Abstract
Background
Coronary artery disease is a leading killer of women. Arterial stiffness predicts myocardial infarction, and postmenopausal women have lower arterial compliance (AC) than men. We hypothesized that lower AC would be associated with greater burden of coronary artery plaque and calcification, and that these associations would be stronger in women than men.
Methods and Results
We evaluated 3639 consecutive adults without coronary artery disease history who had coronary computed tomography between 2006 and 2014. Coronary artery calcification was calculated using the Agatston method. Plaque extent was assessed by the number of arterial segments with visible plaque divided by the number of visualized segments ×100 (percent plaque score). Indexed AC was calculated as stroke volume index/central pulse pressure. We used step‐wise multivariable linear regression to assess associations of log indexed AC with log (percent plaque score+1) and log (coronary artery calcification+1). Sex‐specific models were performed if the interaction sex×AC was significant. Mean age was 57±11 years, 53% were men, and 71% were hypertensive. Interaction term sex×AC was significant for both models (P=0.008 for percent plaque score and 0.022 for coronary artery calcification). Lower indexed AC was associated with higher percent plaque score and coronary artery calcification in women (β±SE: −0.231±0.113, P=0.042 and −0.334±0.166, P=0.044, respectively), but not in men (β±SE: −0.062±0.104, P=0.551 and 0.114±0.173, P=0.510, respectively).
Conclusions
Lower AC is associated with greater burden of coronary artery plaque and calcification in women, but not in men. Our findings highlight low AC as a correlate of more‐advanced coronary artery disease and as a potential link to the worse cardiovascular outcomes in women.
Keywords: atherosclerosis, computed tomography, hemodynamics, hypertension, women
Subject Categories: Coronary Circulation, Hemodynamics, Computerized Tomography (CT), Imaging, Women
Clinical Perspective
What Is New?
Lower arterial compliance is independently associated with greater burden of coronary artery plaque and calcification in women.
This finding was specific to women aged >50 years and not observed in younger women.
In men, the burden of coronary artery plaque and calcification was explained by conventional risk factors without additional contribution from arterial compliance.
What Are the Clinical Implications?
Based on our findings, lower arterial compliance, implying more‐advanced arterial aging, may be a correlate of more advanced coronary atherosclerosis in older women.
Because lower arterial compliance is known to predict adverse cardiovascular events, our findings may help explain the poorer outcomes observed in women with coronary artery disease, which is amenable to testing in future prospective studies.
Our findings also highlight low arterial compliance as a potential target for prevention of coronary artery disease in women.
Introduction
Ischemic heart disease is the principal cause of death worldwide,1 accounting for 17.3 million deaths per year, a number that is expected to rise to >23.6 million by 2030.2 In women, the risk of coronary artery disease (CAD) is often underestimated, attributed to the misperception that females are protected against cardiovascular disease. However, it is now well established that CAD is the most common cause of death of both men and women in North America.3 Importantly, over the past 2 decades the prevalence of myocardial infarction (MI) has increased among middle‐aged women, while declining among similarly aged men.4 This ominous temporal trend in women calls for an intensification of efforts aimed at screening and treating vascular risk factors, as well as early identification of individuals at higher risk of events, with the goal of improving overall vascular health and preventing adverse outcomes.
When addressing cardiovascular risk in women, hypertension has a pivotal role. Incidence of hypertension in women has been increasing at a rate double that of men5 and is more prevalent in older (aged ≥65 years) women than men.3 Impairment in the conduit artery's pressure‐buffering capacity caused by low arterial compliance (AC) leads to increases in pulse pressure (PP), which not only contributes to hypertension, but also impairs coronary perfusion,6 promotes end‐organ damage,7 and predicts MI and death.8, 9 In the search for causes of cardiovascular diseases in women, we have previously shown that proximal aortic stiffness and lower AC are associated with several cardiac derangements such as left ventricular (LV) diastolic dysfunction,10 altered ventricular‐arterial coupling,10 LV concentric remodeling,11 and worse coronary artery microvascular function12 in women, but not in men. These findings support a notion that abnormalities in AC may play a greater role in the pathogenesis of cardiovascular diseases in women than in men.13 Therefore, better understanding the contribution of low AC to CAD may help elucidate the basis for the worse CAD outcomes in women. Thus, we hypothesized that lower AC, leading to greater pulsatile arterial load, would be associated with a greater burden of coronary artery plaque and calcification, and that these associations would be stronger in women than men.
Methods
Study Participants
All participants provided informed consent to have their clinical and demographic data recorded as part of the University of Ottawa Heart Institute's (Ottawa, Ontario, Canada) cardiac computed tomography (CT) database, and to have these data used for research purposes. We obtained additional approval by the Research Ethics Board to perform the present analyses. We then accessed the University of Ottawa Heart Institute's cardiac CT database, identifying subjects who had undergone a coronary CT angiography between 2006 and 2014. Because we needed LV volumes to estimate AC (see below), we restricted our search to subjects with a retrospectively ECG‐gated CT. Subjects without a past history of MI, unstable angina, and surgical or percutaneous coronary revascularization were eligible (n=3639). A flow chart depicting the process for participant selection is presented in Figure 1.
Figure 1.
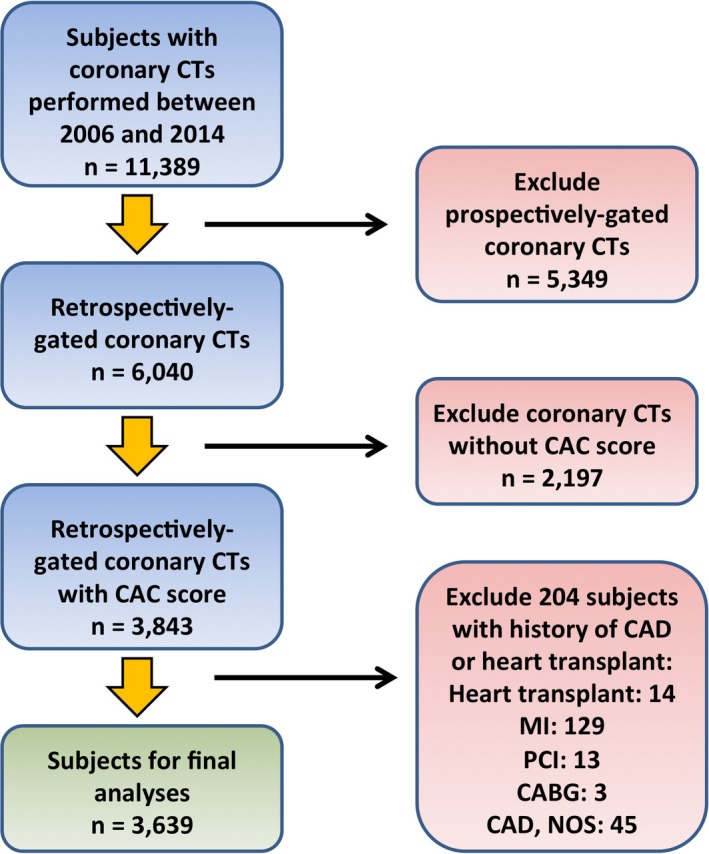
Flow chart for selection of participants for the present analyses. CABG indicates coronary artery bypass grafting; CAC, coronary artery calcium; CAD, coronary artery disease; CT, computerized tomography; MI, myocardial infarction; NOS, not otherwise specified; PCI, percutaneous coronary intervention.
Medical History, Vital Signs, and Anthropometric Measures
Data on age, past medical history, cardiovascular risk factors, medications, laboratory tests, anthropometrics, and vital signs were retrieved from the database. Body mass index was calculated in kg/m2. Body surface area was calculated using the Gehan method.14 Resting blood pressure and heart rate were measured with an automated blood pressure cuff while participants were at rest, before administration of beta‐blockers and nitrates. Mean arterial pressure was calculated as 2/3 diastolic blood pressure+1/3 systolic blood pressure. PP was calculated as systolic blood pressure−diastolic blood pressure. Hypertension was defined based on previous diagnosis of hypertension and/or current treatment with medications for hypertension. Diabetes mellitus was defined as previous diagnosis of diabetes mellitus and/or treatment with insulin or oral hypoglycemic agents. Smoking was defined as previous or current history of tobacco use.
Assessment of Coronary Artery Plaque and Calcification
After baseline documentation of vital signs, metoprolol (oral and/or intravenous) was administered to achieve a target heart rate ≤65 beats/min, and nitroglycerin 0.8 mg was administered sublingually. A biphasic timing bolus was used (Visipaque 320 or Omnipaque 350; GE Healthcare, Princeton, NJ).15 Final images were acquired with a triphasic intravenous contrast administration protocol (100% contrast, 40%/60% contrast/saline, and saline [40 cc]).15, 16 Retrospective ECG‐gated data sets were acquired with the GE Volume CT (GE, Milwaukee, WI) with 64×0.625 mm slice collimation and gantry rotation of 350 ms (mA=400–800, kV=120) using ECG‐gated X‐ray tube modulation. Pitch (0.16–0.24) was individualized based on the patient's heart rate. For coronary assessment, CT data sets were reconstructed with an increment of 0.4 mm using the cardiac phase(s) with the least cardiac motion.
Images were processed using the GE Advantage Volume Share Workstation (GE Healthcare) and visually interpreted by expert observers blinded to clinical data. A 17‐segment model of coronary arteries17 was used for the evaluation. Total burden of coronary artery plaque (calcified and soft) was quantified by total plaque score (TPS), a validated measure of coronary plaque burden known to be an independent predictor of death and nonfatal MI.15 TPS was determined by adding the number of coronary segments with calcific, noncalcific, or mixed plaque. Because not all coronary segments may be visualized, TPS was normalized for the total number of coronary segments observed (percent plaque score; PPS). In addition, we measured the burden of coronary artery calcification (CAC) using the Agatston method.18 These analyses were performed by staff physicians reading CT. For TPS and PPS, intra‐ (n=20) and interobserver (n=2564) concordance were excellent, with intraclass correlation coefficients (95% confidence interval) of 0.936 (0.846–0.974) and 0.904 (0.897–0.911) for intra‐ and interobserver analyses for TPS, respectively, and 0.937 (0.849–0.975) and 0.901 (0.893–0.908) for intra‐ and interobserver analyses for PPS, respectively. There were no changes in CT equipment, protocol, or postprocessing software for the duration of the study.
Assessment of AC
AC is the change in arterial blood volume attributed to a given change in pulsatile blood pressure. The stroke volume/PP ratio is a good estimate of AC (r=0.98; P<0.0001),19 which predicts cardiovascular events and mortality;20 thus, it was used as a measure of AC in this study.
Cardiac volumes were estimated with cardiac CT by staff physicians. This technique has been shown to closely correlate with invasive ventriculography21 and cardiac magnetic resonance imaging.22 Retrospectively ECG‐gated CT angiography images were reconstructed at 10 phase (5–95%) with 1.25 mm slice collimation with an increment of 0.625 mm. Using a semiautomated algorithm, LV volumes were measured at end‐diastole and end‐systole excluding the papillary muscles, and stroke volume was calculated as their difference. Previously published data from our laboratory confirm excellent inter‐ and intraobserver variability for measurement of LV volumes.23, 24
Because of the expected smaller ventricular volumes in women, stroke volume was indexed to body surface area. PP was estimated from brachial blood pressure immediately before the imaging study as outlined above. Indexed AC (iAC) was then estimated as indexed stroke volume/PP. This linear indexation is justified because AC has been shown to have approximately linear relationships to body surface area.25
Statistical Analyses
Continuous variables were reported as mean±SD. Differences between sexes were compared with a t test for normally distributed variables and Wilcoxon rank‐sum test for skewed variables. Categorical variables were reported as number and percentage, and differences between sexes were assessed with the chi‐square test.
Unadjusted associations of iAC with PPS and CAC in the whole group, men, and women were assessed with Spearman correlation coefficients.
Because of skewness, iAC was natural log‐transformed, and PPS and CAC were natural log‐transformed after adding 1. Forward step‐wise multivariable linear regression models were used to assess associations of iAC with the burden of coronary artery plaque and calcification in the whole cohort, using criteria of P≤0.10 to enter and ≤0.05 to stay in the models. Log iAC was forced into models when it did not meet step‐wise criteria. Covariates considered in the models were: age, sex, serum creatinine, heart rate, mean arterial pressure, history of hypertension, diabetes mellitus, dyslipidemia, and smoking, and use of antihypertensives, statins, and aspirin. To determine whether sex modified the associations of iAC with PPS and CAC, we tested the interaction term sex×iAC before the step‐wise procedure. If significant, we repeated the models after stratifying for sex. To better delineate the specific subgroup of women in whom low iAC may be detrimental to coronary health, in exploratory analyses we repeated the multivariable linear regression models in women after stratifying for age groups (younger: ≤50 years; older: >50 years).
All analyses were performed using JMP software (v. 11.2; SAS Institute Inc, Cary, NC). A 2‐tailed P≤0.05 was considered statistically significant.
Results
A summary of participant characteristics is shown in Table 1, and characteristics of women based on age group are shown in Table 2. Fifty‐three percent of the 3639 participants were men. Mean±SD age was 55.1±10.8 years in men and 59.3±10.7 years in women (P<0.001). Seventy‐one percent of participants were hypertensive, and women were more likely to be hypertensive and have isolated systolic hypertension than men. iAC was lower in women than men, even after adjusting for the aforementioned covariates (P<0.0001), confirming greater pulsatile arterial load in women. Men were more likely to have coronary artery plaque and calcification than women, and the burden of total and calcified coronary artery plaque was higher in men.
Table 1.
Participant Characteristics
| Variable | All (n=3639) | Women (n=1723) | Men (n=1916) |
|---|---|---|---|
| Demographics, medications, and vital signs | |||
| Age, y | 57.2±11.0 | 59.3±10.7 | 55.1±10.8b |
| Age >50 y, n (%) | 2636 (72) | 1376 (80) | 1260 (66)b |
| Height, cm | 167.6±10.4 | 159.7±7.0 | 174.7±7.5b |
| Weight, kg | 83.5±19.3 | 76.4±18.1 | 89.9±18.1b |
| Body surface area, m2 | 2.2±0.3 | 2.1±0.3 | 2.3±0.3 b |
| BMI, kg/m2 | 29.7±6.1 | 29.9±6.9 | 29.4±5.4a |
| eGFR, mL/min per 1.73 m2 | 80.9±19.2 | 77.9±19.2 | 83.7±18.8b |
| Heart rate, bpm | 67±12 | 69±11 | 66±11b |
| SBP, mm Hg | 137±20 | 138±21 | 136±18a |
| DBP, mm Hg | 80±10 | 78±10 | 80±10b |
| MAP, mm Hg | 99±11 | 98±12 | 99±11 |
| Brachial pulse pressure, mm Hg | 55±13 | 57±14 | 53±12b |
| Central pulse pressure, mm Hg | 51±8 | 53±9 | 50±7b |
| Hypertension, n (%) | 2571 (71) | 1290 (75) | 1281 (67)b |
| Diabetes mellitus, n (%) | 555 (15) | 254 (15) | 301 (16) |
| Dyslipidemia, n (%) | 2082 (57) | 965 (56) | 1117 (58) |
| Smoking, n (%) | 1965 (54) | 888 (52) | 1077 (56)a |
| Aspirin use, n (%) | 2104 (58) | 969 (56) | 1135 (59) |
| Statin use, n (%) | 1692 (46) | 793 (46) | 899 (47) |
| Antihypertensive use, n (%) | 2313 (64) | 1194 (69) | 1119 (58)b |
| CT data | |||
| Indications for scan | |||
| Chest pain, n (%) | 2166 (60) | 1164 (68) | 1002 (52)b |
| Dyspnea, n (%) | 657 (18) | 379 (22) | 278 (15)b |
| Equivocal stress test result, n (%) | 921 (25) | 471 (27) | 450 (24)a |
| Atrial fibrillation, n (%) | 89 (2) | 26 (2) | 63 (3)a |
| LV EDV, mL | 137.3±39.5 | 121.0±29.8 | 151.9±41.4b |
| LV ESV, mL | 50.6±28.5 | 42.3±22.8 | 58.0±30.9b |
| LV SV, mL | 86.7±21.5 | 78.7±17.1 | 93.9±22.6b |
| LV SVi, mL/m2 | 39.7±8.7 | 38.6±7.9 | 40.5±9.2b |
| LV EF, % | 64±10 | 66±10 | 63±9b |
| CAP present, n (%) | 2583 (71) | 1063 (62) | 1520 (79)b |
| Total plaque score, n | 3.8±3.9 | 2.6±3.2 | 4.8±4.2b |
| Coronary segments visualized, n | 15.9±1.4 | 15.8±1.5 | 16.1±1.2b |
| PPS, % | 23.7±24.6 | 16.9±20.8 | 29.8±26.0b |
| CAC present, n (%) | 2181 (60) | 878 (51) | 1303 (68)b |
| CAC score | 161.2±367.3 | 93.6±213.9 | 222.1±455.3b |
| AC, mL/mm Hg | 1.7±.0.5 | 1.5±0.4 | 1.9±0.5b |
| iAC, (mL/m2)/mm Hg | 0.76±0.25 | 0.73±0.24 | 0.79±0.24b |
AC indicates arterial compliance; BMI, body mass index; CT, computed tomography; CAC, coronary artery calcium; CAP, coronary artery plaque; DBP, diastolic blood pressure; EDV, end‐diastolic volume; eGFR, estimated glomerular filtration rate; ESV, end‐systolic volume; iAC, indexed arterial compliance; LV, left ventricle; MACE, composite end point of death, nonfatal myocardial infarction or coronary revascularization; MAP, mean arterial pressure; PPS, percent plaque score; SBP, systolic blood pressure; SV, stroke volume; SVi, stroke volume index.
P≤0.01.
P<0.001 compared with women.
Table 2.
Women's Characteristics Based on Age Group
| Variable | ≤50 Years Old (n=346) | >50 Years Old (n=1378) | P Value |
|---|---|---|---|
| Demographics, medications, and vital signs | |||
| Age, y | 44.3±5.6 | 63.2±8.1 | <0.0001 |
| Height, cm | 162.22±7.2 | 159.2±6.82 | <0.0001 |
| Weight, kg | 80.9±21.6 | 75.2±16.9 | <0.0001 |
| Body surface area, m2 | 2.1±0.3 | 2.0±0.2 | <0.0001 |
| BMI, kg/m2 | 30.8±8.1 | 29.7±6.4 | 0.02 |
| eGFR, mL/min per 1.73 m2 | 86.7±20.5 | 75.7±18.3 | <0.0001 |
| Heart rate, bpm | 71±12 | 68±11 | 0.0003 |
| SBP, mm Hg | 130±19 | 140±21 | <0.0001 |
| DBP, mm Hg | 79±11 | 78±10 | 0.14 |
| MAP, mm Hg | 96±13 | 99±12 | 0.0002 |
| Brachial pulse pressure, mm Hg | 51±11 | 58±15 | <0.0001 |
| Central pulse pressure, mm Hg | 45±6 | 55±8 | <0.0001 |
| Hypertension, n (%) | 134 (39) | 844 (61) | <0.0001 |
| Diabetes mellitus, n (%) | 37 (11) | 221 (16) | 0.01 |
| Dyslipidemia, n (%) | 126 (36) | 849 (62) | <0.0001 |
| Smoking, n (%) | 194 (56) | 693 (50) | 0.05 |
| Aspirin use, n (%) | 137 (40) | 830 (60) | <0.0001 |
| Statin use, n (%) | 94 (27) | 705 (51) | <0.0001 |
| Antihypertensive use, n (%) | 178 (51) | 1023 (74) | <0.0001 |
| CT data | |||
| LV EDV, mL | 129.7±31.5 | 118.6±28.8 | <0.0001 |
| LV ESV, mL | 46.9±22.3 | 41.1±22.8 | <0.0001 |
| LV SV, mL | 82.8±19.2 | 77.6±16.3 | <0.0001 |
| LV SVi, mL/m2 | 39.3±9.0 | 38.4±7.6 | 0.07 |
| LV EF, % | 64±9 | 66±10 | 0.0003 |
| CAP present, n (%) | 102 (29) | 962 (70) | <0.0001 |
| Total plaque score, n | 0.9±2.1 | 3.0±3.3 | <0.0001 |
| Coronary segments visualized, n | 15.8±1.5 | 15.8±1.5 | 0.85 |
| PPS, % | 5.9±13.2 | 19.6±21.5 | <0.0001 |
| CAC present, n (%) | 59 (17) | 820 (60) | <0.0001 |
| CAC score | 15.3±58.7 | 113.5±233.4 | <0.0001 |
| AC, mL/mm Hg | 1.7±0.6 | 1.4±0.4 | <0.0001 |
| iAC, (mL/m2)/mm Hg | 0.8±0.3 | 0.7±0.2 | <0.0001 |
AC indicates arterial compliance; BMI, body mass index; CT, computed tomography; CAC, coronary artery calcium; CAP, coronary artery plaque; DBP, diastolic blood pressure; EDV, end‐diastolic volume; eGFR, estimated glomerular filtration rate; ESV, end‐systolic volume; iAC, indexed arterial compliance; LV, left ventricle; MAP, mean arterial pressure; PPS, percent plaque score; SBP, systolic blood pressure; SV, stroke volume; SVi, stroke volume index.
Associations of AC With Coronary Artery Plaque and Calcification
Spearman correlation coefficients are depicted in Table 3, showing that while the associations of iAC with PPS and CAC were modest, they were twice as strong in women than in men. Results of the final multivariable linear regression models in the whole cohort are depicted in Tables 4 and 5 for PPS and CAC, respectively. Log iAC was not independently associated with tter log(PPS+1) or log(CAC+1) in the whole cohort.
Table 3.
Spearman Correlation Coefficients for the Associations of Arterial Compliance With Coronary Artery Plaque and Calcification Burden
| All (n=3639) Spearman's ρ (P Value) | Women (n=1723) Spearman's ρ (P Value) | Men (n=1916) Spearman's ρ (P Value) | ||||
|---|---|---|---|---|---|---|
| CAC | PPS, % | CAC | PPS, % | CAC | PPS, % | |
| iAC, (mL/m2)/mm Hg | −0.10 (<0.0001) | −0.09 (<0.0001) | −0.20 (<0.0001) | −0.20 (<0.0001) | −0.09 (<0.0001) | −0.10 (<0.0001) |
CAC indicates coronary artery calcium; iAC, indexed arterial compliance; PPS, percent plaque score.
Table 4.
Multivariable Linear Regression Models for Coronary Artery Plaque Burden (log [PPS+1])
| Variable | All (β±SE) | Women (β±SE) | Men (β±SE) |
|---|---|---|---|
| Age, y | 0.058±0.002, P<0.0001 | 0.051±0.004, P<0.0001 | 0.064±0.003, P<0.0001 |
| Female sex | −0.512±0.024, P<0.0001 | ··· | ··· |
| eGFR, mL/min per 1.73 m2 | ··· | −0.004±0.002, P=0.0228 | 0.004±0.002, P=0.0285 |
| Heart rate, bpm | −0.006±0.002, P=0.0059 | −0.006±0.003, P=0.0384 | ··· |
| MAP, mm Hg | 0.005±0.002, P=0.0089 | ··· | 0.005±0.002, P=0.0685 |
| Hypertension | 0.134±0.025, P<0.0001 | 0.177±0.036, P<0.0001 | 0.114±0.0320, P=0.0004 |
| Hyperlipidemia | ··· | ··· | ··· |
| Diabetes mellitus | 0.113±0.033, P=0.0006 | 0.145±0.050, P=0.0040 | ··· |
| Smoking | 0.206±0.023, P<0.0001 | 0.227±0.034, P<0.0001 | 0.173±0.030, P<0.0001 |
| Antihypertensive use | ··· | ··· | ··· |
| Apirin use | ··· | ··· | 0.073±0.033, P=0.0255 |
| Statin use | 0.206±0.024, P<0.0001 | 0.182±0.036, P<0.0001 | 0.206±0.032, P<0.0001 |
| Log iAC | −0.105±0.077, P=0.1747 | −0.231±0.113, P=0.0418 | −0.062±0.104, P=0.5512 |
Results of step‐wise multivariable linear regression models with forward elimination, with criteria of P≤0.10 to enter and ≤0.05 to stay in the models. eGFR indicates estimated glomerular filtration rate; iAC, indexed arterial compliance; MAP, mean arterial pressure; PPS, percent plaque score.
Table 5.
Multivariable Linear Regression Models for Coronary Artery Calcification Burden (log [CAC+1])
| Variable | All (β±SE) | Women (β±SE) | Men (β±SE) |
|---|---|---|---|
| Age, y | 0.102±0.003, P<0.0001 | 0.089±0.005, P<0.0001 | 0.120±0.005, P<0.0001 |
| eGFR, mL/min per 1.73 m2 | −0.746±0.037, P<0.0001 | ··· | 0.006±0.003, P=0.0227 |
| Heart rate, bpm | ··· | ··· | ··· |
| MAP, mm Hg | ··· | ··· | 0.007±0.005, P=0.0960 |
| Hypertension | 0.008±0.003, P=0.0120 | 0.267±0.054, P<0.0001 | 0.155±0.053, P=0.0034 |
| Hyperlipidemia | 0.138±0.044, P=0.0016 | ··· | ··· |
| Diabetes mellitus | ··· | 0.140±0.073, P=0.0572 | ··· |
| Smoking | 0.140±0.051, P=0.0064 | 0.373±0.050, P<0.0001 | 0.291±0.050, P<0.0001 |
| Antihypertensive use | 0.342±0.035, P<0.0001 | ··· | ··· |
| Apirin use | 0.084±0.044, P=0.0576 | −0.101±0.053, P=0.0618 | ··· |
| Statin use | −0.746±0.037, P<0.0001 | 0.284±0.056, P<0.0001 | 0.368±0.051, P<0.0001 |
| Log iAC | −0.109±0.121, P=0.3700 | −0.334±0.166, P=0.0442 | −0.114±0.173, P=0.5103 |
Results of step‐wise multivariable linear regression models with forward elimination, with criteria of P≤0.10 to enter and ≤0.05 to stay in the models. CAC indicates coronary artery calcium; eGFR, estimated glomerular filtration rate; iAC, indexed arterial compliance; MAP, mean arterial pressure; SV, stroke volume; SVi, stroke volume index.
The interaction term sex×iAC was significant in the prediction of coronary artery plaque and calcification (P=0.0077 and 0.0222, respectively). Thus, we repeated regression models after stratifying for sex (Tables 4 and 5 for PPS and CAC, respectively). We found that lower iAC was associated with greater burden of coronary artery plaque and calcification in women, but not in men. Illustrative cases of women with high and low AC are shown in Figure 2. In addition, because AC is the main determinant of pressure pulsatility, we repeated the models while using brachial or central PP as independent variables instead of log iAC, and inferences remained unchanged (analyses not shown).
Figure 2.
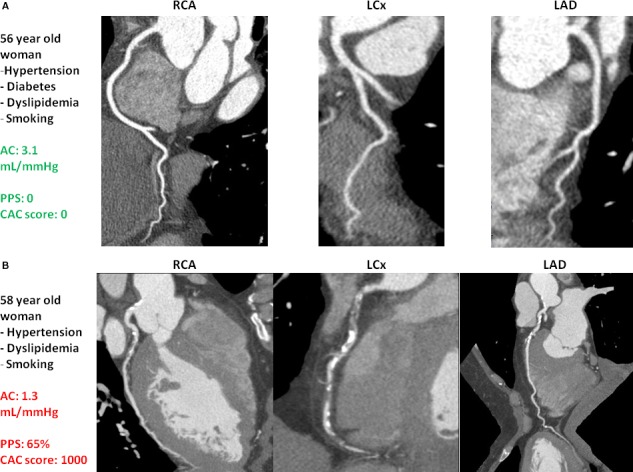
Coronary computed tomography angiography findings of women with high and low arterial compliance. A, 56‐year‐old woman with hypertension, diabetes mellitus, dyslipidemia, and smoking. She had high arterial compliance (3.1 mL/mm Hg) and no visible coronary artery plaque or calcification. B, 58‐year‐old woman with hypertension, dyslipidemia, and smoking. She had low arterial compliance (1.3 mL/mm Hg) and a high burden of coronary plaque and calcification. AC indicates arterial compliance; CAC, coronary artery calcium; LAD, left anterior descending; LCx, left circumflex artery; PPS, percent plaque score; RCA, right coronary artery.
In analyses stratified by age groups, we found that the associations of low log iAC with higher log (PPS+1; β±SE: −0.303±0.133; P=0.0227) and with higher log(CAC+1; −0.446±0.201; P=0.0268) were only present in older women, but not in younger women (P=0.9934 and 0.5974, respectively). However, because women younger than age 50 were under‐represented (20% of all women), we cannot exclude the possibility that the sample size of younger women was underpowered to evaluate this association.
Discussion
In a large cohort of subjects without a history of MI or coronary revascularization, we have demonstrated that iAC is lower in women than men and associated with greater burden of coronary artery plaque and calcification in women (specifically older women), but not men. To the best of our knowledge, this is the first study to report sex‐specific associations of AC with burden of epicardial CAD, contributing to our understanding of the pathogenesis of CAD in middle‐aged women. Our findings highlight low AC as a correlate of more advanced CAD in women, which may have a potential link to women's worse cardiovascular outcomes.
Women are more likely to die after an MI than men.3 Although the higher mortality in women has conventionally been attributed to women's older age at presentation, these sex differences are present throughout age groups.3 In addition, women of all age groups are also more likely than men to develop heart failure and have a recurrent fatal or nonfatal MI.3 These ominous statistics call for an intensification of efforts aimed at understanding potential mechanisms contributing to CAD in women, in order to identify women at risk, treat contributing factors, and improve their health and outcomes. One of the most powerful predictors of coronary events and mortality from CAD is the burden of coronary artery plaque26 and calcification.27 Thus, identifying correlates of more extensive CAD may give insights into the worse CAD outcomes in women. This motivated the present investigations.
In the search for factors correlating to more advanced CAD in women, early or accelerated vascular aging is an attractive candidate. Normal aging is characterized by gradual changes in conduit artery structure and function, leading to lower AC with aging. However, in some individuals, the arterial aging process occurs more rapidly, reflecting their lifetime's exposures to adverse lifestyle habits and cardiovascular risk factors.28 This accelerated vascular aging, marked by arterial stiffening/lower AC, culminates in cardiovascular manifestations such as hypertension,29 MI,8, 9 stroke,30 and vascular cognitive dysfunction.31 In addition, greater arterial stiffness independently predicts all‐cause and cardiovascular mortality.8, 9 Importantly, emerging evidence suggests that arterial aging may be more exacerbated in postmenopausal women than men, given that older women exhibit greater proximal aortic stiffness and lower AC than men of similar age,10, 11, 12, 32 which is not fully explained by smaller aortic size in women.33 These data led to our hypothesis that lower AC would correlate with greater burden of epicardial CAD in women. Indeed, we found that lower iAC was associated with greater burden of coronary artery plaque and calcification in women independently of conventional cardiovascular risk factors. However, in men, CAD burden could be explained by risk factors alone, without additional contribution from AC.
Mechanisms linking lower AC to CAD burden in women may include excessive pressure pusatility delivered to the coronary arterial bed. Increased pressure pulsatility is detrimental to arterial health,34 especially at the level of the end organs, causing greater arterial stretch, shear stress, and alterations in arterial structure,35 therefore promoting endothelial dysfunction.36 These changes, in turn, initiate a cascade that culminates in athero‐ and arteriosclerosis. In a healthy arterial system, compliant conduit arteries buffer the pressure pulsatility generated by the beating left ventricle, thereby containing the amount of pulsatile pressure delivered to the end organs.34 However, as AC decreases, so does this pressure‐buffering mechanism, which results in greater pulsatile pressure delivered to the distal circulation. This sequence of decreased AC, excessive pressure pulsatility penetrating the coronary circulation, and arterial damage and endothelial dysfunction may explain the association of lower AC with coronary atherosclerotic burden observed in older women in our study. Indeed, human studies confirm that excessive pressure pulsatility is detrimental to coronary health. In subjects with MI, increased coronary artery pulsatility is associated with coronary systolic flow reversal and greater risk of death and reinfarction.37
Taking our findings into account, it is possible that lower AC, increasing pressure pulsatility delivered to the coronary bed, may detrimentally affect coronary artery health in older women. Alternatively, it is also possible that these changes occur in parallel, with systemic abnormalities associated with hypertension and other comorbidities simultaneously impairing coronary artery structure and AC in women. Our cross‐sectional study cannot infer causality or temporality of the associations. However, it provides a novel description and a solid foundation on which to base future mechanistic and prospective studies.
Limitations
The main strengths of our study are the large, well‐characterized cohort and the novelty of our findings. However, our study has limitations: (1) Participants included in this cohort represent a referral population. However, the prevalence of hypertension, dyslipidemia, and diabetes mellitus in our cohort is very similar to the prevalence of these conditions in similarly aged adults from the general population,38 which increases the external validity of our study; (2) direct measurement of arterial stiffness and load measures would be the ideal assessment. Because this was a retrospective study, these measures were not available as part of the CT database. However, our estimate of pulsatile AC is well validated19 and predictive of cardiovascular events and death,20 providing robust insights into pulsatile arterial load and its clinical consequences; (3) to precisely assess coronary plaque burden, estimation of coronary plaque volume with angiography and intravascular ultrasound would be ideal. However, coronary CT has been shown to correlate well with findings from coronary intravascular ultrasound,39 and we used well‐established measures of coronary plaque and calcification burden that have been prospectively validated and known to confer prognostic information, allowing robust inferences of plaque burden; (4) the cross‐sectional nature of our study prevents inferences about causality and temporality of the associations found; and (5) in this retrospective study, we did not have data on menopausal status among women. However, we attempted to overcome this by exploring associations of AC with plaque burden based on age younger or older than 50 years, given that mean age at menopause is 51 years.
Conclusions
Our study showed that lower AC is associated with the presence of isolated systolic hypertension and with more‐extensive CAD in older women, whereas in men isolated systolic hypertension and CAD burden are independent of AC. Our novel findings are relevant for highlighting pathological mechanisms that may contribute to advanced CAD and its adverse outcomes in women and motivate further prospective studies aimed at targeting arterial dysfunction in the early detection, prevention, and treatment of CAD in women.
Sources of Funding
Coutinho is a Clinician Scientist supported by a Heart and Stroke Foundation of Ontario Clinician Scientist Phase I Award. Chow holds the Saul and Edna Goldfarb Chair in Cardiac Imaging Research.
Disclosures
Chow receives research support from GE Healthcare and educational support from TeraRecon Inc. None of the other authors have any conflicts of interest to disclose.
(J Am Heart Assoc. 2017;6:e006079 DOI: 10.1161/JAHA.117.006079.)28862955
References
- 1. World Health Organization . World Health Organization Fact Sheet—The Top 10 Causes of Death. 2012. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed Aug 8, 2017.
- 2. World Health Organization . Global Status Report on Noncommunicable Diseases. 2010. Available at: http://www.who.int/nmh/publications/ncd_report2010/en/. Accessed Aug 8, 2017.
- 3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 4. Towfighi A, Zheng L, Ovbiagele B. Sex‐specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med. 2009;169:1762–1766. [DOI] [PubMed] [Google Scholar]
- 5. Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988‐1994 and 1999‐2004. Hypertension. 2008;52:818–827. [DOI] [PubMed] [Google Scholar]
- 6. Cusma‐Piccione M, Zito C, Khandheria BK, Pizzino F, Di Bella G, Antonini‐Canterin F, Vriz O, Bello VA, Zimbalatti C, La Carrubba S, Oreto G, Carerj S. How arterial stiffness may affect coronary blood flow: a challenging pathophysiological link. J Cardiovasc Med. 2014;15:797–802. [DOI] [PubMed] [Google Scholar]
- 7. Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging. 2011;4:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 9. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton‐Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17 635 subjects. J Am Coll Cardiol. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coutinho T, Pellikka PA, Bailey KR, Turner ST, Kullo IJ. Sex differences in the associations of hemodynamic load with left ventricular hypertrophy and concentric remodeling. Am J Hypertens. 2016;29:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coutinho T, Srivaratharajah K, Beanlands R, deKemp R, Mielniczuk L. Associations of hemodynamic load with impaired myocardial flow reserve: role of sex and hypertension. Eur Heart J. 2015;36(suppl 1):854. [Google Scholar]
- 13. Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol. 2014;30:756–764. [DOI] [PubMed] [Google Scholar]
- 14. Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–235. [PubMed] [Google Scholar]
- 15. Chow BJ, Wells GA, Chen L, Yam Y, Galiwango P, Abraham A, Sheth T, Dennie C, Beanlands RS, Ruddy TD. Prognostic value of 64‐slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol. 2010;55:1017–1028. [DOI] [PubMed] [Google Scholar]
- 16. Chow BJ, Abraham A, Wells GA, Chen L, Ruddy TD, Yam Y, Govas N, Galbraith PD, Dennie C, Beanlands RS. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging. 2009;2:16–23. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann U, Moselewski F, Cury RC, Ferencik M, Jang IK, Diaz LJ, Abbara S, Brady TJ, Achenbach S. Predictive value of 16‐slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: patient‐versus segment‐based analysis. Circulation. 2004;110:2638–2643. [DOI] [PubMed] [Google Scholar]
- 18. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 19. Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume‐to‐aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. [DOI] [PubMed] [Google Scholar]
- 20. Fagard RH, Pardaens K, Staessen JA, Thijs L. The pulse pressure‐to‐stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol. 2001;38:227–231. [DOI] [PubMed] [Google Scholar]
- 21. Mochizuki T, Murase K, Higashino H, Koyama Y, Doi M, Miyagawa M, Nakata S, Shimizu K, Ikezoe J. Two‐ and three‐dimensional CT ventriculography: a new application of helical CT. Am J Roentgenol. 2000;174:203–208. [DOI] [PubMed] [Google Scholar]
- 22. Wu YW, Tadamura E, Kanao S, Yamamuro M, Okayama S, Ozasa N, Toma M, Kimura T, Kita T, Marui A, Komeda M, Togashi K. Left ventricular functional analysis using 64‐slice multidetector row computed tomography: comparison with left ventriculography and cardiovascular magnetic resonance. Cardiology. 2008;109:135–142. [DOI] [PubMed] [Google Scholar]
- 23. Khatri PJ, Tandon V, Chen L, Yam Y, Chow BJ. Can left ventricular end‐diastolic volumes be estimated with prospective ECG‐gated CT coronary angiography? Eur J Radiol. 2012;81:226–229. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Yam Y, Chen L, Aljizeeri A, Aliyary Ghraboghly S, Al‐Harbi I, Pen A, Ruddy TD, Chow BJ. Assessment of left ventricular ejection fraction using low radiation dose computed tomography. J Nucl Cardiol. 2016;23:414–421. [DOI] [PubMed] [Google Scholar]
- 25. Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, Segers P; Asklepios Investigators . Arterial load and ventricular‐arterial coupling: physiologic relations with body size and effect of obesity. Hypertension. 2009;54:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, Berman DS, Min JK. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2‐center study of 2583 patients undergoing 64‐detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58:510–519. [DOI] [PubMed] [Google Scholar]
- 27. Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy IP, Peyser PA, Schwartz RS. Long‐term prognostic value of coronary calcification detected by electron‐beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412–417. [DOI] [PubMed] [Google Scholar]
- 28. Thijssen DH, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries? J Physiol. 2016;594:2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pereira T, Maldonado J, Pereira L, Conde J. Aortic stiffness is an independent predictor of stroke in hypertensive patients. Arq Bras Cardiol. 2013;100:437–443. [DOI] [PubMed] [Google Scholar]
- 31. Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–1040. [DOI] [PubMed] [Google Scholar]
- 32. Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age‐related increase in proximal aortic stiffness than men. J Hypertens. 2001;19:2205–2212. [DOI] [PubMed] [Google Scholar]
- 33. Dart AM, Kingwell BA, Gatzka CD, Willson K, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, MacDonald GJ, Morgan TO, West MJ, Cameron JD. Smaller aortic dimensions do not fully account for the greater pulse pressure in elderly female hypertensives. Hypertension. 2008;51:1129–1134. [DOI] [PubMed] [Google Scholar]
- 34. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end‐organ damage. J Appl Physiol. 2008;105:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baumbach GL. Is pulse pressure a stimulus for altered vascular structure in chronic hypertension? Hypertension. 1991;18:728–729. [DOI] [PubMed] [Google Scholar]
- 36. Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross‐sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. [DOI] [PubMed] [Google Scholar]
- 37. Gibson CM, Karha J, Murphy SA, de Lemos JA, Morrow DA, Giugliano RP, Roe MT, Harrington RA, Cannon CP, Antman EM, Califf RM, Braunwald E; TIMI Study Group . Association of a pulsatile blood flow pattern on coronary arteriography and short‐term clinical outcomes in acute myocardial infarction. J Am Coll Cardiol. 2004;43:1170–1176. [DOI] [PubMed] [Google Scholar]
- 38. McDonald M, Hertz RP, Unger AN, Lustik MB. Prevalence, awareness, and management of hypertension, dyslipidemia, and diabetes among United States adults aged 65 and older. J Gerontol. 2009;64:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, Ohnesorge B, Fayad ZA, Becker CR, Reiser M, Steinbeck G, Boekstegers P. Accuracy of 64‐slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672–677. [DOI] [PubMed] [Google Scholar]


