Abstract
Background
Atrial fibrillation and heart failure are 2 of the most common diseases, yet ready means to identify individuals at risk are lacking. The 12‐lead ECG is one of the most accessible tests in medicine. Our objective was to determine whether a premature atrial contraction observed on a standard 12‐lead ECG would predict atrial fibrillation and mortality and whether a premature ventricular contraction would predict heart failure and mortality.
Methods and Results
We utilized the CHS (Cardiovascular Health) Study, which followed 5577 participants for a median of 12 years, as the primary cohort. The ARIC (Atherosclerosis Risk in Communities Study), the replication cohort, captured data from 15 792 participants over a median of 22 years. In the CHS, multivariable analyses revealed that a baseline 12‐lead ECG premature atrial contraction predicted a 60% increased risk of atrial fibrillation (hazard ratio, 1.6; 95% CI, 1.3–2.0; P<0.001) and a premature ventricular contraction predicted a 30% increased risk of heart failure (hazard ratio, 1.3; 95% CI, 1.0–1.6; P=0.021). In the negative control analyses, neither predicted incident myocardial infarction. A premature atrial contraction was associated with a 30% increased risk of death (hazard ratio, 1.3; 95% CI, 1.1–1.5; P=0.008) and a premature ventricular contraction was associated with a 20% increased risk of death (hazard ratio, 1.2; 95% CI, 1.0–1.3; P=0.044). Similarly statistically significant results for each analysis were also observed in ARIC.
Conclusions
Based on a single standard ECG, a premature atrial contraction predicted incident atrial fibrillation and death and a premature ventricular contraction predicted incident heart failure and death, suggesting that this commonly used test may predict future disease.
Keywords: atrial fibrillation, heart failure, mortality, premature atrial contractions, premature ventricular contractions
Subject Categories: Arrhythmias, Risk Factors, Heart Failure
Clinical Perspective
What Is New?
Among participants in 2 large, community‐based cohort studies, the presence of a premature atrial contraction detected from a single, standard 12‐lead ECG predicted a statistically significant elevated risk of both incident atrial fibrillation and death.
Similarly, a premature ventricular contraction from a single, standard ECG predicted statistically significant increased risks of incident heart failure, decline in left ventricular ejection fraction, and death.
What Are the Clinical Implications?
In combination with other risk markers, ectopy on a single, standard 12‐lead ECG may provide valuable information regarding an individual's cardiovascular risk and serve as a broadly available tool for the prediction and prevention of atrial fibrillation, heart failure, and death.
Introduction
Atrial fibrillation (AF) and heart failure (HF) remain 2 of the most common cardiovascular diseases, each affecting millions of individuals and contributing to significant morbidity and mortality,1 yet available treatments remain suboptimal.1, 2 Identification of high‐risk groups and strategies to facilitate primary prevention of AF and HF using widely accessible methods are critical.3, 4
Recent studies using wearable continuous Holter monitoring have shown that frequent premature atrial contractions (PACs) are associated with AF, stroke, and mortality.5, 6 Similarly, frequent premature ventricular contractions (PVCs) quantified by Holter studies are associated with incident HF and mortality.7, 8 Given evidence that successful ablation of PACs can eradicate AF9 and that successful ablation of frequent PVCs can normalize systolic dysfunction,10, 11 experts in the field now refer to ectopy as a cause of myopathy.12, 13, 14, 15, 16
The standard 12‐lead ECG is the most widely used cardiac diagnostic test and is less expensive and burdensome for patients than wearable recording devices. Previous studies investigating the association between ectopic beats and incident disease have relied on quantifying the number of ectopic beats using long‐term ECG monitoring.5, 6, 7, 17 We sought to investigate whether atrial or ventricular ectopy on a single, standard 10‐second 12‐lead ECG is associated with an increased risk of incident AF, HF, and mortality in 2 large, prospective cohort studies.
Methods
We hypothesized that PACs are associated with incident AF and that PVCs are associated with incident HF in 2 prospective cohort studies. For this study, based on current literature and biological plausibility, “myopathy” was considered to manifest as AF in the atria and as HF in the ventricles (and is meant to distinguish these clinically evident processes from diseases of the vasculature, heart valves, conduction system, or pericardium rather than to necessarily connote grossly evident structural changes).
Cardiovascular Health Study (Primary Cohort)
Detailed methods describing the CHS (Cardiovascular Health Study) have been previously published.18 Because previous publications from this cohort have revealed the burden of ectopy determined by continuously worn devices as predictors of incident AF and HF in the CHS,5, 7 we utilized the CHS as the primary cohort. Briefly, the CHS is a prospective, community‐based cohort study to investigate risk factors for stroke and coronary heart disease. Initially, from 1989 to 1990, 5201 adults aged 65 and older were recruited (original cohort) from the Medicare eligibility lists from 4 US communities: Forsyth County (North Carolina), Sacramento County (California), Washington County (Maryland), and Pittsburgh (Pennsylvania). From 1992 to 1993, an additional 687 black participants (new cohort) were recruited. Participants underwent a comprehensive baseline assessment, including a medical history, physical exam, laboratory testing, and resting 12‐lead ECG. Prescription medication use was collected from prescription bottles for any medication used within the past 2 weeks. Participants were followed semiannually with alternating telephone calls and clinic visits until 1999, with resting 12‐lead ECGs performed at every annual visit, after which semiannual telephone contact was continued. During follow‐up through 2008, medical records were obtained for all hospitalizations, and resting 12‐lead ECGs were performed at every annual visit through June 1999. Incident AF was ascertained from annual resting ECGs, hospital discharge diagnosis codes, and inpatient Medicare claims data. Incident HF and myocardial infarction (MI) were identified through self‐report, hospital diagnosis codes, and inpatient and outpatient medical records adjudicated by the CHS Cardiac Events Subcommittee.19 Death was ascertained by reviewing medical records, death certificates, autopsy exams, coroner's reports, obituaries, and search of the National Death Index.
ARIC (Replication Cohort)
Detailed methods regarding the ARIC (Atherosclerosis Risk in Communities) Study have been previously published.20 ARIC is a prospective, community‐based cohort study to investigate the etiology of atherosclerotic disease. Beginning in 1987, 15 792 adults aged 45 to 64 years were enrolled and underwent a comprehensive medical history, physical examination, laboratory testing, ultrasound imaging, and resting 12‐lead ECG. Participants were selected by probability sampling from 4 US communities: Forsyth County (North Carolina), Jackson (Mississippi), the northwestern suburbs of Minneapolis (Minnesota), and Washington County (Maryland). Only blacks were recruited at the Jackson location. Medication use was ascertained by self‐reported use within the past 2 weeks and review of medication bottles. Participants were contacted annually by telephone with repeated clinic visits every 3 years until 1998 followed by a fifth visit from 2011 to 2013. During follow‐up through 2011, incident AF was identified from study visit ECGs, hospital discharge diagnosis, and death certificates. Incident HF was diagnosed from hospital discharge diagnosis codes and death certificates. Incident MI was identified by self‐report, hospital discharge codes, discharge summaries, and evidence of MI on study visit ECGs. Death was ascertained through annual follow‐up, hospital surveillance, and death registries and verified by death certificate review.
For both studies: Participants provided written informed consent and the study protocols were approved by the institutional review board of each center.
ECG Assessment
In both studies, resting 10‐second 12‐lead ECGs were recorded in a uniform fashion in all participants using MAC PC ECG Machines (Marquette Electronics Inc, Milwaukee, WI). ECG abnormalities were classified per the Minnesota Code. We excluded participants with poor quality data, artificial pacing, wandering atrial pacemaker, or missing ECG data (n=298 in the CHS; n=263 in the ARIC).
Echocardiographic Evaluation
Echocardiographic assessment of participants in the CHS has been described previously.21 In brief, at baseline, M‐mode, qualitative 2‐dimensional echocardiography, and Doppler imaging were acquired from each participant in the original cohort recruited from 1989 to 1990. Measurements were recorded by field technicians at each study center on super‐VHS tape using Toshiba SSH‐160A echocardiographic machines (Toshiba America, Tustin, CA) equipped with 2.5‐ and 3.75‐MHz transducers. Imaging was performed at the highest MHz that provided adequate tissue penetration for 2‐dimensional imaging. Videotapes were mailed weekly to the Echocardiography Reading Center at the University of California, Irvine for interpretation. Left ventricular dysfunction was assessed from 2‐dimensional echocardiography using digitized images in which at least 80% of the endocardium was visualized. The average of 3 or more beats was utilized. Repeat echocardiograms were obtained 5 years after study entry. Quality control measures included standardized training of staff, assessment of inter‐reader agreement (94%) and intrareader agreement (92%) of paired studies, and quality‐control audits.22
Left ventricular ejection fractions (LVEFs) were subjectively scored as normal, abnormal, or borderline. A decline in LVEF was defined as a change on baseline echocardiogram of “normal” or “borderline” to “moderate” or “severe” systolic dysfunction on 5‐year echocardiograms. HF was considered to be associated with systolic dysfunction if the LVEF assessed as part of that participant's clinical care within 30 days of the event was <45%.
Statistical Analysis
Normally distributed continuous variables are presented as means±SD and were compared using t tests, and continuous variables with skewed distributions are presented as medians and interquartile ranges and were compared using the Wilcoxon rank‐sum test. Categorical variables were compared using the chi‐squared test.
The primary predictors were the presence of at least 1 PAC or at least 1 PVC on the baseline ECG. Statistically significant associations found in the primary cohort (CHS) were examined in the replication cohort (ARIC). Visually confirmed counts of PACs and PVCs were available in ARIC; we examined the number of each in separate categories as predictors of the primary outcomes as well as progressive numbers of each to determine whether a “dose response” was present. Participants with prevalent AF (n=177 in CHS; n=33 in ARIC) were excluded from the incident AF analyses, and those with prevalent HF (n=275 in CHS; n=730 in ARIC) were excluded from the incident HF analyses. To adjust for LVEF in CHS HF outcome analyses, those without baseline echocardiography were excluded (n=736); a sensitivity analysis including all CHS participants was conducted and failed to reveal any meaningful differences. In the ARIC study, PVC and diabetes mellitus did not satisfy the proportional hazards assumption; therefore, the hazard ratios (HRs) are reported for the first 10 years of follow‐up (comparable to length of follow‐up in the CHS) and the probability of incident HF was graphed using an adjusted Kaplan–Meier curve. In the PVC‐AF model in the ARIC, we stratified by sex, race, and time‐dependent HF to meet the proportional hazards assumption.
Incident events were analyzed using Cox proportional hazards models. Covariates were identified a priori based on biological plausibility and previous literature and included: age, sex, race (white/nonwhite), body mass index, hypertension, diabetes mellitus, coronary disease, MI, and study center (Table S1). Body mass index was dichotomized into normal (<25 kg/m2) and abnormal (≥25 kg/m2) to meet model linearity assumptions. Prevalent HF was included as a covariate in the multivariable model examining AF as the outcome, and AF, baseline LVEF (available in the CHS only), and beta‐blocker use were included in the HF outcome analysis. Plots for the probability of incident AF and HF stratified by the presence or absence of PACs or PVCs were based on multivariate Cox models with covariates centered at their mean values. The proportional hazards assumption was evaluated using the Schoenfeld test and graphically (log‐minus‐log survival plots).
We evaluated the association between ectopy and incident MI as a “negative control.” For these analyses, we excluded individuals with prevalent MI (n=562 in the CHS) and adjusted for age, sex, race, body mass index, hypertension, diabetes mellitus, coronary disease, AF, and study center.
To examine whether ectopy of a given chamber (atrial versus ventricular) led to a chamber‐specific myopathy, we examined whether PACs predicted HF and whether PVCs predicted AF. We adjusted for time‐dependent AF in the multivariate Cox model evaluating the PAC‐HF association and for time‐dependent HF in the PVC‐AF model.
For mediation analyses (to determine how much ectopy‐associated mortality was statistically explained by interim AF or HF), we calculated the “proportion of effect explained” as the percentage reduction in the adjusted regression coefficient after additional adjustment for the candidate mediator, with a 95% bias‐corrected percentile bootstrap confidence interval.
Data analyses were performed using Stata software (version 14; StataCorp LP, College Station, TX). We considered a 2‐tailed P<0.05 statistically significant.
Results
After exclusion criteria were applied in the CHS cohort, 5577 participants were available for the PAC‐AF analyses and 4710 for the PVC‐HF analyses. After exclusion criteria were applied in the ARIC cohort, data from 15 132 participants were available for the PAC‐AF analyses and from 14 200 for the PVC‐HF analyses.
Baseline Characteristics and Premature Beats
At study entry, 234 participants (4.2%) had a PAC on their standard baseline ECG in the CHS and 221 (1.5%) in the ARIC study. Participants with a PAC were older and, in the CHS, were more likely to be male and nonwhite compared with those without a PAC (Table 1). At least 1 PVC was detected on the standard ECG in 243 participants (5.2%) in the CHS and 252 (1.8%) in the ARIC study. Participants with a PVC were more likely to be older in both cohorts. In the CHS, participants with at least 1 PVC were also more likely to be male and have prevalent AF, whereas in ARIC they were more likely to have a history of hypertension, coronary artery disease, and MI.
Table 1.
Baseline Characteristics of Participants Stratified by Ectopy Status on 12‐Lead ECG
| Characteristic | CHS | ARIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAC Status | PVC Status | PAC Status | PVC Status | |||||||||
| No PAC (n=5343) | PAC (n=234) | P Value | No PVC (n=4467) | PVC (n=243) | P Value | No PAC (n=14 911) | PAC (n=221) | P Value | No PVC (n=13 948) | PVC (n=252) | P Value | |
| Median age, y | 71 (68–76) | 75 (71–80) | <0.001 | 71 (68–76) | 73 (70–78) | <0.001 | 54 (49–59) | 58 (51–62) | <0.001 | 54 (49–59) | 57 (52–61) | <0.001 |
| Male, % | 2207 (41) | 118 (50) | 0.006 | 1838 (41) | 149 (61) | <0.001 | 6658 (45) | 111 (50) | 0.12 | 6324 (46) | 120 (48) | 0.49 |
| White, % | 4479 (84) | 171 (73) | <0.001 | 4242 (95) | 224 (92) | 0.057 | 10 879 (74) | 158 (72) | 0.52 | 10 265 (74) | 173 (69) | 0.066 |
| Mean BMI, kg/m2 | 26.7±5 | 26.6±4 | 0.85 | 26.3±4 | 26.8±4 | 0.091 | 27.7±5 | 27.5±5 | 0.54 | 27.5±5 | 28.2±5 | 0.056 |
| Hypertension, % | 2361 (44) | 113 (48) | 0.22 | 1835 (41) | 113 (47) | 0.086 | 5121 (35) | 77 (35) | 0.89 | 4473 (32) | 114 (46) | <0.001 |
| Diabetes mellitus, % | 839 (16) | 43 (18) | 0.31 | 624 (14) | 41 (17) | 0.22 | 1765 (12) | 23 (11) | 0.48 | 1534 (11) | 35 (14) | 0.16 |
| CAD, % | 1018 (19) | 52 (22) | 0.23 | 764 (17) | 51 (21) | 0.12 | 715 (5) | 15 (7) | 0.18 | 548 (4) | 34 (14) | <0.001 |
| MI, % | 493 (9) | 28 (12) | 0.16 | 355 (8) | 28 (12) | 0.047 | 608 (4) | 11 (5) | 0.54 | 458 (3) | 31 (12) | <0.001 |
| Beta‐blocker use, % | 689 (13) | 20 (9) | 0.05 | 594 (13) | 28 (12) | 0.64 | 1417 (10) | 18 (8) | 0.49 | 1235 (9) | 26 (10) | 0.42 |
| Heart failure, % | 210 (4) | 8 (3) | 0.69 | ··· | ··· | ··· | 686 (5) | 15 (7) | 0.12 | ··· | ··· | ··· |
| AF, % | ··· | ··· | ··· | 10 (0.2) | 3 (1.2) | 0.003 | ··· | ··· | ··· | 0 | 0 | ··· |
Values are median (interquartile range), n (%), or mean±SD. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; CAD, coronary artery disease; CHS, Cardiovascular Health Study; MI, myocardial infarction; PAC, premature atrial contraction; PVC, premature ventricular contraction.
A PAC on the Standard ECG and Incident AF
In CHS, 1534 participants (27.5%) developed AF during a median follow‐up of 12 years. In ARIC, 2059 participants (13.6%) developed AF during a median follow‐up of 22 years. The presence of a PAC was associated with an ≈2‐fold increased risk of developing AF in the CHS (HR, 1.9; 95% CI, 1.5–2.4; P<0.001); this relationship was attenuated to a 60% increased risk after multivariate adjustment (HR, 1.6; 95% CI, 1.3–2.0; P<0.001; Figure 1). Similarly, in ARIC, a PAC was associated with greater than a 2‐fold increased risk of AF in both unadjusted (HR, 2.8; 95% CI, 2.2–3.6; P<0.001) and adjusted (HR, 2.4; 95% CI, 1.9–3.1; P<0.001; Figure 1) analyses. The association between a PAC and AF was comparable or greater in magnitude to well‐established AF risk factors (Figure 2). In ARIC, where a PAC count on each 12‐lead ECG was available, no dose‐response relationship was observed between the number of 12‐lead ECG PACs and AF risk (Table 2).
Figure 1.

Probability of incident outcomes by the presence of ectopy on the baseline standard ECG. Ectopy was defined as the presence of at least 1 PAC (in the AF analysis) and at least 1 PVC (in the HF analysis). Models were adjusted for age, sex, race, hypertension, diabetes mellitus, myocardial infarction, coronary artery disease, body mass index, study center, and heart failure (in AF outcome analyses) and AF, baseline left ventricular ejection fraction, and beta‐blocker use (in heart failure outcome analyses). AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities study; CHS, Cardiovascular Health Study; HF, heart failure; PAC, premature atrial contraction; PVC, premature ventricular contraction.
Figure 2.
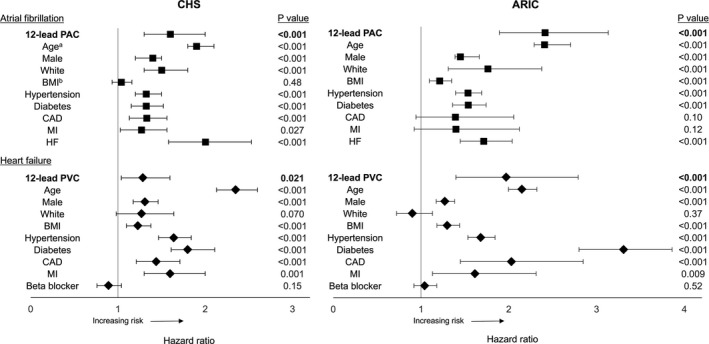
Multivariate‐adjusted hazard ratios for the association between baseline characteristics and incident outcomes. Models were adjusted for study center and atrial fibrillation (in HF analyses) and baseline ejection fraction (in HF analyses in CHS) in addition to the listed covariates. *For every 10‐year increase in age. †Risk in individuals with abnormal BMI (≥25 kg/m2) compared to normal (<25 kg/m2). Error bars represent 95% CI. ARIC indicates Atherosclerosis Risk in Communities study; BMI, body mass index; CAD, coronary artery disease; CHS, Cardiovascular Health Study; HF, heart failure; MI, myocardial infarction; PAC, premature atrial contraction; PVC, premature ventricular contraction.
Table 2.
Number of 12‐Lead ECG Ectopic Beats as a Predictor of Outcomes in ARIC
| Risk of Incident Atrial Fibrillation | ||||||
|---|---|---|---|---|---|---|
| Number of PACs on the Baseline 12‐Lead ECG | Unadjusted Analyses | Multivariable Adjusted Analysesa | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| 1 | 2.8 | 2.2 to 3.6 | <0.001 | 2.5 | 1.9 to 3.2 | <0.001 |
| 2 | 2.4 | 1.3 to 4.3 | 0.004 | 1.8 | 1.0 to 3.2 | 0.075 |
| >3 | 3.6 | 2.3 to 5.7 | <0.001 | 3.0 | 1.9 to 4.8 | <0.001 |
| Risk of Incident Heart Failure | ||||||
|---|---|---|---|---|---|---|
| Number of PVCs on the Baseline 12‐Lead ECG | Unadjusted Analyses | Multivariable Adjusted Analysesb | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| 1 | 2.5 | 2.0 to 3.1 | <0.001 | 1.8 | 1.5 to 2.3 | <0.001 |
| 2 | 2.3 | 1.5 to 3.6 | <0.001 | 1.6 | 1.0 to 2.5 | 0.032 |
| >3 | 2.6 | 1.8 to 3.7 | <0.001 | 2.0 | 1.4 to 2.9 | <0.001 |
HR indicates hazard ratio; PAC, premature atrial contraction; PVC, premature ventricular contraction.
Adjusted for age, sex, race, body mass index, hypertension, diabetes mellitus, myocardial infarction, coronary artery disease, heart failure, and study site.
Adjusted for age, sex, race, body mass index, hypertension, diabetes mellitus, myocardial infarction, coronary artery disease, atrial fibrillation, and study site.
A PVC on the Standard ECG and Incident HF
In the CHS cohort, 1391 participants (30%) developed HF, and, in ARIC, 2449 participants (17%) developed HF. In unadjusted analyses, the presence of a PVC on the standard 12‐lead ECG was associated with an almost 2‐fold elevated risk of incident HF in CHS (HR, 1.8; 95% CI, 1.5–2.2; P<0.001); after multivariable adjustment, there was a statistically significant 30% increased risk of incident HF (CHS: HR, 1.3; 95% CI, 1.0–1.6; P=0.021). Similar findings were then observed in the ARIC: A standard ECG PVC was associated with a 3‐fold increased risk before adjustment (HR, 3.1; 95% CI, 2.2–4.3; P<0.001) and a 2‐fold increased risk after multivariable adjustment (HR, 2.0; 95% CI, 1.4–2.8; P<0.001; Figure 2). In ARIC, where a PVC count on each 12‐lead ECG was available, no dose‐response relationship was observed between the number of 12‐lead ECG PVCs and HF risk (Table 2).
Of participants who developed HF in the CHS, 335 (24%) events were associated with systolic dysfunction, 443 (22%) with preserved systolic function, and 613 (44%) had an undetermined assessment of systolic function. The presence of a PVC was associated with incident HF with systolic dysfunction in both unadjusted (HR, 2.8; 95% CI, 2.0–4.0; P<0.001) and adjusted analyses (HR, 1.8; 95% CI, 1.2–2.5; P=0.003). However, a PVC was not associated with incident HF with preserved systolic function (unadjusted HR, 0.97; 95% CI, 0.57–1.6; P=0.01; adjusted HR, 0.68; 95% CI, 0.38–1.2; P=0.19).
A PVC on the Standard ECG and Echocardiographic Changes
Year 5 echocardiograms were available from 3102 CHS participants (66%), 66 (2%) of whom had a reduction in LVEF. Over 5 years, a PVC on the baseline ECG was associated with an ≈3‐fold greater odds of a reduction in LVEF in both unadjusted (odds ratio, 3.4; 95% CI, 1.6–7.0; P=0.001) and adjusted analyses (odds ratio, 2.8; 95% CI, 1.3–6.1; P=0.008).
Ectopy on the Standard ECG and Mortality
The presence of a PAC predicted death both in unadjusted and adjusted analyses in both cohorts (Table 3). Interim AF mediated approximately more than one third of the PAC‐mortality relationship. A PVC on the ECG similarly predicted mortality in both crude and multivariable analyses, again in both cohorts (Table 3); interim HF statistically explained more than half of the PVC‐mortality relationship in the CHS and ≈20% of the association in ARIC.
Table 3.
Baseline 12‐Lead ECG Ectopy and Overall Mortality
| Adjustment | CHS | ARIC | ||||||
|---|---|---|---|---|---|---|---|---|
| Point Estimate | 95% CI | P Value | Point Estimate | 95% CI | P Value | |||
| A 12‐lead PAC as a predictor of mortality | ||||||||
| None | HR | 1.6 | 1.4 to 1.9 | <0.001 | HR | 1.8 | 1.5 to 2.1 | <0.001 |
| a | HR | 1.3 | 1.1 to 1.5 | 0.008 | HR | 1.4 | 1.2 to 1.7 | <0.001 |
| Plusa interim AF | HR | 1.2 | 1.0 to 1.4 | 0.08 | HR | 1.2 | 1.0 to 1.5 | 0.06 |
| % mediated by AF | 35% | −0.2% to 124% | 42% | 16% to 111% | ||||
| A 12‐lead PVC as a predictor of mortality | ||||||||
| None | HR | 1.5 | 1.3 to 1.7 | <0.001 | HR | 2.3 | 1.9 to 2.7 | <0.001 |
| b | HR | 1.2 | 1.0 to 1.3 | 0.044 | HR | 1.7 | 1.4 to 2.0 | <0.001 |
| Plusb interim HF | HR | 1.1 | 0.9 to 1.2 | 0.42 | HR | 1.5 | 1.3 to 1.8 | <0.001 |
| % mediated by HF | 60% | −5% to 709% | 22% | 0% to 45% | ||||
White shaded areas include results regarding ectopy as a predictor of mortality. Gray shaded areas include mediation results (specifically how much the PAC‐mortality relationship is attenuated and therefore statistically explained by interim atrial fibrillation and how much the PVC‐mortality relationship is attenuated and therefore statistically explained by interim heart failure). AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; HF, heart failure; HR, hazard ratio; PAC, premature atrial contraction; PVC, premature ventricular contraction.
Adjusted for age, sex, race, study site, hypertension, diabetes mellitus, myocardial infarction, coronary artery disease, heart failure, and study site.
Adjusted for age, sex, race, study site, hypertension, diabetes mellitus, myocardial infarction, coronary artery disease, beta‐blocker, atrial fibrillation, and study site (in CHS, also adjusted for baseline left ventricular ejection fraction).
Negative Control: Ectopy and Incident MI
To support the hypothesis that ectopy is associated with myopathy rather than a nonspecific marker of cardiovascular disease in general, we assessed the relationship between 12‐lead ECG ectopy and incident MI. In the CHS, neither PACs (adjusted HR, 0.87; 95% CI, 0.61–1.3; P=0.45) nor PVCs predicted incident MI (adjusted HR, 1.2; 95% CI, 0.93–1.6; P=0.15).
Ectopy and “Cross‐Chamber” Myopathy
To assess the chamber specificity of the ectopy‐myopathy relationship, we investigated whether a PAC predicted HF and whether a PVC predicted AF. In the CHS, no statistically significant association between a PAC and incident HF was observed after multivariable adjustment (HR, 1.1; 95% CI, 0.87–1.5; P=0.36). However, the presence of a PVC was associated with an increased risk of AF in CHS (HR, 1.3; 95% CI, 1.1–1.6; P=0.007); this finding was replicated in ARIC (HR, 1.9; 95% CI, 1.2–3.0; P=0.005).
Discussion
In 2 large, prospective, community‐based cohorts, a single ECG with at least 1 PAC was associated with an increased risk of incident AF and death and at least 1 PVC predicted an increased risk of incident HF, a significant decline in LVEF, and death. Each was associated with a substantially increased risk of death, which, in both cases, appeared to be mediated, in large part, by the incident myopathy. We found no evidence of a dose‐response relationship between the number of ectopic beats and the outcomes studied, and even a single PAC predicted AF and a single PVC presaged subsequent HF.
AF is the most common sustained arrhythmia.1 Because of the lack of clear preventive strategies, increasing prevalence, and high morbidity and mortality associated with AF,1, 23 there is increasing interest in identifying clinically accessible and modifiable risk factors.3 PACs have been shown to be critical to AF pathogenesis and AF ablation is built on the premise that triggers or PACs arising in pulmonary veins initiate AF, suggesting that PACs may be a modifiable risk factor for AF in particular subsets of patients.9, 24 In addition, the presence of ectopy of the 12‐lead ECG may identify patients in clinical practice who would benefit from further studies, such as with continuous Holter monitoring, to further assess the burden of ectopy.
Even if no causal relationship between PACs and AF exists in many patients, the ability to identify PACs may yet be important for prediction.5 Specifically, useful predictors may help identify the best candidates for preventive therapies, and/or identify patients who will benefit the most from more‐careful monitoring (such as with continuous or implantable monitors) to determine appropriate candidacy for stroke prevention using anticoagulation.2, 25 If a causal relationship between either PACs and AF or PVCs and HF does indeed exist, ultimately these findings may also help inform trials investigating prophylactic interventions, such as catheter ablation, to prevent AF and/or HF or pharmacological therapy, such as with beta‐blockers, to prevent systolic dysfunction.
In addition to demonstrating replication in our replication analyses, our findings are consistent with previous literature describing a different population: In a secondary analysis of an important recent study of over 60 000 Japanese individuals participating in annual health exams, Murakoshi et al demonstrated a greater risk of AF in those with a supraventricular premature contraction on baseline, standard resting 12‐lead ECG.26 These findings complement ours well. Additionally, our analyses benefit from a longer length of follow‐up (11.7 and 22.4 years compared to 5.8 years), more‐thorough incident AF ascertainment and ascertainment of covariates, a more‐diverse population, and mediation analyses related to mortality.
Consistent with our hypothesis that ventricular ectopy would predict ventricular myopathy, we demonstrated that, after adjustment for potential confounders, ventricular ectopy on a single ECG was associated with a 30% to 100% increased risk of HF and a decline in LVEF. Given that its prevalence increases with an aging population, HF is quickly becoming a major public health burden, making its prevention as well as the identification of reversible causes and high‐risk groups important priorities.4
The HF events associated with the presence of 1 or more PVCs appears to be predominantly associated with systolic dysfunction. This is consistent with studies from multiple cardiac electrophysiology groups demonstrating that successful ablation of frequent PVCs can improve, and even normalize, reduced ejection fractions among patients with severe systolic dysfunction.10, 11, 27
Because 10‐second 12‐lead ECGs are nearly ubiquitous in clinical research studies and clinical practice and are remarkably superior tools to localize and characterize specific ectopic beats, we believe that the current findings substantially broaden opportunities for secondary analyses of pre‐existing data sets and ultimately for clinical practice in ways not feasible when considering Holter or other continuous monitoring data. The purpose of the current research is to enhance awareness of these readily accessible predictors, which can be used to investigate various prevention approaches, to study effect modification of different treatments efficacies (including by retrospective studies), and be considered by clinicians in their practice to identify patients who may benefit from additional monitoring (such as with Holter monitoring). As with PACs and AF, the importance of the relationship between PVCs and HF does not rely on causality. Even if simply a marker of future risk, some patients with PVCs may benefit from more‐frequent monitoring (such as with serial echocardiograms), or preventative therapies (eg, with beta‐blockers or radiofrequency ablation).28 However, additional research studies are needed to provide comparative outcomes in patients treated with these strategies.
Consistent with our hypothesis, neither PACs nor PVCs predicted incident MI, suggesting that ectopy is not simply a nonspecific marker of future adverse cardiovascular events in general. A PAC on a single, standard ECG failed to predict HF, suggesting that PACs predict outcomes specific to the atria. Of interest, however, a PVC predicted incident AF even after adjusting for HF that occurred in the interim. The mechanism underlying this observation remains unknown, but, if causal, may be related to repeated atrioventricular dyssynchrony or, if not causal, may represent a general propensity to cardiac arrhythmias.29
Importantly, a PAC on the standard 12‐lead ECG predicted overall mortality in both cohorts. Whereas this observation is consistent with previous data using Holter monitoring,5, 7 it underscores the impact that ectopy on a standard ECG might deliver. Of interest, much of the relationship between a PAC and subsequent death appeared to be mediated by incident AF. Similarly, a single, standard ECG‐detected PVC was associated with increased mortality, also explained, at least in part, by incident HF.
Our study has several limitations. Our study was not designed to compare the strength of effects between different risk factors or their prognostic values for our outcomes of interest. In addition, it is likely that ectopic beats are clinically meaningful predictors of disease for only a subset of patients who develop AF or HF. Our participants were at least 45 years old, and thus these findings may not be generalizable to younger populations. Echocardiograms were not performed at the baseline visit in the ARIC study; therefore, we cannot exclude residual confounding from undetected left ventricular dysfunction in the replication cohort. Importantly, however, whereas this might help elucidate mechanism and causality, it would not negate the ability of a PVC to predict HF events. We also recognize that the term “myopathy” may not be optimal in describing AF, which itself may not require structural changes; we chose this term to provide common language for both AF and HF and primarily to distinguish from other types of heart disease (such as coronary or valve disease, for example). Finally, although PACs have been shown to initiate AF and PVCs have been found to cause heart failure,9, 11 our study was not designed to prove causality. However, the absence of causality would not negate neither the validity nor the potential importance of the ability of these 12‐lead‐derived ectopic beats to predict each of our incident outcomes.
Conclusions
In 2 prospective, community‐based cohort studies, the presence of at least 1 PAC on a standard 12‐lead ECG was an important predictor of incident AF and at least 1 PVC was an important predictor of incident HF and a decline in LVEF. Either ECG finding alone predicted mortality, which was, at least partly, explained by the interim myopathy. Our findings suggest that even a single ectopic beat might provide valuable information regarding a patient's future. The vast numbers of ECGs already collected in previous research and the availability of this common test should facilitate the identification of optimal approaches to leverage these observations to enhance risk stratification and prevention strategies for AF and HF. Future studies are needed to characterize PAC and PVC morphology and coupling intervals to assess whether certain types of PACs or PVCs are responsible for the associations found, assess the use of 12‐lead ectopy to risk stratify patients and identify patients who may have improved outcomes with additional monitoring, and assess causality and/or the potential benefit of treatment of ectopic beats.
Sources of Funding
This work was made possible by the Joseph Drown Foundation (Marcus), R25MD00683, the National Institute on Minority Health and Health Disparities (Nguyen), and grant 16EIA26410001 from the American Heart Association (Alonso). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Grants and contracts for the Cardiovascular Health Study include: contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with an additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. These grants and contracts supported the data collection and management of CHS data. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). These grants and contracts supported the data collection and management of ARIC data.
Disclosures
Marcus receives research support from the NIH, PCORI, Medtronic, Pfizer, and Rhythm Diagnostic Systems and is a consultant and equity holder of InCarda. None of the other authors have any competing interests.
Supporting information
Table S1. Definitions of Baseline Comorbidities Used in Multivariate Models
(J Am Heart Assoc. 2017;6:e006028 DOI: 10.1161/JAHA.117.006028.)28775064
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; Members AATF . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd‐Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TSM, Van Wagoner DR, Waldo AL, George Wyse D. Prevention of atrial fibrillation: report from an NHLBI workshop. Circulation. 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y; American Heart Association Council on E, Prevention, American Heart Association Council on Clinical C, American Heart Association Council on Cardiovascular N, American Heart Association Council on High Blood Pressure R, Quality of C, Outcomes Research Interdisciplinary Working G, Functional G, Translational Biology Interdisciplinary Working G . Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. [DOI] [PubMed] [Google Scholar]
- 5. Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. [DOI] [PubMed] [Google Scholar]
- 7. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Frederiksen BS, Davanlou M, Hansen JF. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age >or=55 years. Am J Cardiol. 2006;97:1351–1357. [DOI] [PubMed] [Google Scholar]
- 9. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 10. Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, Yamamoto H, Origuchi H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–1265. [DOI] [PubMed] [Google Scholar]
- 11. Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, Jongnarangsin K, Marine JE, Chugh A, Pelosi F, Oral H, Morady F. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. [DOI] [PubMed] [Google Scholar]
- 12. Marcus GM, Dewland TA. Premature atrial contractions: a wolf in sheep's clothing? J Am Coll Cardiol. 2015;66:242–244. [DOI] [PubMed] [Google Scholar]
- 13. Dong M, Liu T, Li G. Atrial cardiomyopathy—a not yet classified cardiomyopathy? Int J Cardiol. 2011;151:394–396. [DOI] [PubMed] [Google Scholar]
- 14. Latchamsetty R, Bogun F. Premature ventricular complexes and premature ventricular complex induced cardiomyopathy. Curr Probl Cardiol. 2015;40:379–422. [DOI] [PubMed] [Google Scholar]
- 15. Sadron Blaye‐Felice M, Hamon D, Sacher F, Pascale P, Rollin A, Duparc A, Mondoly P, Derval N, Denis A, Cardin C, Hocini M, Jais P, Schlaepfer J, Bongard V, Carrie D, Galinier M, Pruvot E, Lellouche N, Haissaguerre M, Maury P. Premature ventricular contraction‐induced cardiomyopathy: related clinical and electrophysiologic parameters. Heart Rhythm. 2016;13:103–110. [DOI] [PubMed] [Google Scholar]
- 16. Lee AK, Deyell MW. Premature ventricular contraction‐induced cardiomyopathy. Curr Opin Cardiol. 2016;31:1–10. [DOI] [PubMed] [Google Scholar]
- 17. Agarwal SK, Simpson RJ Jr, Rautaharju P, Alonso A, Shahar E, Massing M, Saba S, Heiss G. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2012;109:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 19. Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, McKnight B, Ives D, Gottdiener JS, Kuller LH, Longstreth WT Jr. Study of cardiovascular health outcomes in the era of claims data: the Cardiovascular Health Study. Circulation. 2016;133:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 21. Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton‐Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free‐living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. [DOI] [PubMed] [Google Scholar]
- 22. Gardin JM, Siscovick D, Anton‐Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O'Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free‐living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. [DOI] [PubMed] [Google Scholar]
- 23. Marcus GM. Predicting incident atrial fibrillation: an important step toward primary prevention. Arch Intern Med. 2010;170:1874–1875. [DOI] [PubMed] [Google Scholar]
- 24. Kolb C, Nurnberger S, Ndrepepa G, Zrenner B, Schomig A, Schmitt C. Modes of initiation of paroxysmal atrial fibrillation from analysis of spontaneously occurring episodes using a 12‐lead Holter monitoring system. Am J Cardiol. 2001;88:853–857. [DOI] [PubMed] [Google Scholar]
- 25. Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:429S–456S. [DOI] [PubMed] [Google Scholar]
- 26. Murakoshi N, Xu D, Sairenchi T, Igarashi M, Irie F, Tomizawa T, Tada H, Sekiguchi Y, Yamagishi K, Iso H, Yamaguchi I, Ota H, Aonuma K. Prognostic impact of supraventricular premature complexes in community‐based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170–178. [DOI] [PubMed] [Google Scholar]
- 27. Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, Pelosi F Jr, Crawford T, Ebinger M, Oral H, Morady F, Bogun F. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. [DOI] [PubMed] [Google Scholar]
- 28. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL; American College of Cardiology/American Heart Association Task F, European Society of Cardiology Committee for Practice G, European Heart Rhythm A, Heart Rhythm S . ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. [DOI] [PubMed] [Google Scholar]
- 29. Hesselson AB, Parsonnet V, Bernstein AD, Bonavita GJ. Deleterious effects of long‐term single‐chamber ventricular pacing in patients with sick sinus syndrome: the hidden benefits of dual‐chamber pacing. J Am Coll Cardiol. 1992;19:1542–1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions of Baseline Comorbidities Used in Multivariate Models


