Abstract
Background
Several immune‐mediated diseases have been shown to be associated with an increased risk of cardiovascular disease. However, studies evaluating the association between inflammatory bowel disease and risk of cardiovascular disease reported inconsistent results. We assessed the association between inflammatory bowel disease and risk of ischemic heart disease in a meta‐analysis of cohort studies.
Methods and Results
We conducted a literature search of PubMed and Embase up to October 2016 to identify relevant studies. The summary relative risks were calculated using the random‐effects models. To explore the source of heterogeneity, we performed subgroup and sensitivity analysis. We included 10 cohort studies that satisfied our inclusion criteria. Patients with inflammatory bowel disease were associated with an increased risk of ischemic heart disease (relative risk: 1.244; 95% CI, 1.142–1.355). Considerable heterogeneity was observed. Crohn's disease showed a significantly increased risk of ischemic heart disease (relative risk=1.243; 95% CI, 1.042–1.482) and a positive association was also observed in ulcerative colitis (relative risk=1.206; 95% CI, 1.170–1.242).
Conclusions
Based on meta‐analysis of cohort studies, we found an increased risk of ischemic heart disease in patients with inflammatory bowel disease. Large long‐term prospective studies are warranted to confirm our results.
Keywords: Crohn's disease, inflammatory bowel disease, ischemic heart disease, ulcerative colitis
Subject Categories: Meta Analysis, Myocardial Infarction
Clinical Perspective
What Is New?
Our study suggested a modestly increased risk of ischemic heart disease (IHD) in patients with inflammatory bowel disease.
The increased risk of IHD was higher in women and in young patients.
There is insufficient data to evaluate whether treatment with anti‐inflammatory drugs modifies the risk of IHD in inflammatory bowel disease patients.
What Are the Clinical Implications?
Clinicians should be aware of the increased risk of IHD in inflammatory bowel disease patients.
Further studies are needed to explore the medical impact on the risk of IHD in inflammatory bowel disease patients.
Introduction
Ischemic heart diseases (IHD) are associated with substantial morbidity and mortality.1 Traditional cardiovascular risk factors such as hypertension, type 2 diabetes mellitus, family history of coronary artery disease, and obesity are well characterized. A number of epidemiologic studies indicated that inflammation was involved in the development of IHD.2, 3, 4, 5 Several immune‐mediated diseases have been linked to the risk of coronary artery disease, including rheumatoid arthritis and systemic lupus erythematosus.6, 7 However, whether patients with inflammatory bowel disease (IBD) are associated with an elevated risk of arterial thromboembolism and IHD is still debated.
The prevalence and incidence of inflammatory bowel disease has increased rapidly in recent years, especially in Asia. The IBDs, including Crohn's disease (CD) and ulcerative colitis (UC), are chronic inflammatory conditions of the gastrointestinal tract. The disease courses are characterized by episodes of flares and remission.8, 9, 10 The etiology of this disease remains largely unclear.11, 12 Studies evaluating the relation between IBD and risk of IHD reported conflicting results.13, 14 A nationwide population‐based cohort study from Denmark suggested a markedly elevated risk of IHD in patients with IBD.15 In contrast, a study using the General Practice Research Database reported no relation between myocardial infarction (MI) and CD in either unadjusted or adjusted analysis.16 Furthermore, a recent cross‐sectional study from the United States suggested a decreased risk of acute MI in the IBD population when compared with the general population.17
Two meta‐analyses have been conducted with inconsistent results.18, 19 Since the publication of a previous meta‐analysis, several studies have been published that have not been synthesized with existing data.20, 21, 22, 23 We aimed to examine the relation between IBD and risk of IHD by updating the previous meta‐analysis.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines.24
Literature Search
Literature search of PubMed and Embase databases up to October 2016 was conducted by 2 study investigators (W.F. and D.C.), independently. The following key words were used: “ischemic heart disease,” “coronary heart disease,” “coronary artery disease,” “cardiovascular disease,” “myocardial infarction,” “myocardial ischemia,” and “inflammatory bowel disease,” “ulcerative colitis,” “Crohn's disease.” Additionally, we manually searched the reference lists of reviews, previous meta‐analysis, and identified studies for potentially relevant studies.
Selection Criteria
Studies were considered if they satisfied the inclusion criteria as follows: (1) studies of observational cohort design; (2) reported the incidence of IHD; (3) the exposure was IBD (CD and/or UC); (4) the study provided the risk estimates such as relative risk (RR), hazard ratio (HR), or other measures with 95% CIs. Nested case‐control studies conducted within well defined cohorts were also included.
Studies were excluded as follows: (1) animal studies; (2) case report, review, letter to the editor, cross‐sectional and case‐control studies; (3) no sufficient data to calculate the relative risks; (4) if data were derived from the same cohort; and (5) abstract without sufficient data.
Data Extraction and Quality Assessment
The following information was extracted from the included articles: first author's name, year of publication, country, sex, age, and follow‐up duration, sample size, adjusted and unadjusted relative risk and adjustments. Information abstraction was independently collected by 2 observers (W.F. and G.C.). Any disagreements in the article identification were addressed by discussion and referring back to the original articles.
The methodological quality of included studies was evaluated independently by 2 authors (W.F. and D.C.) by using the Newcastle‐Ottawa Scale. The Newcastle‐Ottawa Scale criteria included the following aspects: selection (4 questions), comparability (2 questions), and outcome (3 questions). A total score of ≥7 was considered to indicate a high‐quality study.25, 26
Statistical Analysis
We extracted odds ratios, or RRs, or hazard ratios or incidence rate ratios. We also pooled unadjusted estimates if the studies reported or provided sufficient data to allow calculation. The summary RRs were calculated in both unadjusted and adjusted form. Analysis was done using the random‐effects models. We computed Q and I2 statistics to assess the heterogeneity. P<0.1 indicated heterogeneity. For I2 statistics, a value of >50% was considered significant heterogeneity. To explore the source of heterogeneity, we conducted subgroup analysis on the basis of age, sex, and follow‐up duration.27 Sensitivity analysis was undertaken to estimate the influence of each individual study in the main analysis by removing each study in turn and evaluating the remaining studies.28 We assessed publication bias by conducting Egger's test and Begger's funnel plot (P<0.05 was considered to represent publication bias). All the statistical analysis was conducted with STATA version 12.0. P<0.05 was considered to be statistically significant.
Results
Study Characteristics
We initially identified 978 studies from database search and 4 studies through manually searching reference lists of reviews. Through screening the titles and abstracts, 43 citations were included for the full‐text review. Finally, we included 10 cohort studies that satisfied our inclusion criteria15, 16, 20, 21, 22, 23, 29, 30, 31, 32 McAuliffe et al addressed the rate of myocardial infarction in patients with moderate to severe IBD when compared with mild IBD patients. Close et al conducted 2 cohort studies using the GPRD. A subset of patients with IHD following IBD and their matched controls (cohort B) was used for our analysis. (The details of study search and selection are shown in Figure 1).
Figure 1.
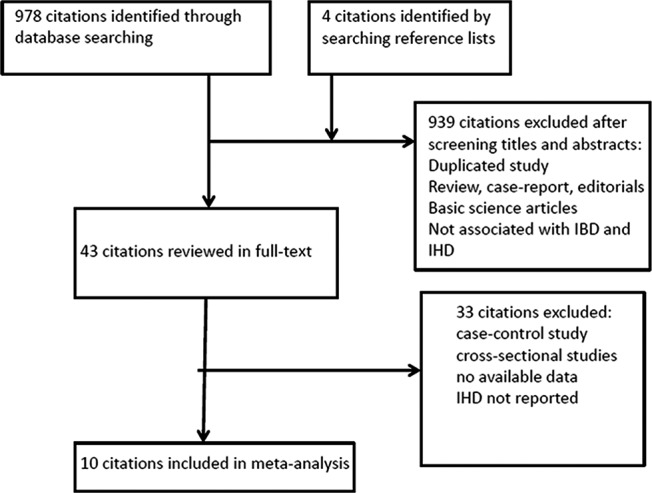
Flow diagram of study selection in the meta‐analysis. IBD indicates inflammatory bowel disease; IHD, ischemic heart disease.
All included studies were published recently; the publication year ranged from 2008 to 2015. Of the 10 studies, 3 were performed in the United Kingdom,16, 20, 23 1 from Taiwan,22 2 from the United States,21, 32 2 from Denmark,15, 30 1 from Canada,29 and 1 from Sweden.31 All identified studies were published in English. Characteristics of included studies are presented in Table 1.
Table 1.
Characteristics of the 10 Studies Included in the Meta‐Analysis
| Source | Country | Data Source | Study Period | No. of IBD Patients | Assessment of IBD | Adjusted RR (95% CI) | Adjustments |
|---|---|---|---|---|---|---|---|
| Zöller et al31 (2012) | Sweden | National Swedish data registers | 1964 to 2008 |
1304CD 2568UC |
ICD‐7 ICD‐8 ICD‐9 ICD‐10 |
CD: 1.06 (1.01–1.12) UC: 1.21 (1.17–1.26) |
Age, period, socioeconomic status, hospitalization of chronic lower respiratory diseases, obesity, alcohol, hypertension, diabetes mellitus, arterial flutter, heart failure, and renal disease. |
| Kristensen et al30 (2013) | Denmark | A nationwide Danish cohort | 1996 to 2009 |
20 795 IBD 199 978 controls |
ICD‐10 |
IBD: 1.17 (1.05–1.31) CD: 1.35 (1.03–1.77) UC: 1.17 (1.03–1.33) |
Age, sex, comorbidity, cardiovascular medication and socioeconomic status. |
| Rungoe et al15 (2013) | Denmark | A nationwide Danish cohort | 1997 to 2009 |
28 833 IBD 4 541 987 controls |
ICD‐8 ICD‐10 |
IBD: 1.22 (1.14–1.30) CD: 1.15 (0.99–1.35) UC: 1.22 (1.13–1.32) |
Age, sex, socioeconomic status and calendar year, use of antidiabetic agents, antihypertensive drugs, cholesterol‐lowering drugs, anticoagulant drugs and antiarrhythmic agents. |
| Yarur et al32 (2011) | United States | A retrospective longitude cohort | 1995 to 2009 |
356 IBD 712 controls |
ICD‐9‐CM and confirmed by review of medical chart | IBD: 4.08 (2.49–6.7) | Hypertension, diabetes mellitus, family history of CAD, dyslipidemia, CKD, and BMI>30. |
| Bernstein et al29 (2008) | Canada | Manitoba health administrative database | 1984 to 2003 |
8060IBD 80 489 controls |
ICD‐9‐CM |
IBD: 1.26 (1.11–1.44) CD: 1.26 (1.04–1.53) UC: 1.26 (1.05–1.51) |
NA. |
| Osterman et al16 (2011) | UK | GPRD (general practice research database) | 4.7 y |
9829 CD 92 987 controls 15 498 UC 144 605 controls |
ICD‐9 |
CD: 1.09 (0.89–1.34) UC: 1.11 (0.98–1.27) |
Age; sex; history of hypertension, diabetes mellitus, smoking status, hypercholesterolemia, BMI, aspirin use. |
| McAuliffe et al21 (2015) | United States | HIRD (healthcore integrated research database) | 2004 to 2011 |
14 733 moderate to severe IBD 29 841 mild IBD |
ICD‐9 | IBD: 1.40 (0.92–2.13) | Age and sex. |
| Dregan et al23 (2014) | UK | CPRD (clinical practice research datalink) | 2002 to 2013 |
7628 CD 12 203UC 373 851 controls |
Read medical codes |
CD: 1.10 (0.84–1.45) UC: 1.13 (0.95–1.35) |
Age, age squared, sex, blood pressure, cholesterol, BMI, smoking, alcohol, serum creatinine levels, glucocorticoids, antihypertensives, and statins. |
| Tsai et al22 (2014) | Taiwan | NHIRD (national health insurance research database) | 1998 to 2010 |
11 822 IBD 47 288 controls |
ICD‐9 |
IBD: 1.72 (1.53–1.94) CD: 1.82 (1.59–2.08) UC: 1.31 (1.11–1.55) |
Age, sex, hypertension, diabetes mellitus, hyperlipidemia, COPD, and heart failure. |
| Close et al20 (2015) | UK | GPRD | 1987 to 2009 |
3928CD 9932UC 61 882 non‐IBD |
ICD‐10 |
IBD: 1.30 (1.20–1.50) CD: 1.20 (1.0–1.60) UC: 1.30 (1.10–1.50) |
None. |
BMI indicates body mass index; CAD, coronary artery disease; CD, Crohn's disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease, IBD, inflammatory bowel disease; ICD, International Classification of Diseases; ICD‐CM, International Classification of Diseases‐Clinical Modification; NA, not available; RR, relative risk; UC, ulcerative colitis.
Using the Newcastle–Ottawa quality tool, most studies were considered high quality, with an average Newcastle–Ottawa Scale score of 8.2 (range, 6–9). Nine studies adjusted for potential confounders, Close et al provided unadjusted RR,20 and hence we included it in the meta‐analysis of crude risk estimates. Among included studies, most studies controlled for traditional cardiac risk factors, such as hypertension, type 2 diabetes mellitus, and hyperlipidemia. Baseline demographic data from the IBD patients included in these studies are presented in Table 2.
Table 2.
Baseline Characteristics of IBD Patients in the Included Studies
| Source | Sex (% Male) | Mean Age or Age Range | BMI (% Obesity) | Hypertension (%) | Diabetes Mellitus (%) | Smoking % Total IBD |
|---|---|---|---|---|---|---|
| Zöller et al31 (2012)a | 39.7% | NA | 0.4% | 3.2% | 8.8% | NA |
| Kristensen et al30 (2013) | 45.5% | 43.8 | NA | 3.1% | 1.8% | NA |
| Rungoe et al15 (2013) | 44% | 38.3 | NA | NA | 6% | NA |
| Yarur et al32 (2011) | 48.31% | 44.62 | 15.45% | 20.51% | 6.18% |
IBD: 30.3% CD: 30.1% UC: 30.6% |
| Bernstein et al29 (2008) | 45% |
CD: 36.51 UC: 42.42 |
NA | NA | NA | NA |
| Osterman et al16 (2011) |
CD: 41% UC: 48.4% |
CD: 44.2 UC: 50 |
CD: 8.2% UC: 11.1% |
CD: 14.5% UC: 18.5% |
CD: 1.9% UC: 3.6% |
CD: 37% UC: 23.2 |
| McAuliffe et al21 (2015) | 50.7% | 18 to 80 | NA | NA | NA | NA |
| Dregan et al23 (2014) |
CD: 44% UC: 51% |
CD: 42 UC: 47 |
CD: 12% UC: 14% |
CD: 17% UC: 23% |
CD: 2% UC: 3% |
CD: 25% UC: 13% |
| Tsai et al22 (2014) | 54.4% | 52.8 | NA | 17.5% | 11.1% | NA |
| Close et al20 (2015) |
CD: 42.9% UC: 47% |
CD: 39.9 UC: 46.2 |
CD: 16.9% UC: 19.9% |
NA |
CD: 3.5% UC: 5.4% |
CD: 39.9% UC: 31.9% |
BMI indicates body mass index; CD, Crohn's disease; IBD, inflammatory bowel disease; NA, not available; UC, ulcerative colitis.
Zöller's study reported the characteristics of patients with coronary heart disease.
Summary Estimates of Outcomes
Patients with IBD were associated with an increased risk of IHD (adjusted RR: 1.244; 95% CI, 1.142–1.355). Considerable heterogeneity was observed in the overall analysis (P for heterogeneity <0.001, I2=87.1%) (Figure 2).
Figure 2.
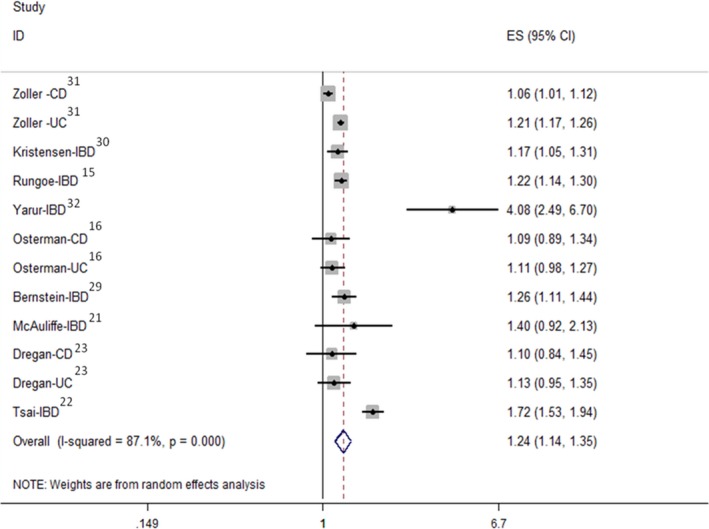
Summary of adjusted ORs evaluating the risk of ischemic heart disease in patients with IBD in all included studies. CD indicates Crohn's disease; ES, effect size; IBD, inflammatory bowel disease; ORs, odds ratios; UC, ulcerative colitis.
We also conducted a meta‐analysis of studies that reported risk of IHD by type of IBD. Patients with CD showed an elevated risk of IHD (RR: 1.243; 95% CI, 1.042–1.482) with substantial heterogeneity (P for heterogeneity <0.001, I2=89.5%), and a positive association was also observed in UC (RR: 1.206; 95% CI, 1.170–1.242), while heterogeneity was eliminated (P for heterogeneity=0.731, I2=0%) (Figure 3).
Figure 3.
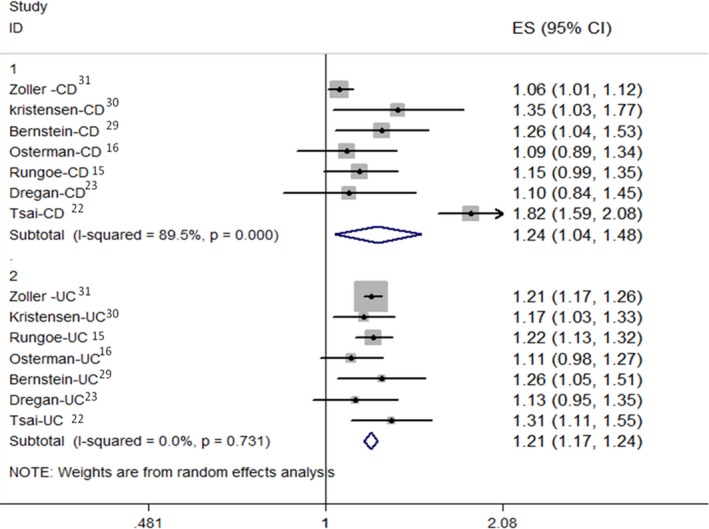
Summary of adjusted ORs evaluating the risk of ischemic heart disease in patients with CD and UC. CD indicates Crohn's disease; ES, effect size; ORs, odds ratios; UC, ulcerative colitis.
Subgroup Analysis
To investigate the source of heterogeneity, we conducted subgroup analysis on the basis of age, sex, and follow‐up duration (Table 3).
Table 3.
Summary Relative Risks and 95% CI of IHD in Patients With IBD, According to the Characteristics of Included Studies
| Relative Risk (95% CI) | Heterogeneity (P for Heterogeneity) | |
|---|---|---|
| All studies | 1.244 (1.142, 1.355) | 0.000 |
| Stratification by type of IBD | ||
| CD | 1.243 (1.042, 1.482) | 0.000 |
| UC | 1.206 (1.170, 1.242) | 0.731 |
| Stratification by age | ||
| Young | 1.354 (1.055, 1.738) | 0.001 |
| Old | 1.265 (1.130, 1.416) | 0.000 |
| Stratification by sex | ||
| Female | 1.351 (1.206, 1.513) | 0.000 |
| Male | 1.189 (1.028, 1.375) | 0.000 |
| Stratification by duration of follow‐up | ||
| <5 y | 1.567 (1.257, 1.954) | 0.000 |
| >5 y | 1.148 (0.991, 1.330) | 0.000 |
| Adjusted for obesity | ||
| Yes | 1.179 (1.057, 1.314) | 0.000 |
| No | 1.329 (1.147, 1.540) | 0.000 |
| Adjusted for smoking | ||
| Yes | 1.110 (1.017, 1.212) | 0.995 |
| No | 1.315 (1.179, 1.466) | 0.000 |
CD indicates Crohn's disease; IBD, inflammatory bowel disease; IHD, ischemic heart disease UC, ulcerative colitis.
Four studies provided risk estimates specific for male and female. In subgroup analysis for sex, the risk for IHD was more pronounced among females (adjusted RR: 1.351; 95% CI, 1.206–1.513) than among males (adjusted RR: 1.189; 95% CI, 1.028–1.375).
The age divided into different categories in included studies; we stratified patients as young group (<50 years old) and old group (>50 years old). Our analysis found that young patients had a higher risk of IHD (adjusted RR: 1.354; 95% CI, 1.055–1.738) compared with old patients (adjusted RR: 1.265; 95% CI, 1.130–1.416).
In the subgroup analysis on the basis of follow‐up duration, we observed that patients with short duration of follow‐up (<5 years) were related to an elevated risk of IHD (adjusted RR: 1.567; 95% CI, 1.257–1.954), whereas there was no such association among patients with long duration of follow‐up (>5 years) (adjusted RR: 1.148; 95% CI, 0.991–1.330).
We also assessed the impact of confounders on the risk estimates. The increased risk of IHD among IBD populations persisted, when restricting analysis to studies that controlled for obesity (adjusted RR: 1.179; 95% CI, 1.057, 1.314). Similar association was found between studies controlled for smoking and those not adjusted (RR: 1.110 and 1.315, 95% CI: 1.110 [1.017–1.212] versus 1.315 [1.179–1.466]).
Pooled Results of Crude Estimates
Three studies provided unadjusted RRs. The results were consistent when using these unadjusted risk estimates, with an elevated risk of IHD in the IBD populations (unadjusted RR: 1.350; 95% CI, 1.107–1.645).16, 20, 32
Sensitivity Analysis and Publication Bias
In the sensitivity analysis, when each study was removed in turn, the pooled RR of the rest of the studies did not alter significantly. McAuliffe et al evaluated the rates of MI in patients with moderate to severe IBD versus those with mild IBD. When this study was removed, the positive association between IBD and IHD remained (RR: 1.189; 95%CI, 1.083–1.31). The pooled RR ranged from 1.229 (95% CI, 1.105–1.367; when the study by Zöller et al was removed) to 1.170 (95% CI, 1.063–1.288; when the study by Tsai et al was removed). No evidence of publication bias was found. (Egger's test: P=0.285 and Begg's test: P=0.837).
Discussion
In this updated meta‐analysis of cohort studies, we observed an elevated risk of IHD among IBD populations in both adjusted and unadjusted analysis. The results were stable across stratified analysis. Consistent with previous studies, our analysis showed the risk for IHD was higher in females than in males and this risk is more pronounced in younger patients.
Our finding was supported by most studies but not all. Among these 10 studies, 7 studies suggested an elevated risk of IHD in patients with IBD.15, 20, 22, 29, 30, 31, 32 However, 3 studies did not find such association.16, 21, 23 Additionally, 2 cross‐sectional studies using a nationwide inpatient database demonstrated lower rates of acute MI among the IBD population than the general population.17, 33
The meta‐analysis conducted by Fumery et al suggested no association between IBD and risk of IHD (RR, 1.23; 95% CI, 0.94–1.62),19 whereas another meta‐analysis including 6 studies found a modestly increased risk of IHD among IBD populations (RR: 1.19; 95% CI, 1.08–1.31).18 The reason for the apparent discrepancy is that a cross‐sectional study that demonstrated reduced risk of IHD in the IBD population was included in the meta‐analysis by Fumery et al.33, 34 Since the previous meta‐analysis was published, another cross‐sectional study has been conducted and also observed lower rates of MI in the IBD population (RR: 0.51; 95% CI, 0.50–0.52).17 In our analysis, inclusion of these 2 cross‐sectional studies, which used the Nationwide Inpatient Sample database, significantly changed the result (adjusted RR: 1.161; 95% CI, 0.878–1.534).17, 33 Results from large long‐term prospective studies may provide precise information about the association of IBD and the risks of IHD.
IBD, including UC and CD, is a group of chronic relapsing inflammatory conditions of the gastrointestinal tract. Despite the shared clinical features, differences in the inflammatory burden, histology findings, and prognosis were observed in UC and CD.35 Considering its frequent systemic inflammation, patients with CD may have a higher risk of developing IHD. Interestingly, our analysis found similar risk of IHD in CD and UC. Low median age of participants, short study duration, and use of anti–tumor necrosis factor‐α (TNF) agents may contribute to this finding.30, 36 Patients with CD are more likely to receive anti‐TNF treatment and surgery than UC patients are. It would be anticipated that more potent therapy in CD patients reduced the inflammatory burden, and hence, significantly decreased the incidence of cardiovascular disease (CVD). Although similar risk was observed, the study found that a higher risk of cardiovascular death was observed in patients with CD than those with UC.30
It is well characterized that tobacco smoking is associated with an increased risk of cardiovascular events. Studies have shown that smoking exerts opposing effects in UC and CD. Smoking is thought to worsen the clinical course of CD and increase the need for steroid use and risk of early surgery. Interestingly, smoking has a protective effect on UC and thus less inflammatory burden.17, 34, 37 Despite the opposing effects of smoking in the inflammatory burden of CD and UC, our subgroup analysis found similar risk of IHD between studies adjusted for smoking and those not smoking. To better understand the association between IBD and cardiovascular events, further studies should evaluate the effect of smoking on this issue.
Disease severity has an important influence on the risk of CVD.38, 39 Among included studies, disease severity was prospectively assessed using frequency of hospitalization or initiation of biological and glucocorticoids treatment. Their observations suggested that the inflammatory activity was associated with an increased risk of CVD.22, 30, 31 A population‐based cohort study reported an elevated risk of MI in the flare and persistent IBD activity period, whereas no increased risk of MI was observed during the remission period.30 However, another study reported no significantly increased rates of MI in patients with moderate or severe IBD versus mild IBD. This study may have been limited by the potential for misclassification and lack of generalizability.21
Given the significant role of chronic inflammation in the risk of IHD, it may be expected that therapy for IBD might reduce the incidence of IHD by reducing the inflammatory burden.40 A nationwide cohort study suggested a markedly lower risk for IHD in users of 5‐aminosalicylates compared with nonusers. They also suggested slightly lower risk of IHD among users than nonusers of azathioprine or TNF‐α antagonist,15 whereas another study found that patients with IBD who used anti‐TNF‐α therapies have no decreased risk of MI compared with patients who had never received anti‐TNF‐α treatment.21 We could not perform the subgroup analysis to evaluate the effect of lowering inflammatory drugs on the IHD risk because of limited data in the primary studies. Moreover, most of the studies included in our analysis did not control for indication, that is, patients with severe IBD, with an increased risk of CVD, are more likely to use the anti‐inflammatory drugs.38, 41
Glucocorticoids are widely used in the treatment of IBD and other immune‐mediated diseases. It is well characterized that glucocorticoids are associated with an increased risk of hypertension, obesity, dyslipidemia, and insulin resistance, which are the risk factors for cardiovascular events. Epidemiological studies suggested that glucocorticoids exposure might increase the risk of CVD.42, 43, 44 A nationwide Danish cohort study found that patients requiring corticosteroids were at increased risk of IHD.15 However, another retrospective cohort study reported that exposure to corticosteroids was not associated with an increased risk of CVD in patients with IBD.32 This association warrants further investigation.
The association between IBD and risk of IHD is biologically plausible. However, the mechanism underlying this association has not been well elucidated.40 Chronic inflammation was thought to play a prominent role in the development of both IBD and IHD.45 Patients with IBD are more likely to develop early atherosclerosis compared with non‐IBD controls. Elevated levels of C‐reactive protein and pro‐inflammatory cytokines, including TNF‐α and interleukin‐6, were observed in both IBD and atherosclerosis. Increasing evidence suggested that activation of coagulation cascade and endothelial injury may also account for the risk of IHD in IBD populations.46 Other possible mechanisms included persistent thrombocytosis, arterial stiffening, coronary microcirculatory dysfunction, and increased carotid intima–media thickness.34, 38, 47, 48
Our analysis has several strengths. It has the advantage of large size, with nearly 155 970 cases of IBD. To explore the potential effect of covariates on the association, we simultaneously evaluated the unadjusted and adjusted risk estimates, and hence, were able to evaluate the potential influence of measured confounders. We also performed the subgroup and sensitivity analyses to investigate the heterogeneity.
The present study had limitations. First, it is impossible to adjust all the covariates; the unmeasured covariates may have an unclear impact on the relation between IBD and risk of IHD. Second, observational studies may suffer from various sources of bias. The cohort studies are susceptible to detection bias because patients with IBD had more frequent contact with professional observation and hence were more likely to be diagnosed with IHD. This bias may affect the true association. Third, great statistical and clinical heterogeneity was observed among studies, which was partly explained by age at diagnosis, disease severity, duration of follow‐up, and covariates. Given the significant heterogeneity, a random‐effects model was used to calculate summary risk estimates and subgroup analyses may offer some clues to explain this heterogeneity.
In conclusion, our meta‐analysis of cohort studies indicates an increased risk of IHD in patients with IBD, but our results should be interpreted with caution given the great heterogeneity among included studies. To confirm the findings of our analysis, large prospective studies well designed to evaluate the association of IBD and IHD are clearly warranted.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005892 DOI: 10.1161/JAHA.117.005892.)28768646
References
- 1. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the global burden of disease 2010 study. Circulation. 2014;129:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiao Z, Pei Z, Yuan M, Li X, Chen S, Xu L. Risk of stroke in patients with inflammatory bowel disease: a systematic review and meta‐analysis. J Stroke Cerebrovasc Dis. 2015;24:2774–2780. [DOI] [PubMed] [Google Scholar]
- 3. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 4. Hansson GK. Atherosclerosis–an immune disease: the Anitschkov lecture 2007. Atherosclerosis. 2009;202:2–10. [DOI] [PubMed] [Google Scholar]
- 5. Dagli N, Poyrazoglu OK, Dagli AF, Sahbaz F, Karaca I, Kobat MA, Bahcecioglu IH. Is inflammatory bowel disease a risk factor for early atherosclerosis? Angiology. 2010;61:198–204. [DOI] [PubMed] [Google Scholar]
- 6. Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first‐time acute myocardial infarction. Am J Cardiol. 2004;93:198–200. [DOI] [PubMed] [Google Scholar]
- 7. Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am J Med. 2008;121:S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV Jr., Tysk C, O'Morain C, Moum B, Colombel JF. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. [DOI] [PubMed] [Google Scholar]
- 9. Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol. 2013;5:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 11. Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–S9. [DOI] [PubMed] [Google Scholar]
- 12. Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- 13. Ha C, Magowan S, Accortt NA, Chen J, Stone CD. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol. 2009;104:1445–1451. [DOI] [PubMed] [Google Scholar]
- 14. Haapamaki J, Roine RP, Turunen U, Farkkila MA, Arkkila PE. Increased risk for coronary heart disease, asthma, and connective tissue diseases in inflammatory bowel disease. J Crohns Colitis. 2011;5:41–47. [DOI] [PubMed] [Google Scholar]
- 15. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62:689–694. [DOI] [PubMed] [Google Scholar]
- 16. Osterman MT, Yang YX, Brensinger C, Forde KA, Lichtenstein GR, Lewis JD. No increased risk of myocardial infarction among patients with ulcerative colitis or Crohn's disease. Clin Gastroenterol Hepatol. 2011;9:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes EL, Beery RM, Schulman AR, McCarthy EP, Korzenik JR, Winter RW. Hospitalizations for acute myocardial infarction are decreased among patients with inflammatory bowel disease using a nationwide inpatient database. Inflamm Bowel Dis. 2016;22:2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh S, Singh H, Loftus EV Jr., Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2014;12:382–393. e381: quiz e322. [DOI] [PubMed] [Google Scholar]
- 19. Fumery M, Xiaocang C, Dauchet L, Gower‐Rousseau C, Peyrin‐Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta‐analysis of observational studies. J Crohns Colitis. 2014;8:469–479. [DOI] [PubMed] [Google Scholar]
- 20. Close H, Mason JM, Wilson DW, Hungin APS, Jones R, Rubin G. Risk of ischaemic heart disease in patients with inflammatory bowel disease: cohort study using the general practice research database. PLoS ONE. 2015;10:e0139745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McAuliffe ME, Lanes S, Leach T, Parikh A, Faich G, Porter J, Holick C, Esposito D, Zhao Y, Fox I. Occurrence of adverse events among patients with inflammatory bowel disease in the healthcore integrated research database. Curr Med Res Opin. 2015;31:1655–1664. [DOI] [PubMed] [Google Scholar]
- 22. Tsai M‐S, Lin C‐L, Chen H‐P, Lee P‐H, Sung F‐C, Kao C‐H. Long‐term risk of acute coronary syndrome in patients with inflammatory bowel disease: a 13‐year nationwide cohort study in an Asian population. Inflamm Bowel Dis. 2014;20:502–507. [DOI] [PubMed] [Google Scholar]
- 23. Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population‐based cohort study. Circulation. 2014;130:837–844. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the prisma statement. Int J Surg. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 25. Wells G, Shea B, O'connell D, Peterson J, Welch V, Losos M, Tugwell P. 2011. The newcastle‐ottawa scale (nos) for assessing the quality of nonrandomised studies in meta‐analyses. Available at : http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Ottawa Hospital Research Institute. [Google Scholar]
- 26. Shen C, Chen F, Zhang Y, Guo Y, Ding M. Association between use of antiepileptic drugs and fracture risk: a systematic review and meta‐analysis. Bone. 2014;64:246–253. [DOI] [PubMed] [Google Scholar]
- 27. Singh S, Garg SK, Singh PP, Iyer PG, El‐Serag HB. Acid‐suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta‐analysis. Gut. 2014; 63:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta‐analysis of observational studies. Am J Gastroenterol 2011;106:1209–1218. quiz 1219. [DOI] [PubMed] [Google Scholar]
- 29. Bernstein CN, Wajda A, Blanchard JF. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: a population‐based study. Clin Gastroenterol Hepatol. 2008;6:41–45. [DOI] [PubMed] [Google Scholar]
- 30. Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp‐Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death–a Danish nationwide cohort study. PLoS ONE. 2013;8:e56944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zöller B, Li X, Sundquist J, Sundquist K. Risk of subsequent coronary heart disease in patients hospitalized for immune‐mediated diseases: a nationwide follow‐up study from Sweden. PLoS ONE. 2012;7:e33442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. [DOI] [PubMed] [Google Scholar]
- 33. Sridhar AR, Parasa S, Navaneethan U, Crowell MD, Olden K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2011;5:287–294. [DOI] [PubMed] [Google Scholar]
- 34. Rungoe C, Andersen NN, Jess T. Inflammatory bowel disease and risk of coronary heart disease. Trends Cardiovasc Med. 2015;25:699–704. [DOI] [PubMed] [Google Scholar]
- 35. Cleynen I, Boucher G, Jostins L, Schumm LP, Zeissig S, Ahmad T, Andersen V, Andrews JM, Annese V, Brand S. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Filimon AM, Negreanu L, Doca M, Ciobanu A, Preda CM, Vinereanu D. Cardiovascular involvement in inflammatory bowel disease: dangerous liaisons. World J Gastroenterol. 2015;21:9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ueno A, Jijon H, Traves S, Chan R, Ford K, Beck PL, Iacucci M, Gasia MF, Barkema HW, Panaccione R. Opposing effects of smoking in ulcerative colitis and Crohn's disease may be explained by differential effects on dendritic cells. Inflamm Bowel Dis. 2014;20:800–810. [DOI] [PubMed] [Google Scholar]
- 38. Singh S, Kullo IJ, Pardi DS, Loftus EV Jr.. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:26–35. [DOI] [PubMed] [Google Scholar]
- 39. Radovits BJ, Popa‐Diaconu DA, Popa C, Eijsbouts A, Laan RF, Van Riel PL, Fransen J. Disease activity as a risk factor for myocardial infarction in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1271–1276. [DOI] [PubMed] [Google Scholar]
- 40. Zuin M, Rigatelli G, del Favero G, Andreotti AN, Picariello C, Zuliani G, Carraro M, Galasso MP, Roncon L. Cardiovascular disease in patients with inflammatory bowel disease: an issue in no guidelines land. Int J Cardiol. 2016;222:984–985. [DOI] [PubMed] [Google Scholar]
- 41. Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, Quinn M, Choy E, Ostor AJ, Edwards CJ. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2011;50:518–531. [DOI] [PubMed] [Google Scholar]
- 42. Souverein P, Berard A, Van Staa T, Cooper C, Egberts A, Leufkens H, Walker B. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case–control study. Heart. 2004;90:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764–770. [DOI] [PubMed] [Google Scholar]
- 44. Davis JM, Maradit Kremers H, Crowson CS, Nicola PJ, Ballman KV, Therneau TM, Roger VL, Gabriel SE. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population‐based cohort study. Arthritis Rheumatol. 2007;56:820–830. [DOI] [PubMed] [Google Scholar]
- 45. Zezos P, Kouklakis G, Saibil F. Inflammatory bowel disease and thromboembolism. World J Gastroenterol. 2014;20:13863–13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schicho R, Marsche G, Storr M. Cardiovascular complications in inflammatory bowel disease. Curr Drug Targets. 2015;16:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caliskan Z, Keles N, Gokturk HS, Ozdil K, Aksu F, Ozturk O, Kahraman R, Kostek O, Tekin AS, Ozgur GT, Caliskan M. Is activation in inflammatory bowel diseases associated with further impairment of coronary microcirculation? Int J Cardiol. 2016;223:176–181. [DOI] [PubMed] [Google Scholar]
- 48. Thapa SD, Hadid H, Imam W, Hassan A, Usman M, Jafri SM, Schairer J. Persistent reactive thrombocytosis may increase the risk of coronary artery disease among inflammatory bowel disease patients. Dig Dis Sci. 2015;60:3062–3068. [DOI] [PubMed] [Google Scholar]


