Abstract
Background
Left ventricular (LV) hypertrophy and subclinical cerebrovascular disease are early manifestations of cardiac and brain target organ damage caused by hypertension. This study aimed to investigate whether intensive office systolic blood pressure (SBP) control has beneficial effects on LV morphology and function and subclinical cerebrovascular disease in elderly patients with hypertension.
Methods and Results
We examined 420 patients treated for hypertension without history of heart failure and stroke from the CABL (Cardiovascular Abnormalities and Brain Lesions) study. All patients underwent 2‐dimensional echocardiographic examination and brain magnetic resonance imaging. Subclinical cerebrovascular disease was defined as silent brain infarcts and white matter hyperintensity volume. Patients were divided into 3 groups: SBP <120 mm Hg (intensive control); SBP 120 to 139 mm Hg (less intensive control); and SBP ≥140 mm Hg (uncontrolled). Prevalence of LV hypertrophy and diastolic dysfunction were lowest in the intensive control, intermediate in the less intensive control, and highest in the uncontrolled groups (12.8%, 31.8%, and 44.7%, respectively [P<0.001], for LV hypertrophy; 46.8%, 61.7%, and 72.6%, respectively [P=0.003], for diastolic dysfunction). Patients with less intensive SBP control had greater risk of LV hypertrophy than those with intensive control (adjusted odds ratio, 3.26; P=0.013). A similar trend was observed for LV diastolic dysfunction but did not reach statistical significance (adjusted odds ratio, 1.65; P=0.144). Conversely, intensive SBP control was not significantly associated with reduced risk of silent brain infarcts and white matter hyperintensity volume compared with less intensive control.
Conclusions
Compared with less intensive control, intensive SBP control may have a stronger beneficial effect on cardiac than cerebral subclinical disease.
Keywords: blood pressure, hypertension, left ventricular diastolic dysfunction, left ventricular hypertrophy, silent brain infarction
Subject Categories: Hypertension, Echocardiography, Magnetic Resonance Imaging (MRI), Cardiovascular Disease, Cerebrovascular Disease/Stroke
Clinical Perspective
What is New?
Among elderly patients with treated hypertension, ≈10% patients met the goal of intensive systolic blood pressure control and 50% met the goal of less intensive systolic blood pressure control.
Intensive systolic blood pressure control had a stronger beneficial effect on left ventricular hypertrophy, and possibly diastolic function, than on subclinical cerebrovascular disease in elderly patients with hypertension.
What are the Clinical Implications?
Intensive office systolic blood pressure control should be considered in elderly patients with hypertension, especially those at higher risk for the development of heart failure.
Introduction
Hypertension is the most common cardiovascular disorder, and its prevalence increases with age, rising steeply after the age of 50, when it affects over 50% of individuals.1 Systolic blood pressure (SBP) is a stronger predictor of adverse cardiovascular outcomes than diastolic blood pressure.2, 3 Long‐standing hypertension serves as a stimulus for the development of left ventricular (LV) hypertrophy (LVH)4, 5 and diastolic dysfunction,6, 7 which may be the earliest manifestations of cardiac target organ damage and likely represent important intermediate steps in the development of heart failure (HF),8, 9, 10, 11, 12, 13 particularly in elderly patients with hypertension. Hypertension is also strongly associated with subclinical cerebrovascular disease, which is associated with an increased risk of subsequent stroke.14, 15 Silent brain infarcts (SBIs) and white matter hyperintensities, both manifestations of subclinical cerebrovascular disease, are commonly seen on brain magnetic resonance imaging of elderly adults,16 especially those with hypertension. However, it is unclear whether the degree of office SBP control is associated with changes in LV morphology and function and with the frequency of subclinical cerebrovascular disease. The SPRINT (Systolic Blood Pressure Intervention Trial) Research Group recently showed that, among elderly patients with hypertension, an office SBP target <120 mm Hg rather than <140 mm Hg reduced major cardiovascular events by 25%, and the beneficial effect of intensive SBP control was mainly attributable to decreased HF development.17 The present study aimed to investigate whether intensive office SBP control is associated with lower LV mass, better LV diastolic function, and less subclinical cerebrovascular disease in elderly patients with hypertension without a history of HF and stroke.
Methods
Study Population
The study population was derived from the CABL (Cardiovascular Abnormalities and Brain Lesions) study, which was designed to assess the cardiovascular predictors of subclinical cerebrovascular disease in a community‐based cohort including participants older than 50 years. the CABL study based its recruitment on the NOMAS (Northern Manhattan Study), an epidemiological study performed in New York City. Extensive details about the population and enrollment of NOMAS have been previously published.18 This study cohort consisted of 420 patients with hypertension treated with at least 1 antihypertensive medication who underwent both 2‐dimensional echocardiographic examination and brain magnetic resonance imaging within 3 months of each other. According to SBP values, patients were divided into 3 groups: an intensive control group (SBP <120 mm Hg), a less intensive control group (SBP between 120 and 139 mm Hg), and an uncontrolled group (SBP ≥140 mm Hg). Written informed consent was obtained from all study participants. The study was approved by the institutional review board of Columbia University Medical Center.
Risk Factor and Office Blood Pressure Assessment
Cardiovascular risk factors were ascertained through direct examination and interviews conducted by trained research assistants. Among the variables used in the analysis, diabetes mellitus was defined by current use of insulin or hypoglycemic agents or a fasting glucose of ≥126 mg/dL tested on ≥2 occasions in each participant. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL or the use of lipid‐lowering medications. Body mass index was calculated using height and weight (kg/m2), and obesity was defined as body mass index ≥30 kg/m2.
Office SBP control status was determined on the basis of the mean value of 2 separate blood pressure (BP) measurements performed within 3 months of each other (mean interval, 3.4 days). At each visit, SBP and diastolic blood pressure were measured on the nondominant arm with the patient in a sitting position after 5 minutes of rest, using a sphygmomanometer calibrated against a reference mercury sphygmomanometer and with an arm cuff of appropriate size. BP was recorded twice with a 5‐minute interval, and the average of the 2 recordings was used.
Two‐Dimensional Echocardiographic Examination
Echocardiographic examination was performed using a commercially available system (iE 33, Philips) by a trained, registered cardiac sonographer blinded to the patient's clinical information according to a standardized protocol. The dimensions of the cardiac chambers were measured in the standard manner.19 LV ejection fraction (LVEF) was obtained using the Simpson's method from apical 4‐ and 2‐chamber views.19 LV mass was calculated with a validated method20 and indexed for body surface area. LVH was defined as greater than the 90th percentile of the participants of the CABL study without conditions associated with LV remodeling such as hypertension, diabetes mellitus, obesity, coronary artery disease, and atrial fibrillation (LV mass index cutoff for LVH: 111.74 g/m2 for women and 126.53 g/m2 for men).
LV diastolic function assessment has been previously described.21, 22 Briefly, transmitral diastolic flow was obtained from an apical 4‐chamber view. Pulsed‐wave Doppler examination of mitral inflow was performed to measure early (E) and late peak velocity (A), and their ratio (E/A) was calculated. Peak early diastolic mitral annular velocity (e′) was also measured from tissue Doppler imaging in the lateral and septal mitral annulus and the average value was used. Diastolic dysfunction was graded as E/A ≤0.7 (impaired relaxation, grade 1); E/A >0.7 and ≤1.5 and e′ <7 cm/s (pseudonormalized pattern, grade 2); or E/A >1.5 and e′ <7 cm/s (restrictive pattern, grade 3).21, 22
Image Acquisition and Interpretation of Brain Magnetic Resonance Imaging
A detailed description of the assessment of subclinical cerebrovascular lesions has been previously published.23, 24 In brief, brain imaging was performed on a 1.5‐T magnetic resonance imaging system (Philips Medical Systems). SBIs were defined as either a cavitation on the fluid‐attenuated inversion recovery sequence of at least 3 mm, distinct from a vessel (owing to the lack of signal void on T2 sequence), and of equal intensity to cerebrospinal fluid in the case of lacunar infarction, or as a wedge‐shaped cortical or cerebellar area of encephalomalacia with surrounding gliosis consistent with infarction attributable to distal arterial branch occlusion. Interobserver agreement for SBI detection was 93.3%.24 White matter hyperintensity volume analysis was based on a fluid‐attenuated inversion recovery image and performed using the Quantum 6.2 package on a Sun Microsystems Ultra 5 workstation. White matter hyperintensity volume was expressed as proportion of total cranial volume to correct for differences in head size and log‐transformed white matter hyperintensity volume (log‐WMHV) to achieve a normal distribution for analysis as a continuous variable. The upper quartile of log‐WMHV was used as the dependent variable in the categorical analyses. All measurements were performed blinded to participant clinical information.
Statistical Analysis
Categorical variables are presented as frequencies and continuous variables as means±SD. For categorical variables, comparison among groups was performed by chi‐square test. For continuous variables, pairwise comparisons between 2 groups were assessed by 2‐sample t test, and overall differences among 3 groups were examined by 1‐way ANOVA. Univariate and multivariate logistic regression models were used to assess the impact of office SBP control on LV morphology and function and subclinical cerebrovascular disease, and corresponding odds ratios along with their 95% CIs were reported. The covariates included in the multivariate logistic regression model were age, sex, diabetes mellitus, history of coronary artery disease, atrial fibrillation, obesity, number of antihypertensive medications, and LVEF A value of P<0.05 was considered significant. Statistical analyses were performed using SAS 9.3 software (SAS Institute).
Results
Patient Characteristics
Patient characteristics are shown in Table 1. Of the 420 patients with hypertension, 47 (11.2%) met intensive SBP control goal, 214 (51.0%) met the less intensive SBP goal, and 159 (37.9%) had uncontrolled SBP. There was a significant difference in age among the 3 groups, whereas no significant difference was observed in traditional cardiovascular risk factors and history of coronary artery disease and atrial fibrillation.
Table 1.
Comparison of Clinical Characteristics and Echocardiographic Parameters According to Degree of SBP Control
| SBP <120 mm Hg (n=47) | SBP 120–139 mm Hg (n=214) | SBP ≥140 mm Hg (n=159) | P Value | |
|---|---|---|---|---|
| Age, y | 68.0±9.1a | 70.6±9.1a | 73.9±9.0 | <0.001 |
| Male sex, No., % | 15 (31.9) | 79 (36.9) | 47 (29.6) | 0.320 |
| Diabetes mellitus, No., % | 15 (31.9) | 78 (36.5) | 60 (37.7) | 0.767 |
| Hypercholesterolemia, No., % | 35 (74.5) | 149 (70.0) | 105 (66.0) | 0.513 |
| History of CAD, No., % | 4 (8.5) | 18 (8.4) | 8 (5.0) | 0.423 |
| Atrial fibrillation, No., % | 6 (12.8) | 14 (6.5) | 9 (5.7) | 0.230 |
| SBP, mm Hg | 113.6±5.4a, b | 129.9±5.6a | 152.7±12.3 | <0.001 |
| DBP, mm Hg | 69.7±7.0a, b | 73.6±7.8a | 78.9±10.1 | <0.001 |
| More than 1 antihypertensive medication, No., % | 12 (25.5)a | 76 (35.5)a | 77 (48.4) | 0.005 |
| ACEI/ARB (n=381) | 19/42 (45.2) | 77/194 (39.7) | 66/145 (45.5) | 0.523 |
| β‐Blocker (n=389) | 20/43 (46.5) | 74/197 (37.6) | 59/149 (39.6) | 0.551 |
| Calcium channel blocker (n=383) | 8/42 (19.1) | 80/195 (41.0) | 74/146 (50.7) | 0.001 |
| Diuretics (n=374) | 7/41 (17.1) | 53/188 (28.2) | 52/145 (35.9) | 0.052 |
| Body mass index, kg/m2 | 28.3±4.9 | 28.7±4.7 | 28.8±5.1 | 0.791 |
| Obesity, No., % | 14 (29.8) | 77 (36.0) | 57 (35.9) | 0.708 |
| Race/ethnicity, No. (%) | 0.531 | |||
| Black | 3 (6.4) | 35 (16.4) | 24 (15.1) | |
| White | 3 (6.4) | 18 (8.4) | 17 (10.7) | |
| Hispanic | 40 (85.1) | 159 (74.3) | 117 (73.6) | |
| Other | 1 (2.1) | 2 (0.9) | 1 (0.6) | |
| LV structure and function | ||||
| LV end‐diastolic diameter, mm | 44.4±4.4 | 45.1±5.0 | 45.6±5.0 | 0.354 |
| LV end‐systolic diameter, mm | 27.7±4.5 | 28.2±5.1 | 28.8±5.3 | 0.297 |
| LV ejection fraction, % | 62.5±6.0 | 63.6±7.2 | 63.9±7.6 | 0.507 |
| Relative wall thickness | 0.50±0.07 | 0.51±0.10 | 0.52±0.10 | 0.469 |
| E wave, cm/s | 68.1±17.3 | 69.7±17.2 | 72.2±18.8 | 0.250 |
| E/A ratio | 0.84±0.24 | 0.82±0.37 | 0.79±0.35 | 0.594 |
| e′, cm/s | 7.8±1.8a, b | 7.1±1.7a | 6.4±1.5 | <0.001 |
| E/e′ ratio | 9.1±2.9a | 10.2±3.1a | 11.7±3.3 | <0.001 |
Values are mean±SD or number (percentage). A indicates late diastolic transmitral flow velocity; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; DBP, diastolic blood pressure; E, early diastolic transmitral flow velocity; e′, early diastolic mitral annular velocity; LV, left ventricular.
P<0.05 vs systolic blood pressure (SBP) ≥140 mm Hg.
P<0.05 vs SBP 120 to 139 mm Hg.
Association of SBP Control With LV Morphology and Function
LV end‐systolic and end‐diastolic diameters and ejection fraction did not differ among the 3 groups (also Table 1). Frequencies of LVH and diastolic dysfunction were lowest in patients who achieved the intensive SBP goal, intermediate in those with the less intensive SBP control, and highest in the uncontrolled group (12.8% versus 31.8% versus 44.7% [P<0.001] for LVH, and 46.8% versus 61.7% versus 72.6% [P=0.003] for diastolic dysfunction) (Figure 1A and 1B). There was a significant difference in e′ between intensive and less intensive control (7.8±1.8 versus 7.1±1.7 cm/s, P=0.031; Table 1). After adjustment for age, sex, diabetes mellitus, history of coronary artery disease, atrial fibrillation, obesity, number of antihypertensive medications, and LVEF, patients with less intensive control had significantly greater risks of LVH than those with intensive SBP control (odds ratio, 3.26; 95% CI, 1.28–8.28 [P=0.013]) (Table 2). A similar trend was observed in LV diastolic dysfunction but did not reach statistical significance (odds ratio, 1.65; 95% CI, 0.84–3.24 [P=0.144]). A significant nonlinear trend was observed among the 3 SBP control groups for LVH even after multivariate adjustment (P=0.026). On the other hand, there was a linear trend for LV diastolic dysfunction in univariate analysis, but it did not reach statistical significance in multivariate analysis (P=0.060).
Figure 1.
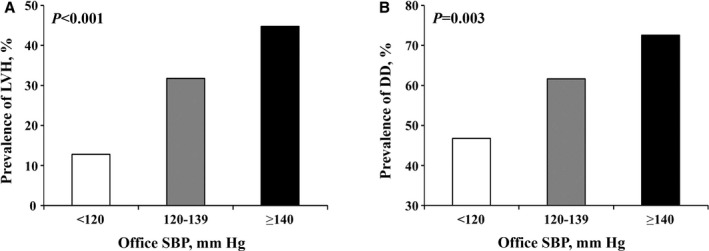
Prevalence of left ventricular hypertrophy (LVH) (A) and diastolic dysfunction (DD) (B) according to office systolic blood pressure (SBP) control.
Table 2.
Association of Office SBP Control With LV Hypertrophy and Diastolic Dysfunction
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| SBP 120–139 mm Hg | SBP ≥140 mm Hg | SBP 120–139 mm Hg | SBP ≥140 mm Hg | |||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| LV hypertrophy | 3.18 (1.29–7.85) | 0.012 | 5.51 (2.21–13.7) | <0.001 | 3.26 (1.28–8.28) | 0.013 | 5.48 (2.11–14.3) | <0.001 |
| Diastolic dysfunction | 1.83 (0.97–3.45) | 0.063 | 3.01 (1.54–5.90) | 0.001 | 1.65 (0.84–3.24) | 0.144 | 2.31 (1.12–4.78) | 0.024 |
Reference: systolic blood pressure (SBP) <120 mm Hg. Multivariate adjusted for age, sex, diabetes mellitus, history of coronary artery disease, atrial fibrillation, obesity, number of antihypertensive medications, and left ventricular (LV) ejection fraction. OR indicates odds ratio.
Association of SBP Control With Subclinical Cerebrovascular Disease
Presence of SBI was detected in 79 patients (18.8%). Mean log‐WMHV was −0.80±0.97 (median −0.96, minimum −5.88, maximum 1.74). The prevalence of subclinical cerebrovascular disease did not differ significantly among the intensive, less intensive, and uncontrolled SBP groups, although a nonsignificant trend was present (12.8% versus 18.7% versus 20.8% [P=0.468] for SBI; 17.0% versus 22.5% versus 30.8% [P=0.076] for the upper quartile of log‐WMHV) (Figure 2A and 2B). Multivariate analysis showed that intensive SBP control was not associated with reduced risk of SBI and white matter hyperintensity volume compared with less intensive control (Table 3). There was no significant linear trend among the 3 SBP control groups for SBI (P=0.167), whereas a significant linear trend was observed for the upper quartile of log‐WMHV (P=0.036) in the multivariate analysis.
Figure 2.
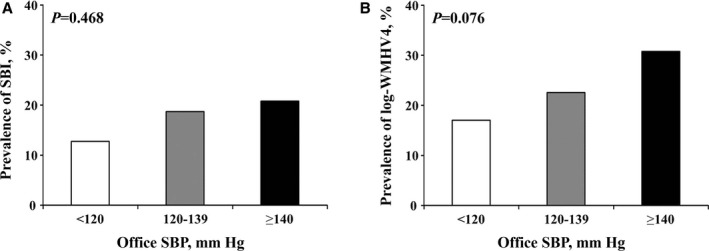
Prevalence of silent brain infarcts (SBIs) (A) and upper quartile of log‐white matter hyperintensity volume (WMHV4) (B) according to office systolic blood pressure (SBP) control.
Table 3.
Association of Office SBP Control With SBI and the Upper Quartile of Log‐WMHV
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| SBP 120–139 mm Hg | SBP ≥140 mm Hg | SBP 120–139 mm Hg | SBP ≥140 mm Hg | |||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| SBI | 1.57 (0.62–3.95) | 0.338 | 1.79 (0.70–4.58) | 0.224 | 1.60 (0.60–4.26) | 0.343 | 1.67 (0.61–4.60) | 0.320 |
| Upper quartile of log‐WMHV | 1.42 (0.62–3.24) | 0.407 | 2.17 (0.95–4.99) | 0.068 | 1.13 (0.47–2.71) | 0.788 | 1.37 (0.56–3.36) | 0.487 |
Reference: systolic blood pressure (SBP) <120 mm Hg. Multivariate adjusted for age, sex, diabetes mellitus, history of coronary artery disease, atrial fibrillation, obesity, number of antihypertensive medications, and left ventricular (LV) ejection fraction. OR indicates odds ratio; SBI, silent brain infarct; WMHV, white matter hyperintensity volume.
Discussion
Our study demonstrates for the first time that intensive SBP control has a stronger association with lower frequency of LVH and, partially, better LV diastolic function than with subclinical cerebrovascular disease in elderly patients with hypertension without a history of HF and stroke.
Office SBP Control and LV Morphology and Function
LVH is an established risk factor for HF8, 9 and a component of the Framingham Heart Failure Risk Score.10 The relationship between hypertension and increased LV mass is well established, as is the notion that controlling BP values with medical treatment may result in regression of LVH.25, 26 However, it is still unknown whether achievement of an intensive SBP goal would further lower the risk of LVH. In the present study, we demonstrated that less intensive SBP control was significantly associated with the risk of LVH compared with intensive SBP control in elderly patients with hypertension, independently of traditional cardiovascular risk factors. Indeed, Soliman et al demonstrated that targeting an SBP of <120 mm Hg produced a greater reduction in LVH evaluated by 12‐lead ECG in 4331 patients with hypertension and diabetes mellitus when compared with <140 mm Hg.27 LV diastolic dysfunction reflects an impairment of the filling properties of the left ventricle, which has been shown to be a predictor of future development of HF11, 12, 13 and is often associated with increased LV mass. Hypertension is strongly associated with diastolic dysfunction6, 7 and is the most prevalent comorbidity in patients with HF, especially in those with HF with preserved LVEF.28 However, the relationship between office SBP control level and LV diastolic function has not been extensively evaluated in elderly patients with hypertension, who are especially prone to developing HF. We demonstrate that significantly lower tissue Doppler e′ (an index of LV diastolic function) was observed in patients with less intensive SBP control than those with intensive control. In addition, less than half of patients in the intensive SBP control group had diastolic dysfunction, compared with nearly two thirds in the less intensive control group, although the difference was not statistically significant after multivariate adjustment. These results are consistent with those of SPRINT, which showed that an office SBP target <120 mm Hg rather than <140 mm Hg significantly reduced the incidence of HF among high‐risk hypertension patients aged at least 50 years.17 Our results on LVH and diastolic dysfunction also provide a potential mechanism for the lower incidence of HF development that was observed in patients with SBP <120 mm Hg in SPRINT.
We observed no significant association between degree of SBP control and LV systolic function. In our study, LVEF was normal in the majority of the cohort, with only 19 participants having LV systolic dysfunction (LVEF <50%). Although a trend was observed toward a greater frequency of reduced LVEF in the uncontrolled group (5.0% versus 4.7% in the less intensive versus 2.1% in the intensive control groups, P=0.694), the overall low frequency of impaired LVEF may have precluded the possibility to detect significant differences.
Office SBP Control and Subclinical Cerebrovascular Disease
SBIs and white matter hyperintensities are important subclinical cerebral abnormalities because of their associations with increased risk of stroke.14, 15 A recent meta‐analysis including 13 studies (14 764 patients) with a mean follow‐up ranging from 25.7 to 174 months confirmed that SBI predicted the occurrence of stroke with a relative risk of 2.94 (95% CI, 2.24–3.86; P<0.001).29 In addition, in a subgroup analysis pooling 9483 stroke‐free individuals from large population‐based studies, SBI remained a strong predictor of future stroke (hazard ratio, 2.06; 95% CI, 1.64–2.59 [P<0.01]). However, no study to date has evaluated the relationship between degree of office SBP control and subclinical cerebrovascular disease, despite hypertension being an established and important risk factor for subclinical cerebrovascular disease.30 In our study, we did not observe a significant association between degree of BP control and frequency of SBI. However, the power of the study was not adequate to conclusively address this issue. With this observed SBI frequency (6 of 47 patients with intensive SBP control, 40 of 214 patients with less‐intensive control), the minimum detectable odds ratio was 3.07 (for the less‐intensive versus the intensive control group, with 80% power at 0.05 significance level), considerably larger than the observed 1.57. Therefore, we cannot conclude that intensive SBP control has no beneficial effects on subclinical cerebrovascular disease, although the relationship of SBP control with subclinical brain disease appears to be less strong than that observed with cardiac parameters in our analyses. In addition, although the duration of hypertension may have had an impact on the observed results, this aspect cannot be addressed in our study because the information was not uniformly available. An association between duration of hypertension and subclinical cerebrovascular disease has been reported31 but not confirmed in a recent study.32 Longitudinal studies are needed to better evaluate this association.
Conflicting results have been reported in the literature regarding the effect of SBP control degree and incidence of stroke. A meta‐analysis of 11 trials with 42 572 participants showed that the relative risk of stroke for those with SBP <130 mm Hg was 0.80 (95% CI, 0.70–0.92) compared with those with SBP ≥130 mm Hg.33 In the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial, which enrolled patients with diabetes mellitus at high cardiovascular risk, more intensive SBP control (SBP <120 mm Hg) compared with standard control (<140 mm Hg) also led to a significant reduction in risk of stroke (HR, 0.59; 95% CI, 0.39–0.89).34 On the other hand, the LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial reported that achieving intensive BP control (<130 mm Hg) was not associated with a reduction in stroke in 9193 patients with hypertension and LVH.35 Furthermore, SPRINT recently reported that intensive SBP control did not reduce the incidence of stroke in elderly patients with hypertension.17 The discordance of these results may be at least partly dependent on differences in patient characteristics and comorbidities among the studies. As such, the current guidelines of the American Heart Association/American Stroke Association concluded that the target for BP reduction may differ by patient characteristics and comorbidities and recommend that patients with hypertension be treated with antihypertensive drugs to a target BP of <140/90 mm Hg for stroke prevention.36
A class effect of antihypertensive medications on LVH and stroke has been reported. Angiotensin‐converting enzyme inhibitors and calcium channel blockers had a similar effect on LV mass reduction,25, 26 and their effect was stronger compared with β‐blocking treatment.37 However, elevation of serum aldosterone level observed in patients receiving long‐term treatment with angiotensin blockade is strongly associated with increased risk of LVH through the profibrotic actions of aldosterone.38 As for stroke prevention, calcium channel blockers appear to have a slightly greater effect on reducing the risk of stroke compared with β‐blockers and angiotensin‐converting enzyme inhibitors.39 A study found that β‐blockers were less effective in reducing stroke risk than angiotensin blockade.40 However, because of sample size limitations in our analyses, we cannot address the class effect of antihypertensive medication on our results. Future studies are needed to elucidate the different class effect of antihypertensive medication on subclinical cardiac and brain organ damage.
Study Limitations
Our study has limitations. The study sample included patients with hypertension older than 50 years who had multiple cardiovascular risk factors, which might preclude the generalization of our findings to populations with different demographic compositions. However, because diastolic HF and stroke are common in the elderly population with hypertension, our study cohort was an ideal setting to explore this topic. Because of the cross‐sectional design of our study, we cannot establish a cause‐effect relationship between the degree of SBP control and subclinical cardiac and brain disease. In addition, the impact of duration of hypertension and antihypertensive treatment on the results could not be assessed, because the information was not uniformly available in our study. However, our observations on the relationship of office SBP control level with LV morphology and function and subclinical cerebrovascular disease in “real‐world” elderly patients with hypertension may provide useful information for treatment strategies for these patients.
Conclusions
In elderly patients with hypertension, intensive SBP control appears to have a stronger beneficial effect on LVH and possibly LV diastolic function than on subclinical cerebrovascular disease.
Sources of Funding
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (R01NS36286 to Di Tullio and R37NS29993 to Sacco/Elkind).
Disclosures
None.
Acknowledgments
The authors wish to thank Rosa (NOMAS project manager), Russo (measurement of diastolic function parameters), and Liu (collection of echocardiographic data).
(J Am Heart Assoc. 2017;6:e006246 DOI: 10.1161/JAHA.117.006246.)28757483
References
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol. 1971;27:335–346. [DOI] [PubMed] [Google Scholar]
- 3. Sagie A, Larson MG, Levy D. The natural history of borderline isolated systolic hypertension. N Engl J Med. 1993;329:1912–1917. [DOI] [PubMed] [Google Scholar]
- 4. Frohlich ED. The heart in hypertension: unresolved conceptual challenges. Special lecture. Hypertension. 1988;11:I19–I24. [DOI] [PubMed] [Google Scholar]
- 5. Weber KT. Cardioreparation in hypertensive heart disease. Hypertension. 2001;38:588–591. [DOI] [PubMed] [Google Scholar]
- 6. Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bountioukos M, Schinkel AF, Bax JJ, Lampropoulos S, Poldermans D. The impact of hypertension on systolic and diastolic left ventricular function. A tissue Doppler echocardiographic study. Am Heart J. 2006;151:e1327–1312. [DOI] [PubMed] [Google Scholar]
- 8. Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure. A clinical mechanistic overview. Arch Intern Med. 1996;156:1789–1796. [PubMed] [Google Scholar]
- 9. Wilson PW. An epidemiologic perspective of systemic hypertension, ischemic heart disease, and heart failure. Am J Cardiol. 1997;80:3J–8J. [DOI] [PubMed] [Google Scholar]
- 10. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. [DOI] [PubMed] [Google Scholar]
- 11. Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2001;37:1042–1048. [DOI] [PubMed] [Google Scholar]
- 12. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. [DOI] [PubMed] [Google Scholar]
- 13. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 14. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 15. Kuller LH, Longstreth WT Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 16. Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. [DOI] [PubMed] [Google Scholar]
- 17. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden‐Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study). J Am Coll Cardiol. 2009;54:2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:e14. [DOI] [PubMed] [Google Scholar]
- 20. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. [DOI] [PubMed] [Google Scholar]
- 21. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of diabetes and hypertension on left ventricular diastolic function in a high‐risk population without evidence of heart disease. Eur J Heart Fail. 2010;12:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a Community‐Based Study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36:1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willey JZ, Moon YP, Paik MC, Yoshita M, Decarli C, Sacco RL, Elkind MS, Wright CB. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology. 2011;76:2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Devereux RB, Palmieri V, Sharpe N, De Quattro V, Bella JN, de Simone G, Walker JF, Hahn RT, Dahlof B. Effects of once‐daily angiotensin‐converting enzyme inhibition and calcium channel blockade‐based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (preserve) trial. Circulation. 2001;104:1248–1254. [DOI] [PubMed] [Google Scholar]
- 26. Terpstra WF, May JF, Smit AJ, de Graeff PA, Havinga TK, van den Veur E, Schuurman FH, Meyboom‐de Jong B, Crijns HJ. Long‐term effects of amlodipine and lisinopril on left ventricular mass and diastolic function in elderly, previously untreated hypertensive patients: the ELVERA trial. J Hypertens. 2001;19:303–309. [DOI] [PubMed] [Google Scholar]
- 27. Soliman EZ, Byington RP, Bigger JT, Evans G, Okin PM, Goff DC Jr, Chen H. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with diabetes mellitus: action to control cardiovascular risk in diabetes blood pressure trial. Hypertension. 2015;66:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I‐PRESERVE trial. Eur J Heart Fail. 2008;10:149–156. [DOI] [PubMed] [Google Scholar]
- 29. Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, Wright C, Beiser AS, Seshadri S, Pandya A, Kamel H. Silent brain infarction and risk of future stroke: a systematic review and meta‐analysis. Stroke. 2016;47:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population‐based cohorts. BMC Med. 2014;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27:2262–2270. [DOI] [PubMed] [Google Scholar]
- 32. McEvoy LK, Fennema‐Notestine C, Eyler LT, Franz CE, Hagler DJ Jr, Lyons MJ, Panizzon MS, Rinker DA, Dale AM, Kremen WS. Hypertension‐related alterations in white matter microstructure detectable in middle age. Hypertension. 2015;66:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee M, Saver JL, Hong KS, Hao Q, Ovbiagele B. Does achieving an intensive versus usual blood pressure level prevent stroke? Ann Neurol. 2012;71:133–140. [DOI] [PubMed] [Google Scholar]
- 34. Barzilay JI, Howard AG, Evans GW, Fleg JL, Cohen RM, Booth GL, Kimel AR, Pedley CF, Cushman WC. Intensive blood pressure treatment does not improve cardiovascular outcomes in centrally obese hypertensive individuals with diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial. Diabetes Care. 2012;35:1401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okin PM, Hille DA, Kjeldsen SE, Dahlof B, Devereux RB. Impact of lower achieved blood pressure on outcomes in hypertensive patients. J Hypertens. 2012;30:802–810. [DOI] [PubMed] [Google Scholar]
- 36. Meschia JF, Bushnell C, Boden‐Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–1462. [DOI] [PubMed] [Google Scholar]
- 38. Struthers AD. The clinical implications of aldosterone escape in congestive heart failure. Eur J Heart Fail. 2004;6:539–545. [DOI] [PubMed] [Google Scholar]
- 39. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta‐blockers for hypertension. Cochrane Database Syst Rev. 2012;11:CD002003. [DOI] [PubMed] [Google Scholar]


