Abstract
Background
Single‐electrode ablation of the main renal artery for renal sympathetic denervation showed mixed blood pressure (BP)‐lowering effects. Further improvement of the technique seems crucial to optimize effectiveness of the procedure. Because sympathetic nerve fibers are closer to the lumen in the distal part of the renal artery, treatment of the distal main artery and its branches has been shown to reduce variability in treatment effects in preclinical studies and a recent randomized trial. Whether this optimized technique improves clinical outcomes remains uncertain. We report a 2‐center experience of main renal artery and combined main renal artery plus branches renal denervation in patients with resistant hypertension using a multielectrode catheter.
Methods and Results
Twenty‐five patients with therapy‐resistant hypertension underwent renal sympathetic denervation with combined main renal artery and renal branch ablation and were compared to matched controls undergoing an ablation of the main renal artery only. BP change was assessed by ambulatory measurement at baseline and after 3 months. At baseline, BP was balanced between the groups. After 3 months, BP changed significantly in the combined ablation group (systolic/diastolic 24‐hour mean and daytime mean BP −8.5±9.8/−7.0±10.7 and −9.4±9.8/−7.1±13.5 mm Hg, P<0.001/0.003 and <0.001/0.016, respectively), but not in patients with main artery treatment (−3.5±11.1/−2.0±7.6 and −2.8±10.9/−1.8±7.7 mm Hg, P=0.19/0.20 and 0.19/0.24, respectively). Systolic daytime BP was significantly more reduced in patients with combined ablation than in patients with main artery ablation (P=0.033).
Conclusions
Combined ablation of the main renal artery and branches appears to improve BP‐lowering efficacy and should be further investigated.
Keywords: branch ablation, combined ablation, hypertension, kidney, renal nerves, renal sympathetic denervation, resistant hypertension
Subject Categories: Treatment, Hypertension
Clinical Perspective
What Is New?
In this balanced cohort of patients with severe therapy‐resistant arterial hypertension, a combined ablation of the main renal artery and its branches appears to improve blood‐pressure–lowering efficacy as compared with an ablation of the main renal artery only.
What Are the Clinical Implications?
The combined ablation approach might help to overcome the lack of procedural reliability of an ablation of the main renal artery only, considered to date the standard approach of renal sympathetic denervation.
Introduction
Single‐electrode ablation of the main renal artery for renal sympathetic denervation (RDN) showed mixed blood‐pressure (BP)–lowering effects.1, 2, 3, 4, 5 The neutral outcome of the Symplicity HTN‐3 trial is part of an ongoing debate as many confounders such as unsatisfactory medication adherence, unfavorable patient selection, and limitations of procedural methods and techniques might have contributed to the results.6 The last includes potential incomplete/insufficient ablation due to a low number of ablation points, especially as patients receiving more complete ablations showed an improved BP response following RDN.6, 7 Consequently, further improvement of the technique and technology appears mandatory to optimize its effectiveness. Because the sympathetic nerve fibers are closer to the lumen in the distal part of the renal vessel,8 ablation of the distal main artery and the side branches could pose a promising approach. This hypothesis has been supported by 2 recent animal studies, showing improved reduction of norepinephrine spillover after combined ablation of the main renal artery and its branches.9, 10 Recent results from a smaller randomized trial suggest an additive effect of a combined ablation approach.11 However, in the latter trial, presence of the 2 most important predictors for response to RDN, baseline BP6, 7, 12, 13 and the presence of isolated systolic hypertension (ISH),7, 13 was not well balanced between the groups. Also, data on overall safety of this revised technique are scarce. Therefore, we aimed to compare the efficacy and safety of a main renal artery ablation and a combined ablation of the main renal artery, side branches, and accessory arteries in a prospective cohort.
Methods
Patient Selection and Follow‐Up
Patients aged between 18 and 75 years were eligible if they were diagnosed with therapy‐resistant hypertension, defined as mean daytime systolic BP ≥135 mm Hg or diastolic BP ≥90 mm Hg in 24‐hour ambulatory blood pressure measurement (ABPM) despite the intake of at least 3 antihypertensive agents, including at least 1 diuretic, for at least 4 weeks before RDN.14 Patients and treating physicians were asked to maintain the antihypertensive medication unchanged for a follow‐up period of at least 3 months, if possible. Patient medication adherence was updated at follow‐up visits, relying on information provided by the patients and general practitioners. Patients with a renal anatomy unsuitable for denervation were excluded. Patients were included into this analysis if treated with a combined ablation of the main renal artery and its branches after treatment practice has been adapted as a consequence of the aforementioned observations. The control group consisted of patients with the same inclusion and exclusion criteria who underwent ablation of the main renal artery only using the same device. Patients were matched by the presence of ISH and by age at the time of the procedure. An age difference of ±5 years between the matched patients was tolerated. All patients provided written informed consent.
Ambulatory Blood Pressure Measurement
ABPM was acertained with a cuff‐based oscillometric device at baseline and after 3 months. BP recordings were performed every 15 minutes during the day (7:00 am to 10:00 pm) and every 30 minutes during the night (10:00 pm to 7:00 am) according to the latest European Society of Cardiology guidelines.15 BP recordings were analyzed with dedicated software.
Renal Denervation
RDN was performed with a multielectrode radiofrequency‐based catheter in both groups (Symplicity Spyral™, Medtronic, Minneapolis, MN) according to a standardized protocol. Multiple ablation runs of 1 minute were delivered to each main renal artery from distal to proximal in both groups (Figures 1A and 2A, 2B). Additionally, all accessory arteries and side branches with an angiographic diameter of at least 3 mm were treated in the combined ablation group, beginning at the distal part of the vessel (Figures 1B and 2C). Lesions were placed outside the kidney's contours on fluoroscopy to prevent renal parenchyma damage. All patients received intravenous remifentanil or morphine for pain control. All procedures were performed by experienced interventional cardiologists.
Figure 1.
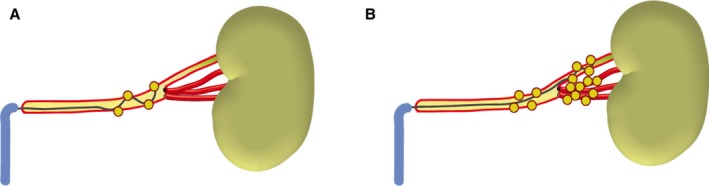
Ablation of the main renal artery only (A) and combined ablation of the main renal artery and its branches (B).
Figure 2.
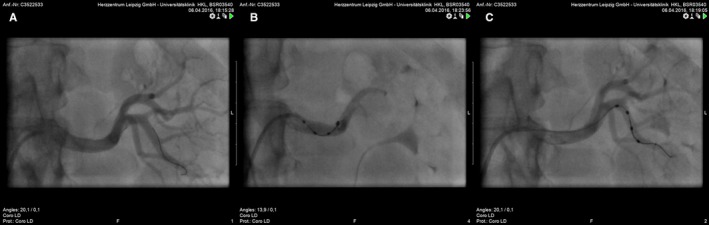
Left renal angiogram before ablation (A) and after ablation of left main artery (B) and branch artery (C).
Safety Assessment
Part of the combined ablation group underwent magnetic resonance imaging before RDN and after 3 months if possible to detect any preexisting or procedure‐related renal artery alterations or stenosis. Repeated renal artery duplex sonography was applied in patients with contraindications for magnetic resonance imaging if possible. Serum creatinine and estimated glomerular filtration rates were assessed at baseline and after 3 months for both groups.
Definitions
Patients with a drop of ≥5 mm Hg in daytime average BP on ABPM after 3 months were defined as responders.16 All other patients were considered nonresponders. ISH was defined as a 24‐hour systolic BP >130 mm Hg and diastolic BP <80 mm Hg in ABPM following the latest recommendations of the European Society of Hypertension.17
Statistical Analysis
Categorical variables are expressed as number and percentage of patients. Continuous data are reported as means and standard deviation. A 2‐tailed paired Student t test was used to compare continuous data, and chi‐squared test was used for categorical variables. Normal distribution of data was verified by the Kolmogorov‐Smirnov‐test.
Results
Baseline Characteristics and Blood Pressure
Baseline characteristics, BP, and medication were comparable in both groups, except for a higher prescription rate of aldosterone antagonists and more patients on maximum tolerated dose of calcium channel antagonists in the combined ablation group (Tables 1, 2 through 3). After 3 months, BP changed significantly in the combined ablation group (systolic/diastolic 24‐hour mean, and daytime mean BP −8.5±9.8/−7.0±10.7 mm Hg and −9.4±9.8/−7.1±13.5 mm Hg, P<0.001/0.003 and <0.001/0.016, respectively, Figure 3), but not in patients with main artery treatment only (−3.5±11.1/−2.0±7.6 and −2.8±10.9/−1.8±7.7 mm Hg, P=0.19/0.20 and 0.19/0.24, respectively, Figure 3). Systolic daytime BP was significantly more reduced in patients with combined ablation than in patients with main artery ablation, and diastolic and 24‐hour BP tended to be lower in patients with combined ablation (P=0.090/0.033 and 0.060/0.090 for 24‐hour/daytime systolic and diastolic BP).
Table 1.
Clinical Baseline Characteristics
| Combined Ablation (n=25) | Main Artery Ablation (n=25) | P Value | |
|---|---|---|---|
| Age, y | 61.8±9.3 | 62.8±9.5 | 0.60 |
| Body mass index, kg/m² | 31.2±5.1 | 30.9±5.1 | 0.86 |
| White, % | 100 (100) | 100 (100) | 1.0 |
| Female, % | 9 (36) | 11 (44) | 0.56 |
| Smoker, % | 13 (52) | 5 (20) | 0.02 |
| Diabetes Mellitus, % | 10 (40) | 11 (44) | 0.77 |
| Peripheral artery disease, % | 1 (4) | 2 (8) | 0.55 |
| Coronary artery disease, % | 12 (48) | 6 (24) | 0.077 |
| History of stroke or transitory ischemic attack, % | 1 (4) | 1 (4) | 1.0 |
| History of myocardial infarction, % | 6 (24) | 1 (4) | 0.042 |
| Hypercholesterolemia, % | 18 (72) | 15 (60) | 0.37 |
| Atrial fibrillation, % | 3 (12) | 4 (16) | 0.68 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 74.1±20.3 | 80.2±18.0 | 0.16 |
| Isolated systolic hypertension, % | 9 (36) | 9 (36) | 1.0 |
Table 2.
Baseline Medication
| Combined Ablation (n=25) | Main Artery Ablation (n=25) | P Value | |
|---|---|---|---|
| Number of drug classes | 5.6±1.2 | 4.9±1.4 | 0.54 |
| Five or more drug classes, % | 20 (80) | 15 (60) | 0.12 |
| Angiotensin‐converting enzyme inhibitors, % | 6 (24) | 10 (40) | 0.23 |
| Maximum dosage, % | 5 (20) | 7 (28) | 0.51 |
| Angiotensin receptor antagonist, % | 19 (76) | 15 (60) | 0.23 |
| Maximum dosage, % | 16 (72) | 13 (68) | 0.39 |
| Renin antagonist, % | 1 (4) | 2 (8) | 0.55 |
| Maximum dosage, % | 0 (0) | 2 (8) | 0.15 |
| β‐Blockers, % | 22 (88) | 23 (92) | 0.64 |
| Maximum dosage, % | 14 (56) | 12 (48) | 0.57 |
| Calcium channel antagonists, % | 22 (88) | 18 (72) | 0.16 |
| Maximum dosage, % | 19 (76) | 12 (48) | 0.041 |
| Diuretics, % | 25 (100) | 22 (88) | 0.08 |
| Maximum dosage, % | 16 (64) | 10 (40) | 0.09 |
| Second diuretic, % | 5 (20) | 2 (8) | 0.22 |
| Maximum dosage, % | 0 (0) | 0 (0) | n/a |
| Aldosterone antagonists, % | 8 (32) | 2 (8) | 0.034 |
| Maximum dosage, % | 7 (28) | 1 (4) | 0.021 |
| Vasodilators, % | 2 (8) | 5 (20) | 0.22 |
| Maximum dosage, % | 1 (4) | 3 (12) | 0.30 |
| α‐Blockers, % | 9 (36) | 6 (24) | 0.36 |
| Maximum dosage, % | 6 (24) | 5 (20) | 0.73 |
| Centrally acting sympathicolytics, % | 17 (68) | 16 (64) | 0.52 |
Table 3.
Baseline BP
| Combined Ablation (n=25) | Main Artery Ablation (n=25) | P Value (Baseline) | P Value (Δ Between Group) | |||
|---|---|---|---|---|---|---|
| Baseline | Δ 3 Months | Baseline | Δ 3 Months | |||
| 24‐h systolic, mm Hg | 152.7±12.4 | −8.5±9.8 | 153.0±17.6 | −3.5±11.1 | 0.93 | 0.091 |
| 24‐h diastolic, mm Hg | 87.7±16.0 | −7.0±10.7 | 84.6±11.5 | −2.0±7.6 | 0.44 | 0.060 |
| Daytime systolic, mm Hg | 155.3±11.7 | −9.4±9.8 | 156.2±16.8 | −2.8±10.9 | 0.82 | 0.033 |
| Daytime diastolic, mm Hg | 89.2±17.4 | −7.1±13.5 | 87.4±12.0 | −1.8±7.7 | 0.68 | 0.090 |
| Nighttime systolic, mm Hg | 144.4±19.8 | −7.4±15.6 | 144.4±23.1 | −5.9±18.5 | 1.00 | 0.75 |
| Nighttime diastolic, mm Hg | 80.6±16.2 | −5.5±10.4 | 76.9±12.3 | −4.0±10.8 | 0.38 | 0.60 |
BP indicates blood pressure.
Figure 3.
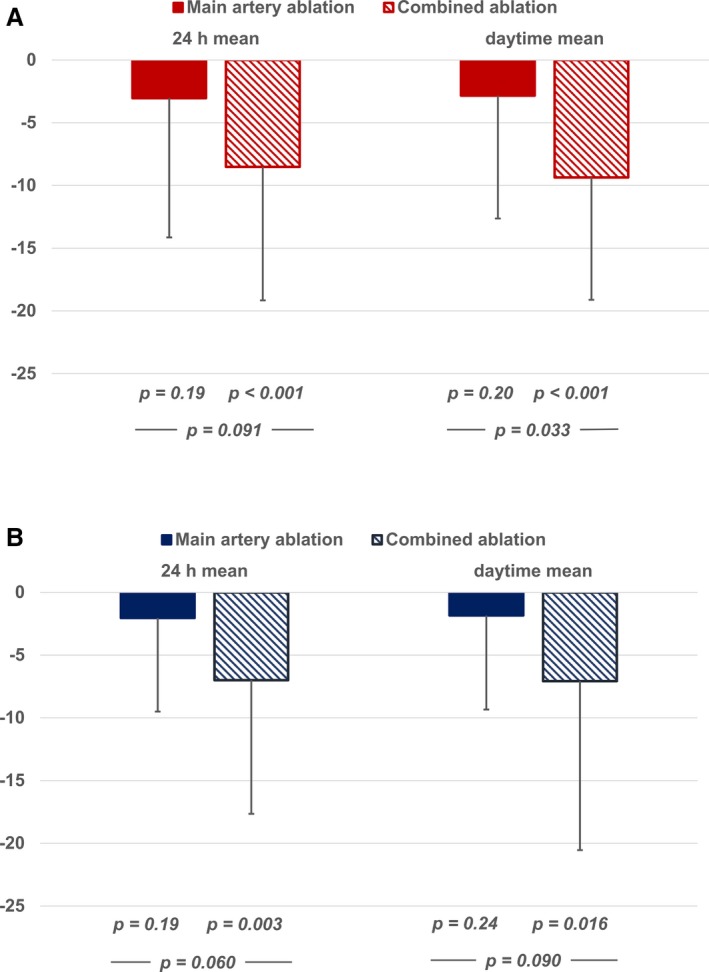
Mean systolic (A) and diastolic (B) blood pressure change in ambulatory blood pressure measurements after 3 months (in mm Hg).
Nocturnal BP dropped significantly in patients with combined ablation but was insignificantly reduced in patients with main artery ablation (−7.4±15.6 and −5.5±10.4 mm Hg versus −5.9±18.5 and −4.0±10.8 mm Hg for systolic and diastolic BP, P=0.027 and 0.014 versus 0.12 and 0.08), and the between‐group comparison was not significant (P=0.75 and 0.60).
The responder rate (≥5 mm Hg daytime BP drop at 3 months) was numerically higher in the combined ablation group (72% versus 48%) but did not reach statistical significance (P=0.083). Antihypertensive medication was reduced in 3 patients in the combined ablation group and remained stable in the main artery ablation group.
Procedural Characteristics and Safety
The number of ablation points was significantly higher in the combined ablation group. The volume of contrast agent administered and fluoroscopy times were significantly higher in the combined ablation group (Table 4).
Table 4.
Procedural Characteristics and Safety
| Combined Ablation (n=25) | Main Artery Ablation (n=25) | P Value | |
|---|---|---|---|
| Ablation points right renal artery | 19.9±6.6 | 9.1±3.7 | <0.001 |
| Ablation points left renal artery | 16.1±6.4 | 8.7±3.6 | <0.001 |
| Contrast agent used, mL | 106.6±43.8 | 70.4±40.7 | 0.010 |
| Irradiation time, min | 14.3±10.2 | 8.8±6.2 | 0.033 |
| Mean change in estimated glomerular filtration rate, μmol/L | −0.5±7.2 | −2.0±6.9 | 0.51 |
Fifteen patients in the combined ablation group underwent renal artery magnetic resonance angiogram at baseline and follow‐up. The remaining 10 patients underwent renal artery duplex sonography instead. No renal artery stenosis was detected at 3 months either in the main artery or in the side branches or any accessory artery. Renal function measured by estimated glomerular filtration rate remained unchanged in both groups (Table 4). No adverse events were observed in any of the groups. One patient in the combined ablation group had to reduce her antihypertensive medication because of symptomatic hypotension (dizziness) after the 3‐month follow‐up.
Discussion
We present data from a balanced cohort of patients with resistant hypertension undergoing a combined ablation approach of the main renal artery, its branches, and accessories. Our findings suggest that ablation of renal artery branches is feasible and safe. Moreover, our results show a significant reduction of BP 3 months after combined ablation in contrast to an insignificant change in the matched control group.
The lack of a significant BP reduction in patients undergoing main artery ablation only highlights that both groups represent severely hypertensive patients at an advanced stage of their disease and, importantly, a per se unfavorable pattern for RDN, with more than a third of the treated patients having ISH, an established predictor for poor BP response.7, 13 Furthermore, ISH is associated with elevated arterial stiffness,18 yet another predictor for poor treatment outcome after RDN.19 Despite that, and against the odds, a significant BP reduction can be achieved using the combined ablation approach. This is especially encouraging, as responder rates in patients undergoing combined ablation also tended to be higher. Renal nerves are located closer to the lumen in the distal sections of the renal arteries and branches as compared with the main artery,8 so it is plausible that limitation in penetration depth can be compensated with this strategy. Therefore, this might indicate a true improvement of procedural efficacy, resulting in a higher success rate as compared with main vessel ablation even in patients with an unfavorable profile at baseline. As the average number of ablation points was significantly higher in the combined ablation group, one could argue that the observed results may in part be explained by the higher overall number of ablations rather than by the location of lesion placement. However, recent preclinical studies were unable to prove a linear dose‐response relationship with increasing numbers of ablations in the main renal artery but documented a superior effect by placement of lesions in the renal artery branches over lesion placement in the main artery.9, 10
Our overall BP effects are below the results of a recently published randomized trial by Pekarskiy et al.11 Compared with this trial, average baseline systolic BP on ABPM was lower in our trial cohort (153 mm Hg versus 170 mm Hg in the combined ablation groups), which is usually associated with a less pronounced BP drop following RDN.6, 7, 12, 13 Further, as baseline systolic BP on ABPM was not well balanced between the randomized groups (170 mm Hg versus 158 mm Hg), it seems possible that the overall results of this trial, especially the enormous BP drop in the combined ablation group, can partly be explained by regression to mean. In contrast to this, our cohort is well balanced regarding these baseline characteristics.
An expanded ablation of the renal arteries is associated with longer fluoroscopy time and higher use of contrast medium. Importantly, renal function remained stable following the procedure, and no new renal artery stenoses were detected. This is in line with the results of 2 recent trials investigating renal artery branch ablation, where no alterations in duplex sonographic renal blood flow11 or catheter‐based renal angiogram (J. Davies, MD, unpublished data, presented at EuroPCR 2016) could be observed at follow‐up. However, long‐term safety of this optimized ablation approach clearly warrants further investigation in a larger prospective cohort. The results of the ongoing SPYRAL‐HTN (Global Clinical Study of Renal Denervation With the Symplicity Spyral™ Multi‐electrode Renal Denervation System in Patients With Uncontrolled Hypertension) trial will allow more definite conclusions.20 Likely, a better patient selection, namely treating patients with less advanced hypertension, combined systolic/diastolic hypertension, and lower arterial stiffness, could improve the outcome after combined RDN of the main arteries and branches even more.
It is important to note that especially in patients with preexisting cardiovascular diseases, a reduction of systolic blood pressure is associated with a marked risk reduction.21, 22 Frequently, these patients present with uncontrolled hypertension despite being under multiple‐drug treatment. Thus, reducing blood pressure to target values is challenging, especially as insufficient adherence to antihypertensive medication is frequently occurring.23 A reliable, reproducible, and effective interventional approach bares the hope to expend the to‐date still very limited armory against uncontrolled hypertension. A combined ablation approach of the main renal artery, its branches and accessories might represent a first step toward such an interventional treatment for arterial hypertension.
Limitations
The sample size is limited; therefore, between‐group changes reached only partial statistical significance. Therefore, our data should be interpreted as hypothesis‐generating. Second, our data are nonrandomized and warrant confirmation in larger, prospective, randomized trials (eg, NCT02920034). Third, patients included herein had relatively advanced hypertension; thus, these results cannot be generalized to the overall hypertensive population.
Conclusions
Combined ablation of the renal artery and its branches seems to be safe and to improve BP reduction over main artery RDN. This is very encouraging, as it might help to overcome the lack of procedural reliability of this to‐date considered standard approach of RDN. If these findings can be confirmed in larger cohorts, renal denervation may become a valuable treatment option for patients with uncontrolled hypertension.
Disclosures
Lurz, Ewen, and Mahfoud are consultants to Medtronic (Minneapolis, MN) and/or ReCor Medical (Palo Alto, CA). No other conflict of interest has been reported for any of the other authors.
(J Am Heart Assoc. 2017;6:e006196 DOI: 10.1161/JAHA.117.006196.)28862930
References
- 1. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier‐Vehier C, Courand PY, Lantelme P, Denolle T, Dourmap‐Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G; Renal Denervation for Hypertension (DENERHTN) Investigators . Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet. 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 2. Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha‐Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN‐3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 3. Desch S, Okon T, Heinemann D, Kulle K, Rohnert K, Sonnabend M, Petzold M, Muller U, Schuler G, Eitel I, Thiele H, Lurz P. Randomized sham‐controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202–1208. [DOI] [PubMed] [Google Scholar]
- 4. Rosa J, Widimsky P, Tousek P, Petrak O, Curila K, Waldauf P, Bednar F, Zelinka T, Holaj R, Strauch B, Somloova Z, Taborsky M, Vaclavik J, Kocianova E, Branny M, Nykl I, Jiravsky O, Widimsky J Jr. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true‐resistant hypertension: six‐month results from the Prague‐15 study. Hypertension. 2015;65:407–413. [DOI] [PubMed] [Google Scholar]
- 5. Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic‐nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. [DOI] [PubMed] [Google Scholar]
- 6. Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O'Neill WW, Patel MR, Rocha‐Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN‐3 trial. Eur Heart J. 2015;36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, Bohm M. Reduced blood pressure‐lowering effect of catheter‐based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN‐3 and the Global SYMPLICITY Registry. Eur Heart J. 2017;38:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, Virmani R, Joner M. Anatomic assessment of sympathetic peri‐arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. [DOI] [PubMed] [Google Scholar]
- 9. Mahfoud F, Tunev S, Ewen S, Cremers B, Ruwart J, Schulz‐Jander D, Linz D, Davies J, Kandzari DE, Whitbourn R, Bohm M, Melder RJ. Impact of lesion placement on efficacy and safety of catheter‐based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766–1775. [DOI] [PubMed] [Google Scholar]
- 10. Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter‐based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens. 2015;28:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pekarskiy SE, Baev AE, Mordovin VF, Semke GV, Ripp TM, Falkovskaya AU, Lichikaki VA, Sitkova ES, Zubanova IV, Popov SV. Denervation of the distal renal arterial branches vs. conventional main renal artery treatment: a randomized controlled trial for treatment of resistant hypertension. J Hypertens. 2017;35:369–375. [DOI] [PubMed] [Google Scholar]
- 12. Bohm M, Mahfoud F, Ukena C, Hoppe UC, Narkiewicz K, Negoita M, Ruilope L, Schlaich MP, Schmieder RE, Whitbourn R, Williams B, Zeymer U, Zirlik A, Mancia G; GSR Investigators . First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension. 2015;65:766–774. [DOI] [PubMed] [Google Scholar]
- 13. Ewen S, Ukena C, Linz D, Kindermann I, Cremers B, Laufs U, Wagenpfeil S, Schmieder RE, Bohm M, Mahfoud F. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension. 2015;65:193–199. [DOI] [PubMed] [Google Scholar]
- 14. ESH/ESC Task Force for the Management of Arterial Hypertension . 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 15. O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring . European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 16. Mahfoud F, Bohm M, Azizi M, Pathak A, Durand Zaleski I, Ewen S, Tsioufis K, Andersson B, Blankestijn PJ, Burnier M, Chatellier G, Gafoor S, Grassi G, Joner M, Kjeldsen SE, Luscher TF, Lobo MD, Lotan C, Parati G, Redon J, Ruilope L, Sudano I, Ukena C, van Leeuwen E, Volpe M, Windecker S, Witkowski A, Wijns W, Zeller T, Schmieder RE. Proceedings from the European Clinical Consensus Conference for Renal Denervation: considerations on future clinical trial design. Eur Heart J. 2015;36:2219–2227. [DOI] [PubMed] [Google Scholar]
- 17. Parati G, Stergiou G, O'Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability . European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366. [DOI] [PubMed] [Google Scholar]
- 18. Wallace SM, Yasmin Y, McEniery CM, Maki‐Petaja KM, Booth AD, Cockcroft JR, Wilkinson IB. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007;50:228–233. [DOI] [PubMed] [Google Scholar]
- 19. Okon T, Rohnert K, Stiermaier T, Rommel KP, Muller U, Fengler K, Schuler G, Desch S, Lurz P. Invasive aortic pulse wave velocity as a marker for arterial stiffness predicts outcome of renal sympathetic denervation. EuroIntervention. 2016;12:e684–e692. [DOI] [PubMed] [Google Scholar]
- 20. Kandzari DE, Kario K, Mahfoud F, Cohen SA, Pilcher G, Pocock S, Townsend R, Weber MA, Bohm M. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF‐MED) and presence (SPYRAL HTN ON‐MED) of antihypertensive medications. Am Heart J. 2016;171:82–91. [DOI] [PubMed] [Google Scholar]
- 21. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 22. Group SR , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–2948. [DOI] [PubMed] [Google Scholar]


