Abstract
Background
Nearly 17% of patients are readmitted within 30 days of discharge after transcatheter aortic valve replacement. Selected patients are discharged to skilled nursing facilities, yet the association between a hospital's practice to discharge home versus to skilled nursing facilities, and readmission remains unclear.
Methods and Results
The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry was used to evaluate readmissions among patients undergoing transcatheter aortic valve replacement (2011‐2015). Hospitals were divided into quartiles (Q1‐Q4) based on the percentage of patients discharged directly home. We assessed patient and hospital level characteristics and used hierarchical logistic regression to analyze the association of discharge disposition with 30‐day readmission. Our cohort included 18 568 transcatheter aortic valve replacement patients at 329 US hospitals, of whom 69% were discharged directly home. Hospitals in the highest quartile of direct home discharge (Q4) compared with hospitals in the lowest (Q1) were more likely to use femoral access (75.2% versus 60.1%, P<0.001), had fewer patients receiving transfusion (26.4% versus 40.9%, P<0.001), and were more likely to be located in the Southern United States (48.8% versus 18.3%, P<0.001). Median 30‐day readmission rate was 17.9%. There was no significant difference in 30‐day readmissions among quartiles (P=0.14), even after multivariable adjustment (odds ratio Q4 versus Q1=0.89, 95%CI 0.76‐1.04; P=0.15). Factors most strongly associated with 30‐day readmission were glomerular filtration rate, in‐hospital stroke or transient ischemic attack, and nonfemoral access.
Conclusions
There was no statistically significant association between hospital practice of direct home discharge post–transcatheter aortic valve replacement and 30‐day readmission. Further research is needed to understand regional variations and optimum strategies for postdischarge care.
Keywords: geriatrics, hospital readmission, registry, transcatheter aortic valve implantation
Subject Categories: Quality and Outcomes, Aortic Valve Replacement/Transcather Aortic Valve Implantation
Clinical Perspective
What Is New?
Our study is the first to use the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry to investigate, among patients undergoing transcatheter aortic valve replacement, the association between the hospital practice of direct‐home discharge and 30‐day readmissions.
What Are the Clinical Implications?
We found that hospital discharge practices (direct‐home versus skilled nursing facility) had little influence on 30‐day readmissions, and readmission events instead appeared to be driven by patient‐level factors.
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as a therapy for patients with symptomatic severe aortic stenosis at high operative risk. In practice, patients undergoing TAVR are elderly and have a high degree of comorbid disease including heart failure, atherosclerosis, and advanced lung disease.1 These factors place patients at high risk for subsequent acute care utilization: in one report, 17% of patients were readmitted to the hospital within 30 days of TAVR, and over half were readmitted within 1 year.1
Because hospital readmissions are costly and can adversely impact patients’ quality of life, reducing preventable readmissions has become a major focus of health systems and payers. The early postdischarge period represents an especially vulnerable time for patients due to factors that include physical deconditioning, sleep disturbances, altered medication regimens, and poor nutritional status.2 Broadly, there are 2 main pathways for postdischarge care: direct‐home discharge, or discharge to a skilled nursing facility (SNF). Although selected patients may benefit from routine use of the more intensive monitoring provided by SNFs,3 data concerning their benefits are mixed.3, 4 It is also possible that patients in the home setting regain mobility more rapidly, are less prone to facility‐acquired infections, and have a less disorienting environment, all of which may hasten the recovery process and reduce preventable readmissions.
Although factors including comorbidity,5 insurance status,6 and social support7 play roles in determining discharge disposition, data suggest that substantial heterogeneity remains and may be due to hospital‐level factors.8 Although one third of patients undergoing TAVR are currently discharged to a SNF,1 there are insufficient data on the degree of hospital‐level variation in routine pathways for discharge disposition. In addition, to our knowledge the association between hospital practice of determining discharge disposition (direct‐home versus SNF) and 30‐day readmission, after adjusting for relevant confounders, has not been described in US clinical practice. Accordingly, we used the national Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) transcatheter valve therapy (TVT) Registry to investigate variations in discharge practices, patient‐ and hospital‐level characteristics associated with discharge to home versus SNF, and 30‐day readmission rates based on case mix of patients discharged to home versus SNF.
Methods
Data Source
The STS/ACC TVT Registry is a joint initiative of the STS and ACC that was started in December 2011 after FDA approval of the Sapien Transcatheter Heart Valve.9 Centers that participate in the Registry submit data including patient demographics, medical comorbidities, functional status, quality of life, and procedural details. Data for 30‐day and 1‐year outcomes (mortality and readmission) are obtained through a linkage with data from the Centers for Medicare and Medicaid Services.
Data are stored at the National Cardiovascular Data Registry Data Warehouse, and analyses are performed at the Duke Clinical Research Institute Analysis Center. Data quality checks are performed at both locations, and feedback to improve completeness and accuracy of reporting is provided to participating sites on an ongoing basis. The institutional review board of record for the STS and ACC is Chesapeake Research Review Incorporated. The Registry has submitted a protocol to this institutional review board, which governs all human subjects research conducted by the Registry. The STS/ACC TVT Registry protocol on file has been granted a waiver of informed consent.
Data Elements
Data elements are reported in the Registry using standard definitions as described previously.9, 10 Discharge to SNF is a discretely coded element that includes discharge to any extended care, transitional care, or rehabilitation facility. Discharge to a permanent nursing home is a separate, mutually exclusive field, and patients with this disposition were not included in the analysis given the inherent differences in permanent nursing home patients from other patients. For purposes of this study, the following covariates were reported: patient demographics, common clinical characteristics, treatment characteristics (femoral access, general anesthesia), complications (transfusion, vascular access complication, hours in intensive care unit), and hospital characteristics (US region, annual TAVR volume, number of beds, teaching status). For all covariates (except the Kansas City Cardiomyopathy Questionnaire [KCCQ] and the 5‐m walk test) there were less than 5% data missing, and we used single imputation (assuming a median value for missing continuous variables and the highest frequency value for missing categorical variables). There were a high proportion of missing data for the KCCQ and 5‐m walk test from the overall study sample, and, therefore, these 2 variables were not included in the primary analysis. However, hospitals with completeness of at least 80% KCCQ and 50% 5‐m walk test were included in a sensitivity analysis. We used multiple imputations to account for residual missing values.
Inclusion and Exclusion Criteria
All patients undergoing TAVR at participating Registry hospitals from November 9, 2011 through March 31, 2015 were initially considered (N=35 221) (Figure 1). We then excluded patients who experienced in‐hospital death and those who were discharged to hospice or to another acute care hospital or a permanent nursing home (which were not considered SNFs for purposes of our study). From the remaining sample we excluded patients without 30‐day follow‐up data available and patients ineligible for Medicare fee‐for‐service. Finally, in order to ensure stability of our effect estimates, we excluded hospitals that performed fewer than 5 TAVR cases during the entire study period. Our final study sample included 18 568 patients at 329 hospitals.
Figure 1.
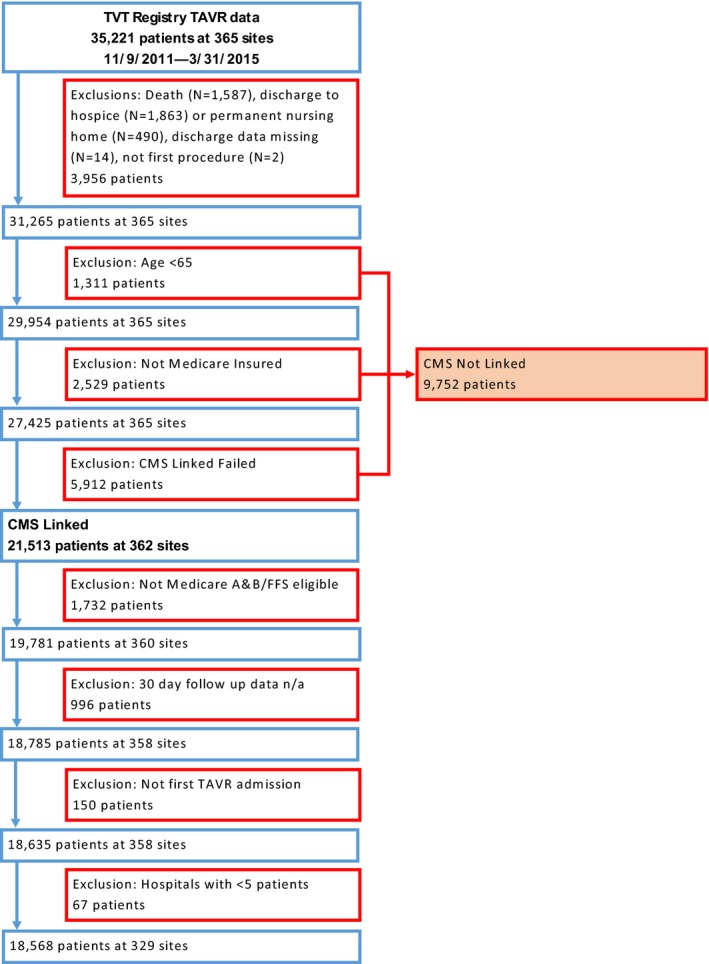
Cohort flow diagram. CMS indicates Centers for Medicare & Medicaid Services; n/a, not available; TAVR, transcatheter aortic valve replacement; TVT, transcatheter valve therapy.
Statistical Analysis
We performed our analysis at the hospital level because our primary interest was variation in institution‐level practice (rather than at the patient level). Accordingly, we first examined the distribution of discharge disposition (direct‐home versus SNF) post‐TAVR among hospitals in our sample. We then separated hospitals into quartiles based on the frequency of direct‐home discharge and compared clinical, treatment, hospital, and regional differences among these quartiles using the Kruskal‐Wallis test for continuous variables or chi‐squared test for categorical variables. Subsequently, we calculated hospital rates of 30‐day readmission (primary outcome) and 30‐day mortality (secondary outcome). We analyzed whether there was an association between direct‐home discharge and these outcomes by comparing rates among hospital quartiles using the Kruskal‐Wallis test.
We then developed a hierarchical logistic regression model for our primary outcome (30‐day readmission). Model covariates were prespecified based on clinically plausible patient‐ or hospital‐level characteristics that might have influenced the risk of 30‐day readmission. In model 1 we included patient‐level fixed effects, and in model 2 we included patient‐ and hospital‐level fixed effects (Table S1). We calculated the interclass correlation coefficients from the covariance parameter estimates of these models. Model fit was assessed using the likelihood‐ratio test. A P value of <0.05 was considered statistically significant. All analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC).
Results
Baseline Patient and Hospital Characteristics
From an initial sample of 35 221 patients (undergoing TAVR at 365 hospitals), there were 18 568 patients (undergoing TAVR at 329 hospitals) who were discharged alive either to home or a SNF with 30‐day follow‐up data available (Figure 1). Overall, 69% of patients were discharged directly home post‐TAVR. The distribution of direct‐home discharge is shown in Figure 2. The range of patients discharged directly home within each hospital quartile was as follows: quartile 1 (N=82 hospitals, 4473 patients) 9.3% to 59.2%; quartile 2 (N=81 hospitals, 5435 patients) 59.3% to 72.1%; quartile 3 (N=84 hospitals, 5011 patients) 72.2% to 81.6%; quartile 4 (N=82 hospitals, 3649 patients) 81.7% to 100.0%.
Figure 2.
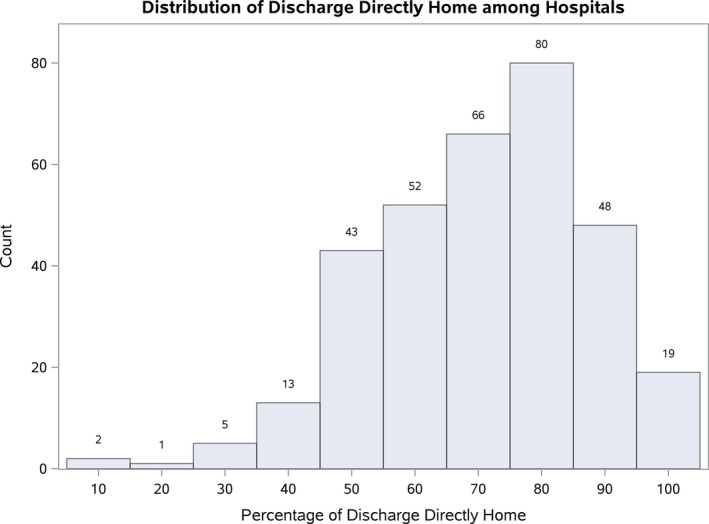
Distribution of direct‐home discharge among hospitals. Shown is the distribution of hospitals (N=329) based on the proportion of patients discharged directly home post‐TAVR. The median percentage direct‐home discharge rate among hospitals was 72.2%. TAVR indicates transcatheter aortic valve replacement.
Patient and hospital characteristics across quartiles are shown in Table. Hospitals in the highest quartile of direct‐home discharge (quartile 4) on average had fewer female patients compared with hospitals in the lowest quartile (quartile 1) (44.6% versus 51.8%, P<0.001) and more nonwhite patients (7.0% versus 3.5%, P<0.001). There were several statistically significant but numerically small differences in baseline comorbidities as shown in Table. For treatment characteristics, hospitals in the highest quartile of direct‐home discharge were more likely to use femoral access (75.2% versus 60.1% in Quartile 1, P<0.001), had fewer patients receiving general anesthesia (93.8% versus 98.0% in Quartile 1, P<0.001), and had fewer patients who received a transfusion (26.4% versus 40.9% in Quartile 1, P<0.001). As a composite measure of risk, median STS predicted mortality was lowest in hospitals with the highest rate of direct‐home discharge (6.3% in quartile 4 versus 6.9% in quartile 1, P<0.001).
Table 1.
Characteristics Across Quartiles of Direct Discharge Home Post‐TAVR
| Direct Discharge Home % (Number of Hospitals) [Number of Patents] | Overall (N=329) [18 568] | Quartile 1 9.3% to 59.2% (N=82) [4473] | Quartile 2 59.3% to 72.1% (N=81) [5435] | Quartile 3 72.2% to 81.6% (N=84) [5011] | Quartile 4 81.7% to 100.0% (N=82) [3649] | P Valuea |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age (median, IQR) | 84.0, 79.0 to 88.0 | 84.0, 79.0 to 88.0 | 84.0, 79.0 to 88.0 | 84.0, 78.0 to 88.0 | 83.0, 78.0 to 87.0 | <0.001 |
| Female sex, N (%) | 9028 (48.6) | 2317 (51.8) | 2655 (48.9) | 2429 (48.5) | 1627 (44.6) | <0.001 |
| Nonwhite race, N (%) | 865 (4.7) | 158 (3.5) | 239 (4.4) | 211 (4.2) | 257 (7.0) | <0.001 |
| Coronary disease, N (%) | 10 684 (57.5) | 2536 (56.7) | 3112 (57.3) | 2958 (59.0) | 2078 (57.0) | 0.129 |
| Atrial fibrillation, N (%) | 7762 (41.8) | 1974 (44.1) | 2298 (42.3) | 2121 (42.3) | 1369 (37.5) | <0.001 |
| Prior stroke, N (%) | 2223 (12.0) | 578 (12.9) | 629 (11.6) | 630 (12.6) | 386 (10.6) | 0.008 |
| Diabetes mellitus, N (%) | 6547 (35.3) | 1498 (33.5) | 1922 (35.4) | 1824 (36.4) | 1303 (35.7) | 0.017 |
| Peripheral arterial disease, N (%) | 5672 (30.6) | 1369 (30.6) | 1718 (31.6) | 1533 (30.6) | 1052 (28.8) | 0.094 |
| Heart failure, N (%) | 14 244 (76.7) | 3500 (78.3) | 4250 (78.2) | 3732 (74.5) | 2762 (75.7) | <0.001 |
| Chronic lung disease, N (%) | 5050 (27.2) | 1125 (25.2) | 1545 (28.4) | 1396 (27.9) | 984 (27.0) | 0.001 |
| Home oxygen, N (%) | 2365 (12.7) | 546 (12.2) | 673 (12.4) | 697 (13.9) | 449 (12.3) | 0.045 |
| Renal function, N (%) | 0.150 | |||||
| GFR ≥30 | 16 837 (90.7) | 4017 (89.8) | 4933 (90.8) | 4557 (90.9) | 3330 (91.3) | |
| GFR <30 | 996 (5.4) | 248 (5.5) | 311 (5.7) | 264 (5.3) | 173 (4.7) | |
| On dialysis | 689 (3.7) | 187 (4.2) | 185 (3.4) | 177 (3.5) | 140 (3.8) | |
| STS predicted mortality rate (median, IQR) | 6.8%, 4.5% to 10.2% | 6.9%, 4.7% to 10.3% | 7.0%, 4.7% to 10.6% | 6.8%, 4.5% to 10.3% | 6.3%, 4.2% to 9.4% | <0.001 |
| Treatment characteristics | ||||||
| LVEF (median, IQR) | 57%, 45% to 63% | 56%, 45% to 63% | 58%, 45% to 65% | 58%, 45% to 63% | 56%, 48% to 63% | 0.011 |
| Femoral access, N (%) | 12 473 (67.2) | 2688 (60.1) | 3468 (67.1) | 3393 (67.7) | 2744 (75.2) | <0.001 |
| General anesthesia, N (%) | 17 461 (95.0) | 4385 (98.0) | 5104 (93.9) | 4728 (94.4) | 3424 (93.8) | <0.001 |
| Transfusion, N (%) | 6300 (33.9) | 1829 (40.9) | 1941 (35.7) | 1568 (31.3) | 962 (26.4) | <0.001 |
| Vascular access complication, N (%) | 953 (8.0) | 220 (7.8) | 279 (7.8) | 240 (7.8) | 214 (8.8) | 0.491 |
| In‐hospital stroke/TIA, N (%) | 347 (1.9) | 81 (1.8) | 124 (2.3) | 89 (1.8) | 53 (1.5) | 0.037 |
| Cardiac arrest, N (%) | 396 (2.1) | 89 (2.0) | 135 (2.5) | 117 (2.3) | 55 (1.5) | 0.012 |
| Aortic dissection, N (%) | 38 (0.2) | 10 (0.2) | 12 (0.2) | 8 (0.2) | 8 (0.2) | 0.873 |
| Hours in ICU (median, IQR) | 37.0, 24.0 to 70.9 | 47.7, 25.0 to 76.0 | 40.7, 24.2 to 72.0 | 40.0, 24.2 to 70.5 | 28.0, 23.3 to 49.9 | <0.001 |
| Hospital characteristics | ||||||
| Region, N (%) | <0.001 | |||||
| Northeast | 76 (23.1) | 33 (40.2) | 26 (32.1) | 13 (15.5) | 4 (4.9) | |
| West | 58 (17.6) | 9 (11.0) | 12 (14.8) | 15 (17.9) | 22 (26.8) | |
| Midwest | 78 (23.7) | 25 (30.5) | 16 (19.8) | 21 (25.0) | 16 (19.5) | |
| South | 117 (35.6) | 15 (18.3) | 27 (33.3) | 35 (41.7) | 40 (48.8) | |
| Annual TAVR volume (median, IQR) | 21.0, 14.0 to 30.0 | 20.0, 13.0 to 25.0 | 24.0, 16.0 to 35.0 | 22.0, 15.0 to 32.5 | 19.0, 13.0 to 29.0 | 0.052 |
| Number of beds (median, IQR) | 533.0, 383.0 to 695.0 | 542.5, 352.0 to 687.0 | 571.0, 409.0 to 729.0 | 526.5, 393.5 to 664.5 | 496.0, 371.0 to 700.0 | 0.518 |
| Teaching hospital, N (%) | 207 (62.9) | 51 (62.2) | 54 (66.7) | 58 (69.1) | 44 (53.7) | 0.183 |
GFR indicates glomerular filtration rate; ICU, intensive care unit, IQR, interquartile range; LVEF, left ventricular ejection fraction; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack.
P value for comparison across hospital quartiles.
There were also significant regional differences among quartiles: hospitals in the highest quartile of direct home discharge were, on average, most likely to be in the Southern United States and least likely to be in the Northeast United States (Table and Figure 3). Patients in the Northeast United States had the highest median STS predicted mortality score, although the absolute difference between regions was small (median STS score: overall 6.8%; Northeast 7.0%.; South 6.6%; Midwest 6.9%; West 6.7%; P<0.001).
Figure 3.
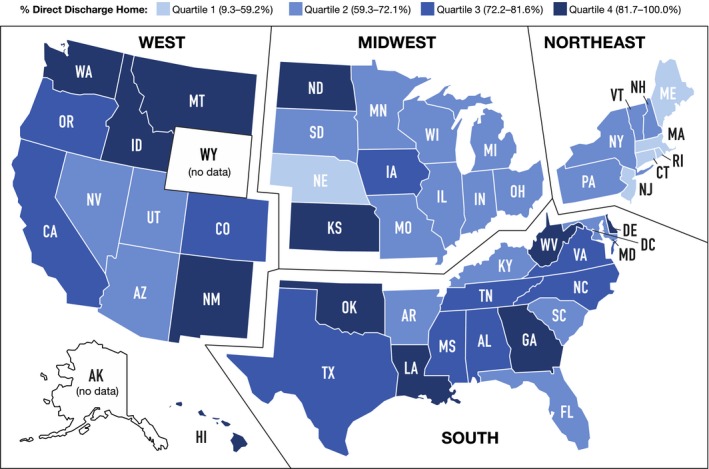
Association of US region with practice of discharge to skilled nursing facility. Shown are the 30‐day direct discharge home rates by state. Overall, the lowest rates of direct home discharge were in the Northeast United States.
Thirty‐Day Readmission and Mortality
The median 30‐day readmission rate among all hospitals post‐TAVR was 17.9% (interquartile range [IQR] 12.5% to 22.2%) (Figure 4). There was no significant difference in 30‐day readmission rate across hospital quartiles (quartile 1, 18.7% [IQR 13.0% to 24.2%]; quartile 2, 18.0% [IQR 13.7% to 20.6%]; quartile 3, 17.4% [IQR 13.3% to 22.2%]; quartile 4, 16.3% [IQR 11.6% to 21.1%]; P=0.14). Mortality within 30 days (among patients who survived to discharge) was rare in our sample; there were 201 patients (1.1%) who died during this period. Median 30‐day mortality rate among all hospitals was 0.0% (IQR 0.0% to 1.7%) and did not differ significantly between quartiles (P=0.39).
Figure 4.
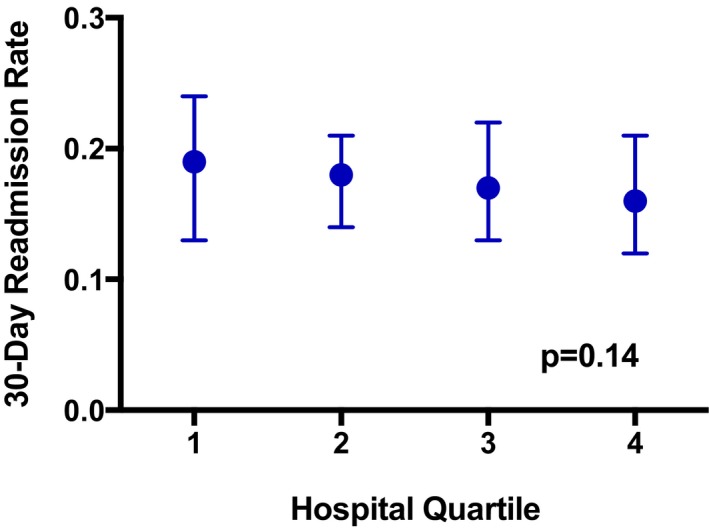
Thirty‐day readmission rates by quartile of direct home discharge. Shown are 30‐day readmission rates (median, interquartile range) for each category of direct discharge home (quartile 1=lowest direct home discharge rate, range 9.3% to 59.2%; quartile 4=highest direct home discharge rate, range 81.7% to 100.0%). There was no significant association between hospital category of direct discharge home and 30‐day readmission (P=0.14).
Adjusted 30‐Day Readmission
We adjusted for 30‐day readmission rates with a hierarchical regression model that accounted for patient‐ and hospital‐level characteristics. After multivariable adjustment, our finding of no statistically significant association between discharge disposition and readmission did not change (odds ratio [OR] quartile 4 versus quartile 1=0.89, 95%CI 0.76‐1.04; P=0.15). This finding persisted when we explored direct home discharge as a continuous variable rather than divided by hospital quartile (OR percentage direct home discharge=1.00, 95%CI=0.99‐1.00, P=0.29). The interclass correlation coefficient before multivariable adjustment was 0.155, implying that 15.5% of the variation in discharge disposition was accounted for by the hospitals, leaving 84.5% to be accounted for by other factors.
The risk factors for 30‐day readmission in our multivariable analysis are shown in Figure 5; the strongest risk factors were glomerular filtration rate (on dialysis versus glomerular filtration rate ≥30; OR=1.61, 95%CI 1.34‐1.93, P<0.001; glomerular filtration rate <30 versus ≥30; OR=1.33, 95%CI 1.14‐1.56, P<0.001), in‐hospital stroke or transient ischemic attack (OR=1.47, 95%CI 1.13‐1.89, P<0.001), and nonfemoral access (OR=1.43, 95%CI 1.31‐1.57, P<0.001). In a separate analysis that included the KCCQ and 5‐m walk test, among the subset of hospitals (N=248 hospitals, 10 055 patients) with sufficient data based on our thresholds for inclusion, we found that KCCQ score was independently associated with readmission (OR per 1‐point increase=0.99, 95%CI 0.99‐1.00, P<0.001), but slow gait speed was not (OR normal versus slow gait=0.90, 95%CI 0.75‐1.08, P=0.26). Including these elements in a multivariable model did not change our findings from the full sample, as there remained no statistically significant association between discharge disposition and readmission (OR quartile 4 versus quartile 1=0.92, 95%CI 0.76‐1.12, P=0.41).
Figure 5.
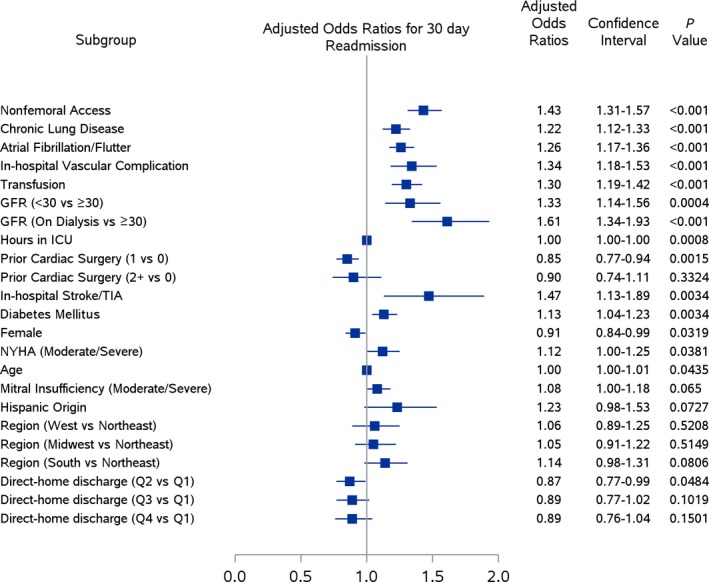
Risk factors for 30‐day readmission. Figure includes selected covariates (with P<0.10) for 30‐day readmission in our multivariable model. After adjustment, direct discharge home was not associated with 30‐day readmission across hospital quartiles (odds ratio quartile 4 vs quartile 1=0.89, 95%CI 0.76‐1.04). The strongest associations with 30‐day readmission were seen among patients with dialysis, in‐hospital stroke or TIA, and nonfemoral access. GFR indicates glomerular filtration rate; ICU, intensive care unit; TIA, transient ischemic attack.
Discussion
This study analyzed data from the national STS/ACC TVT Registry merged with Centers for Medicare and Medicaid Services data to evaluate the association between hospital practice of direct‐home versus SNF discharge and 30‐day hospital readmission among patients undergoing TAVR. There are several key findings. First, direct‐home discharge is common, representing over two thirds of our study sample. Second, we found no significant association between the hospital practice of discharge to home versus to SNF and 30‐day readmission, either before or after adjustment for relevant patient‐ and hospital‐level characteristics. Third, we found considerable differences across categories of direct‐home discharge. As expected, hospitals with the highest rate of direct‐home discharge had patients with a generally less complicated hospital course (eg, lower transfusion rate, fewer hours in intensive care unit). Less expected was the considerable US regional variation in SNF use; for example, over half of hospitals in the highest quartile of direct‐home versus SNF discharge were in the Southern United States, whereas 40% of hospitals in the lowest quartile were in the Northeast United States.
Unanticipated hospital readmissions are costly, can be a marker of poor healthcare quality, and are disruptive for patients.11 In light of these factors, there has been a considerable effort by payers and health systems to understand and prevent the causes of readmissions, especially in the early postdischarge period. In 2012, the Centers for Medicare and Medicaid Services instituted the Hospital Readmissions Reduction Program for selected conditions, whereby hospitals with excessive 30‐day readmission rates were penalized financially.12 Although TAVR does not yet fall under this program, understanding 30‐day readmissions post‐TAVR is critical given the marked growth in the procedure over recent years; for example, in the United States the number of commercial TAVR procedures increased from 4627 in 2012 to 24 808 in 2015.13 In addition, while indications for TAVR are evolving, patients undergoing the procedure have historically been at high or prohibitive risk for traditional surgical aortic valve replacement and therefore have many characteristics known to increase readmission risk including advanced age and comorbidities.1 The 30‐day readmission rate among our sample was 17.9%; a prior study by Holmes et al using the STS/ACC TVT Registry (November 2011 through June 2013) found a comparable rate (17.4%).1 Despite the advanced age and comorbidities of patients undergoing TAVR, these rates are relatively similar to other types of cardiac surgery including isolated surgical aortic valve replacement (19.0%)14 and CABG (17.4%).15 As with these other operations, the exact proportion of post‐TAVR readmissions that are preventable is unclear. Among patients with heart failure, where some of the most extensive work on readmissions has been done, a meta‐analysis estimated that only one‐quarter of readmissions within 30 days were preventable with appropriate interventions.16
Intuitively, hospitals with a higher proportion of patients discharged to SNF would be expected to have patients with a greater degree of comorbidity and less functional independence, as SNF discharge is typically reserved for patients who are either debilitated at baseline or become significantly deconditioned during their hospital stay. This was partially borne out in our data: hospitals with the lowest rate of direct‐home discharge (highest rate of discharge to SNF) had, on average, patients with a higher rate of transfusion, a lower rate of femoral access, and spent more hours in the intensive care unit. Patients in the lowest quartile of direct‐home discharge also had higher rates of selected medical conditions (eg, heart failure, atrial fibrillation), although we did not see a consistent trend across comorbidities. Although the lack of a significant relationship between the hospital practice of direct‐home discharge and 30‐day readmission may suggest that SNF discharge is protective (because patients in this quartile were more complicated), there was still no difference in 30‐day readmission even after adjusting for differences between quartiles.
Based on this finding, it appears that factors other than discharge disposition predominate in determining readmission risk. For example, we found that hemodialysis had a strong association with readmission risk (60% greater odds of readmission compared with nonhemodialysis). Frequent hospitalizations among hemodialysis patients have been previously documented17 and may be due to issues such as infection, volume overload, access site malfunction, or electrolyte disturbances. In‐hospital stroke/transient ischemic attack was another factor associated with 30‐day readmission; plausibly, these patients may have experienced complications postdischarge (eg, falls, aspiration, recurrent stroke) that required hospital readmission. Nonfemoral access also conferred greater readmission risk in our sample. These patients are systematically different from those with femoral access—typically with greater comorbidities—and are at higher generalized risk, highlighted in a recent study by O'Brian et al from the STS/ACC TVT Registry, which found that patients with nonfemoral access had nearly double the risk of in‐hospital mortality.18 Selected readmissions in our sample also may have been largely an event with no clear predictor; previous investigations in other conditions such as heart failure and acute myocardial infarction have documented that predictive risk models for 30‐day readmission have poor discrimination,19 possibly in part due to the discretionary nature of readmission.
Our finding of considerable regional variation within quartiles of SNF discharge (with highest rates in the Northeast United States and lowest rates in the Southern and Western United States) suggests that, for many patients, SNF discharge may be largely based on local institutional practice. SNF placement is clearly needed in selected patients with factors such as significant deconditioning, functional impairments, and/or insufficient social support, prior to transitioning home. The SNF environment can provide more comprehensive physical rehabilitation than available at home, as well as direct supervision of functional recovery, close ascertainment of goals attained, support of nutritional needs, and regular medication administration.4 All of these may contribute to recovery in the most debilitated patients. However, regional variation in SNF use has been documented in other populations.8, 20 For example, a report by Allen among older patients hospitalized for heart failure found that patients in the Northeast United States were discharged to SNF at a 3‐fold higher rate than patients in the Western United States.8 Our study was not designed to investigate this phenomenon in depth, but the discretionary variation in SNF discharge may contribute to its lack of significance in predicting 30‐day readmission.
These findings must be interpreted in the context of the study design. First, not all Registry patients were linked with Centers for Medicare and Medicaid Services data, and therefore, we are unable to comment on the readmission rate in the subset that could not be linked. These patients may have been systematically different from those for whom linkage was possible. However, to our knowledge, our final study population still represents the largest study to date focusing on discharge practices post‐TAVR and 30‐day readmission. Second, although we were able to investigate both gait speed and patient‐reported health status by using the 5‐m walk test and KCCQ, respectively, these data were available for only a limited subset of patients, and incorporating these measures did not change our primary findings. We were also unable to analyze other covariates of potential relevance to older patients, such as frailty or cognitive impairment, because they are not currently collected in the STS/ACC TVT Registry. Third, our study was not designed to investigate what proportion of readmissions were preventable or to identify strategies to reduce readmissions. Readmissions reduction strategies may include closer outpatient follow‐up, intensive physical therapy, patient education, and remote hemodynamic or telemetry monitoring; however, all of these would need to be tested prospectively. Finally, the STS/ACC TVT Registry includes only patients receiving commercially available devices, and we therefore cannot comment on outcomes among individuals who received investigational, newer‐generation transcatheter valves during the study period.
In conclusion, in a large national US cohort of patients with advanced age and multiple comorbidities undergoing TAVR, there was no significant association between hospital practice of discharge disposition to home versus SNF and 30‐day readmission. Further research is necessary to understand reasons for the significant regional variations in the practice of direct‐home discharge as well as what proportion of readmissions are preventable.
Sources of Funding
Dr Dodson receives support from a Patient Oriented Career Development Award (K23 AG052463) from the NIH/National Institute of Aging, and a Mentored Clinical and Population Research Award from the American Heart Association. Dr Blaum receives support from the John A. Hartford Foundation and the Patient Centered Outcomes Research Institute. Dr Cohen receives support from Medtronic, Edwards Lifesciences, and Boston Scientific. The analyses in this article were funded by the STS/ACC TVT Registry. The funding agency was responsible for initial collection of Registry data and reviewed the article prior to submission.
Disclosures
Dr Dodson serves as a Consultant for Novartis Pharmaceuticals on a research project unrelated to the current study. Dr Cohen serves as a Consultant for Medtronic and Edwards Lifesciences. Dr Rumsfeld was Chief Science Officer for the National Cardiovascular Data Registry during this study. Dr Williams has no relevant disclosures. Manandhar has no relevant disclosures. Dr Vemulapalli has no relevant disclosures. Dr Blaum has no relevant disclosures. Dr Zhong has no relevant disclosures. Dr Hochman has no relevant disclosures.
Supporting information
Table S1. Model Elements
(J Am Heart Assoc. 2017;6:e006127 DOI: 10.1161/JAHA.117.006127.)28862964
References
- 1. Holmes DR, Brennan J, Rumsfeld JS, Dai D, O'Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. [DOI] [PubMed] [Google Scholar]
- 2. Krumholz HM. Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Philbin EF, DiSalvo TG. Prediction of hospital readmission for heart failure: development of a simple risk score based on administrative data. J Am Coll Cardiol. 1999;33:1560–1566. [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Ross JS, Carlson MDA, Lin Z, Normand SL, Bernheim SM, Drye EE, Ling SM, Han LF, Rapp MT, Krumholz HM. Skilled nursing facility referral and hospital readmission rates after heart failure or myocardial infarction. Am J Med. 2012;125:100.e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bozic KJ, Wagie A, Naessens JM, Berry DJ, Rubash HE. Predictors of discharge to an inpatient extended care facility after total hip or knee arthroplasty. J Arthroplasty. 2006;21:151–156. [DOI] [PubMed] [Google Scholar]
- 6. Sacks GD, Hill C, Rogers SO. Insurance status and hospital discharge disposition after trauma: inequities in access to postacute care. J Trauma Acute Care Surg. 2011;71:1011–1015. [DOI] [PubMed] [Google Scholar]
- 7. Munin MC, Kwoh CK, Glynn N, Crossett L, Rubash HE. Predicting discharge outcome after elective hip and knee arthroplasty. Am J Phys Med Rehabil. 1995;74:294–301. [DOI] [PubMed] [Google Scholar]
- 8. Allen LA, Hernandez AF, Peterson ED, Curtis LH, Dai D, Masoudi FA, Bhatt DL, Heidenreich PA, Fonarow GC. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, Shahian D, Tuzcu EM, Peterson ED, Rumsfeld JS, Hewitt K, Shewan C, Michaels J, Christensen B, Christian A, O'Brien S, Holmes D. Outcomes following transcatheter aortic valve replacement in the United States. J Am Med Assoc. 2013;310:2069–2077. [DOI] [PubMed] [Google Scholar]
- 10. American College of Cardiology; Society of Thoracic Surgeons . ACC/STS TVT Registry: data collection. Available at: https://www.ncdr.com/webncdr/tvt/home/data-collection. Accessed December 15, 2016.
- 11. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Readmissions reduction program (HRPP): Department of Health and Human Services Centers for Medicare & Medicaid Services. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed December 15, 2016.
- 13. Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE, Jacobs JP, Hanzel G, Bavaria JE, Tuzcu EM, Peterson ED, Fitzgerald S, Kourtis M, Michaels J, Christensen B, Seward WF, Hewitt K, Holmes DR Jr. 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2016;69:1215–1230. [DOI] [PubMed] [Google Scholar]
- 14. Barreto‐Filho J, Wang Y, Dodson JA, Desai MM, Sugeng L, Geirsson A, Krumholz HM. Trends in aortic valve replacement for elderly patients in the United States, 1999–2011. JAMA. 2013;310:2078–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Walraven C, Jennings A, Forster AJ. A meta‐analysis of hospital 30‐day avoidable readmission rates. J Eval Clin Pract. 2012;18:1211–1218. [DOI] [PubMed] [Google Scholar]
- 17. Chan KE, Lazarus M, Wingard RL, Hakim RM. Association between repeat hospitalization and early intervention in dialysis patients following hospital discharge. Kidney Int. 2009;76:331–341. [DOI] [PubMed] [Google Scholar]
- 18. O'Brien SM, Cohen DJ, Rumsfeld JS, Brennan JM, Shahian DM, Dai D, Holmes DR, Hakim RB, Thourani VH, Peterson ED, Edwards FH. Variation in hospital risk‐adjusted mortality rates following transcatheter aortic valve replacement in the United States: a report from the Society of Thoracic Surgeons and American College of Cardiology National Transcatheter Valve Therapies Registry. Circ Cardiovasc Qual Outcomes. 2016;9:560–565. [DOI] [PubMed] [Google Scholar]
- 19. Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, Kripalani S. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368:1465–1468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Model Elements


