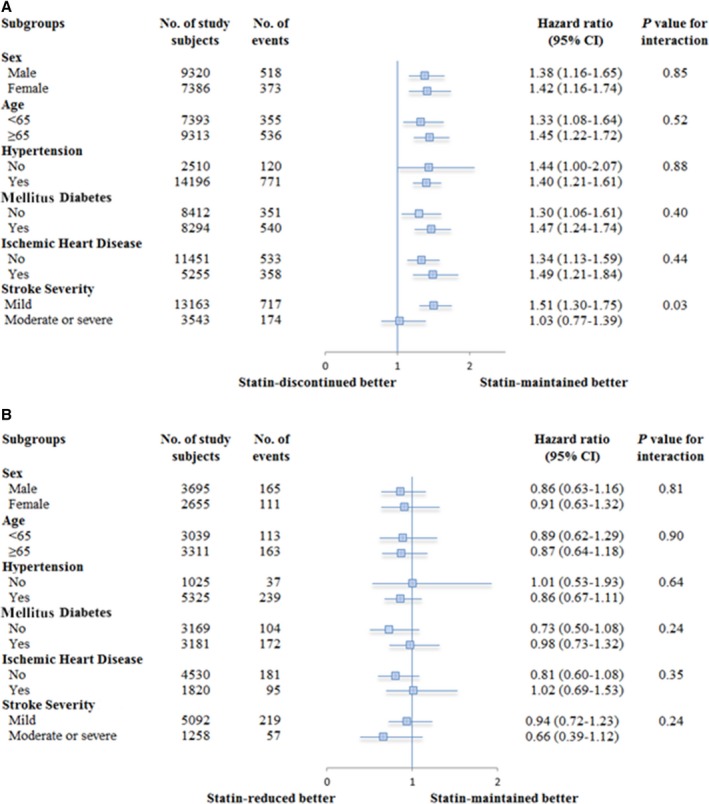
Stratified analysis for recurrent stroke according to baseline characteristics, by propensity score matching: (A) statin‐maintained vs statin‐discontinued; (B) statin‐maintained vs statin‐reduced.
Discussion
The primary finding of this analysis is that discontinuation of statin treatment beyond the first 3 months after an index ischemic stroke was associated with increased risk of recurrent stroke and all‐cause mortality at 1 year after statin discontinuation. Among the one fifth of patients who did discontinue statin therapy in the 3‐ to 6‐month period, the risks of any recurrent stroke (mostly ischemic stroke), increased by 42%, from 4.4% to 6.2% in the 6‐ to 18‐month period of discharge after an index ischemic stroke. Statin discontinuation in the 3‐ to 6‐month period was also associated with a 37% relative risk increase in mortality in the 6‐ to 18‐month time window. On the other hand, a reduction in the dose of statin therapy in the 3‐ to 6‐month period after an ischemic stroke did not affect recurrent stroke or all‐cause mortality rates.
Our findings are consistent with, and further extend, results of a meta‐analysis that revealed higher risks of cardiovascular events and mortality among individuals with poor statin adherence.9 Reasons for statin discontinuation in the current study might be different from those in most prior studies. Potential complications of statin, such as myalgia, rhabdomyolysis, memory/cognitive impairment, and increased liver enzymes, were not different among statin utilization groups and were unlikely to have contributed to discontinuation or reducing intensity of statin therapy. High‐intensity statin use (SPARCL [Stroke Prevention by Aggressive Reduction in Cholesterol Levels] study)2 and moderate‐intensity statin use (post hoc analyses of the HPS [Heart Protection Study])10 have been associated with a reduction in vascular events after stroke, and we are unaware of published data examining the link between low‐intensity statin use and reduced recurrent vascular events after stroke. Nonetheless, we speculated that because both the high‐intensity statin used in SPARCL2 and moderate‐intensity statin used in HPS10 were prescribed over 5‐year follow‐up periods, that the follow‐up period in our study (around 1 year) may have been too short to detect a clinically significant difference between high/moderate‐intensity statin versus maintained yet reduced‐intensity statin dose.
Patients who may have been poorly adherent to overall medical instructions or too sick to comply were likely to have been very few in this study cohort because we purposely excluded patients who discontinued the cornerstone of the recurrent stroke preventive regimen, antithrombotic therapy, from our analyses. As a result, the potential for bias arising from the “healthy user” effect was curtailed in the current study. Using compliance to concomitant therapies to control for the healthy user effect is an established approach in analysis of administrative data sets.11 Reinforcing the notion that the patients stopping statins were not systematically sicker than patients continuing statins was the observation that there were no major differences in the baseline characteristics between the groups, including no differences in the rates of receipt of another key concomitant therapy, antihypertensive agents. Statin discontinuation was unlikely to be due to cost concerns because the National Health Insurance in Taiwan covers all medication costs for eligible patients. However, the Taiwan National Health Bureau during the study period did recommend that physicians should consider stopping statins or reducing statin dosage once LDL‐cholesterol <100 mg/dL or total cholesterol <160 mg/dL was achieved in a stroke patient, and this recommendation might have contributed to the discontinuation of statin in some stroke patients.
Our findings also confirm and build on prior studies of statin discontinuation in patients with ischemic stroke. A randomized trial found that discontinuation of statins in the acute poststroke period, within 3 days after onset, was associated with increased death or dependency at 90 days.12 The current study found adverse effects in patients who did continue statins through the acute period but then were no longer on the therapy more than 3 months after onset. A single‐center study found increased mortality among patients stopping statins in the first year after stroke.6 Our study affirms the deleterious effect of stopping statins after the initial period following a stroke but does so in a population 30‐fold larger, demonstrates increased risk of recurrent stroke, and controls more rigorously for the healthy‐user effect.
Several mechanisms may explain a harmful effect of statin discontinuation. Statins stabilize plaques in arteries supplying brain and heart, reducing the risk of recurrent cerebral infarction and coronary artery disease–related events.13 Also, statin cessation has been associated with reduced flow‐mediated vasodilation, increased atrial fibrillation, and elevated C‐reactive protein serum levels.14, 15, 16
The observation in this study that patients had similar rates of intracerebral hemorrhage among different groups of statin therapy during 1‐year follow‐up is consistent with prior studies. Studies have found that use of statins is associated with neutral effect on risk of intracerebral hemorrhage in persons without prior ischemic stroke,1 neutral effect on early hemorrhagic transformation of acute ischemic stroke,17, 18, 19 and neutral or increased risk of intracerebral hemorrhage when used for long‐term secondary prevention after an ischemic stroke.1, 20, 21 An observational study based on Taiwan NHIRD showed no association between statin use and the risk of intracranial hemorrhage among subjects without a previous history of stroke.22
Our study has both clinical and policy implications. Discontinuation of statins was associated with higher recurrent stroke rate beyond the initial period following an index ischemic stroke. After a stroke due to large or small vessel atherosclerosis, patients are at high risk of recurrent stroke and should be treated aggressively in the absence of clear contraindications. Physicians also need to increase awareness among stroke patients about the potential risk of discontinuing their medications and to encourage greater adherence. It has been shown that statin‐related side effects often lead to statin discontinuation, but most patients who initially experience statin‐provoked symptoms can actually tolerate statins in the long term by either taking a lower dose or by switching to a different statin type. Symptoms occurring while on a statin may not necessarily be related to its use, may eventually be tolerated by the patient, may not recur with a rechallenge, or may be specific to individual statins rather than the entire drug class.23 Ideally, discontinuation of statin treatment in patients with ischemic stroke of large‐ or small‐vessel atherosclerotic origin taking statin who have reached a target LDL‐cholesterol goal should be strongly discouraged. Our study indicated that shifting to low‐intensity statin therapy did not increase recurrent stroke risk and could be an alternative choice for stroke patients not able to receive moderate or high‐intensity statin therapy.
This study has limitations inherent to studies of administrative data sets. First, LDL‐cholesterol values over time are important because achieved cholesterol could influence both the decision to discontinue/reduce dose and long‐term outcomes. However, such information was not available in the Taiwan NHIRD, and we were unable to know exactly whether it potentially biased our results. Second, this is a retrospective study, and not all possible confounders may have been adjusted. Because statin therapy is a proven strategy for secondary stroke prevention,2 it would be unethical to assign certain stroke patients to discontinue their statin therapy. Still, we conducted a propensity core matching analysis, which likely minimized differences of baseline characteristics among statin utilization groups. Third, it was possible that a few patients may have received medications without filing a medication claim, and such data were not captured by Taiwan NHIRD. However, because medication fees are typically covered by national insurance in Taiwan, the numbers of patients who might have done this was likely negligible. Fourth, the current database did not provide information on whether a recurrent stroke was related to a medical procedure or occurred during a hospitalization for a non‐stroke‐related problem.
In conclusion, this nationwide study evaluated outcomes in poststroke patients among whom one‐fifth discontinued the use of prescribed statin therapy 3 to 6 months after discharge for an ischemic stroke, although they continued to receive other standard secondary stroke‐prevention therapies such as antithrombotic agents. Stroke patients no longer on statins beyond the initial period following their index event had increased rates of recurrent stroke and death during the first year after statin discontinuation. These findings suggest that providers and atherosclerotic stroke patients should not discontinue statin therapy unless there is a highly compelling reason for doing so. Additional prospective studies should be carried out to clarify the underlying mechanisms, such as LDL‐cholesterol rebound and/or inflammation, linking statin discontinuation to higher risk of recurrent stroke.
Sources of Funding
Study was funded by grants from Ministry of Science and Technology, Taiwan (MOST103‐2314‐B‐182‐056) and Chang Gung Memorial Hospital, Taiwan (CORPG6D103). The sponsors played no role in the study design, data collection and analysis, or decision to submit the article for publication.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005658 DOI: 10.1161/JAHA.117.005658.)28768645
Contributor Information
Meng Lee, Email: menglee5126@gmail.com.
Pei‐Chun Chen, Email: peichun@mail.cmu.edu.tw.
References
- 1. Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta‐analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. [DOI] [PubMed] [Google Scholar]
- 2. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators . High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 4. Hong KS, Lee JS. Statins in acute ischemic stroke: a systematic review. J Stroke. 2015;17:282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez Sandoval YH, Braganza MV, Daskalopoulou SS. Statin discontinuation in high‐risk patients: a systematic review of the evidence. Curr Pharm Des. 2011;17:3669–3689. [DOI] [PubMed] [Google Scholar]
- 6. Colivicchi F, Bassi A, Santini M, Caltagirone C. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38:2652–2657. [DOI] [PubMed] [Google Scholar]
- 7. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–242. [DOI] [PubMed] [Google Scholar]
- 8. Sung S‐F, Hsieh C‐Y, Kao Yang Y‐H, Lin H‐J, Chen C‐H, Chen Y‐W, Hu Y‐H. Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol. 2015;68:1292–1300. [DOI] [PubMed] [Google Scholar]
- 9. De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins R, Armitage J, Parish S, Sleight P, Peto R; Heart Protection Study Collaborative Group . Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions. Lancet. 2004;363:757–767. [DOI] [PubMed] [Google Scholar]
- 11. Platt AB, Kuna ST, Field SH, Chen Z, Gupta R, Roche DF, Christie JD, Asch DA. Adherence to sleep apnea therapy and use of lipid‐lowering drugs: a study of the healthy‐user effect. Chest. 2010;137:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanco M, Nombela F, Castellanos M, Rodriguez‐Yanez M, Garcia‐Gil M, Leira R, Lizasoain I, Serena J, Vivancos J, Moro MA, Davalos A, Castillo J. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69:904–910. [DOI] [PubMed] [Google Scholar]
- 13. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists’ Collaborators . Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Ren JY, Xing Y, Zhang WL, Liu X, Wu P, Wang RJ, Luo Y. Short‐term withdrawal of simvastatin induces endothelial dysfunction in patients with coronary artery disease: a dose‐response effect dependent on endothelial nitric oxide synthase. Int J Cardiol. 2009;131:313–320. [DOI] [PubMed] [Google Scholar]
- 15. Chang CH, Lee YC, Tsai CT, Chang SN, Chung YH, Lin MS, Lin JW, Lai MS. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis. 2014;232:224–230. [DOI] [PubMed] [Google Scholar]
- 16. Lee KT, Lai WT, Chu CS, Tsai LY, Yen HW, Voon WC, Sheu SH. Effect of withdrawal of statin on C‐reactive protein. Cardiology. 2004;102:166–170. [DOI] [PubMed] [Google Scholar]
- 17. Nardi K, Engelter S, Strbian D, Sarikaya H, Arnold M, Casoni F, Ford GA, Cordonnier C, Lyrer P, Bordet R, Soinne L, Gensicke H, Duriez P, Baumgartner RW, Tatlisumak T, Leys D; Lipid Profile in Thrombolysis Study Group . Lipid profiles and outcome in patients treated by intravenous thrombolysis for cerebral ischemia. Neurology. 2012;79:1101–1108. [DOI] [PubMed] [Google Scholar]
- 18. Spence JD. Statins do not cause intracerebral hemorrhage. Neurology. 2012;79:1076–1077. [DOI] [PubMed] [Google Scholar]
- 19. Rocco A, Sykora M, Ringleb P, Diedler J. Impact of statin use and lipid profile on symptomatic intracerebral haemorrhage, outcome and mortality after intravenous thrombolysis in acute stroke. Cerebrovasc Dis. 2012;33:362–368. [DOI] [PubMed] [Google Scholar]
- 20. Hackam DG, Austin PC, Huang A, Juurlink DN, Mamdani MM, Paterson JM, Hachinski V, Li P, Kapral MK. Statins and intracerebral hemorrhage: a retrospective cohort study. Arch Neurol. 2012;69:39–45. [DOI] [PubMed] [Google Scholar]
- 21. Goldstein LB, Amarenco P, Szarek M, Callahan A III, Hennerici M, Sillesen H, Zivin JA, Welch KM; SPARCL Investigators . Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology. 2008;70:2364–2370. [DOI] [PubMed] [Google Scholar]
- 22. Chang CH, Lin CH, Caffrey JL, Lee YC, Liu YC, Lin JW, Lai MS. Risk of intracranial hemorrhage from statin use in Asians: a nationwide cohort study. Circulation. 2015;131:2070–2078. [DOI] [PubMed] [Google Scholar]
- 23. Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, Turchin A. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]


