Abstract
Background
Patients with a Fontan circulation have reduced exercise capacity and respiratory muscle strength. Inspiratory muscle training (IMT) improves exercise capacity and quality of life in adults with heart failure. We assessed whether 6 weeks of a home‐based program of IMT improves inspiratory muscle strength and the ventilatory efficiency of exercise in adolescent patients with a Fontan circulation.
Methods and Results
Twenty‐three adolescent participants (aged 16±2 years) with a Fontan circulation underwent 6 weeks of IMT for 30 minutes daily. Respiratory muscle strength (maximal inspiratory pressure and expiratory pressure), lung function, and exercise capacity (cardiopulmonary exercise testing) were assessed. Fourteen of 23 participants also underwent exercise cardiac magnetic resonance imaging to examine the effects of IMT on cardiac output and systemic and pulmonary blood flow. Six weeks of IMT improved maximal inspiratory pressure by 36±24 cm H2O (61±46%) with no change in maximal expiratory pressure. Ventilatory efficiency of exercise improved after 6 weeks of IMT (from 34.2±7.8 to 32.2±5.6, P=0.04). In those who underwent exercise cardiac magnetic resonance imaging, IMT increased resting cardiac output (from 4.2±1.2 to 4.5±1.0 L/min, P=0.03) and ejection fraction (from 50.1±4.3 to 52.8±6.1%, P=0.03).
Conclusions
Six weeks of IMT is associated with improved inspiratory muscle strength, ventilatory efficiency of exercise, and resting cardiac output in young Fontan patients. IMT may be a simple beneficial addition to the current management of Fontan patients, potentially reducing exercise intolerance and long‐term morbidity and mortality.
Keywords: exercise, exercise capacity, Fontan procedure, inspiratory muscle strength
Subject Categories: Congenital Heart Disease
Clinical Perspective
What Is New?
Six weeks of inspiratory muscle training in adolescent Fontan patients is associated with improved inspiratory muscle strength and ventilatory efficiency of exercise, an independent predictor of morbidity in patients with congenital heart disease.
Inspiratory muscle training resulted in improvements in resting cardiac output and ejection fraction, highlighting its potential to alter the progressive decline in cardiac function in Fontan patients.
What Are the Clinical Implications?
Inspiratory muscle training is a simple technique that has the potential to improve the clinical outcomes of Fontan patients by improving their exercise tolerance.
This study provides a basis for future larger studies to explore the effects of inspiratory muscle training in conjunction with other exercise regimens such as aerobic or resistance training.
Since its original description in 1971, the “Fontan operation” has undergone a number of modifications and widening of the indications for its use.1 A growing population of children and adults with congenital heart disease are living with a Fontan circulation. In the current era, medium‐term survival after Fontan completion is high. However, these patients still face significant morbidity and higher mortality than the general population.1 Treatments to improve outcomes for Fontan patients are limited.
The Fontan circulation, in which systemic venous return bypasses a subpulmonary ventricle, has inherent limitations of chronic elevation of central venous pressure and restricted ventricular preload.2 These inherent limitations may be compounded by chronotropic incompetence, nonuniform distribution of pulmonary blood flow, elevated pulmonary and systemic vascular resistance2, and reduced skeletal muscle blood flow and strength.3 The limitations of a Fontan circulation manifest during exercise, and various parameters of exercise performance have been associated with adverse outcomes in Fontan patients.4
Respiratory muscle weakness has been demonstrated in adults with congenital heart disease including patients with a Fontan circulation.3 Such weakness has been described previously in adults with heart failure,5 and inspiratory muscle training (IMT) in heart failure patients, for periods of between 4 and 12 weeks, results in improved inspiratory muscle strength, exercise capacity, and quality of life.6, 7, 8 Fatiguing inspiratory muscles activate phrenic afferents, which produce sympathetic peripheral vasoconstriction (the “inspiratory muscle metaboreflex”9), and in adult heart failure patients, IMT attenuates the exaggerated peripheral vasoconstriction in resting and exercising limbs.6
To our knowledge, the effects of IMT have not been reported previously in patients with a Fontan circulation. The aims of the current study in Fontan patients were to test whether IMT improved (1) inspiratory muscle strength and (2) objective measures of exercise capacity. Regarding the latter aim, we specifically tested the hypothesis that IMT improves the ventilatory efficiency of exercise, as measured by the slope of the relationship of minute ventilation (VE) to carbon dioxide production (VCO2). A high VE/VCO2 slope may be determined by 2 factors potentially altered by IMT, namely, physiologic dead space ventilation (dead space/tidal volume ratio [Vd/Vt]) and increased ventilatory drive mediated by a complex metabolic reflex by which peripheral chemoreceptors in muscles register local metabolic byproducts during exercise and initiate a neural reflex that drives hyperventilation.10
Patients and Methods
Patients with a Fontan circulation were identified through databases of the Children's Hospital at Westmead, Sydney, Australia. Details of the underlying cardiac anatomy and previous surgery were obtained. To maintain a relatively homogenous population, only participants with a nonfenestrated extracardiac conduit who were aged 12 to 20 years were included. Exclusion criteria were moderate to severe ventricular dysfunction or atrioventricular valve regurgitation, a history of exercise‐induced syncope, recent arrhythmia or clinical instability, major musculoskeletal disease, or intellectual disability. Written informed consent was obtained from all participants and/or parents, and the study was approved by the institutional ethics committee.
The study consisted of 3 visits over a 6‐week period (Figure 1). Anthropometric data were obtained at each visit. Participants underwent the following tests at visits 1 (week 0, baseline) and 3 (week 6, study end): (1) assessment of physical activity levels (self‐administered standardized questionnaire, International Physical Activity Questionnaire short form, with physical activity rated as low, moderate, or high11); (2) testing of inspiratory and expiratory muscle strength; (3) spirometry; and (4) cardiopulmonary exercise testing (CPET). In an exploratory substudy to evaluate the effects of IMT on resting and exercise pulmonary and systemic blood flow, an exercise cardiac magnetic resonance imaging (CMRI) study was performed at visits 1 and 3. At visit 1, participants were instructed on how to use the IMT device and asked to train for 30 minutes daily. At visit 2 (week 3), participants underwent repeated respiratory muscle and lung function testing. The load of the IMT device was adjusted for any change in maximal inspiratory pressure (MIP) between visits 1 and 2. Between visits, phone contact was made with participants and/or parents to assess compliance with IMT. Compliance with IMT was additionally assessed by twice‐daily entry in each participant's diary. Baseline testing at visit 1 also included a transthoracic echocardiogram in which atrioventricular valve regurgitation and ejection fraction (EF) of the dominant ventricle were measured.
Figure 1.
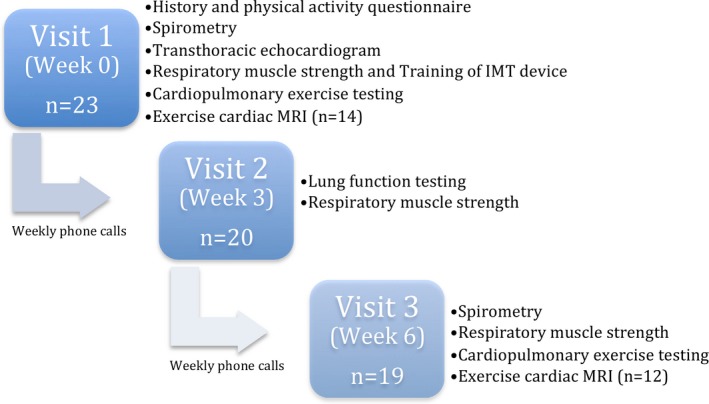
Study outline. IMT indicates inspiratory muscle training; MRI, magnetic resonance imaging.
Sixty‐seven potential participants aged 12 to 20 years were identified from the institutional database. Twenty‐three agreed to participate and underwent baseline testing. Four participants dropped out of the study after visit 1 because of commitments unrelated to the study, with 19 completing 6 weeks of IMT. The first 14 consecutive participants were recruited into the exercise CMRI substudy, 2 of whom withdrew after visit 1.
Inspiratory Muscle Testing and Training
Inspiratory and expiratory muscle strength, measured as MIP and maximal expiratory pressure at the mouth, were assessed using a MicroRPM respiratory pressure meter (Carefusion), as recommended in the 2002 American Thoracic Society guidelines.12 Briefly, maximal inspiratory and expiratory maneuvers were supervised by an experienced respiratory physiotherapist, and measurements were performed with a nose peg in place and the participants sitting with their feet flat on the floor. Up to 10 measurements were performed for MIP and maximal expiratory pressure until 3 consistent results (within 5 cm H2O) were obtained. The maximum value of these 3 readings was recorded. The device was calibrated against a fluid‐filled manometer at the start of the study.
Initial instruction on IMT was provided by an experienced respiratory physiotherapist. The IMT was done using a Philips Threshold IMT device. The inspiratory load on the device was set to 30% of the participant's measured MIP. Technique was checked twice, at visit 1 and then again at visit 2. Each participant kept a diary of usage at home, and the total time using the device was calculated and averaged to minutes per day.
Cardiopulmonary Exercise Testing
CPET was performed on an upright cycle ergometer (Ergoselect 200; Ergoline) using a ramp protocol. The initial workload started at 20 W and increased by 10 W every minute for participants who weighed <50 kg and by 15 W every minute if >50 kg, until exhaustion.13 Baseline heart rate (HR), blood pressure, and oxygen saturation were recorded before test commencement and during exercise. Breath‐by‐breath oxygen consumption (VO2), VCO2, and VE were measured (SentrySuite; Carefusion). VE/VCO2 slope was calculated using the linear relationship between VE and VCO2 between 25% and 75% of maximum VCO2.14 Anaerobic threshold was calculated using the V‐slope method and by confirming the value with the ventilatory equivalent method and respiratory exchange ratio.15 O2 pulse, a surrogate for stroke volume, was calculated by dividing peak VO2 by peak HR. Vd/Vt was calculated noninvasively using the Jones equation, calculating arterial CO2 from end‐tidal CO2.16 Maximal voluntary ventilation (shown as MVV) was estimated using FEV1 (forced expiratory volume in 1 second) ×35, and breathing reserve was then calculated as a percentage using the formula [1−(peak VE/MVV)]×100.17 The Borg score of perceived exertion was recorded at test completion.
Exercise CMRI
Exercise CMRI was performed (1.5T; Phillips Intera) at visits 1 and 3 in a subset of participants (n=14 visit 1, n=12 visit 2). Vectorcardiography, peripheral pulse, and respiratory belt monitoring were applied, and the data were recorded in the scan physiology log file. Resting and exercise nongated real‐time phase contrast flow (200 consecutive dynamics, dynamic scan time 75 ms, corresponding to ≈15 seconds of flow data) was measured in the ascending aorta, descending aorta at the level of the diaphragm, superior vena cava, extracardiac conduit, and left or right pulmonary artery (depending on the presence of a left‐ or right‐sided bidirectional Glenn shunt). Retrospective‐gated (vectorcardiography or peripheral pulse) balanced steady‐state free‐precession cine imaging was performed (axial stack, 2‐ and 4‐chamber, left ventricular outflow tract and 2 left or right pulmonary artery orthogonal views at rest, and a short‐axis stack at rest and during each stage of exercise). Typical imaging parameters were field of view, 350×350 mm (approximately); reconstruction matrix, 240×240; scan percentage, 94%; flip angle, 60°; sensitivity encoding factor, 2; repetition time, 2.5 ms; echo time, 1.3 ms; reconstructed voxel size, 1.46×1.46×10 mm; and temporal resolution, 34 ms.
Exercise was undertaken using an MRI‐compatible stepper. The initial workload was set to 30 W and increased by 20 W every 3 minutes until exhaustion. Flow and cine imaging was acquired from 50% of the participants’ maximum workload based on the prior CPET testing and at every stage thereafter until fatigue.
Ventricular volumes were determined by manual contouring of the endocardial border of each short‐axis slice, at end‐diastole and end‐systole. Papillary muscles and ventricular trabeculae were excluded from the blood pool. Stroke volume was calculated as end‐diastolic volume minus end‐systolic volume and EF as stroke volume divided by end‐diastolic volume.
The flow data for each vessel at each workload were extracted by contouring the vessel of interest in each dynamic, producing a flow‐versus‐time curve over the ≈15 second time period. Using a trapezoidal method, the total area under the flow–time curve was calculated, giving the total flow in each vessel over the sample period. Simultaneously acquired vectorcardiography and peripheral pulse data allowed calculation of each participant's HR, giving vessel flow in mL per beat. Vessel blood flow during inspiration and expiration was determined using simultaneously recorded respiratory data. An in‐house code was written to apply a Butterworth filter to the respiratory data, and the inspiration and expiration states were classified by taking the first derivative of the filtered data. Respective flow values were then calculated by trapezoidal integration on the same time scale. This output the total flow in each vessel during inspiration and expiration. The proportion of blood flow during inspiration was calculated as the ratio of blood flow during inspiration over the blood flow during inspiration plus expiration (I:I+E). This ratio was then compared between baseline and peak workload before and after IMT training and between peak workload before and after IMT training. Pulmonary blood flow was calculated as superior vena cava plus extracardiac conduit flow. Cardiac output was calculated as aortic flow times HR. Aortopulmonary collateral (APC) flow was calculated as ascending aortic flow minus (superior vena cava plus conduit flow), as described previously.18 APC flow indexed to aortic flow (indexed APC flow as a percentage) was also calculated.
Power and Statistical Analysis
For the primary study questions, the following sample size calculations were made.
First, to examine whether 6 weeks of IMT improves MIP, we determined that a sample size of 20 would have 80% power at α=0.05 to detect a change of 16 cm H2O (20% difference), assuming a mean MIP of 80±25 cm H2O and a within‐subject correlation of 0.6, based on previously published literature.19 We allowed only for a conservative estimate of MIP improvement (20%), even though previous literature in adults with heart failure demonstrated substantially greater increases in MIP after 6 weeks of IMT.8, 20, 21
Second, to examine whether 6 weeks of IMT improves VE/VCO2 slope, we determined that a sample size of 14 would have 80% power at α=0.05 to detect a change of 2, assuming a VE/VCO2 slope SD of 3 in adolescents with a Fontan circulation.22 In a recent systematic review, IMT in heart failure was associated with a mean reduction in VE/VCO2 slope of 2.28 (95% confidence interval, 1.3–3.25).23
Statistical analyses were performed using SPSS version 24 (IBM Corp). Data are presented as median (range) or mean±SD, as appropriate. Pre‐ and post‐IMT data were compared using paired t tests for continuous variables. Correlation or linear regression was used to analyze associations with MIP change. In addition, participants were categorized into 2 groups (upper tertile and lower 2 tertiles) based on MIP change and compared by χ2 tests for categorical variables and independent sample t tests for continuous data. A P value of <0.05 was considered to be statistically significant. Mean change was calculated as post‐IMT values minus pre‐IMT values, with a 95% confidence interval.
Results
Baseline demographics are shown in Table 1. The morphological diagnoses of participants were representative of the Fontan population of Australia and New Zealand.1 Overall, 30% of the participants had exposure to cigarette smoke at home or at work, but all were themselves nonsmokers. The majority of participants self‐reported their usual activity levels as moderate or high.
Table 1.
Baseline Characteristics of Participants at Visit 1
| Parameter | Value |
|---|---|
| Patients, N | 23 |
| Age, y, mean±SD (range) | 16±2 (12–20) |
| Sex, male; female, n (%) | 12 (52); 11 (48) |
| Height, cm, mean±SD | 164.9±8.3 |
| Weight, kg, mean±SD | 61.4±15.0 |
| BMI, kg/m2, mean±SD | 22.5±4.4 |
| Dominant ventricle, n (%) | |
| Left | 13 (57) |
| Right | 8 (35) |
| Balanced | 2 (9) |
| Smoke exposure, yes; no, n (%) | 7 (30); 16 (70) |
| Age at Fontan, y, mean±SD (range) | 5±2 (3–9) |
| Lateral thoracotomy, yes; no, n (%) | 12 (52); 11 (48) |
| Diagnosis, n (%) | |
| Pulmonary atresia | 4 (17) |
| Tricuspid atresia | 5 (22) |
| DILV | 2 (9) |
| HLHS | 3 (13) |
| Other | 9 (40) |
| Baseline saturation, %, mean±SD (range) | 93±4 (83–97) |
| IPAQ, n (%) | |
| Low | 3 (13) |
| Moderate | 10 (43) |
| High | 10 (43) |
| % Predicted FEV1, mean±SD | 89±11 |
| % Predicted FVC, mean±SD | 86±11 |
BMI indicates body mass index; DILV, double‐inlet left ventricle; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HLHS, hypoplastic left heart syndrome; IPAQ, International Physical Activity Questionnaire.
Respiratory Muscle Strength
Male participants had higher baseline MIP than female participants (75±28 versus 62±11 cm H2O, P=0.02). IMT improved MIP by 61±46% (P<0.01; Table 2). As expected, there was no significant change in maximal expiratory pressure. The prescribed training time was 30 minutes daily, and based on self‐completed diaries, participants trained for an average of 24±5.7 minutes per day. Figure 2A, demonstrating individual participant MIP, highlights variability in the magnitude and tempo of change. However, the grouped data (Figure 2B) highlight that the largest magnitude change occurred between visits 1 and 2 (mean increase of 26 cm H2O), with a plateau between visits 2 and 3 (mean increase of 9 cm H2O).
Table 2.
Effects of IMT on Respiratory Muscle Strength
| Parameter | Before IMT, Mean±SD | After IMT, Mean±SD | P Value |
|---|---|---|---|
| MIP, cm H2O | 69±22a | 103±32 | <0.001 |
| MEP, cm H2O | 67±23 | 73±33 | 0.10 |
| Change in MIP, cm H2O | 36±24 | … | … |
| Percentage change in MIP, % | 61±46 | … | … |
IMT indicates inspiratory muscle training; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure.
Male, 74±38 cm H2O; female, 62±11 cm H2O.
Figure 2.
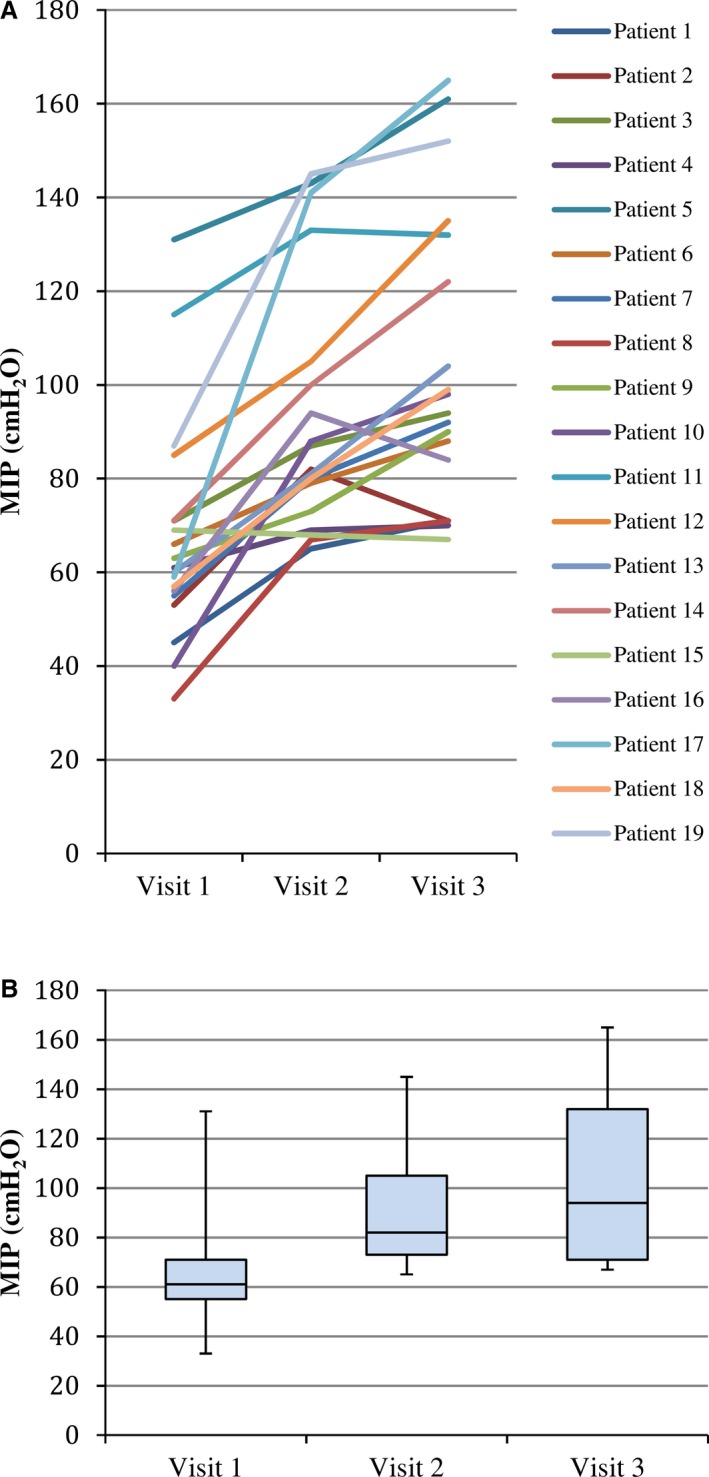
A, Individual change in MIP. B, Grouped change in MIP. MIP indicates maximal inspiratory pressure.
Male participants tended to have a greater change in MIP than female participants (45.7±29 versus 25.8±10 cm H2O, P=0.07). Change in MIP was positively correlated with baseline peak VO2 (R=0.474, P=0.04). Change in MIP was not related to the participants’ baseline MIP, age, self‐reported compliance based on IMT usage in minutes per day, baseline oxygen saturations, predominant ventricle, EF as measured on echocardiogram, previous thoracotomy, or self‐reported physical activity. No adverse effects were reported from IMT.
Exercise Capacity
All participants achieved maximal exertion on CPET both before and after IMT, as shown by a respiratory exchange ratio ≥1.1 and a Borg score ≥15. Consistent with other studies in young Fontan patients, pre‐IMT peak workload was 120±28 W and peak VO2 was 26.8±6.8 mL/min per kilogram. IMT improved the ventilatory efficiency of exercise. as shown by a reduced VE/VCO2 slope (34.2±7.8 versus 32.2±5.6, P=0.04; Table 3). IMT did not alter other exercise parameters including peak VO2, O2 pulse, and Borg score.
Table 3.
Effects of IMT on Cardiopulmonary Exercise Testing Parameters
| Parameter | Before IMT, Mean±SD | After IMT, Mean±SD | P Value | Changea |
|---|---|---|---|---|
| Workload, W | 120±28 | 118±28 | 1.00 | −0.1 (−5.2 to 5.0) |
| Peak HR, beats/min | 174±15 | 171±19 | 0.19 | −4.1 (−10.4 to 2.3) |
| Peak systolic BP, mm Hg | 133±23 | 134±15 | 0.66 | 4.2 (−3.5 to 11.9) |
| Peak diastolic BP, mm Hg | 68±8 | 70±9 | 0.91 | 3.7 (−2.3 to 9.7) |
| Lowest saturation, % | 87±7 | 86±6 | 0.37 | −1.6 (−5.2 to 2.1) |
| RER | 1.3±0.1 | 1.3±0.2 | 0.32 | 0.04 (−0.05 to 0.13) |
| Peak VO2, mL/min per kg | 26.8±6.8 | 26.0±7.2 | 0.05 | −6.04 (−7.66 to 4.43) |
| O2 pulse, mL/beat | 9.2±2.2 | 8.7±2.0 | 0.32 | −0.26 (−0.79 to 0.28) |
| VE/VCO2 slope | 34.2±7.8 | 32.2±5.6 | 0.04 | −2.24 (−4.63 to −0.18) |
| Peak VT, L | 1.6±0.5 | 1.6±0.6 | 0.67 | −0.03 (−0.17 to 0.11) |
| Peak VE, L/min | 64.2±13.5 | 62.2±18.3 | 0.56 | −1.42 (−6.41 to 3.56) |
| Peak VCO2, L/min | 1.7±0.5 | 1.6±0.5 | 0.27 | −0.05 (−0.16 to 0.05) |
| Breathing reserve, % | 33.0±9.6 | 34.5±9.8 | 0.54 | 0.01 (−0.04 to 0.06) |
| Borg score | 17±2 | 18±1 | 0.51 | 0.3 (−0.7 to 1.3) |
BP indicates blood pressure; HR, heart rate; IMT, inspiratory muscle training; RER, respiratory exchange ratio; VCO2, carbon dioxide production; VE, minute ventilation; VO2, oxygen consumption; VT, tidal volume.
Calculated as post‐IMT values minus pre‐IMT values, expressed as mean change (95% confidence interval).
Participants in the upper tertile of MIP change, compared with those in the lower 2 tertiles, had a lower baseline VE/VCO2 slope (27.3±3.4 versus 37.9±7.2, P=0.02; Table 4) but equivalent baseline MIP, peak workload, and peak VO2. Those in the lower 2 tertiles of MIP change tended to have a larger improvement in VE/VCO2 slope (−3.6±5.0 versus 0.1±2.4, P=0.16; Table 5), suggesting that even small changes in MIP may improve VE/VCO2 in those with the lowest baseline ventilatory efficiency of exercise.
Table 4.
Comparison of Baseline Characteristics in Upper Tertile vs Lower 2 Tertiles of MIP Change
| Baseline (Pre‐IMT) Parameter | Upper Tertile of MIP Change, Mean±SD | Lower Tertiles of MIP Change, Mean±SD | P Value |
|---|---|---|---|
| MIP, cmH2O | 67±18 | 67±27 | 0.64 |
| Compliance, min | 22.9±7.1 | 24.5±5.1 | 0.64 |
| Baseline O2 saturation, % | 93±3 | 92±4 | 0.43 |
| Lowest O2 saturation, % | 86±7 | 88±7 | 0.87 |
| Workload, W | 129±29 | 113±24 | 0.52 |
| Peak VO2, mL/min per kg | 33.2±4.7 | 24.8±5.7 | 0.69 |
| VE/VCO2 slope | 27.3±3.4 | 37.9±7.2 | 0.02 |
IMT indicates inspiratory muscle training; MIP, maximal inspiratory pressure; VCO2, carbon dioxide production; VE, minute ventilation; VO2, oxygen consumption.
Table 5.
Comparison of Change in Cardiopulmonary Exercise Testing Parameters in Upper Tertile vs Lower Tertiles of MIP Change
| Parameter | Upper Tertile of MIP Change, Mean±SD | Lower Tertiles of MIP Change, Mean±SD | P Value |
|---|---|---|---|
| Change in workload, W | 1.2±8.2 | −0.7±11.8 | 0.71 |
| Change in peak VO2, L/min | −0.5±−1.7 | −1.9±3.6 | 0.26 |
| Change in VT, L | 139.0±156.5 | −106.3±305.9 | 0.50 |
| Change in VE/VCO2 | 0.1±2.4 | −3.6±5.0 | 0.16 |
MIP indicates maximal inspiratory pressure; VCO2, carbon dioxide production; VE, minute ventilation; VO2, oxygen consumption; VT, tidal volume.
Lung Function
Only 1 participant had spirometry consistent with restrictive lung disease. All other participants had normal baseline lung function (Table 6). There were no statistically significant changes in lung function after IMT; in particular, there was no change in Vd/Vt.
Table 6.
Effects of IMT on Lung Function
| Parameter | Before IMT, Mean±SD | After IMT, Mean±SD | P Value | Changea |
|---|---|---|---|---|
| FEV1, L | 2.9±0.7 | 2.7±0.7 | 0.48 | −0.04 (−0.02 to 0.10) |
| FVC, L | 3.3±0.8 | 3.3±0.8 | 0.10 | 0.07 (−0.02 to 0.16) |
| FEV1/FVC, % | 87.8±5.6 | 83.3±7.5 | 0.06 | −3.30 (−7.32 to 0.73) |
| IC (rest), L | 2.3±0.5 | 2.3±0.7 | 0.31 | −0.10 (−0.30 to 0.10) |
| Vd/Vt | 0.2±0.04 | 0.2±0.10 | 0.98 | −0.02 (−0.03 to 0.01) |
FEV1 indicates forced expiratory volume in 1 s; FVC, forced vital capacity; IC, inspiratory capacity; IMT, inspiratory muscle training; Vd/Vt, dead space/tidal volume ratio.
Calculated as post‐IMT values minus pre‐IMT values; expressed as mean change (95% confidence interval).
Exercise CMRI
In participants who underwent exercise CMRI, neither resting or peak HR nor aortic or pulmonary blood flow changed significantly after IMT (Table 7). However, resting cardiac output improved following IMT (4.2±1.2 versus 4.5±1.0 L/min, P=0.03). Cardiac output at peak exercise remained unchanged. The relative proportion of aortic flow during inspiration (ratio of inspiration to inspiration plus expiration), both at rest and peak exercise, did not change significantly after IMT (Table 7). The relative proportion of resting pulmonary blood flow during inspiration also remained unchanged, but there was a trend toward an increase of the inspiratory proportion of pulmonary blood flow at peak exercise after IMT (47 to 54%, P=0.06). Both stroke volume and EF increased significantly from rest to peak exercise before and after IMT (Table 8). Although IMT did not change either rest or peak exercise stroke volume, it resulted in increased resting EF (Table 7). APC flow at rest and at peak exercise did not change significantly after IMT.
Table 7.
Effects of IMT on Blood Flow, Cardiac Output, and Ejection Fraction Measured by CMRI
| Parameter | Before IMT, Mean±SD | After IMT, Mean±SD | P Value | Changea |
|---|---|---|---|---|
| Resting heart rate, beats/min | 73.3±15.1 | 75.7±14.4 | 0.41 | 2.33 (−3.66 to 8.33) |
| Peak heart rate, beats/min | 121.0±20.0 | 118.8±19.6 | 0.53 | −2.25 (−9.88 to 5.38) |
| Resting aortic flow, mL/beat | 58.5±19.2 | 60.0±14.3 | 0.64 | 1.45 (−5.20 to 8.11) |
| Peak aortic flow, mL/beat | 55.3±14.6 | 59.3±11.5 | 0.17 | 4.03 (−2.06 to 10.11) |
| Resting cardiac output, L/min | 4.2±1.2 | 4.5±1.0 | 0.03 | 0.29 (0.03 to 0.54) |
| Peak cardiac output, L/min | 6.6±1.5 | 7.0±1.4 | 0.16 | 0.36 (−0.22 to 0.95) |
| Resting pulmonary flow, mL/beat | 52.2±11.2 | 55.1±12.2 | 0.37 | 2.82 (−3.82 to 9.45) |
| Peak pulmonary flow, mL/beat | 55.4±11.6 | 54.8±6.5 | 0.81 | −0.52 (−5.29 to 4.24) |
| Resting stroke volume, mL | 60.6±7.6 | 63.7±10.4 | 0.10 | 3.08 (−0.65 to 6.81) |
| Peak stroke volume, mL | 70.5±11.5 | 68.2±9.0 | 0.26 | −2.33 (−6.70 to 2.03) |
| Resting ejection fraction, % | 50.1±4.3 | 52.8±6.1 | 0.03 | 2.67 (0.41 to 4.92) |
| Peak ejection fraction, % | 57.6±6.0 | 55.3±5.1 | 0.14 | −2.25 (−5.40 to 0.90) |
| Resting APC flow, mL/beat | 9.4±9.4 | 6.9±6.0 | 0.24 | −2.45 (−6.79 to 1.89) |
| Indexed resting APC, % | 13.8±11.9 | 10.9±8.8 | 0.32 | −2.90 (−9.02 to 3.23) |
| Peak APC flow, mL/beat | 4.2±5.8 | 6.0±6.1 | 0.25 | 1.80 (−1.47 to 5.07) |
| Indexed peak APC, % | 6.3±7.7 | 9.0±8.6 | 0.23 | 2.71 (−1.97 to 7.40) |
| I+E ratiob | ||||
| Resting aortic flow, % | 48.6±6.5 | 48.4±7.6 | 0.94 | −0.18 (−4.76 to 4.41) |
| Peak exercise aortic flow, % | 54.5±7.3 | 55.1±4.8 | 0.81 | 0.99 (−4.81 to 6.78) |
| Resting pulmonary flow, % | 55.1±10.2 | 56.1±10.2 | 0.84 | 1.07 (−10.1 to 12.2) |
| Peak exercise pulmonary flow, % | 47.1±10.1 | 54.1±16.2 | 0.06 | 6.98 (−0.40 to 14.36) |
APC indicates aortopulmonary collateral; CMRI, cardiac magnetic resonance imaging; I, inspiratory; E, expiratory; IMT, inspiratory muscle training.
Calculated as post‐IMT values minus pre‐IMT values; expressed as mean change (95% confidence interval).
Inspiration percentage of aortic and pulmonary blood flow at rest and at peak exercise.
Table 8.
Change in Stroke Volume and Ejection Fraction Between Rest and Peak Exercise
| Parameter | Rest, Mean±SD | Peak Exercise, Mean±SD | P Value |
|---|---|---|---|
| Pre‐IMT stroke volume, mL | 64.0±12.7 | 72.4±12.6 | 0.003 |
| Pre‐IMT ejection fraction, % | 50.1±3.9 | 56.7±6.1 | 0.001 |
| Post‐IMT stroke volume, mL | 63.7±10.4 | 68.2±9.0 | 0.03 |
| Post‐IMT ejection fraction, % | 52.8±6.1 | 55.3±2.1 | 0.02 |
IMT indicates inspiratory muscle training.
Discussion
To our knowledge, this study is the first report of the effects of IMT in patients with a Fontan circulation. The 2 principal findings are that a 6‐week home‐based program of IMT increases inspiratory muscle strength by an average of 61% and is associated with improvement in the ventilatory efficiency of exercise, as shown by a reduced VE/VCO2 slope, in young Fontan patients. We also found an increase in resting cardiac output and EF after IMT.
A number of lines of evidence suggested a rationale for IMT in Fontan patients. First, adults with congenital heart disease, including those with a Fontan circulation, have been shown to have reduced respiratory muscle strength compared with controls.3 Second, pulmonary blood flow in the Fontan circulation may be significantly influenced by breathing and the respiratory pump.24, 25 Last, Fontan physiology overlaps with that of adults with heart failure, a patient group for which IMT has been shown to have benefit.23, 26
Improvement in MIP
We demonstrated a magnitude of improvement in MIP similar to that shown in studies of IMT in adults with heart failure.20, 27, 28, 29 The majority of these IMT trials in adult heart failure have utilized targeted threshold trainers, set at inspiratory loads of between 20% and 60% of measured MIP with training durations of between 1 session and 12 months.30, 31 We based our program of IMT on the most frequently reported protocols of 6 to 12 weeks of training for 30 minutes per day, 5 to 7 days per week, and with inspiratory loads of 30% of measured MIP. Most7, 32, 33 but not all8 studies of IMT in adults with heart failure, either alone or in combination with aerobic training, have shown improvements in measures of exercise capacity, functional status, and quality of life.
Improvement in VE/VCO2
We also demonstrated a magnitude of improvement in VE/VCO2 similar to that shown in studies of IMT in adults with heart failure.23 Potentially, IMT could influence one or more of the major mechanisms contributing to elevated VE/VCO2, namely Vd/Vt, early lactic acidosis, and impaired breathing control related to activation of peripheral or central chemoreceptors. Although we cannot exclude some effect of IMT on ventilation–perfusion mismatch, we did not demonstrate a change in Vd/Vt or tidal volume to suggest an effect of IMT on dead space ventilation. Although blood lactate during exercise was not measured in the current study, we were unable to demonstrate significant changes after IMT in O2 pulse or O2 saturation on CPET or peak exercise cardiac output on exercise CMRI, pointing against an effect mediated by reduced lactic acid production. Studies in both healthy controls and adults with heart failure have suggested an effect of IMT on reducing activation of peripheral chemoreceptors by inspiratory muscle fatigue (“inspiratory muscle metaboreflex”). Witt et al, in a study of 16 healthy participants who undertook 5 weeks of either IMT (n=8) or sham IMT (n=8), demonstrated a blunted increase in HR and mean blood pressure induced by eucapnic resistive breathing after IMT.34 Chiappa et al, in study of 18 patients with heart failure and inspiratory muscle weakness, demonstrated improved resting calf blood flow and exercise forearm blood flow after a 4‐week program of IMT.6 Although yet to be proven, our data point to a similar mechanism in patients with a Fontan circulation, in whom IMT may attenuate vasoconstriction to exercising peripheral muscles induced by fatiguing inspiratory muscle.
In adults with heart failure, VE/VCO2 slope is independently associated with severe cardiovascular events including death, with perhaps a better ability to predict risk than peak VO2.35 In adults with all forms of congenital heart disease, including those with a Fontan circulation, VE/VCO2 slope is higher than in healthy control participants. In examining the association between exercise parameters and mortality, in a large population of adults with congenital heart disease, Dimopoulos et al demonstrated that the strongest independent association in noncyanotic patients was with VE/VCO2 (hazard ratio: 1.076 per unit; 95% confidence interval, 1.038–1.115; P<0.05).36 Consequently, improvements in ventilatory efficiency of the order of magnitude that we have documented after just 6 weeks of IMT, if sustained over longer durations, hold the promise of translating into improved morbidity and mortality for Fontan patients.
Improvement in Resting Cardiac Output and EF
We have documented increased resting cardiac output and EF after IMT in a substudy of participants who had exercise CMRI. EF is a load‐dependent measure of cardiac function and may be influenced by preload, afterload, and contractility. Resting cardiac output in the Fontan circulation has been thought to be, importantly, dependent on ventricular preload and thus on low resistance to pulmonary blood flow.37 We were unable to detect a change in either resting pulmonary blood flow or APC flow after IMT, suggesting a mechanism other than an effect on ventricular preload. The effects of IMT on afterload, ventricular–vascular coupling, and myocardial contractility, all which have been reported as abnormal in patients with a Fontan circulation,38, 39 may be of interest in future studies. Consequently, IMT holds the promise of arresting the progressive decline in resting cardiac output brought about by the inherent limitations of the Fontan circulation.
Future Directions
To limit underlying patient heterogeneity, we included only young participants with a nonfenestrated extracardiac conduit. We demonstrated a positive association between MIP change and baseline peak VO2 and greatest improvements in MIP in those with the lowest baseline VE/VCO2. These data suggest that improvements in MIP may be greatest in those with higher baseline exercise capacity. However, we also demonstrated a trend for the largest improvements in VE/VCO2 slope in those in the lowest 2 tertiles of MIP change, suggesting that IMT may be most beneficial in those with the highest baseline VE/VCO2 slope. Taken together, these findings imply that the greatest benefit of IMT may occur in Fontan patients who are the least fit and that, in this group, even small changes in MIP may be of value. Conversely, although Fontan patients who are more fit may have greater improvement in MIP, their benefit in terms of lowering VE/VCO2 appears to be smaller. Because both peak VO2 and VE/VCO2 slope show gradual declines with age in patients with congenital heart disease,22, 40 and because VE/VCO2 may be higher in non–extracardiac conduit types of Fontan circulation, we speculate that IMT could have an even greater benefit in older adults with either a lateral tunnel or atriopulmonary–type Fontan circulation.
We did not demonstrate any improvement in exercise duration, workload, or peak VO2 related to IMT. Peak VO2 is influenced by a number of potential factors that limit the ability of the circulation to increase cardiac output during exercise, including preload inadequacy, excessive afterload, systolic and diastolic dysfunction, and chronotropic incompetence. Although studies in adults with heart failure have demonstrated improvements in peak VO2 related to IMT, it is possible that our study was either underpowered to detect such a change and/or that the interaction between IMT and cardiac output during exercise is substantially different in Fontan and heart failure patients. The effects of combining IMT with aerobic and/or resistance training on peak VO2 and other exercise parameters will be a focus of future trials.
Several potential explanations are possible for why we did not demonstrate any improvement in lung function or pulmonary blood flow after IMT. First, baseline lung function may have been better preserved in our cohort than that reported in other Fontan patients. In a study of 52 adult atriopulmonary Fontan patients (mean age: 26.5 years [range: 18–45 years]; mean age at Fontan: 17.5 years [range: 5–36 years]), Fredriksen et al reported a percentage of predicted forced vital capacity (FVC) of 77% with much lower baseline exercise capacity (maximum VO2 of 15.9 mL/kg per minute) than in our study.41 Opotowsky et al, in a study of 260 young Fontan patients (aged 13.2±3.0 years, 57 extracardiac conduit, 152 lateral tunnel, and 43 atriopulmonary connection) reported a percentage of predicted FVC of 79±14.8%, with FVC correlating with peak VO2.42 Ohuchi et al, in a study of 101 younger Fontan patients (aged 13.6±4.6 years, 29 atriopulmonary type, and 72 lateral tunnel or extracardiac conduit) found lung function closer to that in our cohort (percentage of predicted FVC: 80±20%).26 Similarly, in a study of 33 Norwegian children with a Fontan circulation (mean age: 12.6 years), Matthews et al reported a percentage of predicted FVC of 86±17% and a percentage of predicted FEV1 of 94±19%.43 In our study of younger patients with an extracardiac conduit Fontan, higher baseline lung function and self‐reported levels of physical activity may have limited the scope for IMT to produce further improvements in lung function and pulmonary blood flow. Second, trials of IMT in adults with heart failure have not demonstrated improvement in either FEV1 or FVC, despite significant improvements in inspiratory muscle strength and exercise capacity,7, 32 suggesting that in heart failure, the beneficial effects of IMT may be independent of changes in lung function. Finally, although there was not even a trend toward improvement, we acknowledge that we did not power our study to detect a change in either lung function or pulmonary blood flow.
Potential Study Limitations
We used an IMT program that was home‐based and feasible for adolescent participants and young adults to undertake. Although the training was not fully supervised by the investigators, participants demonstrated their use of the IMT device at the end of each study visit, and parents were encouraged to supervise their child's training. Compliance was checked by diary entry and by weekly phone contact with participants. All of our participants were young and had a nonfenestrated extracardiac Fontan; therefore, our results cannot necessarily be extrapolated to the general Fontan population, in which a larger clinical trial may be required. The duration of IMT in our study was based on adult heart failure trials, which demonstrate the greatest magnitude of MIP improvement over the first 6 weeks and plateauing thereafter. We are thus unable to draw firm conclusions about the longer term effects of IMT or the effects of detraining after ceasing IMT.
Our study was powered to meet our primary outcomes of change in MIP and VE/VCO2 slope. Consequently, the study may have been insufficiently powered to detect differences after IMT in other exercise parameters. To limit the likelihood of type II statistical error, we did not undertake adjustment for multiple comparisons in the results from the exploratory exercise CMRI component of our study. Although we demonstrated interesting findings in relation to the effects of IMT on resting cardiac output and EF, we acknowledge that our results may be subject to type I statistical error and require confirmation in other trials of IMT in patients with a Fontan circulation.
In this short‐term study, we also were not able to examine the effects of IMT on potentially clinically meaningful end points, such as Fontan failure and mortality. Although maximal exertion was achieved in all CPET studies (respiratory exchange ratio ≥1.1, Borg score >15), the ventilatory compensation point was not clearly identifiable in all participants in the plots of VE versus VCO2; therefore, we chose to determine VE/VCO2 slope as occurring between 25% and 75% of maximum VCO2, as previously described,14, 44 rather than across the whole test. In those participants who had a clear ventilatory compensation point, we performed a correlation between the VE/VCO2 slope calculated by the 2 methods, demonstrating that the 2 values were highly correlated (R=0.894, P<0.01).
Conclusion
Six weeks of IMT is associated with improved inspiratory muscle strength, ventilatory efficiency of exercise, and resting cardiac output in young patients with a Fontan circulation. Although the mechanisms of these improvements and the effects of IMT combined with other forms of exercise prescription remain to be determined, our data suggest that IMT may be a simple beneficial addition to the current long‐term management of Fontan patients, potentially improving exercise capacity and late morbidity and mortality.
Sources of Funding
This work was supported by Heart Kids Australia (Grant‐in‐Aid 2013).
Disclosures
Dr d'Udekem receives consultancy fees from MSD and Actelion. All other authors have no relevant conflicts of interest to disclose.
Acknowledgments
Brendan Kennedy was involved in lung function testing. Anna Middleton was involved in exercise and lung function testing and supervision of inspiratory muscle training. Terri Walker was involved in collection of cardiac magnetic resonance imaging data. Danyi Zhu was involved in analysis of flow data during respiration.
(J Am Heart Assoc. 2017;6:e005750 DOI: 10.1161/JAHA.117.005750.)28862962
References
- 1. Iyengar AJ, Winlaw DS, Galati JC, Celermajer DS, Wheaton GR, Gentles TL, Grigg LE, Weintraub RG, Bullock A, Justo RN, d'Udekem Y. Trends in Fontan surgery and risk factors for early adverse outcomes after Fontan surgery: the Australia and New Zealand Fontan Registry experience. J Thorac Cardiovasc Surg. 2014;148:566–575. [DOI] [PubMed] [Google Scholar]
- 2. Jolley M, Colan SD, Rhodes J, DiNardo J. Fontan physiology revisited. Anesth Analg. 2015;121:172–182. [DOI] [PubMed] [Google Scholar]
- 3. Greutmann M, Le TL, Tobler D, Biaggi P, Oechslin EN, Silversides CK, Granton JT. Generalised muscle weakness in young adults with congenital heart disease. Heart. 2011;97:1164–1168. [DOI] [PubMed] [Google Scholar]
- 4. Fernandes SM, Alexander ME, Graham DA, Khairy P, Clair M, Rodriguez E, Pearson DD, Landzberg MJ, Rhodes J. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis. 2011;6:294–303. [DOI] [PubMed] [Google Scholar]
- 5. Hammond MD, Bauer KA, Sharp JT, Rocha RD. Respiratory muscle strength in congestive heart failure. Chest. 1990;98:1091–1094. [DOI] [PubMed] [Google Scholar]
- 6. Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. 2008;51:1663–1671. [DOI] [PubMed] [Google Scholar]
- 7. Dall'Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol. 2006;47:757–763. [DOI] [PubMed] [Google Scholar]
- 8. Johnson PH, Cowley AJ, Kinnear WJ. A randomized controlled trial of inspiratory muscle training in stable chronic heart failure. Eur Heart J. 1998;19:1249–1253. [DOI] [PubMed] [Google Scholar]
- 9. Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise‐induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151:242–250. [DOI] [PubMed] [Google Scholar]
- 10. Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole‐Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. [DOI] [PubMed] [Google Scholar]
- 11. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 12. American Thoracic Society/European Respiratory S . ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. [DOI] [PubMed] [Google Scholar]
- 13. Connuck DM. The role of exercise stress testing in pediatric patients with heart disease. Prog Pediatr Cardiol. 2005;20:45–52. [Google Scholar]
- 14. Ingle L, Goode K, Carroll S, Sloan R, Boyes C, Cleland JG, Clark AL. Prognostic value of the VE/VCO2 slope calculated from different time intervals in patients with suspected heart failure. Int J Cardiol. 2007;118:350–355. [DOI] [PubMed] [Google Scholar]
- 15. Wasserman K. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 16. Jones NL, Robertson DG, Kane JW. Difference between end‐tidal and arterial PCO2 in exercise. J Appl Physiol Respir Environ Exerc Physiol. 1979;47:954–960. [DOI] [PubMed] [Google Scholar]
- 17. American Thoracic S, American College of Chest P . ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 18. Grosse‐Wortmann L, Al‐Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI. Circ Cardiovasc Imaging. 2009;2:219–225. [DOI] [PubMed] [Google Scholar]
- 19. Smyth RJ, Chapman KR, Rebuck AS. Maximal inspiratory and expiratory pressures in adolescents. Normal values. Chest. 1984;86:568–572. [DOI] [PubMed] [Google Scholar]
- 20. Bosnak‐Guclu M, Arikan H, Savci S, Inal‐Ince D, Tulumen E, Aytemir K, Tokgozoglu L. Effects of inspiratory muscle training in patients with heart failure. Respir Med. 2011;105:1671–1681. [DOI] [PubMed] [Google Scholar]
- 21. Martinez A, Lisboa C, Jalil J, Munoz V, Diaz O, Casanegra P, Corbalan R, Vasquez AM, Leiva A. [Selective training of respiratory muscles in patients with chronic heart failure]. Rev Med Chil. 2001;129:133–139. [PubMed] [Google Scholar]
- 22. Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–1687. [DOI] [PubMed] [Google Scholar]
- 23. Smart NA, Giallauria F, Dieberg G. Efficacy of inspiratory muscle training in chronic heart failure patients: a systematic review and meta‐analysis. Int J Cardiol. 2013;167:1502–1507. [DOI] [PubMed] [Google Scholar]
- 24. Hsia TY, Khambadkone S, Redington AN, Migliavacca F, Deanfield JE, de Leval MR. Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. Circulation. 2000;102:III148–III153. [DOI] [PubMed] [Google Scholar]
- 25. Penny DJ, Redington AN. Doppler echocardiographic evaluation of pulmonary blood flow after the Fontan operation: the role of the lungs. Br Heart J. 1991;66:372–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohuchi H, Ohashi H, Takasugi H, Yamada O, Yagihara T, Echigo S. Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after Fontan operation. Pediatr Cardiol. 2004;25:513–521. [DOI] [PubMed] [Google Scholar]
- 27. Mello PR, Guerra GM, Borile S, Rondon MU, Alves MJ, Negrao CE, Dal Lago P, Mostarda C, Irigoyen MC, Consolim‐Colombo FM. Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. J Cardiopulm Rehabil Prev. 2012;32:255–261. [DOI] [PubMed] [Google Scholar]
- 28. Palau P, Dominguez E, Nunez E, Schmid JP, Vergara P, Ramon JM, Mascarell B, Sanchis J, Chorro FJ, Nunez J. Effects of inspiratory muscle training in patients with heart failure with preserved ejection fraction. Eur J Prev Cardiol. 2014;21:1465–1473. [DOI] [PubMed] [Google Scholar]
- 29. Stein R, Chiappa GR, Güths H, Dall'Ago P, Ribeiro JP. Inspiratory muscle training improves oxygen uptake efficiency slope in patients with chronic heart failure. J Cardiopulm Rehabil Prev. 2009;29:392–395. [DOI] [PubMed] [Google Scholar]
- 30. Cahalin LP, Arena R, Guazzi M, Myers J, Cipriano G, Chiappa G, Lavie CJ, Forman DE. Inspiratory muscle training in heart disease and heart failure: a review of the literature with a focus on method of training and outcomes. Expert Rev Cardiovasc Ther. 2013;11:161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montemezzo D, Fregonezi GA, Pereira DA, Britto RR, Reid WD. Influence of inspiratory muscle weakness on inspiratory muscle training responses in chronic heart failure patients: a systematic review and meta‐analysis. Arch Phys Med Rehabil. 2014;95:1398–1407. [DOI] [PubMed] [Google Scholar]
- 32. Laoutaris I, Dritsas A, Brown MD, Manginas A, Alivizatos PA, Cokkinos DV. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2004;11:489–496. [DOI] [PubMed] [Google Scholar]
- 33. Winkelmann ER, Chiappa GR, Lima CO, Viecili PR, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am Heart J. 2009;158:768.e1–768.e7. [DOI] [PubMed] [Google Scholar]
- 34. Witt JD, Guenette JA, Rupert JL, McKenzie DC, Sheel AW. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J Physiol. 2007;584:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poggio R, Arazi HC, Giorgi M, Miriuka SG. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: a meta‐analysis of the published literature. Am Heart J. 2010;160:1004–1014. [DOI] [PubMed] [Google Scholar]
- 36. Dimopoulos K, Okonko DO, Diller G‐P, Broberg CS, Salukhe TV, Babu‐Narayan SV, Li W, Uebing A, Bayne S, Wensel R, Piepoli MF, Poole‐Wilson PA, Francis DP, Gatzoulis MA. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation. 2006;113:2796–2802. [DOI] [PubMed] [Google Scholar]
- 37. Gewillig MH, Lundstrom UR, Bull C, Wyse RK, Deanfield JE. Exercise responses in patients with congenital heart disease after Fontan repair: patterns and determinants of performance. J Am Coll Cardiol. 1990;15:1424–1432. [DOI] [PubMed] [Google Scholar]
- 38. Cheung YF, Penny DJ, Redington AN. Serial assessment of left ventricular diastolic function after Fontan procedure. Heart. 2000;83:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Senzaki H, Masutani S, Ishido H, Taketazu M, Kobayashi T, Sasaki N, Asano H, Katogi T, Kyo S, Yokote Y. Cardiac rest and reserve function in patients with Fontan circulation. J Am Coll Cardiol. 2006;47:2528–2535. [DOI] [PubMed] [Google Scholar]
- 40. Muller J, Ewert P, Hager A. Only slow decline in exercise capacity in the natural history of patients with congenital heart disease: a longitudinal study in 522 patients. Eur J Prev Cardiol. 2015;22:113–118. [DOI] [PubMed] [Google Scholar]
- 41. Fredriksen PM, Therrien J, Veldtman G, Warsi MA, Liu P, Siu S, Williams W, Granton J, Webb G. Lung function and aerobic capacity in adult patients following modified Fontan procedure. Heart. 2001;85:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Opotowsky AR, Landzberg MJ, Earing MG, Wu FM, Triedman JK, Casey A, Ericson DA, Systrom D, Paridon SM, Rhodes J. Abnormal spirometry after the Fontan procedure is common and associated with impaired aerobic capacity. Am J Physiol Heart Circ Physiol. 2014;307:H110–H117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matthews IL, Fredriksen PM, Bjornstad PG, Thaulow E, Gronn M. Reduced pulmonary function in children with the Fontan circulation affects their exercise capacity. Cardiol Young. 2006;16:261–267. [DOI] [PubMed] [Google Scholar]
- 44. Arena R, Humphrey R, Peberdy MA. Prognostic ability of VE/VCO2 slope calculations using different exercise test time intervals in subjects with heart failure. Eur J Cardiovasc Prev Rehabil. 2003;10:463–468. [DOI] [PubMed] [Google Scholar]


