Abstract
Background
We sought to examine the mortality impact of appropriate implantable cardioverter defibrillator (ICD) therapy between patients who received ICD for primary versus secondary prevention purposes.
Methods and Results
From a prospective, population‐based registry, we identified 7020 patients who underwent de novo ICD implantation between February 2007 and May 2012 in Ontario, Canada. The primary outcome was all‐cause mortality. We used multivariable Cox proportional hazard modeling to adjust for differences in baseline characteristics and analyzed the mortality impact of first appropriate ICD therapy (shock and antitachycardia pacing [ATP]) as a time‐varying covariate. There were 1929 (27.5%) patients who received ICDs for secondary prevention purposes. The median follow‐up period was 5.02 years. Compared with those with secondary prevention ICDs, patients with primary prevention ICDs had more medical comorbidities, and lower ejection fraction. Patients who experienced appropriate ICD shock or ATP had greater risk of death compared with those who did not, irrespective of implant indication. In the primary prevention group, the adjusted hazard ratios of death for appropriate shock and ATP were 2.00 (95% CI: 1.72–2.33) and 1.73 (95% CI: 1.52–1.97), respectively. In the secondary prevention group, the adjusted hazard ratios of death for appropriate ICD shock and ATP were 1.46 (95% CI: 1.20–1.77) and 1.38 (95% CI: 1.16–1.64), respectively.
Conclusions
Despite having a more favorable clinical profile, occurrence of appropriate ICD shock or ATP in patients with secondary prevention ICDs was associated with similar magnitudes of mortality risk as those with primary prevention ICDs. A heightened degree of care is warranted for all patients who experience appropriate ICD shock or ATP therapy.
Keywords: cardiac arrhythmia, death, implantable cardioverter‐defibrillator, sudden, ventricular arrhythmia, ventricular fibrillation
Subject Categories: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Sudden Cardiac Death, Ventricular Fibrillation
Clinical Perspective
What Is New?
The mortality implications of appropriate implantable cardioverter defibrillator (ICD) therapy between primary and secondary prevention patients is provided from a prospective registry of ≈7020 de novo ICD patients not in a clinical trial setting.
Secondary prevention ICD patients have a lower clinical profile of risk with better left ventricular ejection fraction and fewer medical comorbidities, but have greater burden of ventricular arrhythmia and exhibit similar long‐term mortality as their primary prevention counterparts following therapy.
Appropriate ICD shocks for ventricular arrhythmia are associated with an adjusted ≈2‐fold increase of death in primary prevention patients and a 46% increase in secondary prevention patients.
Appropriate ICD antitachycardia pacing therapy for ventricular arrhythmia is associated with an adjusted 1.73‐fold increase of death in primary prevention patients and a 38% increase of death in secondary prevention patients.
What Are the Clinical Implications?
Ventricular arrhythmia treated by the ICD by means of shock or antitachycardia pacing is a marker of increased risk of death, irrespective of implant indication of primary or secondary prophylaxis.
Strategies to prevent the occurrence of ventricular arrhythmia are warranted.
Introduction
Implantable cardioverter defibrillators (ICD) are the mainstay for preventing sudden cardiac death in patients with structural heart disease and impaired left ventricular ejection fraction, as demonstrated in multiple randomized trials that employed ICD as either a primary or secondary prevention strategy.1, 2, 3 On the other hand, occurrence of an appropriate ICD shock is associated with a subsequent 3‐ to 5‐fold increased risk of death among patients with primary prevention ICDs.4, 5, 6, 7 Whether this association can be extended to patients with secondary prevention ICD is not well defined. Since the frequency of appropriate ICD shock is 2 to 3 times greater among patients with secondary prevention ICD,8 delineation of the association between appropriate ICD therapy and its subsequent impact on survival will have important implications for clinicians when communicating the expected outcomes of ICD therapy with these patients. Elucidating the mortality impact of ICD therapy in this population may enhance decision‐making when allocating healthcare resources for this presumably high‐risk patient subset, given their propensity for experiencing ventricular arrhythmias post‐ICD implant.
To address this question, we analyzed data from a prospective, population‐based registry of consecutive de novo ICD patients in Ontario, Canada in which healthcare costs are provided by a single payer. Clinical outcomes are ascertained by linkage with administrative databases. As this registry also collected data on all first appropriate ICD therapies (shock or antitachycardia pacing [ATP]), we were able to examine the association between ICD therapy (shock or ATP) and its impact on patients’ subsequent survival in relation to their implant indication.
Methods
Study Population
The Ontario ICD registry was a population‐based, prospective, multicenter registry that included all patients who underwent de novo ICD implant in the province between February 2007 and May 2012. Design, implementation, and maintenance of the Ontario ICD database have been previously published elsewhere.9 All patients referred for evaluation of ICD implantation were enrolled into the registry. The Ontario Ministry of Health and Long‐Term Care mandated this registry. This study was approved by the Institutional Review Board at Sunnybrook Health Sciences Centre (Toronto, Ontario, Canada) and complies with the Declaration of Helsinki.
All patients ≥18 years old and residents of Ontario were enrolled if they underwent de novo ICD implantation between February 2007 and May 2012, and informed consent was waived because it was a mandatory registry. The database accrued patients at the time of arrhythmia clinic assessment. For the purposes of this study, the date of implantation served as the index date (“time zero”) for the analysis. Patients were followed until death or until the end of the prespecified follow‐up period on March 31, 2015. Patients were excluded from this analysis if 1 of the following conditions was met: <18 years old; non‐Ontario residency or death before implant (n=19); received a secondary prevention ICD for an inherited arrhythmia syndrome, hypertrophic cardiomyopathy, or complex congenital heart disease (n=243); or if they had missing covariates for the multivariate analysis (n=587).
Data Sources
ICD data source
Trained personnel collected ICD data from all implantation centers in the province. Data included baseline clinical characteristics, ICD implant indications, and subsequent ICD therapies. Details on first appropriate ICD shocks and ATP were collected from patients’ ICD follow‐up visits, and they were adjudicated by electrophysiologists with high interobserver agreement.8 Primary prevention patients were defined as those who received ICD on a prophylactic basis without a prior history of sudden cardiac death, cardiac arrest, or sustained ventricular arrhythmia. Secondary prevention patients were defined as those who experienced resuscitated sudden cardiac death, cardiac arrest, or sustained ventricular arrhythmia before ICD implantation. Standardized programming was not mandated in this study given its observational design. Further details pertaining to data collection and quality assurance have been described elsewhere.9
Outcome data source
The primary outcome was all‐cause mortality, which was collected using each patient's unique, encoded health card number where ICD data were linked to Ontario provincial administrative databases for vital statistics, namely, the Registered Persons Database for death events. Vital statistics information was ascertained in all study patients until the end of study follow‐up. Database linkage using unique encoded identifiers was performed at the Institute for Clinical Evaluative Sciences in Ontario, Canada.
Statistical Analysis
We reported serum creatinine with median (interquartile range), and the Kruskal–Wallis test was used to compare the values. The other continuous variables were reported using mean±SD and comparisons were performed using ANOVA test. Categorical variables were reported as proportions and were compared using the χ2 statistic. There were 2 main exposures of interest in this study. First, we were interested in comparing outcomes between patients who received primary versus secondary prevention ICD. Second, we compared outcomes between patients who experienced ICD shock or ATP versus those who did not during study follow‐up. The primary analysis was a comparison of the rates of death between patients who experienced appropriate ICD therapy (shock or ATP) versus those who did not. Furthermore, we examined whether the rates of death differed between primary versus secondary prevention ICD patients who experienced appropriate shock or ATP. In order to conduct these analyses, we performed multivariable Cox proportional hazard modeling in which the occurrence of appropriate ICD therapy (shock or ATP) was analyzed as a time‐varying covariate. The date of the ICD implantation was assigned as the date of start of follow‐up and the end of the follow‐up was death or March 31, 2015, whichever happened first. When the cumulative incidence function curves were plotted, death was considered as a competing event, and the end of the follow‐up was death date or appropriate shock/ATP date or March 31, 2015, whichever happened first.
The evaluation of appropriate ATP was performed separately from the analysis of ICD shock. According to the design of the Ontario ICD Database, only the first appropriate ATP was recorded, and ATPs were not recorded if there was a preceding appropriate ICD shock. We included the following covariates in the model: implant indication (primary versus secondary), left ventricular ejection fraction, and the individual components of a validated mortality prediction score for ICD patients that we previously published (age, ischemic heart disease, previous revascularization procedure, previous heart failure hospitalization, New York Heart Association status III–IV, pre‐existing permanent pacemaker system, systolic blood pressure, diabetes mellitus, smoker, chronic obstructive lung disease, home oxygen therapy, cancer, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker treatment, creatinine, serum sodium, and hemoglobin).10 To examine whether the risk of death differed between primary and secondary prevention ICD patients, we added an interaction term between the occurrence of appropriate ICD therapy (shock or ATP) and implant indication. Statistical measures of significance were reported with hazard ratios (HR) with exact 95% CI. For all analyses, a 2‐sided P<0.05 was considered to be statistically significant. All analyses were performed with SAS 9.4 (Cary, NC, USA).
Results
Baseline Clinical Characteristics
Our study cohort consisted of 7020 patients and there were 5091 (72.5%) and 1929 (27.5%) patients who received primary and secondary prevention ICD, respectively (Table 1). Compared with those with secondary prevention ICDs, patients in the primary prevention group had lower left ventricular ejection fraction, higher New York Heart Association class, were more likely to have heart failure or diabetes mellitus, and more likely to receive cardiac resynchronization therapy. The use of heart failure medications was more frequent in the primary prevention group compared with the secondary prevention. On the other hand, amiodarone use at baseline was 4 times more common in the secondary group than the primary group. The median follow‐up period of the entire cohort was 5.02 years (interquartile range: 3.8–6.3 years).
Table 1.
Baseline Patient Characteristics by ICD Indication
| Primary Prevention | Secondary Prevention | P Value | |
|---|---|---|---|
| N=5091 | N=1929 | ||
| Demographics | |||
| Male, n (%) | 4037 (79.3) | 1560 (80.9) | 0.143 |
| Age at ICD implant date, n (%) | 64.51±11.93 | 65.47±12.58 | 0.003 |
| Cardiomyopathy details | |||
| Ischemic, n (%) | 4738 (93.1) | 1712 (88.8) | <0.001 |
| Ischemic+previous revascularization, n (%) | 3320 (65.2) | 1377 (71.4) | … |
| Previous heart failure, n (%) | 1886 (37.0) | 439 (22.8) | <0.001 |
| Device details | |||
| Cardiac resynchronization‐defibrillator, n (%) | 1678 (33.0) | 178 (9.2) | <0.001 |
| Dual‐chamber ICD, n (%) | 1311 (25.8) | 831 (43.1) | … |
| Single‐chamber ICD, n (%) | 2100 (41.2) | 918 (47.6) | … |
| Medical comorbidities | |||
| Atrial fibrillation, n (%) | 1506 (29.6) | 609 (31.6) | 0.105 |
| Diabetes mellitus, n (%) | 1571 (30.9) | 472 (24.5) | <0.001 |
| Current cigarette smoking, n (%) | 751 (14.8) | 325 (16.8) | 0.03 |
| Hypertension, n (%) | 2900 (57.0) | 1209 (62.7) | <0.001 |
| Stroke or transient ischemic attack, n (%) | 184 (3.6) | 87 (4.5) | 0.082 |
| Peripheral vascular disease, n (%) | 514 (10.1) | 219 (11.4) | 0.124 |
| COPD, n (%) | 636 (12.5) | 219 (11.4) | 0.192 |
| Clinical variables | |||
| Reported NYHA class | |||
| III or IV | 1778 (34.9%) | 283 (14.7%) | … |
| Systolic blood pressure, mean±SD | 121.46±19.91 | 124.62±20.08 | <0.001 |
| Mean QRS duration, mean±SD | 132.93±35.62 | 122.47±33.18 | <0.001 |
| Testing | |||
| Serum creatinine, median (IQR) | 96.00 (80.00–120.00) | 95.00 (80.00–117.00) | 0.092 |
| Hb <110 g/L, n (%) | 385 (7.6) | 422 (21.9) | <0.001 |
| LVEF | |||
| LVEF ≤20, n (%) | 1033 (20.3) | 201 (10.4) | <0.001 |
| LVEF: 21 to 30, n (%) | 2695 (52.9) | 471 (24.4) | <0.001 |
| LVEF: >30, n (%) | 1208 (23.7) | 1079 (55.9) | <0.001 |
| Medications | |||
| β‐Adrenoreceptor antagonist, n (%) | 4388 (86.2) | 1616 (83.8) | 0.01 |
| ACEI, n (%) | 3614 (71.0) | 1273 (66.0) | <0.001 |
| ARB, n (%) | 904 (17.8) | 253 (13.1) | <0.001 |
| Spironolactone, n (%) | 1556 (30.6) | 287 (14.9) | <0.001 |
| Loop diuretics, n (%) | 3144 (61.8) | 753 (39.0) | <0.001 |
| Digoxin, n (%) | 1202 (23.6) | 238 (12.3) | <0.001 |
| Amiodarone, n (%) | 499 (9.8) | 696 (36.1) | <0.001 |
| Statin, n (%) | 3632 (71.3) | 1386 (71.9) | 0.673 |
| Aspirin, n (%) | 3012 (59.2) | 1376 (71.3) | <0.001 |
| Clopidogrel, n (%) | 915 (18.0) | 586 (30.4) | <0.001 |
Baseline characteristics of subjects. Patients with ICDs for primary prevention had lower LVEF, had more previous history of heart failure, higher prevalence of ICM and diabetes mellitus, and were more likely to have a cardiac resynchronization therapy defibrillator than the secondary prevention group and more frequently on heart failure medications. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction; NICM, nonischemic cardiomyopathy; NYHA, New York Heart Association.
Appropriate ICD Therapy
In the primary prevention group, 395 (8.4%) patients experienced appropriate ICD shock and 617 (12.1%) patients had appropriate ATP during follow‐up. In the secondary prevention group, 310 patients experienced appropriate ICD shock (16.1%) and 445 patients (23.1%) received ATP. The cumulative incidence of shock at 1, 2, 3, and 4 years of follow‐up was (primary prevention versus secondary prevention): 4.5% versus 11.3%; 6.3% versus 14.3%; 7.4% versus 15.7%; and 7.7% versus 16.0% (P<0.001 for all comparisons). The cumulative incidence of ATP at 1, 2, 3, and 4 years of follow‐up was (primary prevention versus secondary prevention): 7.5% versus 17.2%; 10.4% versus 20.9%; 11.7% versus 22.5%; and 12.1% versus 22.9% (P<0.001 for all comparisons).
The rate of first occurrence of appropriate ICD shock was 1.7 per 100 person‐years in the primary prevention group and 4.0 per 100 person‐years in the secondary prevention group (P<0.001) (Figure 1). On univariable analysis, patients in the primary prevention group who received an ICD shock were more likely male, had history of atrial fibrillation, had lower left ventricular ejection fraction, and were more frequently treated with loop diuretics and digoxin compared with those who did not receive an ICD shock (Table 2). On univariable analysis, patients in the secondary prevention group who experienced appropriate ICD shock were more likely to be male, had a history of peripheral vascular disease, chronic obstructive pulmonary disease, and had higher baseline hemoglobin levels (Table 3). In this group, the rate of amiodarone use at baseline was similar between patients with or without appropriate ICD shock.
Figure 1.
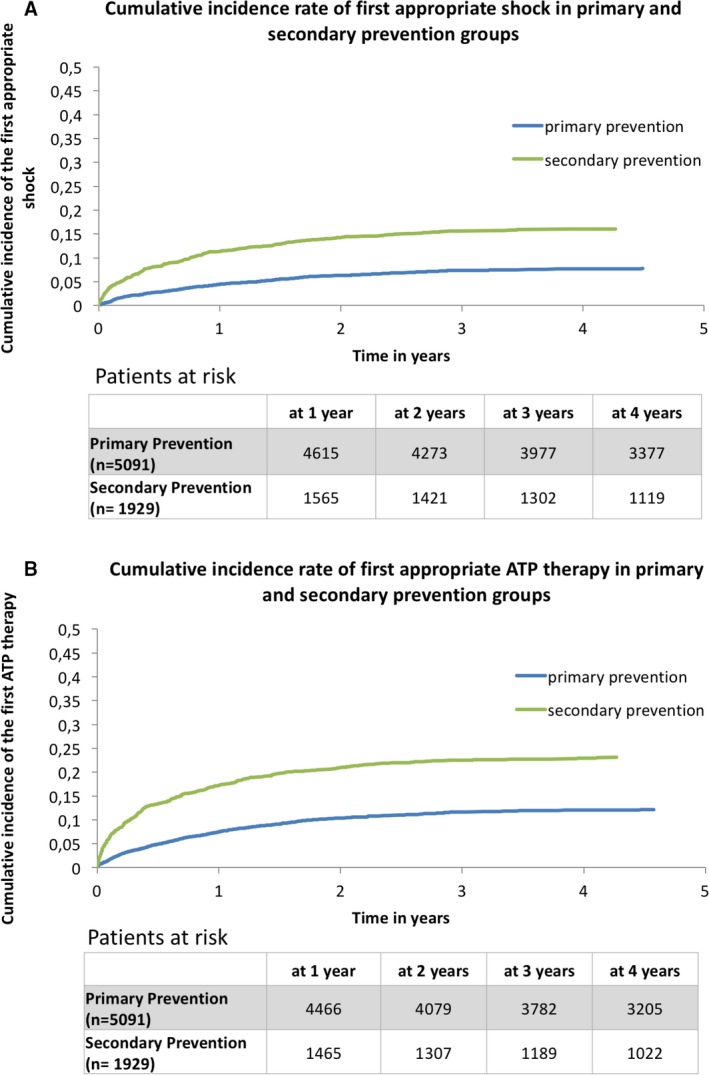
A, Cumulative incidence rate of first appropriate shock in primary and secondary prevention groups. B, Cumulative incidence rate of first ATP in primary and secondary prevention groups. ATP indicates anti‐tachycardia pacing.
Table 2.
Clinical Characteristics of Primary Prevention ICD Patients With and Without Appropriate Shock
| No Appropriate Shock | Appropriate Shock | P Value | |
|---|---|---|---|
| N=4696 | N=395 | ||
| Demographics | |||
| Male, n (%) | 3691 (78.6) | 346 (87.6) | <0.001 |
| Age, y, mean±SD | 64.59±11.93 | 63.61±12.01 | 0.118 |
| Cardiomyopathy details | |||
| Ischemic, n (%) | 4360 (92.8) | 378 (95.7) | … |
| Ischemic+previous revascularization, n (%) | 3066 (65.3) | 254 (64.3) | 0.044 |
| Previous heart failure, n (%) | 1726 (36.8) | 160 (40.5) | 0.138 |
| Device details | |||
| Cardiac resynchronizator‐defibrillator, n (%) | 1553 (33.1) | 125 (31.6) | 0.876 |
| Dual‐chamber ICD, n (%) | 1204 (25.6) | 107 (27.1) | … |
| Single‐chamber ICD, n (%) | 1937 (41.2) | 163 (41.3) | … |
| Medical comorbidities | |||
| Atrial fibrillation, n (%) | 1355 (28.9) | 151 (38.2) | <0.001 |
| Diabetes mellitus, n (%) | 1459 (31.1) | 112 (28.4) | 0.262 |
| Current cigarette smoking, n (%) | 683 (14.5) | 68 (17.2) | 0.151 |
| Hypertension | 2690 (57.3%) | 210 (53.2%) | 0.112 |
| Stroke or transient ischemic attack, n (%) | 169 (3.6) | 15 (3.8) | 0.839 |
| Peripheral vascular disease, n (%) | 479 (10.2) | 35 (8.9) | 0.396 |
| Any cancer, n (%) | 397 (8.5) | 35 (8.8) | 0.792 |
| COPD, n (%) | 590 (12.6) | 46 (11.6) | 0.596 |
| Clinical variables | |||
| Reported NYHA class | |||
| III or IV, n (%) | 1638 (34.9) | 140 (35.4) | |
| Systolic blood pressure, mean±SD | 121.62±19.90 | 119.64±19.98 | 0.058 |
| QRS duration in ms, mean±SD | 132.77±35.44 | 134.86±37.72 | 0.265 |
| Testing | |||
| Serum creatinine, median (IQR) | 96.00 (80.00–120.00) | 100.00 (84.00–122.00) | 0.017 |
| Hb <110 g/L, n (%) | 353 (7.5) | 32 (8.1) | 0.177 |
| LVEF | |||
| LVEF ≤20, n (%) | 935 (19.9) | 98 (24.8) | 0.02 |
| LVEF: 21 to 30, n (%) | 2484 (52.9) | 211 (53.4) | 0.842 |
| LVEF: >30, n (%) | 1133 (24.1) | 75 (19.0) | 0.021 |
| Medications | |||
| β‐Adrenoreceptor antagonist, n (%) | 4046 (86.2) | 342 (86.6) | 0.815 |
| ACEI, n (%) | 3318 (70.7) | 296 (74.9) | 0.072 |
| ARB, n (%) | 842 (17.9) | 62 (15.7) | 0.264 |
| Spironolactone, n (%) | 1420 (30.2) | 136 (34.4) | 0.082 |
| Loop diuretics, n (%) | 2875 (61.2) | 269 (68.1) | 0.007 |
| Digoxin, n (%) | 1075 (22.9) | 127 (32.2) | <0.001 |
| Amiodarone, n (%) | 458 (9.8) | 41 (10.4) | 0.687 |
| Statin, n (%) | 3359 (71.5) | 273 (69.1) | 0.308 |
| Aspirin, n (%) | 2798 (59.6) | 214 (54.2) | 0.036 |
| Clopidogrel, n (%) | 852 (18.1) | 63 (15.9) | 0.275 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Table 3.
Clinical Characteristics of Patients With Secondary Prevention Indication by Presence or Absence of Shock
| No Appropriate Shock | Appropriate Shock | P Value | |
|---|---|---|---|
| N=1619 | N=310 | ||
| Demographics | |||
| Male, n (%) | 1291 (79.7) | 269 (86.8) | 0.004 |
| Age, mean±SD | 65.40±12.69 | 65.82±12.00 | 0.589 |
| Cardiomyopathy details | |||
| Ischemic, n (%) | 1437 (88.8) | 275 (88.7) | … |
| Ischemic+previous revascularization, n (%) | 1163 (71.8) | 214 (69.0) | 0.491 |
| Previous heart failure, n (%) | 366 (22.6) | 73 (23.5) | 0.717 |
| Device details | |||
| Cardiac resynchronizator‐defibrillator, n (%) | 146 (9.0) | 32 (10.3) | 0.82 |
| Dual‐chamber ICD, n (%) | 700 (43.2) | 131 (42.3) | … |
| Single‐chamber ICD, n (%) | 771 (47.6) | 147 (47.4) | … |
| Medical comorbidities | |||
| Atrial fibrillation, n (%) | 510 (31.5) | 99 (31.9) | 0.88 |
| Diabetes mellitus, n (%) | 419 (25.9) | 53 (17.1) | <0.001 |
| Current cigarette smoking, n (%) | 271 (16.7) | 54 (17.4) | 0.769 |
| Hypertension, n (%) | 1018 (62.9) | 191 (61.6) | 0.673 |
| Stroke or transient ischemic attack, n (%) | 71 (4.4) | 16 (5.2) | 0.546 |
| Peripheral vascular disease, n (%) | 172 (10.6) | 47 (15.2) | 0.021 |
| Any cancer, n (%) | 133 (7.4) | 33 (9.4) | 0.195 |
| COPD, n (%) | 173 (10.7) | 46 (14.8) | 0.035 |
| Clinical variables | |||
| Reported NYHA class | |||
| III or IV, n (%) | 237 (14.6) | 46 (14.8) | … |
| Systolic blood pressure, mean±SD | 124.60±19.98 | 124.70±20.66 | 0.94 |
| QRS duration in ms, mean±SD | 121.91±33.63 | 125.37±30.59 | 0.095 |
| Testing | |||
| Serum creatinine level, median (IQR) | 95.00 (79.00–117.00) | 95.00 (81.00–117.00) | 0.763 |
| Hb <110 g/L, n (%) | 360 (22.2) | 62 (20.0) | 0.004 |
| LVEF | |||
| LVEF ≤20, n (%) | 162 (10.0) | 39 (12.6) | 0.174 |
| LVEF: 21 to 30, n (%) | 400 (24.7) | 71 (22.9) | 0.498 |
| LVEF: >30, n (%) | 913 (56.4) | 166 (53.5) | 0.355 |
| Medications | |||
| β‐Adrenoreceptor antagonist, n (%) | 1353 (83.6) | 263 (84.8) | 0.579 |
| ACEI, n (%) | 1059 (65.4) | 214 (69.0) | 0.218 |
| ARB, n (%) | 218 (13.5) | 35 (11.3) | 0.299 |
| Spironolactone, n (%) | 245 (15.1) | 42 (13.5) | 0.473 |
| Loop diuretics, n (%) | 631 (39.0) | 122 (39.4) | 0.9 |
| Digoxin, n (%) | 198 (12.2) | 40 (12.9) | 0.741 |
| Amiodarone, n (%) | 574 (35.5) | 122 (39.4) | 0.19 |
| Statin, n (%) | 1163 (71.8) | 223 (71.9) | 0.971 |
| Aspirin, n (%) | 1160 (71.6) | 216 (69.7) | 0.482 |
| Clopidogrel, n (%) | 494 (30.5) | 92 (29.7) | 0.77 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Mortality Rates
In the overall cohort, 2360 (33.6%) patients died. There were 1697 (33.3%) patients who died in the primary prevention group and 663 (34.4%) patients died in the secondary prevention group. The unadjusted incidence rate of death was 6.87 per 100‐person years in the primary prevention group and 7.31 per 100‐person years in the secondary prevention group (P=0.178) (Figure 2).
Figure 2.
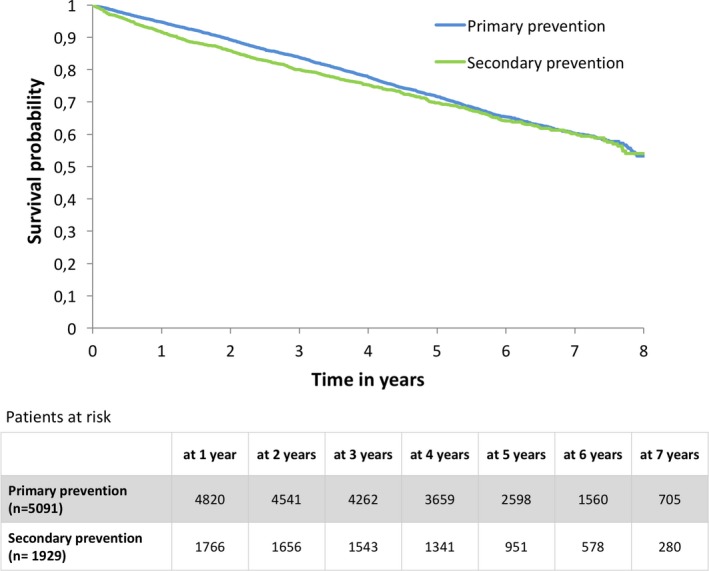
Kaplan–Meier survival curve showing occurrence of all‐cause mortality in primary vs secondary prevention groups.
Impact of Appropriate ICD Shock on Mortality
In the overall cohort, the crude mortality rate was higher among patients who received appropriate ICD shock when compared with those who did not (9.33 versus 6.72 deaths per 100‐person years, P<0.001). The crude mortality rate was higher among primary prevention ICD patients who experienced appropriate shock when compared with secondary prevention ICD patients who had appropriate ICD shock (13.02 versus 9.94 deaths per 100 person‐years, P=0.0187). The median post‐ICD shock follow‐up periods for the primary and secondary prevention groups were 4.16 and 4.48 years, respectively.
Impact of ATP Therapy on Mortality
Patients who experienced appropriate ATP had higher crude mortality rates when compared with those who did not (8.56 versus 6.69 deaths per 100‐person years, P<0.001). Survival rates were similar between primary and secondary prevention patients who experienced appropriate ICD shocks (10.58 versus 9.95 deaths per 100 person‐years, P=0.52).
Risk of Death After Appropriate ICD Shock or ATP
After an appropriate ICD shock, the unadjusted HR of death was 1.96 (95% CI 1.68–2.27, P<0.001) in the primary prevention group and 1.42 (95% CI 1.17–1.72, P<0.001) in the secondary prevention group. After appropriate ATP, the unadjusted HR of death was 1.60 (95% CI 1.40–1.82, P<0.001) in the primary prevention group and 1.48 (95% CI 1.25–1.75, P<0.001) in the secondary prevention group.
After adjustment of baseline differences with multivariable regression, patients who had an appropriate ICD shock had a 74% increase in their risk of death relative to those who did not have an ICD shock (HR 1.74, 95% CI 1.54–1.96, P<0.001) in the overall cohort. In the primary prevention group, patients who experienced appropriate ICD shock had a 2‐fold increase in their risk of death when compared with those who did not have appropriate ICD shock (adjusted HR 2.00, 95% CI 1.72–2.33, P<0.001) (Figure 3A). Patients who experienced appropriate ICD shock in the secondary prevention group had a 46% increase in their risk of death when compared with those who did not have ICD shock (adjusted HR 1.46, 95% CI 1.20–1.77, P<0.001) (Figure 3A). We did not observe a statistically significant interaction between implant indication (primary versus secondary) and occurrence of appropriate ICD shock on mortality (P=0.13). This suggested that the impact on mortality of an appropriate ICD shock did not differ based on the implant indication.
Figure 3.
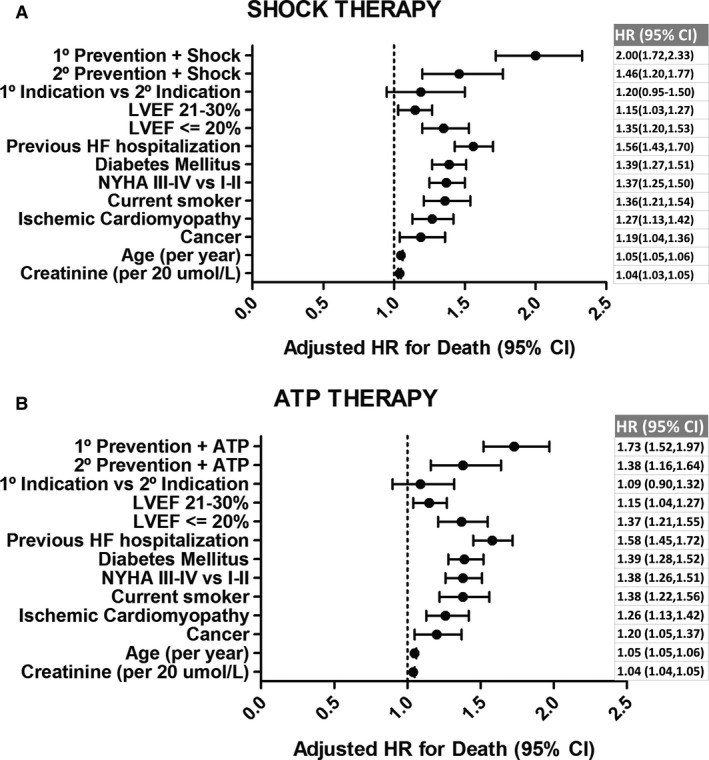
Forest plot with adjusted hazard ratios of death of variables included in the Cox model. A, HR of risk of death in patients with shock therapy vs no shock therapy. B, HR of risk of death in patients with antitachycardia therapy (ATP) therapy vs no ATP therapy. HF indicates heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
In the primary prevention group, patients who experienced appropriate ATP had a 73% increased risk of death when compared with those who did not have ATP (adjusted HR 1.73, 95% CI 1.52–1.97, P<0.001) (Figure 3B). In the secondary prevention group, patients who experienced appropriate ATP had a 38% increase in their risk of death when compared with those who did not have ATP (adjusted HR 1.38, 95% CI 1.16–1.64, P<0.001) (Figure 3B). We did not observe a statistically significant interaction between implant indication (primary versus secondary) and occurrence of appropriate ATP on mortality (P=0.4).
Discussion
There are 3 main findings from this prospective, population‐based registry of ≈7020 patients who underwent de novo ICD implantation in Ontario, Canada with long‐term follow‐up. First, we observed that the incidence of first appropriate shock or ATP was twice as common in patients with secondary prevention ICD relative to those with primary prevention ICD. Second, occurrence of either an appropriate ICD shock or ATP was associated with a substantial increase in patients’ subsequent risk of dying irrespective of implant indication. Third, despite having twice as much appropriate ICD therapies, patients in the secondary prevention group had similar survival rates as those with primary prevention ICDs.
Incidence of Appropriate ICD Therapy
The incidence of shocks was higher in the secondary prevention population compared with primary prevention patients, and this is in keeping with the findings of previous smaller registries.11, 12 It is indeed not surprising that patients who receive an ICD after symptomatic ventricular arrhythmia are at higher risk of experiencing an appropriate shock from their devices than those who receive it prophylactically. Our data suggest that treatment measures to avoid ICD therapies in the secondary prevention group are of paramount importance since both ATP and shocks carry a significant mortality risk in a population otherwise thought to exhibit a lower risk profile.
Mortality Analysis
The fact that the adjusted mortality of primary and secondary prevention patients after receiving a shock or ATP is similar could be interpreted as somewhat unexpected. One would expect that therapies in the primary prevention population reflect a much sicker myocardium since the baseline characteristics of patients showed a significantly higher risk profile. In contrast, patients with a secondary indication seem to exhibit a better clinical profile at the time of implant and for instance should not exhibit the same long‐term mortality. One possible hypothesis is that secondary prevention patients have higher rates of ventricular arrhythmia during follow‐up and that probably has an impact on their mortality. Other authors’ data have also shown similar findings.13
The negative impact on mortality of ventricular tachycardia or ventricular fibrillation needing shocks has been consistently seen in different primary prevention clinical trials.6, 7 Studies to define predictors of shocks and mortality have been done in primary prevention patients with aims to shift clinicians’ focus toward mitigating such risks.10, 14 Although it has been consistently demonstrated that secondary prevention patients have higher incidence of shocks, there is not a large amount of comparative data11, 12, 13, 15 with primary prevention patients’ outcomes. In that respect, only left ventricular ejection fraction <35% as a cut point as a predictor for secondary prevention has been highlighted in meta‐analysis of major secondary prevention trials.1 However, the largest secondary prevention trial—AVID16—randomized more than 1000 patients and in those patients, ICD shocks were not associated with increased mortality,17 although findings in that population may be because of important differences of baseline characteristics from subjects in our registry.
With regard to ATP therapies, recent data suggest increased mortality risk in patients who experience ATP therapies,18 and our findings also point in that direction. This is in contrast with previous data from subanalysis of MADIT‐RIT,19 where ATP had a neutral effect in mortality risk. Our population of patients is different from the MADIT‐RIT population in a few aspects: (1) It is larger; (2) It has a longer follow‐up period; (3) It includes primary and secondary prevention patients; and (4) It has a higher occurrence of primary outcome in patients treated with ATP. Comparability of both studies is arguable based on these facts. Our findings indicate that experiencing arrhythmia that merits treatment from the ICD is an independent marker of risk of death no matter how the device treats it.
Our study with a larger number of patients and a longer follow‐up in a real‐world setting provides clinicians caring for ICD patients information that could potentially benefit them if strategies could be developed to mitigate the increased risk.
Clinical Implications
The clinical implications of our study are as follows. The first implication is that prophylaxis of shocks and ATP by recommending a strategy that mitigates the likelihood of ventricular arrhythmia in patients with ICDs may have mortality implications. Two randomized trials have evaluated the effect of prophylactic ventricular tachycardia catheter ablation as an addition to ICD in the ischemic cardiomyopathy population.20, 21 Although the findings of those trials cannot be extrapolated to our population, one could hypothesize that decreasing the burden of shocks or delaying the appearance of ventricular arrhythmia could translate into a better survival. The second clinical implication of our study is that patients presenting with an appropriate shock or ATP whether they received an automatic implantable cardioverter/defibrillator for primary or secondary indication has mortality implications. It is a novel finding that there is increased mortality both in primary and secondary prevention even in patients with a less severe cardiomyopathy in terms of LV dysfunction but with greater probability of therapy in the secondary prevention group. Consideration for advanced heart failure therapies or antiarrhythmic drugs or ventricular tachycardia ablation should be taken and optimization of heart function treatment should be done accordingly.
Limitations
Programming strategies for primary prevention patients were not standardized in the implanting centers, but previous reports of our group have shown that the shocks and ATP incidence rates were similar to the contemporary studies with delayed detection therapy groups,22, 23 so an impact on mortality risk based on this is very unlikely. The score used to control for baseline differences was designed and validated on a sample derived from a primary prevention cohort and was extended to the secondary prevention sample in the analysis.10 Our analysis was focused on assessing outcome after a single ICD shock or ATP and did not evaluate multiple shocks or ATPs. We did not pursue analysis of repeated therapies because during the study follow‐up, changes in device programming may occur and were not standardized24, 25, 26 and so will antiarrhythmic drugs and utilization of catheter ablation. We believe that the first ICD therapy is the one with the highest impact in the management of patients and that analyzing further therapies includes several possible biases that would be extremely difficult to account for in the analysis of the data. Inappropriate ICD therapies and the specific type of mortality were not analyzed in our study and are beyond the scope of this prospective registry. This analysis is especially pertinent to the ischemic cardiomyopathy patients because nonischemic cardiomyopathy patients only comprised a very small minority of the group studied. The use of antiarrhythmic drugs and their possible impact on the mortality of patients who received shocks has not been analyzed in this study.
Conclusion
The implant indication of primary versus secondary prevention did not have a differential impact on mortality following ICD therapies irrespective of the worse baseline clinical profile in the primary prevention group. Though the ICD shock aborted sudden death in the secondary prevention patient who had malignant arrhythmia and had ICD implantation on this basis, this study highlights an unrecognized patient issue that suggests that event has mortality implications. The issue highlighted by this work is that, though the ICD therapy in the secondary prevention patient achieved its goal of aborting sudden death, the work of the clinician is not complete. Strategies should be implemented for caring for these patients even after a single appropriate ICD therapy, and the impact of such strategies on altering survival will require further characterization.
Sources of Funding
Dr Porta‐Sánchez's collaboration was partially funded via a ‘la Caixa’ Foundation grant, Barcelona (Spain). Dr Austin is supported in part by a Career Investigator Award from the Heart and Stroke Foundation of Canada (Ontario Office). Dr. Nanthakumar laboratory was supported by an operating grant (CIHR MOP 142272). Dr Lee is the Ted Rogers Chair in Heart Function Outcomes. This Ontario ICD Database was supported by an operating grant (CIHR MOP 111150) and a Foundation Grant (FDN 148446) from the Canadian Institutes of Health Research, and an operating grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). This study was supported by Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC).
Disclosures
None.
Acknowledgments
The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by Institute for Clinical Evaluative Sciences or the Ontario Ministry of Health and Long‐Term Care is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute of Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of CIHI.
(J Am Heart Assoc. 2017;6:e006220 DOI: 10.1161/JAHA.117.006220.)28862957
Contributor Information
Douglas S. Lee, Email: dlee@uhn.ca.
Kumaraswamy Nanthakumar, Email: kumar.nanthakumar@uhn.ca.
References
- 1. Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, Greene HL, Boczor S, Domanski M, Follmann D, Gent M, Roberts RS. Meta‐analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. [DOI] [PubMed] [Google Scholar]
- 2. Hohnloser SH, Israel CW. Current evidence base for use of the implantable cardioverter‐defibrillator. Circulation. 2013;128:172–183. [DOI] [PubMed] [Google Scholar]
- 3. Domanski MJ, Epstein A, Hallstrom AL, Saksena S, Zipes DP. Survival of antiarrhythmic or implantable cardioverter defibrillator treated patients with varying degrees of left ventricular dysfunction who survived malignant ventricular arrhythmias. J Cardiovasc Electrophysiol. 2002;13:580–583. [DOI] [PubMed] [Google Scholar]
- 4. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, Klein H, Andrews ML, Hall WJ, Moss AJ. Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: Frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. [DOI] [PubMed] [Google Scholar]
- 5. Pedersen SS, Van Den Broek KC, Van Den Berg M, Theuns DA. Shock as a determinant of poor patient‐centered outcomes in implantable cardioverter defibrillator patients: is there more to it than meets the eye? Pacing Clin Electrophysiol. 2010;33:1430–1436. [DOI] [PubMed] [Google Scholar]
- 6. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sweeney MO, Sherfesee L, DeGroot PJ, Wathen MS, Wilkoff BL. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter‐defibrillator patients. Heart Rhythm. 2010;7:353–360. [DOI] [PubMed] [Google Scholar]
- 8. Zaman S, Sivagangabalan G, Chik W, Stafford W, Hayes J, Denman R, Young G, Sanders P, Kovoor P. Ventricular tachyarrhythmia recurrence in primary versus secondary implantable cardioverter‐defibrillator patients and role of electrophysiology study. J Interv Card Electrophysiol. 2014;41:195–202. [DOI] [PubMed] [Google Scholar]
- 9. Lee DS, Birnie D, Cameron D, Crystal E, Dorian P, Gula LJ, Healey JS, Janmohammed A, Khaykin Y, Krahn AD, LeFeuvre C, Simpson CS, Yee R, Hardy J, Slaughter PM, Chen Z, Alter DA, Laupacis A, Tu JV. Design and implementation of a population‐based registry of Implantable Cardioverter Defibrillators (ICDs) in Ontario. Heart Rhythm. 2008;5:1250–1256. [DOI] [PubMed] [Google Scholar]
- 10. Lee DS, Hardy J, Yee R, Healey JS, Birnie D, Simpson CS, Crystal E, Mangat I, Nanthakumar K, Wang X, Krahn AD, Dorian P, Austin PC, Tu JV; Investigators of the Ontario ICDD . Clinical risk stratification for primary prevention implantable cardioverter defibrillators. Circ Heart Fail. 2015;8:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabbag A, Suleiman M, Laish‐Farkash A, Samania N, Kazatsker M, Goldenberg I, Glikson M, Beinart R. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real‐world setting: from the Israeli ICD Registry. Heart Rhythm. 2015;12:2426–2433. [DOI] [PubMed] [Google Scholar]
- 12. van Welsenes GH, van Rees JB, Borleffs CJ, Cannegieter SC, Bax JJ, van Erven L, Schalij MJ. Long‐term follow‐up of primary and secondary prevention implantable cardioverter defibrillator patients. Europace. 2011;13:389–394. [DOI] [PubMed] [Google Scholar]
- 13. Konstantino Y, Shafat T, Novack V, Novack L, Amit G. Incidence of implantable cardioverter defibrillator therapy and mortality in primary and secondary prevention of sudden cardiac death. Isr Med Assoc J. 2015;17:760–763. [PubMed] [Google Scholar]
- 14. Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, Alter DA, Laupacis A. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–2415. [DOI] [PubMed] [Google Scholar]
- 15. Manuchehry A, Agusala K, Montevecchi M, Kadish A, Passman R. Ventricular tachyarrhythmias in patients receiving an implantable cardioverter‐defibrillator for primary versus secondary prophylaxis indications. Pacing Clin Electrophysiol. 2011;34:571–576. [DOI] [PubMed] [Google Scholar]
- 16. A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. The antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–1583. [DOI] [PubMed] [Google Scholar]
- 17. Sony J, Apurva B, Ankit R, Palaniappan M, Mohammad K, Aditya B, Luis A. Prognostic importance of defibrillator shocks in survivors of sudden cardiac death. J Clin Exp Cardiolog. 2010;01:105 Doi:10.4172/2155‐9880.1000105. Available at https://www.omicsonline.org/prognostic‐importance‐of‐defibrillator‐shocks‐in‐survivors‐of‐sudden‐cardiac‐death‐2155‐9880.1000105.php?aid=453. Accessed July 5, 2017. [Google Scholar]
- 18. Sun S, Johnson J, Degroot P, Brown ML, Obel O. Effect of ICD therapies on mortality in the Omni trial. J Cardiovasc Electrophysiol. 2016;27:192–199. [DOI] [PubMed] [Google Scholar]
- 19. Ruwald AC, Schuger C, Moss AJ, Kutyifa V, Olshansky B, Greenberg H, Cannom DS, Estes NA, Ruwald MH, Huang DT, Klein H, McNitt S, Beck CA, Goldstein R, Brown MW, Kautzner J, Shoda M, Wilber D, Zareba W, Daubert JP. Mortality reduction in relation to implantable cardioverter defibrillator programming in the multicenter automatic defibrillator implantation trial‐reduce inappropriate therapy (MADIT‐RIT). Circ Arrhythm Electrophysiol. 2014;7:785–792. [DOI] [PubMed] [Google Scholar]
- 20. Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz E, Pitschner HF, Kautzner J, Schumacher B, Hansen PS; Group Vs . Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. [DOI] [PubMed] [Google Scholar]
- 21. Kuck K‐H, Tilz RR, Deneke T, Hoffmann BA, Ventura R, Hansen PS, Zarse M, Hohnloser SH, Kautzner J, Willems S. Impact of substrate modification by catheter ablation on implantable cardioverter–defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease. Results From the Multicenter Randomized Controlled SMS (Substrate Modification Study). Circ: Arrhythm Electrophysiol. 2017;10:e004422 https://doi.org/10.1161/CIRCEP.116.004422 [DOI] [PubMed] [Google Scholar]
- 22. Yung D, Birnie D, Dorian P, Healey JS, Simpson CS, Crystal E, Krahn AD, Khaykin Y, Cameron D, Chen Z, Lee DS. Survival after implantable cardioverter‐defibrillator implantation in the elderly. Circulation. 2013;127:2383–2392. [DOI] [PubMed] [Google Scholar]
- 23. Yung D, Birnie D, Dorian P, Healey JS, Simpson CS, Crystal E, Krahn AD, Cameron D, Lee DS. Response to letter regarding article, “survival after implantable cardioverter‐defibrillator implantation in the elderly”. Circulation. 2014;129:e337–e338. [DOI] [PubMed] [Google Scholar]
- 24. Howlett JG, Chan M, Ezekowitz JA, Harkness K, Heckman GA, Kouz S, Leblanc MH, Moe GW, O'Meara E, Abrams H, Ducharme A, Grzeslo A, Hamilton PG, Koshman SL, Lepage S, McDonald M, McKelvie R, Rajda M, Swiggum E, Virani S, Zieroth S. The Canadian Cardiovascular Society heart failure companion: bridging guidelines to your practice. Can J Cardiol. 2016;32:296–310. [DOI] [PubMed] [Google Scholar]
- 25. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al‐Khatib SM, Almendral J, Aguinaga L, Berger RD, Cuesta A, Daubert JP, Dubner S, Ellenbogen KA, Estes NA III, Fenelon G, Garcia FC, Gasparini M, Haines DE, Healey JS, Hurtwitz JL, Keegan R, Kolb C, Kuck KH, Marinskis G, Martinelli M, McGuire M, Molina LG, Okumura K, Proclemer A, Russo AM, Singh JP, Swerdlow CD, Teo WS, Uribe W, Viskin S, Wang CC, Zhang S. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter‐defibrillator programming and testing. J Arrhythm. 2016;32:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. [DOI] [PubMed] [Google Scholar]


