Abstract
Background
The major determinants and prognostic importance of self‐reported health in patients with stable coronary heart disease are uncertain.
Methods and Results
The STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial randomized 15 828 patients with stable coronary heart disease to treatment with darapladib or placebo. At baseline, 98% of participants completed a questionnaire that included the question, “Overall, how do you feel your general health is now?” Possible responses were excellent, very good, good, average, and poor. Adjudicated major adverse cardiac events, which included cardiovascular death, myocardial infarction, and stroke, were evaluated by Cox regression during 3.7 years of follow‐up for participants who reported excellent or very good health (n=2304), good health (n=6863), and average or poor health (n=6361), before and after adjusting for 38 covariates. Self‐reported health was most strongly associated with geographic region, depressive symptoms, and low physical activity (P<0.0001 for all). Poor/average compared with very good/excellent self‐reported health was independently associated with major adverse cardiac events (hazard ratio [HR]: 2.30 [95% confidence interval (CI), 1.92–2.76]; adjusted HR: 1.83 [95% CI, 1.51–2.22]), cardiovascular mortality (HR: 4.36 [95% CI, 3.09–6.16]; adjusted HR: 2.15 [95% CI, 1.45–3.19]), and myocardial infarction (HR: 1.87 [95% CI, 1.46–2.39]; adjusted HR: 1.68 [95% CI, 1.25–2.27]; P<0.0002 for all).
Conclusions
Self‐reported health is strongly associated with geographical region, mood, and physical activity. In a global coronary heart disease population, self‐reported health was independently associated with major cardiovascular events and mortality beyond what is measurable by established risk indicators.
Clinical Trial Registration
URL: http://www.ClinicalTrials.gov. Unique identifier: NCT00799903.
Keywords: coronary artery disease, general health, prognostic studies
Subject Categories: Aging, Risk Factors, Chronic Ischemic Heart Disease, Mortality/Survival
Clinical Perspective
What Is New?
In patients with stable coronary heart disease, geographic region, mood, and lifestyle risk factors are strong determinants of self‐reported health.
Self‐reported general health is a strong independent predictor of cardiovascular mortality, noncardiovascular mortality, and myocardial infarction.
What Are the Clinical Implications?
General health should be considered in addition to traditional risk factors when estimating the risk of adverse cardiovascular events.
It is possible that interventions that improve general health, either at the individual or general population level, will also lower cardiovascular risk.
Introduction
An important goal of management of patients with stable coronary heart disease (CHD) is to improve physical, mental, and/or social well‐being. Consequently, it is relevant to consider the overall general health of the patient when deciding on a management strategy. Better understanding of the determinants of general health and of how general health may influence the risk of adverse clinical outcomes has the potential to improve clinical decisions.
General health is usually considered to be a patient‐centered measure.1 It can be assessed by asking patients about their physical, mental, and social functioning and other aspects of quality of life using questionnaires such as the Short Form–362 and the EQ‐5D.3 The simplest assessment of health can be obtained by asking a single question: “How is your overall health?” In large epidemiological studies, the response to this simple question predicted both total and cardiovascular mortality.4, 5, 6 Few studies, however, have evaluated the clinical importance of this simple indicator of overall health in CHD patients.7, 8, 9 In addition, the primary determinants of self‐reported health in patients with stable CHD are uncertain.
The aims of this study were to describe the most important factors associated with self‐reported health and to evaluate its independent association with outcomes in patients with stable CHD who participated in the global STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial.10, 11
Methods
Study Population
The STABILITY trial (ClinicalTrials.gov identifier NCT00799903) was a global outcomes trial designed to determine whether darapladib, a specific inhibitor of lipoprotein‐associated phospholipase A2, would reduce the risk of cardiovascular death, myocardial infarction, and stroke in patients with chronic CHD.10 In total, 15 828 participants from 39 countries were randomized. All patients had chronic stable CHD, defined as prior myocardial infarction, prior coronary revascularization, or multivessel CHD confirmed by coronary angiography. In addition, patients had to meet at least one of the following cardiovascular risk criteria: age ≥60 years; diabetes mellitus requiring pharmacotherapy; high‐density lipoprotein cholesterol <1.03 mmol/L; current or previous smoker, defined as ≥5 cigarettes per day on average; significant renal dysfunction (estimated glomerular filtration rate ≥30 and <60 mL/min per 1.73 m2 or urine albumin:creatinine ratio ≥30 mg albumin/g creatinine); or polyvascular disease (CHD and cerebrovascular disease or CHD and peripheral arterial disease). Darapladib did not influence the risk of major adverse cardiac events (MACE).10, 11 More detailed descriptions of the study design and population were published previously.10, 11, 12 All patients provided written informed consent, and the relevant ethics committees in each participating country approved the study, in accordance with the Helsinki Declaration.
Baseline Clinical Assessment and Questionnaire
At baseline, 15 528 participants (98%) completed a lifestyle questionnaire. This included the following question: “Overall, how do you feel your general health is now?” Possible responses were excellent, very good, good, average, and poor. Because only a small proportion of participants responded with excellent and poor, results are presented for 3 groups (1) excellent or very good, (2) good, and (3) average or poor.
In addition to the cardiovascular risk criteria needed for study inclusion, the following information was recorded at baseline: age, sex, diagnosis of hypertension, history of congestive heart failure, prior myocardial infarction, prior percutaneous coronary intervention or coronary artery bypass grafting, and New York Heart Association functional class. Body mass index was calculated in kg/m2. Geographic regions of enrollment were North America, South America including Mexico, Western Europe including Australia and New Zealand, Eastern Europe and Asia including South Africa. These country groupings were chosen primarily by geographic location but also considered the similarity of race, culture, and gross domestic product of countries.
On the lifestyle questionnaire, patients indicated whether they lived alone and the number of years of education completed, classified as <8 years, 8 to 12 years, trade school, or college or university. Financial stress and home‐ and work‐related stress were graded as never or rarely, sometimes, often, or always. Depressive symptoms were assessed from the questions “How is your current mood?” (denoted as “depressed mood”) and “Have you lost interest in activities or hobbies that normally give you pleasure?” (denoted as “loss of interest”), with possible responses of never or rarely, sometimes, often, or always.13 Physical activity was assessed from the total hours of moderate‐intensity (4 metabolic equivalents) and vigorous‐intensity (8 metabolic equivalents) physical activity during an average week.14, 15 A healthy diet was classified using a Mediterranean diet score based on responses to a simple food‐frequency questionnaire.16 The number of remaining teeth was recorded. Tooth loss was classified as ≤25 teeth.17
Hemoglobin, white blood cell count, creatinine, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, trigylcerides, and C‐reactive protein were measured at a Quest Diagnostics Clinical Laboratory. In addition, blood samples were obtained from 14 577 patients at baseline and stored until biochemical analysis. Lipoprotein‐associated phospholipase A2 was measured at diaDexus Inc. High‐sensitivity troponin T, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), cystatin C, interleukin 6, and growth differentiation factor 15 were measured by an electrochemiluminescence immunoassay using a Cobas e 601 analyzer (Roche Diagnostics) at the Uppsala Clinical Research Center Laboratory in Uppsala, Sweden.
Clinical Outcomes
The primary end point of MACE was the composite of cardiovascular death, myocardial infarction, or stroke. Secondary end points were total mortality, cardiovascular mortality, noncardiovascular mortality, myocardial infarction, stroke, major coronary events, and hospital admission for heart failure. Clinical outcomes were adjudicated by a committee. The event definitions and main results of the study were presented elsewhere.10, 11 The median follow‐up time was 3.7 years (interquartile range: 3.5–3.8 years). Vital status was complete for 99.3% of patients and 99.6% of total possible follow‐up time.
Statistical Analysis
The baseline characteristics were summarized, with categorical variables presented as count and proportion and continuous variables presented as mean and standard deviation. To investigate differences across the 3 groups of patients, the categorical variables were compared with the χ2 test. Continuous variables were compared with Mann–Whitney nonparametric tests.
To investigate the associations between different covariates and self‐reported health, multivariable ordinal logistic regressions were used due to the ordinal nature of the dependent variable (excellent or very good, good, average or poor). The results of the regression analyses are presented as adjusted odds ratios (ORs) and 95% confidence intervals. The ORs describe the associations of different covariates with decreasing levels of self‐reported health. Standardized ORs are presented for all continuous variables. All biomarkers were based on a 1‐SD increase, ORs for body mass index and Mediterranean diet score were based on a 1‐U increase, and ORs for physical activity (metabolic equivalent in hours per week) is based on a 50% increase. To evaluate the relative strength of association of different covariates with self‐reported health, χ2−df was plotted.18 This statistic allows comparison of predictors with different parameters, with a higher number indicating a stronger association.
Associations between self‐reported health and the study outcomes were assessed using Cox proportional hazards regression models and expressed with hazard ratio and 95% confidence interval. The underlying proportional hazards assumptions of the Cox proportional hazards models were verified by Schoenfeld residual tests. Using a multivariate model, we adjusted for demographic variables (age at randomization, sex, geographic region), psychosocial measures (depressed mood, loss of interest in hobbies, financial stress, stress at work or home, years of education), lifestyle risk factors (body mass index, smoking status, physical activity,15 Mediterranean diet score,16 attendance at cardiac rehabilitation), disease markers at baseline (diagnosis of hypertension, congestive heart failure, significant renal dysfunction, prior myocardial infarction, prior coronary revascularization [percutaneous coronary intervention or coronary artery bypass grafting]), prior multivessel chronic heart disease, diabetes mellitus, polyvascular disease, tooth loss, New York Heart Association functional class), and biomarkers (low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, hemoglobin, high‐sensitivity troponin T, interleukin 6, growth differentiation factor 15, triglycerides, estimated glomerular filtration rate [using the Chronic Kidney Disease Epidemiology Collaboration formula], creatinine, white blood cell count, high‐sensitivity C‐reactive protein, NT‐proBNP, cystatin C, and lipoprotein‐associated phospholipase A2 activity). The covariates included in the model were prespecified based on previous analyses from the STABILITY trial.16, 17, 19, 20 Kaplan–Meier curves were constructed for MACE by self‐reported health groups.
All analyses were performed using SAS software version 9.4 (SAS Institute). For all statistical analyses, a 2‐sided P<0.05 was considered statistically significant.
Results
The baseline characteristics of the study population are presented for participants reporting excellent or very good, good, and average or poor self‐reported health in Table 1. A broad range of demographic, geographic, psychosocial, lifestyle‐related, and conventional cardiovascular risk factors, disease markers, and biomarkers were associated with self‐reported health. Thirty‐two of these 38 covariates were associated with self‐reported health in univariate analysis, with P<0.0001.
Table 1.
Demographic and Baseline Characteristics by Self‐Reported General Health Levels
| Excellent or Very Good (n=2304) | Good (n=6863) | Average or Poor (n=6361) | Total (N=15 528) | Odds Ratio for one category decrease in health OR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y | ||||||
| Mean (SD) | 65.2 (8.9) | 64.7 (9.3) | 63.7 (9.5) | 64.4 (9.3) | 0.88 (0.85–0.91) | <0.0001§ |
| Sex | ||||||
| Female | 294 (12.8) | 1228 (17.9) | 1378 (21.7) | 2900 (18.7) | 1.44 (1.34–1.56) | <0.0001 |
| Race | ||||||
| Black | 40 (1.7) | 157 (2.3) | 164 (2.6) | 361 (2.3) | 1.40 (1.15–1.71) | <0.0001 |
| Central, South, or Southeast Asian | 104 (4.5) | 531 (7.7) | 528 (8.3) | 1163 (7.5) | 1.45 (1.30–1.63) | |
| East Asian or Japanese | 150 (6.5) | 421 (6.1) | 951 (15.0) | 1522 (9.8) | 2.59 (2.33–2.88) | |
| Other | 53 (2.3) | 168 (2.4) | 112 (1.8) | 333 (2.1) | 0.89 (0.72–1.09) | |
| White | 1957 (84.9) | 5586 (81.4) | 4606 (72.4) | 12 149 (78.2) | Reference group | |
| Geographic region | ||||||
| Asia/Pacific | 234 (10.2) | 890 (13.0) | 1429 (22.5) | 2553 (16.4) | 3.37 (3.05–3.71) | <0.0001 |
| Eastern Europe | 177 (7.7) | 1436 (20.9) | 1849 (29.1) | 3462 (22.3) | 3.30 (3.02–3.61) | |
| South America | 217 (9.4) | 662 (9.6) | 440 (6.9) | 1319 (8.5) | 1.40 (1.25–1.58) | |
| Western Europe | 737 (32.0) | 2071 (30.2) | 1546 (24.3) | 4354 (28.0) | 1.48 (1.36–1.60) | |
| North America | 939 (40.8) | 1804 (26.3) | 1097 (17.2) | 3840 (24.7) | Reference group | |
| Psychosocial measures | ||||||
| Depressed mood | ||||||
| Often/always | 100 (4.4) | 524 (7.9) | 1148 (18.7) | 1772 (11.7) | 4.25 (3.82–4.74) | <0.0001 |
| Sometimes | 820 (36.1) | 3000 (45.0) | 3023 (49.2) | 6843 (45.4) | 1.84 (1.73–1.97) | |
| Never/rarely | 1354 (59.5) | 3142 (47.1) | 1976 (32.1) | 6472 (42.9) | Reference group | |
| Loss of interest | ||||||
| Often/always | 84 (3.7) | 508 (7.6) | 1107 (18.1) | 1699 (11.3) | 4.09 (3.67–4.55) | <0.0001 |
| Sometimes | 472 (20.8) | 1979 (29.7) | 2236 (36.6) | 4687 (31.2) | 2.00 (1.87–2.15) | |
| Never/rarely | 1715 (75.5) | 4174 (62.7) | 2768 (45.3) | 8657 (57.5) | Reference group | |
| Financial stress | ||||||
| Often/always | 205 (9.1) | 895 (13.4) | 1386 (22.6) | 2486 (16.5) | 2.44 (2.23–2.67) | <0.0001 |
| Sometimes | 633 (28.0) | 2165 (32.5) | 2114 (34.5) | 4912 (32.6) | 1.47 (1.38–1.57) | |
| Never/rarely | 1424 (63.0) | 3603 (54.1) | 2630 (42.9) | 7657 (50.9) | Reference group | |
| Lives alone | ||||||
| Yes | 302 (13.2) | 897 (13.1) | 862 (13.6) | 2061 (13.3) | 1.03 (0.95–1.13) | 0.4556 |
| No | 1991 (86.8) | 5941 (86.9) | 5480 (86.4) | 13 412 (86.7) | Reference group | |
| Stress at work or at home | ||||||
| Often/always | 312 (13.8) | 1156 (17.3) | 1513 (24.6) | 2981 (19.8) | 1.96 (1.80–2.14) | <0.0001 |
| Sometimes | 1027 (45.4) | 3126 (46.8) | 2898 (47.2) | 7051 (46.7) | 1.33 (1.24–1.43) | |
| Never/rarely | 925 (40.9) | 2400 (35.9) | 1732 (28.2) | 5057 (33.5) | Reference group | |
| Years of education | ||||||
| 9–12 y | 710 (30.9) | 2125 (31.1) | 1897 (30.0) | 4732 (30.6) | 0.75 (0.69–0.82) | <0.0001 |
| College/university | 858 (37.3) | 1923 (28.2) | 1572 (24.9) | 4353 (28.2) | 0.60 (0.55–0.66) | |
| Trade school | 347 (15.1) | 1262 (18.5) | 1205 (19.1) | 2814 (18.2) | 0.87 (0.79–0.95) | |
| <8 y | 384 (16.7) | 1513 (22.2) | 1651 (26.1) | 3548 (23.0) | Reference group | |
| Lifestyle risk factors | ||||||
| Body mass index, kg/m2 | ||||||
| Mean (SD) | 28.5 (4.4) | 28.9 (4.8) | 29.2 (5.4) | 28.9 (5.0) | 1.09 (1.01–1.02) | <0.0001‡ |
| Smoking status | ||||||
| Current smoker | 269 (11.7) | 1172 (17.1) | 1362 (21.4) | 2803 (18.1) | 1.44 (1.32–1.58) | <0.0001 |
| Former smoker | 1298 (56.3) | 3589 (52.3) | 3060 (48.1) | 7947 (51.2) | 0.92 (0.86–0.99) | |
| Never smoked | 737 (32.0) | 2102 (30.6) | 1939 (30.5) | 4778 (30.8) | Reference group | |
| Mediterranean diet score | ||||||
| Mean (SD) | 12.8 (3.1) | 12.0 (3.0) | 11.7 (3.1) | 12.0 (3.1) | 0.93 (0.92–0.94) | <0.0001‡ |
| Physical activity (MET h/wk) | ||||||
| Mean (SD) | 60.7 (50.6) | 55.2 (49.7) | 46.8 (45.6) | 52.6 (48.5) | 0.90 (0.89–0.92) | <0.0001¶ |
| Attended cardiac rehabilitation | ||||||
| Yes | 966 (42.0) | 2409 (35.3) | 2037 (32.3) | 5412 (35.1) | 0.781 (0.734–0.831) | <0.0001 |
| Disease markers | ||||||
| Diagnosis of hypertension | ||||||
| Yes | 1512 (65.6) | 4841 (70.5) | 4759 (74.8) | 11 112 (71.6) | 1.34 (1.25–1.43) | <0.0001 |
| Congestive heart failure at baseline | ||||||
| Yes | 223 (9.7) | 1255 (18.3) | 1857 (29.2) | 3335 (21.5) | 2.24 (2.08–2.41) | <0.0001 |
| Significant renal dysfunction | ||||||
| Yes | 581 (25.2%) | 2011 (29.3%) | 2094 (32.9%) | 4686 (30.2%) | 1.27 (1.19–1.35) | <0.0001 |
| Prior myocardial infarction | ||||||
| Yes | 1288 (55.9) | 4069 (59.3) | 3784 (59.5) | 9141 (58.9) | 1.07 (1.01–1.14) | 0.0226 |
| Prior coronary revasculation (PCI or CABG) | ||||||
| Yes | 1852 (80.4) | 5222 (76.1) | 4574 (71.9) | 11 648 (75.0) | 0.74 (0.70–0.80) | <0.0001 |
| Prior multivessel chronic heart disease | ||||||
| Yes | 266 (11.5) | 988 (14.4) | 1084 (17.0) | 2338 (15.1) | 1.32 (1.22–1.44) | <0.0001 |
| Diabetes mellitus | ||||||
| Yes | 677 (29.4) | 2537 (37.0) | 2801 (44.0) | 6015 (38.7) | 1.50 (1.41–1.60) | <0.0001 |
| Polyvascular disease | ||||||
| Yes | 235 (10.2) | 999 (14.6) | 1101 (17.3) | 2335 (15.0) | 1.40 (1.30–1.53) | <0.0001 |
| Tooth loss | ||||||
| ≤25 teeth | 1593 (69.4) | 5294 (77.9) | 5193 (82.6) | 12 080 (78.5) | 1.61 (1.50–1.73) | <0.0001 |
| 26–32 (all) | 701 (30.6) | 1505 (22.1) | 1093 (17.4) | 3299 (21.5) | Reference group | |
| NYHA functional class | ||||||
| Class II, III, or IV | 120 (5.2) | 856 (12.5) | 1467 (23.1) | 2443 (15.7) | 2.63 (2.42–2.87) | <0.0001 |
| Medications at randomization | ||||||
| Aspirin | ||||||
| Yes | 2181 (94.7) | 6385 (93.0) | 5779 (90.9) | 14 345 (92.4) | 0.687 (0.613–0.770) | <0.0001 |
| ACEI or ARB | ||||||
| Yes | 1632 (70.8) | 5291 (77.1) | 5036 (79.2) | 11 959 (77.0) | 1.301 (1.212–1.396) | <0.0001 |
| Statin | ||||||
| Yes | 2243 (97.4) | 6689 (97.5) | 6174 (97.1) | 15 106 (97.3) | 0.892 (0.743–1.071) | 0.2213 |
| Beta blocker | ||||||
| Yes | 1728 (75.0) | 5413 (78.9) | 5117 (80.4) | 12 258 (78.9) | 1.206 (1.122–1.297) | <0.0001 |
| P2Y12 inhibitor | ||||||
| Yes | 718 (31.2) | 2214 (32.3) | 2319 (36.5) | 5251 (33.8) | 1.203 (1.129–1.281) | <0.0001 |
| Biomarkers | ||||||
| LDL‐C, mmol/L | ||||||
| Mean (SD) | 2.1 (0.8) | 2.2 (0.8) | 2.3 (0.9) | 2.2 (0.9) | 1.18 (1.14–1.21) | <0.0001∥ |
| HDL‐C, mmol/L | ||||||
| Mean (SD) | 1.3 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 0.88 (0.86–0.91) | <0.0001∥ |
| Hemoglobin, g/L | ||||||
| Mean (SD) | 144.5 (12.3) | 142.7 (13.8) | 141.8 (14.7) | 142.6 (14.0) | 0.89 (0.87–0.92) | <0.0001∥ |
| High‐sensitivity troponin T, ng/L | ||||||
| Mean (SD) | 10.8 (9.3) | 11.9 (12.2) | 13.7 (23.2) | 12.4 (17.2) | 1.19 (1.14–1.25) | <0.0001∥ |
| Interleukin 6, ng/L | ||||||
| Mean (SD) | 2.4 (2.0) | 2.8 (2.9) | 3.1 (3.2) | 2.8 (2.9) | 1.18 (1.14–1.22) | <0.0001∥ |
| GDF‐15, ng/L | ||||||
| Mean (SD) | 1415.2 (974.0) | 1532.0 (1081.4) | 1669.5 (1302.6) | 1569.2 (1164.0) | 1.17 (1.13–1.21) | <0.0001∥ |
| Triglycerides, mmol/L | ||||||
| Mean (SD) | 1.6 (1.0) | 1.8 (1.2) | 1.9 (1.5) | 1.8 (1.3) | 1.17 (1.13–1.21) | <0.0001∥ |
| eGFR (CKD‐EPI), mL/min/1.73 m2 | ||||||
| Mean (SD) | 73.9 (16.3) | 73.3 (17.1) | 73.8 (18.2) | 73.6 (17.4) | 1.01 (0.98–1.04) | 0.4384∥ |
| Creatinine | ||||||
| Mean (SD) | 93.5 (19.4) | 94.1 (24.1) | 93.7 (23.7) | 93.9 (23.3) | 1.00 (0.97–1.03) | 0.8168∥ |
| White blood cell count (1×109/L) | ||||||
| Mean (SD) | 6.4 (1.7) | 6.8 (1.9) | 7.0 (1.9) | 6.8 (1.9) | 1.19 (1.156–1.232) | <0.0001∥ |
| hs‐CRP, mg/L | ||||||
| Mean (SD) | 2.4 (6.6) | 2.8 (5.9) | 3.4 (7.2) | 3.0 (6.6) | 1.12 (1.08–1.16) | <0.0001∥ |
| NT‐proBNP, ng/L | ||||||
| Mean (SD) | 267.6 (490.9) | 345.4 (636.3) | 431.9 (989.6) | 368.2 (783.1) | 1.21 (1.16–1.26) | <0.0001∥ |
| Cystatin C, ng/L | ||||||
| Mean (SD) | 1.0 (0.3) | 1.0 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 1.13 (1.10–1.17) | <0.0001∥ |
| Lp‐PLA2 activity, μmol/min/L | ||||||
| Mean (SD) | 173.6 (44.4) | 175.4 (47.2) | 176.5 (50.0) | 175.6 (47.9) | 1.04 (1.01–1.07) | 0.0177∥ |
Values are mean (SD) and n (%) for categorical variables. Percentages refer to the percentage of patients in each level of self‐reported health and of all patients who have the given characteristic. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CI, confidence interval; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor‐15; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; LpPLA2, lipoprotein‐associated phospholipase A2; MET, metabolic equivalent; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; OR, odds ratio; PCI, percutaneous coronary intervention.
OR for 1‐category decrease in self‐reported health category: ‡OR based on 1‐U increase, §OR based on 10‐U increase, ∥OR based on 1‐SD increase, ¶OR based on 50% increase.
Patients who self‐reported poorer health were slightly more likely to be on an angiotensin‐converting enzyme inhibitor or angiotensin II receptor antagonist, a beta‐blocker, or a P2Y12 antagonist compared with those with better self‐reported health (Table 1). Aspirin use was slightly less in patients reporting poorer general health. Statin use was similar by self‐reported health. There was no difference in self‐reported health at baseline according to allocation to darapladib or placebo, as expected by randomization.
Variables independently associated with self‐reported health in a multivariable adjusted model are displayed in Table 2. The statistical strength of associations between different variables and self‐reported health is compared in Figure 1. The strongest independent associations were with geographic region, depressed mood, and low physical activity. The geographic region with the best self‐reported health was North America, followed by South America, Western Europe, Eastern Europe, and Asia (Table 1). After adjusting for other covariates associated with self‐reported health, persons from South America and North America had the best self‐reported health, participants from Western Europe reported slightly worse general health, and those from Eastern Europe and Asia were much more likely to report poorer health (Table 2). A less healthy diet, obesity, smoking, diabetes mellitus, hypertension, exertional dyspnea, cardiovascular disease markers, and tooth loss were each associated with poorer self‐reported health. Blood markers independently associated with poorer self‐reported health were high‐sensitivity cardiac troponin T, growth differentiation factor 15, interleukin 6, low‐density lipoprotein cholesterol, and low hemoglobin. Age and sex were not associated with self‐reported health in the fully adjusted model. Attendance at cardiac rehabilitation was not associated with self‐reported health after adjusting for covariates (Table 2).
Table 2.
Covariates Independently Associated With Self‐Reported Health in a Model Adjusting for All Other Covariates
| Variables | Contrast | OR (95% CI) | Overall P Value |
|---|---|---|---|
| Demographics | |||
| Age at randomization, y | 1.080 (1.006–1.159)† | 0.0330 | |
| Sex | Female vs male | 1.175 (0.989–1.395) | 0.0664 |
| Geographic region | Asia/Pacific vs North America | 4.795 (4.142–5.549) | <0.0001 |
| Eastern Europe vs North America | 3.015 (2.651–3.427) | ||
| South America vs North America | 0.866 (0.732–1.025) | ||
| Western Europe vs North America | 1.647 (1.477–1.837) | ||
| Psychosocial measures | |||
| Depressed mood | Often/always vs never/rarely | 2.214 (1.897–2.583) | <0.0001 |
| Sometimes vs never/rarely | 1.408 (1.292–1.535) | ||
| Lost interest in hobbies | Often/always vs never/rarely | 2.625 (2.270–3.036) | <0.0001 |
| Sometimes vs never/rarely | 1.502 (1.378–1.638) | ||
| Financial stress | Often/always vs never/rarely | 1.455 (1.287–1.645) | <0.0001 |
| Sometimes vs never/rarely | 1.150 (1.054–1.255) | ||
| Lives alone | Yes vs no | 1.009 (0.905–1.126) | 0.8676 |
| Stress at work or at home | Often/always vs never/rarely | 1.222 (1.081–1.382) | 0.0024 |
| Sometimes vs never/rarely | 1.136 (1.040–1.242) | ||
| Years of education | 9–12 vs <8 y | 0.873 (0.783–0.974) | <0.0001 |
| Trade school vs <8 y | 0.968 (0.856–1.094) | ||
| College/university vs <8 y | 0.785 (0.700–0.881) | ||
| Lifestyle risk factors | |||
| Body mass index, kg/m2 | 1.028 (1.019–1.037)* | <0.0001 | |
| Smoking status | Current smoker vs never/former smoker | 1.285 (1.153–1.432) | <0.0001 |
| Mediterranean diet score | 0.959 (0.947–0.972)* | <0.0001 | |
| Physical activity, MET h/wk | 0.916 (0.901–0.931)§ | <0.0001 | |
| Cardiac rehabilitation | Yes vs no | 0.962 (0.888–1.043) | 0.3514 |
| Disease markers | |||
| Diagnosis of hypertension | Yes vs no | 1.285 (1.181–1.399) | <0.0001 |
| Congestive heart failure at baseline | Yes vs no | 1.141 (1.001–1.301) | 0.0481 |
| Significant renal dysfunction | Yes vs no | 1.061 (0.965–1.165) | 0.2194 |
| Prior myocardial infarction | Yes vs no | 0.895 (0.825–0.971) | 0.0076 |
| Prior coronary revascularization (PCI or CABG) | Yes vs no | 0.876 (0.797–0.962) | 0.0055 |
| Prior multivessel chronic heart disease | Yes vs no | 1.228 (1.098–1.372) | 0.0003 |
| Diabetes mellitus | Yes vs no | 1.229 (1.128–1.338) | <0.0001 |
| Polyvascular disease | Yes vs no | 1.272 (1.144–1.415) | <0.0001 |
| Tooth loss | ≤25 vs 26–32 (all) teeth | 1.387 (1.262–1.524) | <0.0001 |
| NYHA functional class | Class II,III, or IV vs no class/class I | 1.554 (1.337–1.806) | <0.0001 |
| Biomarkers | |||
| LDL‐C, mmol/L | 1.092 (1.040–1.146)‡ | 0.0004 | |
| HDL‐C, mmol/L | 0.939 (0.898–0.982)‡ | 0.0062 | |
| Hemoglobin, g/L | 0.951 (0.909–0.995)‡ | 0.0307 | |
| High‐sensitivity troponin T, ng/L | 1.081 (1.026–1.140)‡ | 0.0036 | |
| Interleukin 6, ng/L | 1.070 (1.023–1.119)‡ | 0.0033 | |
| GDF‐15, ng/L | 1.070 (1.017–1.124)‡ | 0.0083 | |
| Triglycerides, mmol/L | 1.050 (0.995–1.109)‡ | 0.0767 | |
| eGFR (CKD‐EPI), mL/min/1.73 m2 | 1.117 (0.971–1.286)‡ | 0.1219 | |
| Creatinine | 1.131 (0.979–1.307)‡ | 0.0949 | |
| White blood cell count (1×109/L) | 0.988 (0.949–1.028)‡ | 0.5541 | |
| High‐sensitivity C‐reactive protein, mg/L | 1.009 (0.967–1.053)‡ | 0.6678 | |
| NT‐proBNP, ng/L | 1.049 (0.995–1.105)‡ | 0.0750 | |
| Cystatin C, ng/L | 0.943 (0.885–1.006)‡ | 0.0750 | |
| Lp‐PLA2 activity, μmol/min/L | 0.965 (0.918–1.013)‡ | 0.1506 | |
CABG indicates coronary artery bypass grafting; CI, confidence interval; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor‐15; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LpPLA2, lipoprotein‐associated phospholipase A2; MET, metabolic equivalent; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; OR, odds ratio; PCI, percutaneous coronary intervention.
OR for 1‐category decrease in self‐reported health category: *OR based on 1‐U increase, †OR based on 10‐U increase, ‡OR based on 1‐SD increase, §OR based on 50% increase.
Figure 1.
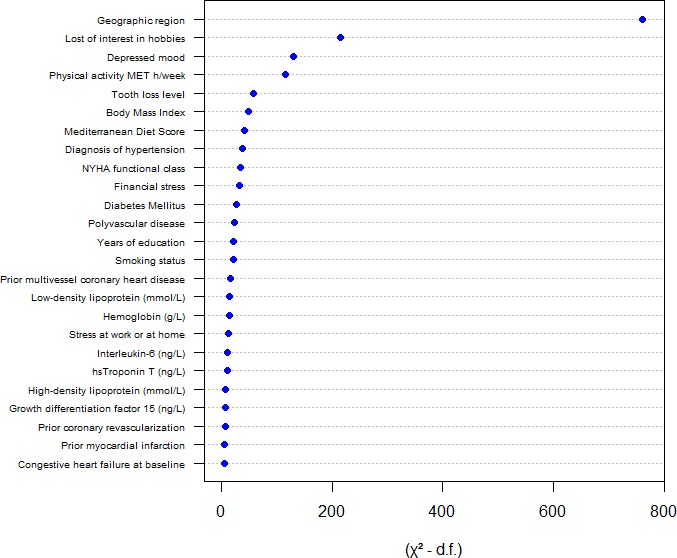
Relative strength of association with self‐reported health of each variable included in the full model, as measured by the Wald χ2 test minus the predictor degrees of freedom: (χ2−df)=χ2−predictor df. Higher values on the x‐axis indicate a stronger association with self‐reported health. hs indicates high sensitivity; MET, metabolic equivalent; NYHA, New York Heart Association.
There was a stepwise increase in the risk of MACE with worsening self‐reported health (Figure 2). The hazard ratios for adverse outcomes by self‐reported health before and after adjustment for covariables are displayed in Figure 3A and 3B, respectively. Poorer self‐reported health was associated with increased cardiovascular and total mortality, myocardial infarction, stroke, and hospital admission for heart failure (Figure 3A). After adjustment for all covariates, these associations were maintained for all outcomes except heart failure hospitalization and stroke (Figure 3B).
Figure 2.
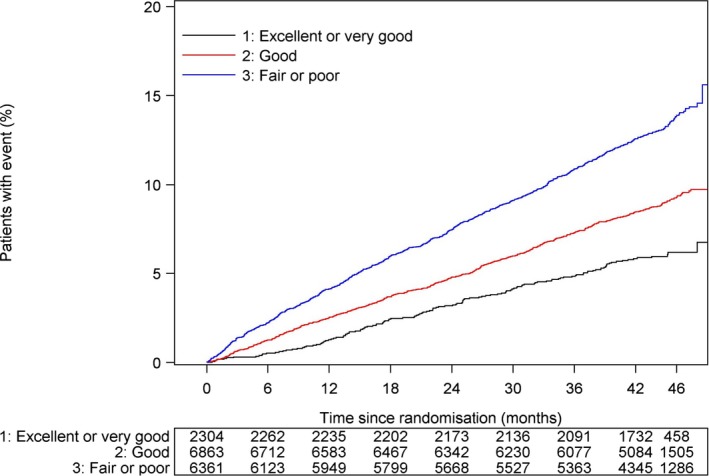
Kaplan–Meier plot of major adverse cardiovascular events by self‐reported health at baseline.
Figure 3.
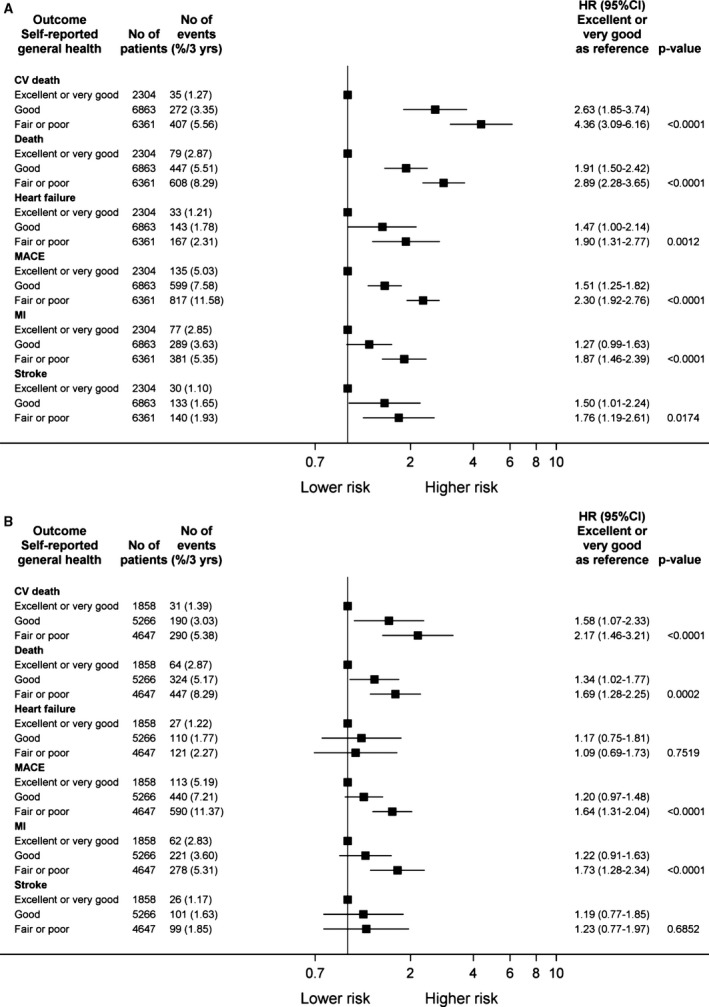
Adverse events by self‐reported health at baseline adjusted (A) for treatment allocation only and (B) for all covariates. Hazard ratios (HRs) and 95% confidence intervals (CIs) are presented for each outcome for patients reporting good and average or poor health compared with the reference group that reported very good or excellent health. Heart failure refers to hospitalization for heart failure. CV indicates cardiovsacular; MACE, major adverse cardiac events; MI, myocardial infarction.
Discussion
In this study, which evaluated a global population of patients with stable CHD on optimal secondary prevention treatment, average or poor self‐reported health was independently associated with a 2‐ to 3‐fold increased risk of cardiovascular mortality and myocardial infarction compared with patients reporting very good or excellent health. These observations indicate that self‐reported health is an important incremental risk indicator of myocardial infarction and cardiovascular mortality in patients with stable CHD, despite optimal secondary prevention treatment. The association with a large number of prognostically important variables is consistent with the conclusion that self‐reported health is a global health measure that both reflects the cumulative effects of a broad range of known risk indicators and indicates the importance of additional risk indicators not measurable by conventional methods.
A number of large studies have evaluated associations between self‐reported health and mortality in general populations.4, 6, 21 In the UK Biobank cohort, which included nearly 500 000 volunteers and evaluated multiple clinical, biomarker, and genetic risk factors, self‐reported health was the strongest single predictor of all‐cause mortality in men and the third strongest mortality predictor in women after a cancer diagnosis and illness or injury.4 Meta‐analyses of smaller studies have also been consistent in reporting associations between poorer self‐reported health and cardiovascular and all‐cause mortality.5, 22 In a large Swedish general population cohort, poorer self‐reported health was associated with a higher prevalance of multiple cardiovascular risk factors, and with an increased risk of myocardial infarction during follow‐up over ≈13 years.21
A systematic review of studies reporting the relationship between self‐reported health and fatal and nonfatal cardiovascular outcomes22 identified 3 studies8, 9, 23 including 10 648 patients with known cardiovascular or ischemic heart disease. In this meta‐analysis, patients with poor health compared with good or excellent health had a ≈2.4 times higher risk of cardiovascular death, consistent with the current study. However these studies have limitations, including poor measurement of baseline risk factors and cardiovascular disease status, lack of detail on study methods, and poor ascertainment of disease status or severity.
Most previous studies reporting self‐reported health have been undertaken in a single country. In the current study, which included patients from 39 countries and multiple regions of the world, self‐reported health was strongly associated with geographic region and country of residence. These observations suggest that cultural or regional norms need to be considered when interpreting self‐reported health. In addition, the large geographic differences in self‐reported health changed after adjustment for covariates, suggesting that geographic differences in self‐reported health reflect both differences in the burden of disease or symptoms and different perceptions of their impact on health. Socioeconomic24 and international gradients25 in adverse outcomes for patients with CHD persist despite adjustment for conventional cardiovascular risk factors and are well described. It is possible these could be explained in part by differences in general health.
Both psychosocial and conventional cardiovascular risk factors have been associated with self‐reported health in previous studies.7, 21, 22 In these studies, however, information on multiple covariates was more limited, and a detailed analysis of the relative importance of different factors was not undertaken. In the current study, depressive symptoms were strongly associated with self‐reported health, consistent with an effect of mood on the perception of health and the impact of poorer health on mood. Stress from various causes and fewer years of education were also associated with poorer self‐reported health. Other indicators of socioeconomic status were not assessed in this study. Lifestyle risk factors, including current smoking, physical activity, diet, and body weight, were relatively strong predictors of self‐reported health.
The presence of clinical markers of cardiovascular disease or risk, including prior myocardial infarction, stroke, polyvascular disease, diabetes mellitus, and renal dysfunction, was associated with poorer self‐reported health; however, the strength of these associations, as assessed by the χ2 statistic, was generally less than that of psychosocial and lifestyle measures. Associations between self‐reported health and blood biomarkers, some of which are powerful risk predictors, were generally weaker than those of clinical variables. Associations of self‐reported health with low‐density lipoprotein cholesterol, hemoglobin, interleukin 6, growth differentiation factor 15, and troponin T are consistent with an influence of multiple pathophysiological pathways.
There were modest differences in use of several evidence‐based medications by self‐reported health, but the reasons for these differences cannot be determined reliably in this observational study. Differences in medication use could be explained by treatment indication, such as impaired left ventricular function, which is a stronger indication for beta blockers and angiotensin‐converting enzyme inhibitors or angiotensin receptor antagonists, or effects of treatment on general health (eg, if beta blockers caused fatigue). A common association between medication use and other factors that influence health, such as socioeconomic conditions, geography, or medical care, is also possible.12
Study Limitations
The current study has a number of limitations. The degree to which the observed associations are causal cannot be established in this observational study. Despite the availability of a wide array of clinical and biochemical risk indicators and risk factors, there are still additional unmeasured risk factors, for example, details on all comorbidities, extent of coronary lesions, and genetic factors that were not included in the database. Furthermore, participants selected to participate in a clinical trial may not be representative of all CHD patients; however, the large geographically and culturally diverse study population and the internal consistency of the data suggest that results are likely to be broadly applicable. Additional strengths of the current analysis include the standardized assessment of multiple clinical, psychosocial, and lifestyle variables and near‐complete ascertainment of outcomes.
This study described associations between self‐reported health and a broad range of prognostically important variables. Our observation that many of these associations remained statistically significant after multivariable adjustment indicates that self‐reported health captures a broad range of conditions affecting sense of well‐being.
Broad geographic regions were prespecified for the analysis, but chosen groupings are relatively crude indicators of the importance of cultural, medical, and socioeconomic differences between and within countries—a limitation that is likely to diminish estimates of the importance of geographic factors. Despite this, geographic region was a strong independent predictor of both self‐reported health and MACE.
Study Implications
An assessment of self‐reported health can be undertaken simply as part of almost any consultation. Poor or worsening self‐reported health may occur for many reasons, both cardiac and noncardiac. This study suggests that poorer general health, whatever the reason, is associated with a higher risk of MACE in patients with stable CHD. This raises the possibility that interventions that improve overall health could reduce cardiovascular risk.
Disease‐specific patient‐centered outcome measures, such as the Quality of Life After Myocardial Infarction Questionnaire26 or the Seattle Angina Questionnaire,27 may be more sensitive to the impact of cardiovascular disease on quality of life than on overall health.1 Self‐reported overall health is influenced by many factors. Compared with disease‐specific tools, general health may be less sensitive to changes in symptoms directly related to, for example, CHD. Nevertheless, disease‐specific measures have some disadvantages. They generally include more questions, are less comparable for patients with different medical problems, are more time consuming to administer, and may not capture the impact of non–disease‐related factors on general health.
Because self‐reported overall health is a powerful risk marker, considering it could better inform decisions about treatment or delivery of health care and discussions about risks to future health. Few studies have evaluated whether and how response to different treatments relates to self‐reported health. Further research is needed to evaluate the effectiveness of targeting health care based on assessment of self‐reported general health.
Conclusions
In a global stable CHD population, self‐reported health was strongly associated with geographical region, psychosocial variables, and lifestyle risk factors. Self‐reported health was independently associated with MACE when adjusting for baseline characteristics including a wide array of prognostic biomarkers. These data support the conclusion that self‐perceived health and psychosocial and lifestyle‐related factors contribute to cardiovascular events beyond what is measurable by established risk indicators.
Author Contributions
Stewart, Held, Wallentin, and White designed the lifestyle questionnaire administered to STABILITY trial participants, which included the question on self‐reported health, and proposed the study analysis. Statistical analysis was undertaken by Hadziosmanovic. The manuscript was drafted by Stewart. All coauthors contributed to the design and management of the STABILITY trial. All authors contributed to critical review of the manuscript. Additional Contributions: Editorial assistance was provided by Susanna Thörnqvist, PhD, Uppsala Clinical Research Center (UCR), Uppsala University, Sweden, through funds from GlaxoSmithKline and secretarial assistance was provided by Michelle D'Souza, Auckland City Hospital.
Sources of Funding
This work was supported by the STABILITY trial funded by GlaxoSmithKline. The design, statistical analysis, and drafting of the manuscript were all undertaken independently by the study investigators.
Disclosures
Stewart has received nonfinancial support from GlaxoSmithKline. Hagström is an expert committee member, receiving lecture fees, and institutional research grants from Sanofi, and Amgen; institutional research grants from AstraZeneca, and GlaxoSmithKline; he is also an expert committee member for Ariad and MSD. Held has received an institutional research grant and speaker's bureau from AstraZeneca; institutional research grants from Bristol‐Myers Squibb, Merck & Co, GlaxoSmithKline, Roche. Armstrong has received grants from Merck, Sanofi‐Aventis, Bayer; lecture fees from AstraZeneca; consulting fees from Merck, Bayer, Axio/Orexigen, Eli Lilly, Bayer, Mast Therapeutics Inc. Aylward has received grants, personal fees and other from GlaxoSmithKline, AstraZeneca, Merck; grants and personal fees from Sanofi‐Aventis; personal fees from Boehringer Ingelheim, Pfizer, Eli Lilly, The Medicines Company, Amgen. Cannon has received grants and personal fees from AstraZeneca, Takeda, Boerhinger Ingelheim, Merck, Bristol‐Myers Squibb, Arisaph, GlaxoSmithKline; personal fees from Alnylam, Pfizer, Kowa, Lipimedix, Regeneron, Sanofi, Amgen, Boehringer Ingelheim/Eli Lilly; grants from Janssen. Koenig has received lecture and consultancy fees from Novartis, Amgen, AstraZeneca; lecture fees from Actavis, Berlin‐Chemie; consultancy fees from GlaxoSmithKline, The Medicines Company, Pfizer, Merck Sharpe & Dohme and Kowa; research grants from Roche Diagnostics, Abbott, Singulex, Beckmann. López‐Sendón has received grants and personal fees from Pfizer, Novartis, Servier, Menarini, and Sanofi; personal fees from Boehringer Ingelheim, grants from Bayer, grants from GlaxoSmithKline. Mohler has received grants and honoraria from GlaxoSmithKline. Hadziosmanovic has received institutional research grant from GlaxoSmithKline. Krug‐Gourley is an employee of and having stock ownership in GlaxoSmithKline. Siddique has received personal fees and non‐financial support from GlaxoSmithKline, Novartis, Bayer, Pfizer, Sanofi‐Aventis, Servier; non‐financial support from Ferozsons/Boston Scientific, Pharmevo, Horizon Pharma, Highnoon, Atco Laboratories. Steg has received personal fees from GlaxoSmithKline, Amarin, Bayer, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, Daiichi‐Sankyo, Eli Lilly, Merck‐Sharpe‐Dohme, Novartis, Pfizer, The Medicines Company, CLS‐Behring, Janssen; grants, personal fees and other from Sanofi and Servier; personal fees and other from AstraZeneca. White has received research grants and personal fees from GlaxoSmithKline, research grants from Sanofi‐Aventis, Eli Lilly, National Institute of Health, George Institute, Omthera Pharmaceuticals, Pfizer New Zealand, Intarcia Therapeutics Inc., Elsai Inc., Dal‐GenE and research grants and advisory board member for AstraZeneca, Honoraria and lecture fees from Sirtex and Acetilion. Wallentin has received institutional research grants, consultancy fees, lecture fees, and travel support from Bristol‐Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim; institutional research grants from Merck & Co, Roche; consultancy fees from Abbott; holds 2 patents involving GDF‐15. The remaining authors have no disclosures to report.
Supporting information
Appendix S1. STABILITY Trial—Organizational.
(J Am Heart Assoc. 2017;6:e006096 DOI: 10.1161/JAHA.117.006096.)28862971
References
- 1. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. [DOI] [PubMed] [Google Scholar]
- 2. Ware JE; New England Medical Center H, Health I . SF‐36 Physical and Mental Health Summary Scales: A User's Manual. Boston: Health Institute, New England Medical Center; 1994. [Google Scholar]
- 3. Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ‐5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ganna A, Ingelsson E. 5 year mortality predictors in 498,103 UK Biobank participants: a prospective population‐based study. Lancet. 2015;386:533–540. [DOI] [PubMed] [Google Scholar]
- 5. DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self‐rated health question. A meta‐analysis. J Gen Intern Med. 2006;21:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schnittker J, Bacak V. The increasing predictive validity of self‐rated health. PLoS One. 2014;9:e84933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olson KL, Stiefel M, Ross C, Stadler S, Hornak R, Sandhoff B, Merenich JA. Self‐rated health among patients with coronary artery disease enrolled in a cardiovascular risk reduction service. Popul Health Manag. 2016;19:24–30. [DOI] [PubMed] [Google Scholar]
- 8. Bosworth HB, Siegler IC, Brummett BH, Barefoot JC, Williams RB, Clapp‐Channing NE, Mark DB. The association between self‐rated health and mortality in a well‐characterized sample of coronary artery disease patients. Med Care. 1999;37:1226–1236. [DOI] [PubMed] [Google Scholar]
- 9. Rutledge T, Linke SE, Johnson BD, Bittner V, Krantz DS, Whittaker KS, Eastwood JA, Eteiba W, Cornell CE, Pepine CJ, Vido DA, Olson MB, Shaw LJ, Vaccarino V, Bairey Merz CN. Self‐rated versus objective health indicators as predictors of major cardiovascular events: the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation. Psychosom Med. 2010;72:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White HD, Held C, Stewart RA, Watson D, Harrington R, Budaj A, Steg G, Cannon C, Tarka E, Krug‐Gourley S, Wittes J, Trivedi T, Wallentin L; on behalf of the STABILITY Steering Committee . Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilisation of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with clinical coronary heart disease). Am Heart J. 2010;160:655–661. [DOI] [PubMed] [Google Scholar]
- 11. White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, Lopez‐Sendon J, Manolis AJ, Mohler ER III, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos‐Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 12. Vedin O, Hagstrom E, Stewart R, Brown R, Krug‐Gourley S, Davies R, Wallentin L, White H, Held C. Secondary prevention and risk factor target achievement in a global, high‐risk population with established coronary heart disease: baseline results from the STABILITY study. Eur J Prev Cardiol. 2013;20:678–685. [DOI] [PubMed] [Google Scholar]
- 13. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craig CL, Marshall AJ, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF. International Physical Activity Questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 15. Stewart R, Held C, Brown R, Vedin O, Hagstrom E, Lonn E, Armstrong P, Granger CB, Hochman J, Davies R, Soffer J, Wallentin L, White H. Physical activity in patients with stable coronary heart disease: an international perspective. Eur Heart J. 2013;34:3286–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart RA, Wallentin L, Benatar J, Danchin N, Hagstrom E, Held C, Husted S, Lonn E, Stebbins A, Chiswell K, Vedin O, Watson D, White HD. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high‐risk patients with stable coronary heart disease. Eur Heart J. 2016;37:1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vedin O, Hagstrom E, Budaj A, Denchev S, Harrington RA, Koenig W, Soffer J, Sritara P, Stebbins A, Stewart RH, Swart HP, Viigimaa M, Vinereanu D, Wallentin L, White HD, Held C. Tooth loss is independently associated with poor outcomes in stable coronary heart disease. Eur J Prev Cardiol. 2016;23:839–846. [DOI] [PubMed] [Google Scholar]
- 18. Harrell F. Regression Modeling Strategies, With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2015. [Google Scholar]
- 19. Hagstrom E, Held C, Stewart RA, Aylward PE, Budaj A, Cannon CP, Koenig W, Krug‐Gourley S, Mohler ER III, Steg PG, Tarka E, Ostlund O, White HD, Siegbahn A, Wallentin L. Growth differentiation factor 15 predicts all‐cause morbidity and mortality in stable coronary heart disease. Clin Chem. 2017;63:325–333. [DOI] [PubMed] [Google Scholar]
- 20. Wallentin L, Held C, Armstrong PW, Cannon CP, Davies RY, Granger CB, Hagstrom E, Harrington RA, Hochman JS, Koenig W, Krug‐Gourley S, Mohler ER III, Siegbahn A, Tarka E, Steg PG, Stewart RA, Weiss R, Ostlund O, White HD. Lipoprotein‐associated phospholipase A2 activity is a marker of risk but not a useful target for treatment in patients with stable coronary heart disease. J Am Heart Assoc. 2016;5:e003407 DOI: 10.1161/JAHA.116.003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waller G, Janlert U, Norberg M, Lundqvist R, Forssén A. Self‐rated health and standard risk factors for myocardial infarction: a cohort study. BMJ Open. 2015;5:110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mavaddat N, Parker RA, Sanderson S, Mant J, Kinmonth AL. Relationship of self‐rated health with fatal and non‐fatal outcomes in cardiovascular disease: a systematic review and meta‐analysis. PLoS One. 2014;9:e103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Idler E, Leventhal H, McLaughlin J, Leventhal E. In sickness but not in health: self‐ratings, identity, and mortality. J Health Soc Behav. 2004;45:336–356. [DOI] [PubMed] [Google Scholar]
- 24. Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 25. Nichols M, Townsend N, Scarborough P, Rayner M. Trends in age‐specific coronary heart disease mortality in the European Union over three decades: 1980–2009. Eur Heart J. 2013;34:3017–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valenti L, Lim L, Heller RF, Knapp J. An improved questionnaire for assessing quality of life after acute myocardial infarction. Qual Life Res. 1996;5:151–161. [DOI] [PubMed] [Google Scholar]
- 27. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. STABILITY Trial—Organizational.


